From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules
Abstract
1. Introduction
2. Brief Historical Context for PDRN/PN Biopolymers
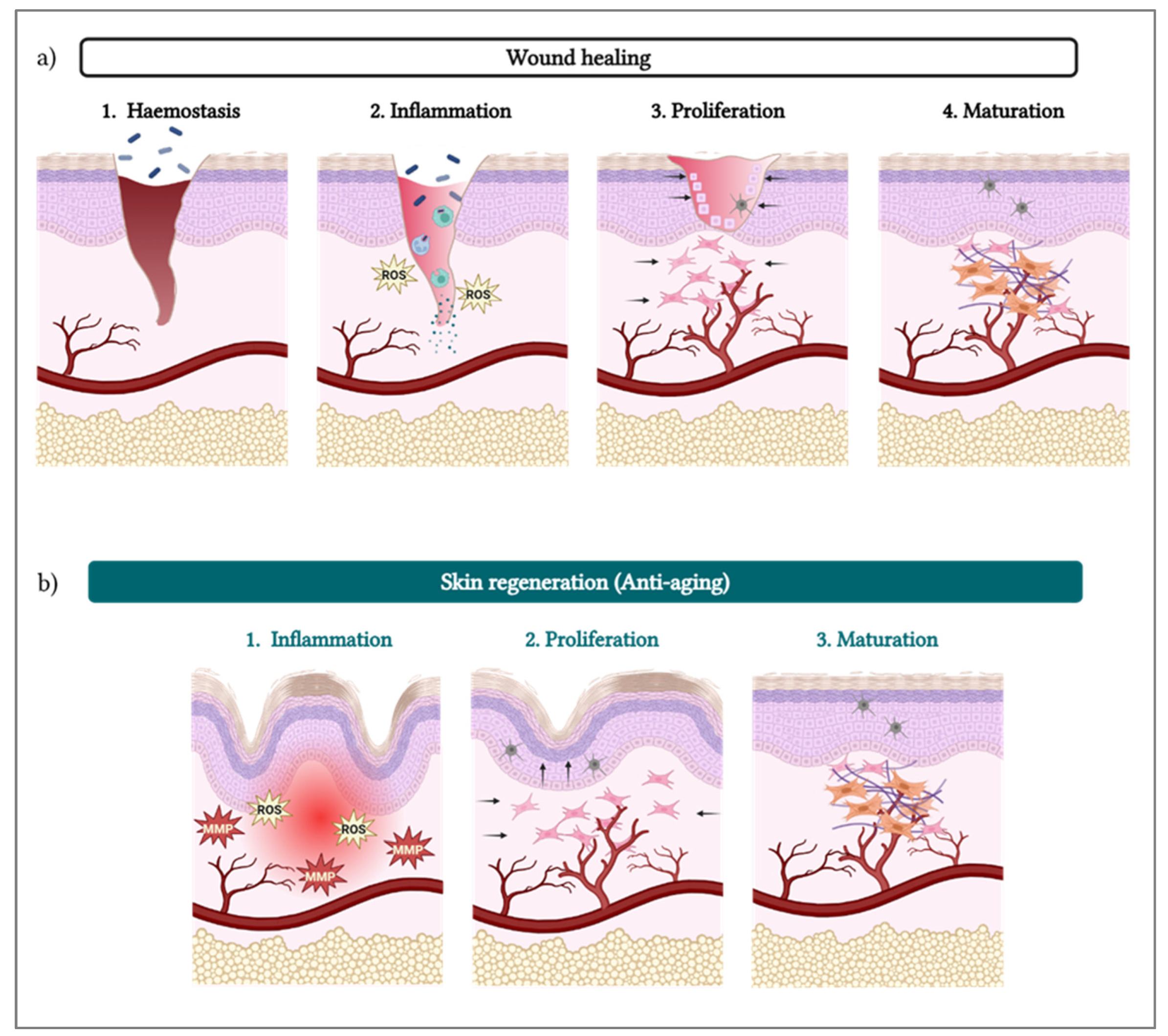
3. PDRN/PN and Skin Regeneration
- •
- Inflammation resolution: The resulting cascade reaction leads to a decrease in the levels of pro-inflammatory cytokines (e.g., TNF-α, IL-6, IL-8) [7] and an increase in their anti-inflammatory counterparts (e.g., IL-10), decreasing the overall inflammatory status. The cascade also notably inhibits the synthesis and secretion of collagenase by synovial fibroblasts [7].
- •
- •
- Remodeling: With decreased inflammation, increased blood support, and cell growth stimulation, the cells (e.g., fibroblasts) are surrounded by optimal conditions to produce collagen (i.e., types I and III) [37,38,39], elastin, and fibrinogen [18,33]. Those proteins then contribute to form the ECM, providing mechanical and structural support to fibroblasts, generating new tissue [27].
