Acral Melanoma: A Review of Its Pathogenesis, Progression, and Management
Abstract
1. Introduction
2. Clinical Features and Epidemiology
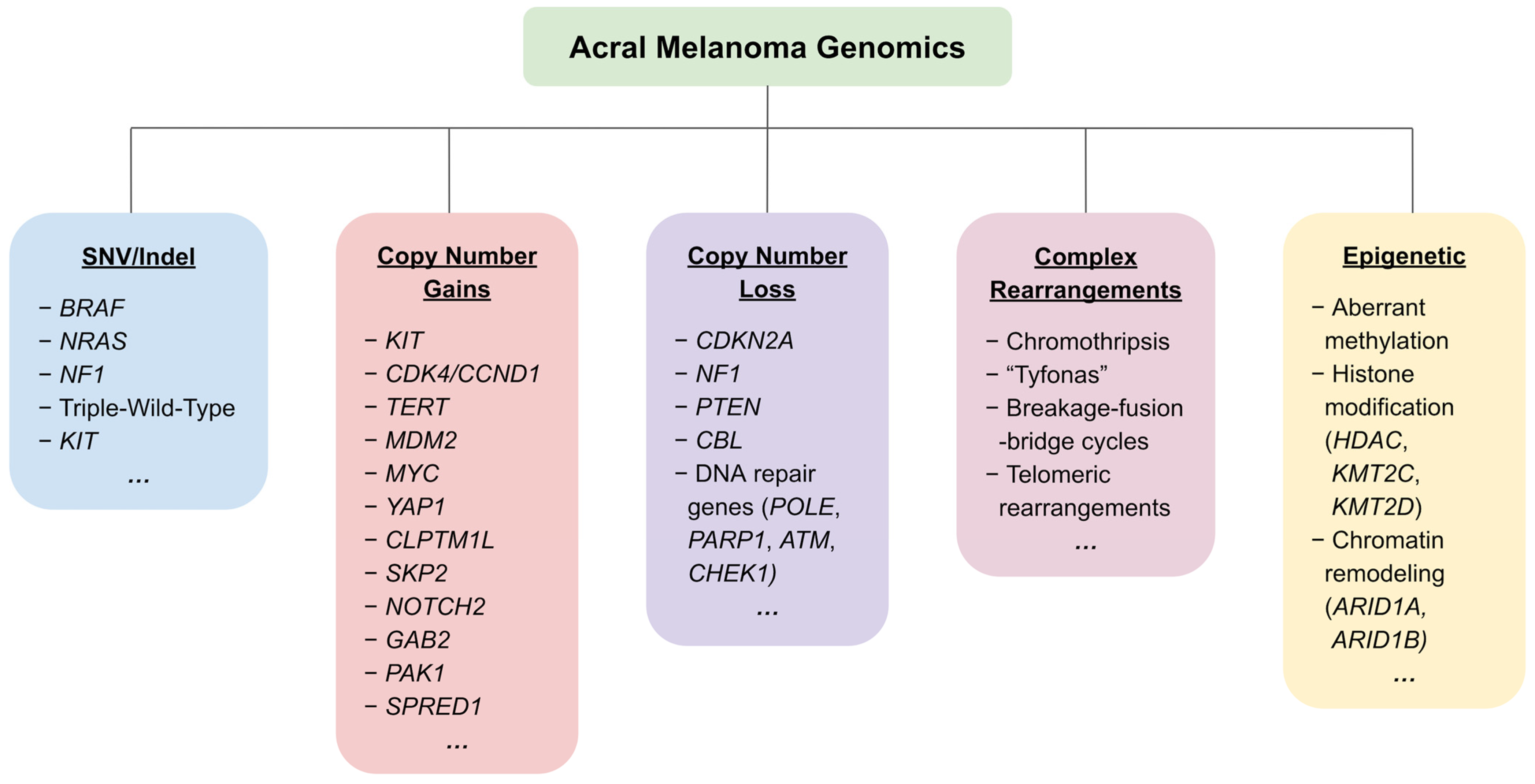
3. Genetic and Genomic Aberrations in Acral Melanoma
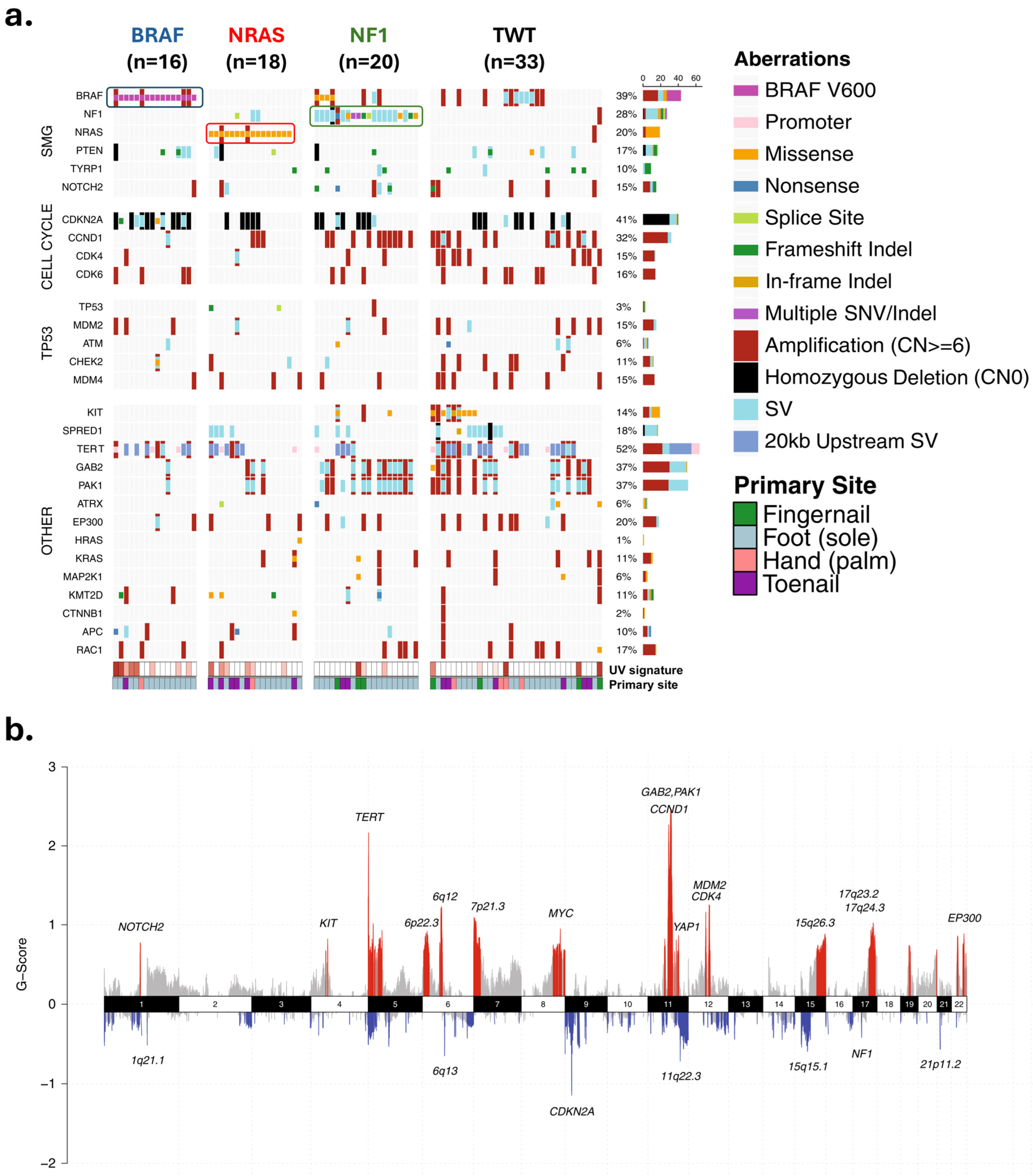
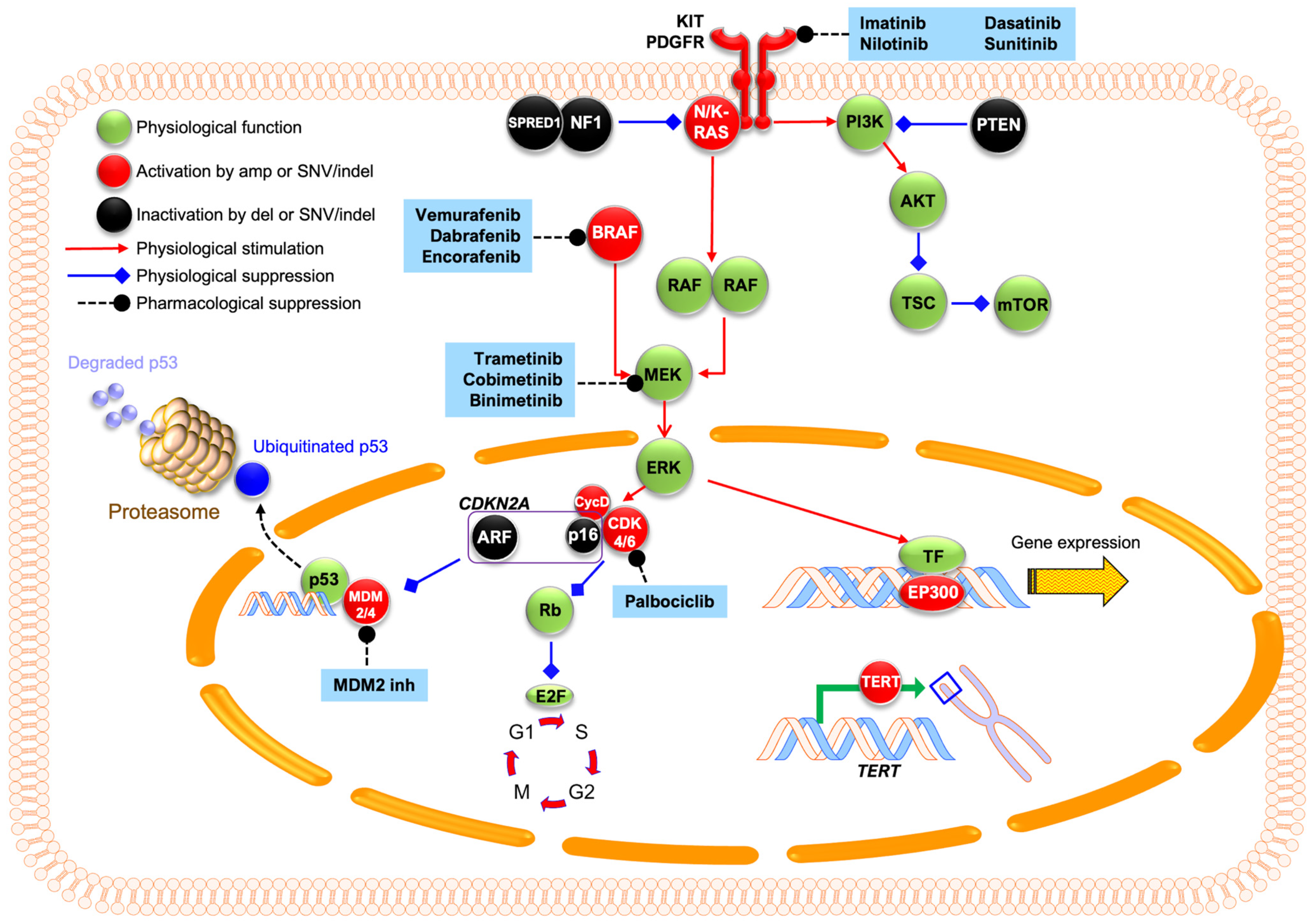
3.1. Single Nucleotide Variants and Indels in the MAPK Pathway
3.2. Structural Variants/Gene Amplification
3.2.1. Cell Cycle Regulation
3.2.2. TERT: Copy Number Increases and Promoter Mutations
3.2.3. MDM2 Amplification and p53 Alterations
3.3. Other Recent Genetic Findings
3.4. Markers for Metastasis and Prognosis
3.5. Anatomical Specificity
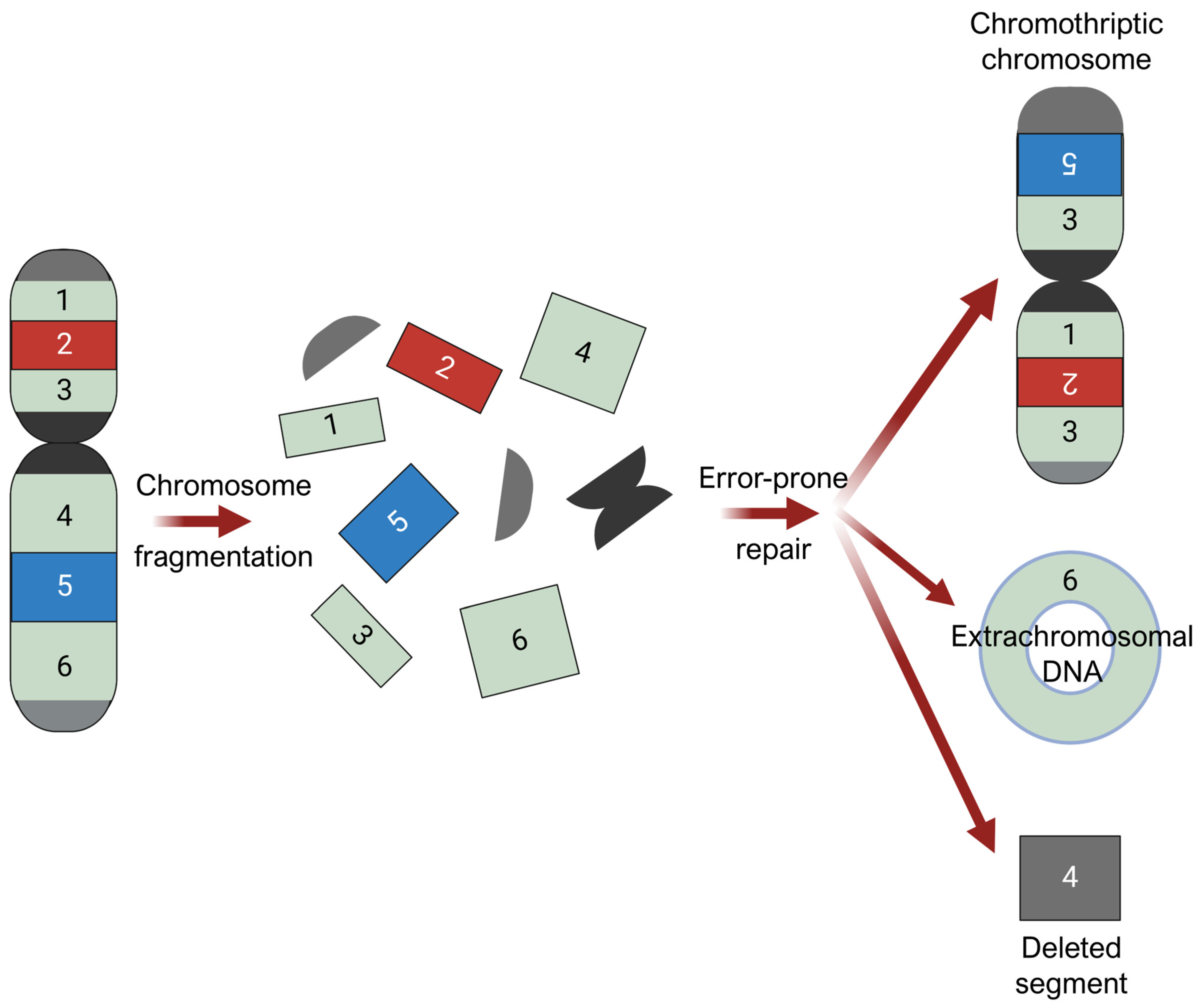
3.6. Mechanical Stress and Chromothripsis
4. Current Therapies and Clinical Implications
4.1. Immune Checkpoint Blockade
4.2. Adjuvant Therapies
4.3. Molecular Therapies
4.3.1. c-Kit Inhibitors
4.3.2. BRAF/MEK Inhibitors
4.3.3. CDK4/6 Inhibitors
4.4. Clinical Implications
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.A.; Teer, J.K.; Eroglu, Z.; Wu, J.-Y.; Koomen, J.M.; Karreth, F.A.; Messina, J.L.; Smalley, K.S.M. Translational Pathology, Genomics and the Development of Systemic Therapies for Acral Melanoma. Semin. Cancer Biol. 2020, 61, 149–157. [Google Scholar] [CrossRef]
- Basurto-Lozada, P.; Molina-Aguilar, C.; Castaneda-Garcia, C.; Vázquez-Cruz, M.E.; Garcia-Salinas, O.I.; Álvarez-Cano, A.; Martínez-Said, H.; Roldán-Marín, R.; Adams, D.J.; Possik, P.A.; et al. Acral Lentiginous Melanoma: Basic Facts, Biological Characteristics and Research Perspectives of an Understudied Disease. Pigment. Cell Melanoma Res. 2021, 34, 59–71. [Google Scholar] [CrossRef]
- Park, H.S.; Cho, K.H. Acral Lentiginous Melanoma in Situ: A Diagnostic and Management Challenge. Cancers 2010, 2, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.P.; Loria, P.R.; Reed, R.J.; Krementz, E.T. Acral Lentiginous Melanoma. Arch. Dermatol. 1980, 116, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Haugh, A.M.; Zhang, B.; Quan, V.L.; Garfield, E.M.; Bubley, J.A.; Kudalkar, E.; Verzi, A.E.; Walton, K.; VandenBoom, T.; Merkel, E.A.; et al. Distinct Patterns of Acral Melanoma Based on Site and Relative Sun Exposure. J. Investig. Dermatol. 2018, 138, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Dai, B.; Kong, Y.; Shen, X.; Kong, J. Acral Melanoma in Chinese: A Clinicopathological and Prognostic Study of 142 Cases. Sci. Rep. 2016, 6, 31432. [Google Scholar] [CrossRef] [PubMed]
- Druskovich, C.; Kelley, J.; Aubrey, J.; Palladino, L.; Wright, G.P. A Review of Melanoma Subtypes: Genetic and Treatment Considerations. J. Surg. Oncol. 2024. [Google Scholar] [CrossRef]
- Huang, K.; Fan, J.; Misra, S. Acral Lentiginous Melanoma: Incidence and Survival in the United States, 2006–2015, an Analysis of the SEER Registry. J. Surg. Res. 2020, 251, 329–339. [Google Scholar] [CrossRef]
- Chang, J.W.-C. Acral Melanoma: A Unique Disease in Asia. JAMA Dermatol. 2013, 149, 1272–1273. [Google Scholar] [CrossRef]
- Dugan, M.M.; Perez, M.C.; Karapetyan, L.; Zager, J.S. Management of Acral Lentiginous Melanoma: Current Updates and Future Directions. Front. Oncol. 2024, 14, 1323933. [Google Scholar] [CrossRef]
- Asgari, M.M.; Shen, L.; Sokil, M.M.; Yeh, I.; Jorgenson, E. Prognostic Factors and Survival in Acral Lentiginous Melanoma. Br. J. Dermatol. 2017, 177, 428–435. [Google Scholar] [CrossRef]
- Alicea, G.M.; Rebecca, V.W. Un-Fair Skin: Racial Disparities in Acral Melanoma Research. Nat. Rev. Cancer 2022, 22, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, A.; Murali, R.; Jansen, P.; Möller, I.; Sucker, A.; Paschen, A.; Zimmer, L.; Livingstone, E.; Brinker, T.J.; Hadaschik, E.; et al. Clinical and Genetic Analysis of Melanomas Arising in Acral Sites. Eur. J. Cancer 2019, 119, 66–76. [Google Scholar] [CrossRef]
- Tod, B.M.; Schneider, J.W.; Bowcock, A.M.; Visser, W.I.; Kotze, M.J. The Tumor Genetics of Acral Melanoma: What Should a Dermatologist Know? JAAD Int. 2020, 1, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.A.; Fridlyand, J.; Kageshita, T.; Patel, H.N.; Busam, K.J.; Kutzner, H.; Cho, K.-H.; Aiba, S.; Bröcker, E.-B.; LeBoit, P.E.; et al. Distinct Sets of Genetic Alterations in Melanoma. N. Engl. J. Med. 2005, 353, 2135–2147. [Google Scholar] [CrossRef]
- Farshidfar, F.; Rhrissorrakrai, K.; Levovitz, C.; Peng, C.; Knight, J.; Bacchiocchi, A.; Su, J.; Yin, M.; Sznol, M.; Ariyan, S.; et al. Integrative Molecular and Clinical Profiling of Acral Melanoma Links Focal Amplification of 22q11.21 to Metastasis. Nat. Commun. 2022, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Newell, F.; Johansson, P.A.; Wilmott, J.S.; Nones, K.; Lakis, V.; Pritchard, A.L.; Lo, S.N.; Rawson, R.V.; Kazakoff, S.H.; Colebatch, A.J.; et al. Comparative Genomics Provides Etiologic and Biological Insight into Melanoma Subtypes. Cancer Discov. 2022, 12, 2856–2879. [Google Scholar] [CrossRef]
- Rawson, R.V.; Johansson, P.A.; Hayward, N.K.; Waddell, N.; Patch, A.-M.; Lo, S.; Pearson, J.V.; Thompson, J.F.; Mann, G.J.; Scolyer, R.A.; et al. Unexpected UVR and Non-UVR Mutation Burden in Some Acral and Cutaneous Melanomas. Lab. Investig. 2017, 97, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Newell, F.; Wilmott, J.S.; Johansson, P.A.; Nones, K.; Addala, V.; Mukhopadhyay, P.; Broit, N.; Amato, C.M.; Van Gulick, R.; Kazakoff, S.H.; et al. Whole-Genome Sequencing of Acral Melanoma Reveals Genomic Complexity and Diversity. Nat. Commun. 2020, 11, 5259. [Google Scholar] [CrossRef]
- Broit, N.; Johansson, P.A.; Rodgers, C.B.; Walpole, S.T.; Hayward, N.K.; Pritchard, A.L. Systematic Review and Meta-analysis of Genomic Alterations in Acral Melanoma. Pigment Cell Melanoma Res. 2022, 35, 369. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.S.; Hendricks, W.; Kiefer, J.; Schmidt, J.; Sekar, S.; Carpten, J.; Craig, D.W.; Adkins, J.; Cuyugan, L.; Manojlovic, Z.; et al. Integrated Genomic Analyses Reveal Frequent TERT Aberrations in Acral Melanoma. Genome Res. 2017, 27, 524. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-Genome Landscapes of Major Melanoma Subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Akbani, R.; Akdemir, K.C.; Aksoy, B.A.; Albert, M.; Ally, A.; Amin, S.B.; Arachchi, H.; Arora, A.; Auman, J.T.; Ayala, B.; et al. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.T.C.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; et al. Mechanism of Activation of the RAF-ERK Signaling Pathway by Oncogenic Mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, I.V.; Gibney, G.T.; Smalley, K.S.M. NRAS Mutant Melanoma: Biological Behavior and Future Strategies for Therapeutic Management. Oncogene 2012, 32, 3009. [Google Scholar] [CrossRef]
- Kiuru, M.; Busam, K.J. The NF1 Gene in Tumor Syndromes and Melanoma. Lab. Investig. J. Tech. Methods Pathol. 2017, 97, 146. [Google Scholar] [CrossRef]
- Rabbie, R.; Ferguson, P.; Molina-Aguilar, C.; Adams, D.J.; Robles-Espinoza, C.D. Melanoma Subtypes: Genomic Profiles, Prognostic Molecular Markers and Therapeutic Possibilities. J. Pathol. 2019, 247, 539. [Google Scholar] [CrossRef]
- Shtivelman, E.; Davies, M.A.; Hwu, P.; Yang, J.; Lotem, M.; Oren, M.; Flaherty, K.T.; Fisher, D.E. Pathways and Therapeutic Targets in Melanoma. Oncotarget 2014, 5, 1701–1752. [Google Scholar] [CrossRef]
- Grichnik, J.M.; Burch, J.A.; Burchette, J.; Shea, C.R. The SCF/KIT Pathway Plays a Critical Role in the Control of Normal Human Melanocyte Homeostasis. J. Investig. Dermatol. 1998, 111, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.D.M.; Guhan, S.; Tsao, H. KIT and Melanoma: Biological Insights and Clinical Implications. Yonsei Med. J. 2020, 61, 562–571. [Google Scholar] [CrossRef]
- Steeb, T.; Wessely, A.; Petzold, A.; Kohl, C.; Erdmann, M.; Berking, C.; Heppt, M.V. C-Kit Inhibitors for Unresectable or Metastatic Mucosal, Acral or Chronically Sun-Damaged Melanoma: A Systematic Review and One-Arm Meta-Analysis. Eur. J. Cancer 2021, 157, 348–357. [Google Scholar] [CrossRef]
- Garrido, M.C.; Bastian, B.C. KIT as a Therapeutic Target in Melanoma. J. Investig. Dermatol. 2010, 130, 20–27. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef]
- Torres-Cabala, C.A.; Wang, W.-L.; Trent, J.; Yang, D.; Chen, S.; Galbincea, J.; Kim, K.B.; Woodman, S.; Davies, M.; Plaza, J.A.; et al. Correlation between KIT Expression and KIT Mutation in Melanoma: A Study of 173 Cases with Emphasis on the Acral-Lentiginous/Mucosal Type. Mod. Pathol. 2009, 22, 1446–1456. [Google Scholar] [CrossRef]