4. PDRN/PN Sourcing and Extraction Methodologies
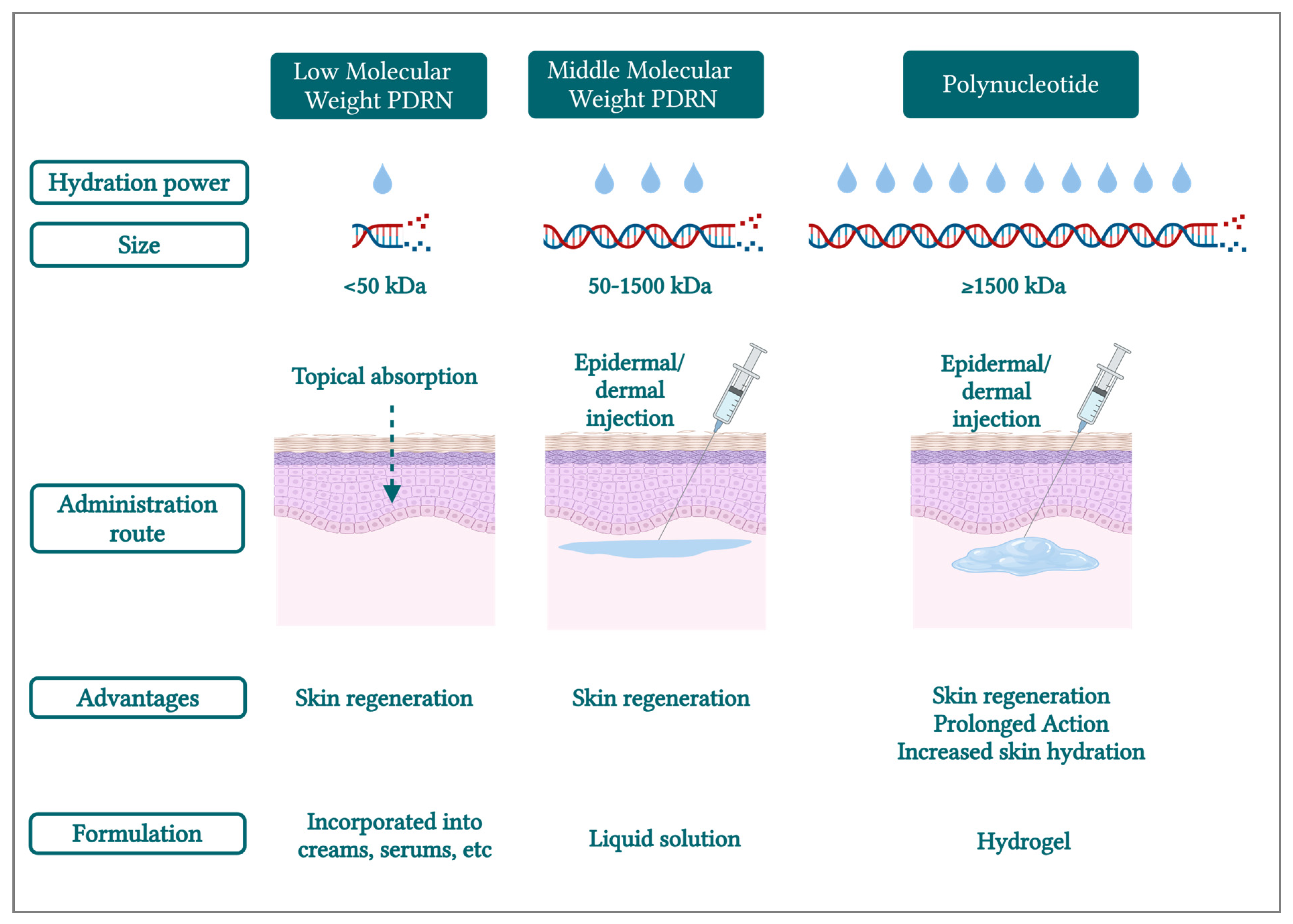
5. PDRN/PN Molecular Weights Linked to Properties and Actions on the Skin
6. Properties and Potential Applications of PN-Based Hydrogels
7. Conclusions
- •
- “PDRN” (i.e., low-MW and medium-MW [“classic”]) for the description of small and medium chains of deoxyribonucleotides, with MW < 50 kDa and between 50 and 1500 kDa, respectively;
- •
- “PN” should specifically refer to long chains of deoxyribonucleotides (≥1500 kDa).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| bp | base pairs |
| CaHA | calcium hydroxylapatite |
| DNA | deoxyribonucleic acid |
| ECM | extracellular matrix |
| IL | interleukin |
| kDa | kilodalton |
| LED | light-emitting diode |
| MMP | matrix metalloproteinase |
| MW | molecular weight |
| PCL | polycaprolactone |
| PDRN | polydeoxyribonucleotide |
| PLLA | poly-L-lactic acid |
| PN | polynucleotide |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| SEM | scanning electron microscopy |
| TNF | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
References
- Rho, N.K.; Han, K.H.; Cho, M.; Kim, H.S. A Survey on the Cosmetic Use of Injectable Polynucleotide: The Pattern of Practice Among Korean Dermatologists. J. Cos. Dermatol. 2024, 23, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Wang, S.-L.; Nguyen, V.B. Recent Advances on Polydeoxyribonucleotide Extraction and Its Novel Application in Cosmeceuticals. Int. J. Biol. Macromol. 2024, 282, 137051. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wang, G.; Zhou, F.; Gong, L.; Zhang, J.; Qi, L.; Cui, H. Polydeoxyribonucleotide: A Promising Skin Anti-Aging Agent. Chin. J. Plast. Reconstruct. Surg. 2022, 4, 187–193. [Google Scholar] [CrossRef]
- Kim, T.-H.; Heo, S.-Y.; Oh, G.-W.; Heo, S.-J.; Jung, W.-K. Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering. Marine Drugs 2021, 19, 296. [Google Scholar] [CrossRef] [PubMed]