- Woodman, S.E.; Davies, M.A. Targeting KIT in Melanoma: A Paradigm of Molecular Medicine and Targeted Therapeutics. Biochem. Pharmacol. 2010, 80, 568. [Google Scholar] [CrossRef]
- Yun, J.; Lee, J.; Jang, J.; Lee, E.J.; Jang, K.T.; Kim, J.H.; Kim, K.-M. KIT Amplification and Gene Mutations in Acral/Mucosal Melanoma in Korea. APMIS 2011, 119, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F., III; Azar, S.; et al. KIT Gene Mutations and Copy Number in Melanoma Subtypes. Clin. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef]
- Ashida, A.; Takata, M.; Murata, H.; Kido, K.; Saida, T. Pathological Activation of KIT in Metastatic Tumors of Acral and Mucosal Melanomas. Int. J. Cancer 2009, 124, 862–868. [Google Scholar] [CrossRef]
- Alexeev, V.; Yoon, K. Distinctive Role of the cKit Receptor Tyrosine Kinase Signaling in Mammalian Melanocytes. J. Investig. Dermatol. 2006, 126, 1102–1110. [Google Scholar] [CrossRef]
- Merkel, E.A.; Gerami, P. Malignant Melanoma of Sun-Protected Sites: A Review of Clinical, Histological, and Molecular Features. Lab. Investig. 2017, 97, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Posch, C.; Moslehi, H.; Sanlorenzo, M.; Green, G.; Vujic, I.; Panzer-Grümayer, R.; Rappersberger, K.; Ortiz-Urda, S. Pharmacological Inhibitors of C-KIT Block Mutant c-KIT Mediated Migration of Melanocytes and Melanoma Cells in Vitro and in Vivo. Oncotarget 2016, 7, 45916. [Google Scholar] [CrossRef]
- Bastian, B.C.; Kashani-Sabet, M.; Hamm, H.; Godfrey, T.; Moore, D.H.; Bröcker, E.B.; LeBoit, P.E.; Pinkel, D. Gene Amplifications Characterize Acral Melanoma and Permit the Detection of Occult Tumor Cells in the Surrounding Skin. Cancer Res. 2000, 60, 1968–1973. [Google Scholar] [PubMed]
- Furney, S.J.; Turajlic, S.; Stamp, G.; Thomas, J.M.; Hayes, A.; Strauss, D.; Gavrielides, M.; Xing, W.; Gore, M.; Larkin, J.; et al. The Mutational Burden of Acral Melanoma Revealed by Whole-Genome Sequencing and Comparative Analysis. Pigment Cell Melanoma Res. 2014, 27, 835–838. [Google Scholar] [CrossRef]
- Wang, M.; Banik, I.; Shain, A.H.; Yeh, I.; Bastian, B.C. Integrated Genomic Analyses of Acral and Mucosal Melanomas Nominate Novel Driver Genes. Genome Med. 2022, 14, 65. [Google Scholar] [CrossRef]
- Garutti, M.; Targato, G.; Buriolla, S.; Palmero, L.; Minisini, A.M.; Puglisi, F. CDK4/6 Inhibitors in Melanoma: A Comprehensive Review. Cells 2021, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Marine, J.-C.; Lozano, G. Mdm2-Mediated Ubiquitylation: P53 and Beyond. Cell Death Differ. 2010, 17, 93–102. [Google Scholar] [CrossRef]
- McConnell, B.B.; Gregory, F.J.; Stott, F.J.; Hara, E.; Peters, G. Induced Expression of p16INK4a Inhibits Both CDK4- and CDK2-Associated Kinase Activity by Reassortment of Cyclin-CDK-Inhibitor Complexes. Mol. Cell. Biol. 1999, 19, 1981. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Jorgenson, E.; Shen, L.; Xu, M.; North, J.P.; Shain, A.H.; Reuss, D.; Wu, H.; Robinson, W.A.; Olshen, A.; et al. Targeted Genomic Profiling of Acral Melanoma. JNCI J. Natl. Cancer Inst. 2019, 111, 1068. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Sheng, X.; Wu, X.; Yan, J.; Ma, M.; Yu, J.; Si, L.; Chi, Z.; Cui, C.; Dai, J.; et al. Frequent Genetic Aberrations in the CDK4 Pathway in Acral Melanoma Indicate the Potential for CDK4/6 Inhibitors in Targeted Therapy. Clin. Cancer Res. 2017, 23, 6946–6957. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Bryan, T.M.; Reddel, R.R. Increased Copy Number of the TERT and TERC Telomerase Subunit Genes in Cancer Cells. Cancer Sci. 2008, 99, 1092–1099. [Google Scholar] [CrossRef]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly Recurrent TERT Promoter Mutations in Human Melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Zhang, L.; Ma, L.; Jiang, K.; Yao, G.; Zhu, L. TERT Promoter Mutations and Telomerase in Melanoma. J. Oncol. 2022, 2022, 6300329. [Google Scholar] [CrossRef]
- de Lima Vazquez, V.; Vicente, A.L.; Carloni, A.; Berardinelli, G.; Soares, P.; Scapulatempo, C.; Martinho, O.; Reis, R.M. Molecular Profiling, Including TERT Promoter Mutations, of Acral Lentiginous Melanomas. Melanoma Res. 2016, 26, 93. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fukushima, S.; Sheen, Y.-S.; Ramelyte, E.; Cruz-Pacheco, N.; Shi, C.; Liu, S.; Banik, I.; Aquino, J.D.; Sangueza Acosta, M.; et al. The Genetic Evolution of Acral Melanoma. Nat. Commun. 2024, 15, 6146. [Google Scholar] [CrossRef] [PubMed]
- Elefanti, L.; Zamuner, C.; Del Fiore, P.; Stagni, C.; Pellegrini, S.; Dall’Olmo, L.; Fabozzi, A.; Senetta, R.; Ribero, S.; Salmaso, R.; et al. The Molecular Landscape of Primary Acral Melanoma: A Multicenter Study of the Italian Melanoma Intergroup (IMI). Int. J. Mol. Sci. 2021, 22, 3826. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef]
- Forschner, A.; Hilke, F.-J.; Bonzheim, I.; Gschwind, A.; Demidov, G.; Amaral, T.; Ossowski, S.; Riess, O.; Schroeder, C.; Martus, P.; et al. MDM2, MDM4 and EGFR Amplifications and Hyperprogression in Metastatic Acral and Mucosal Melanoma. Cancers 2020, 12, 540. [Google Scholar] [CrossRef]
- Garraway, L.A.; Widlund, H.R.; Rubin, M.A.; Getz, G.; Berger, A.J.; Ramaswamy, S.; Beroukhim, R.; Milner, D.A.; Granter, S.R.; Du, J.; et al. Integrative Genomic Analyses Identify MITF as a Lineage Survival Oncogene Amplified in Malignant Melanoma. Nature 2005, 436, 117–122. [Google Scholar] [CrossRef]
- Hartman, M.L.; Czyz, M. MITF in Melanoma: Mechanisms behind Its Expression and Activity. Cell. Mol. Life Sci. 2014, 72, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Sun, W.; Shen, K.; Zhong, J.; Liu, W.; Gao, Z.; Xu, Y.; Wang, L.; Hu, T.; Ren, M.; et al. Delineating the Early Dissemination Mechanisms of Acral Melanoma by Integrating Single-Cell and Spatial Transcriptomic Analyses. Nat. Commun. 2023, 14, 8119. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.S.; Tan, K.T.; Tse, K.P.; Liao, Y.H.; Lin, M.H.; Chen, J.S.; Liau, J.Y.; Tseng, Y.J.; Lee, C.H.; Hong, C.H.; et al. Genetic Alterations in Primary Melanoma in Taiwan. Br. J. Dermatol. 2020, 182, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yu, J.; Wu, X.; Xu, T.; Yu, H.; Dai, J.; Ma, M.; Tang, H.; Xu, L.; Chi, Z.; et al. Increased AURKA Gene Copy Number Correlates with Poor Prognosis and Predicts the Efficacy of High-Dose Interferon Therapy in Acral Melanoma. J. Cancer 2018, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xu, T.; Dai, J.; Ma, M.; Tang, H.; Chi, Z.; Si, L.; Cui, C.; Sheng, X.; Kong, Y.; et al. TERT Copy Gain Predicts the Outcome of High-Dose Interferon α-2b Therapy in Acral Melanoma. Onco. Targets Ther. 2018, 11, 4097–4104. [Google Scholar] [CrossRef]
- Weiss, J.M.; Hunter, M.V.; Cruz, N.M.; Baggiolini, A.; Tagore, M.; Ma, Y.; Misale, S.; Marasco, M.; Simon-Vermot, T.; Campbell, N.R.; et al. Anatomic Position Determines Oncogenic Specificity in Melanoma. Nature 2022, 604, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, A.; Omodaka, T.; Okuyama, R. Melanomas and Mechanical Stress Points on the Plantar Surface of the Foot. N. Engl. J. Med. 2016, 374, 2404–2406. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.M.; Pittelkow, M.R.; Mangold, A.R. Acral Melanoma and Mechanical Stress on the Plantar Surface of the Foot. N. Engl. J. Med. 2017, 377, 395–396. [Google Scholar] [CrossRef]
- Galica, A.M.; Hagedorn, T.J.; Dufour, A.B.; Riskowski, J.L.; Hillstrom, H.J.; Casey, V.A.; Hannan, M.T. Hallux Valgus and Plantar Pressure Loading: The Framingham Foot Study. J. Foot Ankle Res. 2013, 6, 42. [Google Scholar] [CrossRef]
- Stucke, S.; McFarland, D.; Goss, L.; Fonov, S.; McMillan, G.R.; Tucker, A.; Berme, N.; Cenk Guler, H.; Bigelow, C.; Davis, B.L. Spatial Relationships between Shearing Stresses and Pressure on the Plantar Skin Surface during Gait. J. Biomech. 2012, 45, 619–622. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, J.H.; Cho, M.Y.; Chung, K.Y.; Roh, M.R. PTEN Promoter Hypermethylation Is Associated with Breslow Thickness in Acral Melanoma on the Heel, Forefoot, and Hallux. Ann. Dermatol. 2020, 33, 18. [Google Scholar] [CrossRef]
- Roh, M.R.; Gupta, S.; Park, K.-H.; Chung, K.Y.; Lauss, M.; Flaherty, K.T.; Jönsson, G.; Rha, S.Y.; Tsao, H. Promoter Methylation of PTEN Is a Significant Prognostic Factor in Melanoma Survival. J. Investig. Dermatol. 2016, 136, 1002–1011. [Google Scholar] [CrossRef]