- Galeano, M.; Pallio, G.; Irrera, N.; Mannino, F.; Bitto, A.; Altavilla, D.; Vaccaro, M.; Squadrito, G.; Arcoraci, V.; Colonna, M.R.; et al. Polydeoxyribonucleotide: A Promising Biological Platform to Accelerate Impaired Skin Wound Healing. Pharmaceuticals 2021, 14, 1103. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. The Effects of Polydeoxyribonucleotide on Wound Healing and Tissue Regeneration: A Systematic Review of the Literature. Regen. Med. 2020, 15, 1801–1821. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Gadangi, P.; Longaker, M.; Sung, J.; Levine, J.; Nilsen, D.; Reibman, J.; Li, M.; Jiang, C.-K.; Hirschhorn, R.; et al. Wound Healing Is Accelerated by Agonists of Adenosine A2 (Gαs-Linked) Receptors. J. Exp. Med. 1997, 186, 1615–1620. [Google Scholar] [CrossRef]
- Tonello, G.; Daglio, M.; Zaccarelli, N.; Sottofattori, E.; Mazzei, M.; Balbi, A. Characterization and Quantitation of the Active Polynucleotide Fraction (PDRN) from Human Placenta, a Tissue Repair Stimulating Agent. J. Pharm. Biomed. Anal. 1996, 14, 1555–1560. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Kim, H.H.; Kyu, P.J.; Bae, H.W.; Choi, B.K.; Choi, W.G.; Yoon, H.E.; Kim, M.S. Method for Preparing of Composition for Anti-Inflammatory and Skin Regeneration Using Polydeoxyribonucleotide. KR20180048520A, 10 May 2018. Available online: https://patents.google.com/patent/KR20180048520A/en?q=(PDRN+extraction)&oq=PDRN+extraction (accessed on 14 January 2025).
- Baek, S.G.; Lee, S.H.; Kim, J.; Lee, S.O.; Kim, S.H.; Kim, S. Skin Wrinkle Improvement Composition Comprising Fragmented DNA Mixtures with Increased Skin Permeability. KR102415344B1, 30 June 2022. Available online: https://patents.google.com/patent/KR102415344B1/en?q=(polynucleotide)&assignee=pharmaresearch&oq=polynucleotide+pharmaresearch (accessed on 14 January 2025).
- Proskurina, A.; Nikolin, V.; Popova, N.; Varaksin, N.; Ryabicheva, T.; Ershova, E.; Kostyuk, S.; Leplina, O.; Ostanin, A.; Chernykh, E.; et al. Comparing the Biological Properties of Double-Stranded DNA Extracted from Human and Porcine Placenta and Salmon Sperm. Rep. Biochem. Mol. Biol. 2023, 11, 577. [Google Scholar] [CrossRef]
- Mastelli, L.C.; Mastelli, G.C.; Mastelli, S.C. Dermocosmetic Filler and Uses Thereof for Aesthetic Purposes. EP3107587B1, 27 March 2019. Available online: https://patents.google.com/patent/EP3107587B1/en?oq=EP3107587B1 (accessed on 14 January 2025).