- Seo, J.; Kim, H.; Min, K.I.; Kim, C.; Kwon, Y.; Zheng, Z.; Kim, Y.; Park, H.-S.; Ju, Y.S.; Roh, M.R.; et al. Weight-Bearing Activity Impairs Nuclear Membrane and Genome Integrity via YAP Activation in Plantar Melanoma. Nat. Commun. 2022, 13, 2214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA Damage in Micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, L.A.; Abascal, F.; Abeshouse, A.; Aburatani, H.; Adams, D.J.; Agrawal, N.; Ahn, K.S.; Ahn, S.-M.; Aikata, H.; Akbani, R.; et al. Pan-Cancer Analysis of Whole Genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef]
- Kneissig, M.; Keuper, K.; de Pagter, M.S.; van Roosmalen, M.J.; Martin, J.; Otto, H.; Passerini, V.; Campos Sparr, A.; Renkens, I.; Kropveld, F.; et al. Micronuclei-Based Model System Reveals Functional Consequences of Chromothripsis in Human Cells. eLife 2019, 8, e50292. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, S.M.; Yao, X.; Rosiene, J.; Tian, H.; Behr, J.; Bosco, N.; Takai, K.K.; de Lange, T.; Imieliński, M. Structural Variant Evolution after Telomere Crisis. Nat. Commun. 2021, 12, 2093. [Google Scholar] [CrossRef] [PubMed]
- Oscar Sánchez Solorzano, C.; Pascual-Montano, A.; Sánchez de Diego, A.; Martínez-A, C.; van Wely, K.H.M. Chromothripsis: Breakage-Fusion-Bridge over and over Again. Cell Cycle 2013, 12, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.M.; Fischer, A.H.; Deerinck, T.J.; Hetzer, M.W. Catastrophic Nuclear Envelope Collapse in Cancer Cell Micronuclei. Cell 2013, 154, 47–60. [Google Scholar] [CrossRef]
- Liu, S.; Kwon, M.; Mannino, M.; Yang, N.; Renda, F.; Khodjakov, A.; Pellman, D. Nuclear Envelope Assembly Defects Link Mitotic Errors to Chromothripsis. Nature 2018, 561, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.M.; Hetzer, M.W. Linking Micronuclei to Chromosome Fragmentation. Cell 2015, 161, 1502–1504. [Google Scholar] [CrossRef] [PubMed]
- Krupina, K.; Goginashvili, A.; Cleveland, D.W. Causes and Consequences of Micronuclei. Curr. Opin. Cell Biol. 2021, 70, 91–99. [Google Scholar] [CrossRef]
- Voronina, N.; Wong, J.K.L.; Hübschmann, D.; Hlevnjak, M.; Uhrig, S.; Heilig, C.E.; Horak, P.; Kreutzfeldt, S.; Mock, A.; Stenzinger, A.; et al. The Landscape of Chromothripsis across Adult Cancer Types. Nat. Commun. 2020, 11, 2320. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechano-Transduction and YAP-Dependent Matrix Remodelling Is Required for the Generation and Maintenance of Cancer Associated Fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Leask, A.; Nguyen, J.; Naik, A.; Chitturi, P.; Riser, B.L. The Role of Yes Activated Protein (YAP) in Melanoma Metastasis. iScience 2024, 27, 109864. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Szeto, P.; Abali, G.K.; Zhang, Y.; Kulkarni, A.; Amarasinghe, K.; Li, J.; Vergara, I.A.; Molania, R.; et al. The Hippo Pathway Oncoprotein YAP Promotes Melanoma Cell Invasion and Spontaneous Metastasis. Oncogene 2020, 39, 5267–5281. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Su, J.; Liu, J.; Yi, X.; Zhou, F.; Zhang, J.; Wang, J.; Meng, X.; Si, L.; Wu, C. YAP Activation in Promoting Negative Durotaxis and Acral Melanoma Progression. Cells 2022, 11, 3543. [Google Scholar] [CrossRef]
- Hadi, K.; Yao, X.; Behr, J.M.; Deshpande, A.; Xanthopoulakis, C.; Tian, H.; Kudman, S.; Rosiene, J.; Darmofal, M.; DeRose, J.; et al. Distinct Classes of Complex Structural Variation Uncovered across Thousands of Cancer Genome Graphs. Cell 2020, 183, 197–210.e32. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Munhoz, R.R.; Kuk, D.; Ott, P.A.; Johnson, D.B.; Tsai, K.K.; Rapisuwon, S.; Eroglu, Z.; Sullivan, R.J.; Luke, J.J.; et al. Efficacy of Anti-PD-1 Agents in Acral and Mucosal Melanoma. Cancer 2016, 122, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohara, K.; Kishi, A.; Teramoto, Y.; Sato, S.; Fujisawa, Y.; Fujimoto, M.; Otsuka, F.; Hayashi, N.; Yamazaki, N.; et al. Effects of Non-Amputative Wide Local Excision on the Local Control and Prognosis of in Situ and Invasive Subungual Melanoma. J. Dermatol. 2015, 42, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Le, M.; Gabrielli, S.; Zloty, D. Mohs Micrographic Surgery Is Equivalent to Nail Unit Excision or Amputation for Melanoma In Situ of the Nail Unit: A Systematic Review and Meta-Analysis. Dermatol. Surg. 2023, 49, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ishitsuka, Y.; Tanaka, R.; Okiyama, N.; Saito, A.; Watanabe, R.; Fujisawa, Y. Acral Lentiginous Melanoma and Mucosal Melanoma Expressed Less Programmed-Death 1 Ligand than Cutaneous Melanoma: A Retrospective Study of 73 Japanese Melanoma Patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e424–e426. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Yoshino, K.; Nagai, K.; Oaku, S.; Kato, M.; Hiura, A.; Hata, H. Efficacy of Nivolumab Monotherapy against Acral Lentiginous Melanoma and Mucosal Melanoma in Asian Patients. Br. J. Dermatol. 2019, 180, 1230–1231. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.A.D.; Aguiar, F.C.; Smalley, K.S.; Possik, P.A. Acral Melanoma: New Insights into the Immune and Genomic Landscape. Neoplasia 2023, 46, 100947. [Google Scholar] [CrossRef]
- Li, J.; Smalley, I.; Chen, Z.; Wu, J.-Y.; Phadke, M.S.; Teer, J.K.; Nguyen, T.; Karreth, F.A.; Koomen, J.M.; Sarnaik, A.A.; et al. Single-Cell Characterization of the Cellular Landscape of Acral Melanoma Identifies Novel Targets for Immunotherapy. Clin. J. Am. Assoc. Cancer Res. 2022, 28, 2131–2146. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, H.; Yang, T.; Li, T.; Liu, X.; Wang, J.; Liao, Z.; Wei, J.; Lu, J.; Liu, H.; et al. A Single-Cell Analysis Reveals Tumor Heterogeneity and Immune Environment of Acral Melanoma. Nat. Commun. 2022, 13, 7250. [Google Scholar] [CrossRef]
- Si, L.; Zhang, X.; Shu, Y.; Pan, H.; Wu, D.; Liu, J.; Mao, L.; Wang, X.; Wen, X.; Gu, Y.; et al. Pembrolizumab in Chinese Patients with Advanced Melanoma: 3-Year Follow-up of the KEYNOTE-151 Study. Front. Immunol. 2022, 13, 882471. [Google Scholar] [CrossRef]
- Nakamura, Y.; Namikawa, K.; Yoshino, K.; Yoshikawa, S.; Uchi, H.; Goto, K.; Nakamura, Y.; Fukushima, S.; Kiniwa, Y.; Takenouchi, T.; et al. Anti-PD1 Checkpoint Inhibitor Therapy in Acral Melanoma: A Multicenter Study of 193 Japanese Patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chi, Z.; Chen, Y.; Liu, X.; Wu, D.; Chen, J.; Song, X.; Wang, W.; Dong, L.; Song, H.; et al. Safety, Efficacy, and Biomarker Analysis of Toripalimab in Previously Treated Advanced Melanoma: Results of the POLARIS-01 Multicenter Phase II Trial. Clin. Cancer Res. 2020, 26, 4250–4259. [Google Scholar] [CrossRef]
- Si, L.; Zhang, X.; Shu, Y.; Pan, H.; Wu, D.; Liu, J.; Lou, F.; Mao, L.; Wang, X.; Wen, X.; et al. A Phase Ib Study of Pembrolizumab as Second-Line Therapy for Chinese Patients with Advanced or Metastatic Melanoma (KEYNOTE-151). Transl. Oncol. 2019, 12, 828–835. [Google Scholar] [CrossRef]
- Wen, X.; Ding, Y.; Li, J.; Zhao, J.; Peng, R.; Li, D.; Zhu, B.; Wang, Y.; Zhang, X.; Zhang, X. The Experience of Immune Checkpoint Inhibitors in Chinese Patients with Metastatic Melanoma: A Retrospective Case Series. Cancer Immunol. Immunother. 2017, 66, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.; Ascierto, P.A.; Haanen, J.; Espinosa, E.; Demidov, L.; Garbe, C.; Guida, M.; Lorigan, P.; Chiarion-Sileni, V.; Gogas, H.; et al. Safety and Efficacy of Nivolumab in Patients with Rare Melanoma Subtypes Who Progressed on or after Ipilimumab Treatment: A Single-Arm, Open-Label, Phase II Study (CheckMate 172). Eur. J. Cancer 2019, 119, 168–178. [Google Scholar] [CrossRef]
- Nakamura, Y.; Namikawa, K.; Kiniwa, Y.; Kato, H.; Yamasaki, O.; Yoshikawa, S.; Maekawa, T.; Matsushita, S.; Takenouchi, T.; Inozume, T.; et al. Efficacy Comparison between Anti-PD-1 Antibody Monotherapy and Anti-PD-1 plus Anti-CTLA-4 Combination Therapy as First-Line Immunotherapy for Advanced Acral Melanoma: A Retrospective, Multicenter Study of 254 Japanese Patients. Eur. J. Cancer 2022, 176, 78–87. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Bhave, P.; Ahmed, T.; Lo, S.N.; Shoushtari, A.; Zaremba, A.; Versluis, J.M.; Mangana, J.; Weichenthal, M.; Si, L.; Lesimple, T.; et al. Efficacy of Anti-PD-1 and Ipilimumab Alone or in Combination in Acral Melanoma. J. Immunother. Cancer 2022, 10, e004668. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Lipson, E.J.; Hodi, F.S.; Ascierto, P.A.; Larkin, J.; Lao, C.; Grob, J.-J.; Ejzykowicz, F.; Moshyk, A.; Garcia-Horton, V.; et al. First-Line Nivolumab Plus Relatlimab Versus Nivolumab Plus Ipilimumab in Advanced Melanoma: An Indirect Treatment Comparison Using RELATIVITY-047 and CheckMate 067 Trial Data. J. Clin. Oncol. 2024, 42, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Li, D.; Wen, X.; Ding, Y.; Liu, X.; Jiang, H.; Huang, F.; Zhang, X. Adjuvant PD-1 Inhibitor versus High-Dose Interferon α-2b for Chinese Patients with Cutaneous and Acral Melanoma: A Retrospective Cohort Analysis. Dermatol. Ther. 2021, 34, e15067. [Google Scholar] [CrossRef]
- Muto, Y.; Kambayashi, Y.; Kato, H.; Mizuhashi, S.; Ito, T.; Maekawa, T.; Ishizuki, S.; Uchi, H.; Matsushita, S.; Yamamoto, Y.; et al. Three-Year Analysis of Adjuvant Therapy in Postoperative Melanoma Including Acral and Mucosal Subtypes. Cancers 2024, 16, 2755. [Google Scholar] [CrossRef] [PubMed]
- Bloem, M.; van Not, O.J.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; Blank, C.U.; Blokx, W.A.M.; Boers-Sonderen, M.J.; Bonenkamp, J.J.; de Groot, J.-W.B.; Haanen, J.B.; et al. Adjuvant Treatment with Anti-PD-1 in Acral Melanoma: A Nationwide Study. Int. J. Cancer 2024, 155, 1455–1465. [Google Scholar] [CrossRef]
- Sun, W.; Xu, Y.; Yan, W.; Wang, C.; Hu, T.; Luo, Z.; Zhang, X.; Liu, X.; Chen, Y. A Real-world Study of Adjuvant anti-PD -1 Immunotherapy on Stage III Melanoma with BRAF, NRAS, and KIT Mutations. Cancer Med. 2023, 12, 15945–15954. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, P.K.; Patel, A.; Hossain, S.; Kaufman, H.L. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am. J. Clin. Dermatol. 2017, 18, 1–15. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Li, Z.; Gao, S.; Mao, L.; Dai, J.; Li, C.; Cui, C.; Chi, Z.; Sheng, X.; et al. Neoadjuvant Oncolytic Virus Orienx010 and Toripalimab in Resectable Acral Melanoma: A Phase Ib Trial. Signal Transduct. Target. Ther. 2024, 9, 318. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 Recommendations for Treating Chronic Myeloid Leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Armstrong, E.; Wei, A.Z.; Ye, F.; Lee, A.; Carlino, M.S.; Sullivan, R.J.; Carvajal, R.D.; Shoushtari, A.N.; Johnson, D.B. Clinical and Genomic Correlates of Imatinib Response in Melanomas with KIT Alterations. Br. J. Cancer 2022, 127, 1726–1732. [Google Scholar] [CrossRef]
- Wei, X.; Mao, L.; Chi, Z.; Sheng, X.; Cui, C.; Kong, Y.; Dai, J.; Wang, X.; Li, S.; Tang, B.; et al. Efficacy Evaluation of Imatinib for the Treatment of Melanoma: Evidence from a Retrospective Study. Oncol. Res. 2019, 27, 495–501. [Google Scholar] [CrossRef]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Friedlander, P.; Gonzalez, R.; Weber, J.S.; Gajewski, T.F.; et al. Imatinib for Melanomas Harboring Mutationally Activated or Amplified KIT Arising on Mucosal, Acral, and Chronically Sun-Damaged Skin. J. Clin. Oncol. 2013, 31, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.; Natoli, A.; Hui, Y.; Conus, N.; Jackson, S.; Brüggen, J.; Manley, P.W.; McArthur, G.A. Preclinical Evaluation of Nilotinib Efficacy in an Imatinib-Resistant KIT-Driven Tumor Model. Mol. Cancer Ther. 2010, 9, 1461–1468. [Google Scholar] [CrossRef]
- Larkin, J.; Marais, R.; Porta, N.; de Castro, D.G.; Parsons, L.; Messiou, C.; Stamp, G.; Thompson, L.; Edmonds, K.; Sarker, S.; et al. Nilotinib in KIT-Driven Advanced Melanoma: Results from the Phase II Single-Arm NICAM Trial. Cell Rep. Med. 2024, 5, 101435. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, K.; Lee, S.; Rubin, K.; Lawrence, D.P.; Iafrarte, A.J.; Borger, D.R.; Margolin, K.A.; Leitao, M.M.; Tarhini, A.A.; Koon, H.B.; et al. A Phase II Trial of Dasatinib in Patients with Locally Advanced or Stage IV Mucosal, Acral and Vulvovaginal Melanoma: A Trial of the ECOG-ACRIN Cancer Research Group (E2607). Cancer 2017, 123, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Dudek, A.Z.; McCann, C.; Ritacco, J.; Southard, N.; Jilaveanu, L.B.; Molinaro, A.; Sznol, M. A Phase II Trial of Dasatinib in Advanced Melanoma. Cancer 2011, 117, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Minor, D.R.; Kashani-Sabet, M.; Garrido, M.; O’Day, S.J.; Hamid, O.; Bastian, B.C. Sunitinib Therapy for Melanoma Patients with KIT Mutations. Clin. Cancer Res. 2012, 18, 1457–1463. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Sosman, J.A.; Lawrence, D.P.; McDermott, D.F.; Ramaiya, N.H.; Van den Abbeele, A.D.; Linette, G.P.; Giobbie-Hurder, A.; Hodi, F.S. Phase 2 Study of Sunitinib in Patients with Metastatic Mucosal or Acral Melanoma. Cancer 2015, 121, 4007–4015. [Google Scholar] [CrossRef]
- Fernandes, M.; Barcelos, D.; Comodo, A.N.; Guimarães, D.P.; Lopes Carapeto, F.C.; Cardili, L.; de Sousa Morães, L.; Cerutti Ap, J.; Landman Ap, G. Acral Lentiginous Melanomas Harbour Intratumor Heterogeneity in BRAF Exon 15, with Mutations Distinct from V600E/V600K. Am. J. Dermatopathol. 2019, 41, 733–740. [Google Scholar] [CrossRef]
- Bai, X.; Mao, L.L.; Chi, Z.H.; Sheng, X.N.; Cui, C.L.; Kong, Y.; Dai, J.; Wang, X.; Li, S.M.; Tang, B.X.; et al. BRAF Inhibitors: Efficacious and Tolerable in BRAF-Mutant Acral and Mucosal Melanoma. Neoplasma 2017, 64, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Ito, T.; Kato, H.; Irie, H.; Kaji, T.; Maekawa, T.; Asai, J.; Yamamoto, Y.; Fujimura, T.; Nakai, Y.; et al. Outcome of Combination Therapy Using BRAF and MEK Inhibitors among Asian Patients with Advanced Melanoma: An Analysis of 112 Cases. Eur. J. Cancer 2021, 145, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, S.; Kim, K.; Heo, M.H.; Lee, H.; Cho, J.; Kim, N.K.D.; Park, W.; Lee, S.J.; Kim, J.H.; et al. Efficacy of BRAF Inhibitors in Asian Metastatic Melanoma Patients: Potential Implications of Genomic Sequencing in BRAF-Mutated Melanoma. Transl. Oncol. 2016, 9, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ding, Y.; Bai, X.; Sheng, X.; Dai, J.; Chi, Z.; Cui, C.; Kong, Y.; Fan, Y.; Xu, Y.; et al. Overall Survival of Patients With Unresectable or Metastatic BRAF V600-Mutant Acral/Cutaneous Melanoma Administered Dabrafenib Plus Trametinib: Long-Term Follow-Up of a Multicenter, Single-Arm Phase IIa Trial. Front. Oncol. 2021, 11, 720044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, S.; Wu, D. Advanced Acral Melanoma Therapies: Current Status and Future Directions. Curr. Treat. Options Oncol. 2022, 23, 1405–1427. [Google Scholar] [CrossRef] [PubMed]
- Kaszubski, J.; Gagat, M.; Grzanka, A.; Wawrzyniak, A.; Niklińska, W.; Łapot, M.; Żuryń, A. Cyclin-Dependent Kinase Inhibitors in the Rare Subtypes of Melanoma Therapy. Molecules 2024, 29, 5239. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Xin, Q.; Zhang, Y.; Wang, K.; Li, M. Recent Progress of CDK4/6 Inhibitors’ Current Practice in Breast Cancer. Cancer Gene Ther. 2024, 31, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 Inhibition Triggers Anti-Tumor Immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef]
- Mao, L.; Dai, J.; Cao, Y.; Bai, X.; Sheng, X.; Chi, Z.; Cui, C.; Kong, Y.; Zhang, Y.; Wu, L.; et al. Palbociclib in Advanced Acral Melanoma with Genetic Aberrations in the Cyclin-Dependent Kinase 4 Pathway. Eur. J. Cancer 2021, 148, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Jagirdar, K.; Portuallo, M.E.; Wei, M.; Wilhide, M.; Bravo Narula, J.A.; Robertson, B.M.; Alicea, G.M.; Aguh, C.; Xiao, M.; Godok, T.; et al. ERK Hyperactivation Serves as a Unified Mechanism of Escape in Intrinsic and Acquired CDK4/6 Inhibitor Resistance in Acral Lentiginous Melanoma. Oncogene 2024, 43, 395–405. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Tsao, H. Acral Melanoma: A Review of Its Pathogenesis, Progression, and Management. Biomolecules 2025, 15, 120. https://doi.org/10.3390/biom15010120
Kim SH, Tsao H. Acral Melanoma: A Review of Its Pathogenesis, Progression, and Management. Biomolecules. 2025; 15(1):120. https://doi.org/10.3390/biom15010120
Chicago/Turabian StyleKim, Soo Hyun, and Hensin Tsao. 2025. "Acral Melanoma: A Review of Its Pathogenesis, Progression, and Management" Biomolecules 15, no. 1: 120. https://doi.org/10.3390/biom15010120
APA StyleKim, S. H., & Tsao, H. (2025). Acral Melanoma: A Review of Its Pathogenesis, Progression, and Management. Biomolecules, 15(1), 120. https://doi.org/10.3390/biom15010120