- Pray, L. Discovery of DNA Structure and Function: Watson and Crick. Nat. Edu. 2008, 1, 100. [Google Scholar]
- Clancy, S. Chemical Structure of RNA. Nat. Edu. 2008, 7, 60. [Google Scholar]
- Clancy, S.; Brown, W. Translation: DNA to mRNA to Protein. Nat. Edu. 2008, 1, 101. [Google Scholar]
- Bianchini, P.; Tellini, N.; Morani, A.M.; Folloni, M.G. Pharmacological Data on Polydeoxyribonucleotide of Human Placenta. Int. J. Tissue React. 1981, 3, 151–154. [Google Scholar]
- Sini, P.; Denti, A.; Cattarini, G.; Daglio, M.; Tira, M.E.; Balduini, C. Effect of Polydeoxyribonucleotides on Human Fibroblasts in Primary Culture. Cell Biochem. Funct. 1999, 17, 107–114. [Google Scholar] [CrossRef]
- Thellung, S.; Florio, T.; Maragliano, A.; Cattarini, G.; Schettini, G. Polydeoxyribonucleotides Enhance the Proliferation of Human Skin Fibroblasts: Involvement of A2 Purinergic Receptor Subtypes. Life Sci. 1999, 64, 1661–1674. [Google Scholar] [CrossRef]
- Bianchini, P. Process for Obtaining Non Informational Substantially Pure Polydesoxyribonucleotides Having Biologic Activities, and Respective Product. EP0226254B1, 8 December 1993. Available online: https://patents.google.com/patent/EP0226254B1/fr?oq=EP0226254B1 (accessed on 14 January 2025).
- Placentex, Banca Dati Farmaci dell’AIFA. Available online: https://medicinali.aifa.gov.it/it/#/it/dettaglio/0000001170 (accessed on 14 November 2024).
- Kim, M.J.; Park, H.-J.; Jung, R.-J.; Won, C.-Y.; Ohk, S.-O.; Kim, H.-T.; Roh, N.-K.; Yi, K.-H. High-Resolution 3-D Scanning Electron Microscopy (SEM) Images of DOTTM Polynucleotides (PN): Unique Scaffold Characteristics and Potential Applications in Biomedicine. Skin Res. Technol. 2024, 30, e13667. [Google Scholar] [CrossRef]
- Park, K.Y.; Seok, J.; Rho, N.K.; Kim, B.J.; Kim, M.N. Long-Chain Polynucleotide Filler for Skin Rejuvenation: Efficacy and Complications in Five Patients. Dermatol. Ther. 2016, 29, 37–40. [Google Scholar] [CrossRef]
- Bartoletti, E.; Cavallini, M.; Maioli, L.; Massirone, A.; Palmieri, I.P.; Papagni, M. Introduction to Polynucleotides Highly Purified Technology. Aesth. Med. 2020, 6, 43–47. [Google Scholar]
- Cavallini, M.; Bartoletti, E.; Maioli, L.; Massirone, A.; Pia Palmieri, I.; Papagni, M.; Priori, M.; Trocchi, G. Consensus Report on the Use of PN-HPTTM (Polynucleotides Highly Purified Technology) in Aesthetic Medicine. J. Cosmet. Dermatol. 2021, 20, 922–928. [Google Scholar] [CrossRef]
- Valdatta, L.; Thione, A.; Mortarino, C.; Buoro, M.; Tuinder, S. Evaluation of the Efficacy of Polydeoxyribonucleotides in the Healing Process of Autologous Skin Graft Donor Sites: A Pilot Study. Curr. Med. Res. Opin. 2004, 20, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide Stimulates Angiogenesis and Wound Healing in the Genetically Diabetic Mouse. Wound Rep. Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef]
- Gennero, L.; De Siena, R.; Denysenko, T.; Roos, M.A.; Calisti, G.F.; Martano, M.; Fiobellot, S.; Panzone, M.; Reguzzi, S.; Gabetti, L.; et al. A Novel Composition for in Vitro and in Vivo Regeneration of Skin and Connective Tissues. Cell Biochem. Funct. 2011, 29, 311–333. [Google Scholar] [CrossRef] [PubMed]
- De Caridi, G.; Massara, M.; Acri, I.; Zavettieri, S.; Grande, R.; Butrico, L.; de Franciscis, S.; Serra, R. Trophic Effects of Polynucleotides and Hyaluronic Acid in the Healing of Venous Ulcers of the Lower Limbs: A Clinical Study. Int. Wound J. 2016, 13, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lee, J.Y. Polydeoxyribonucleotide Improves Wound Healing of Fractional Laser Resurfacing in Rat Model. J. Cosmet. Laser Ther. 2017, 19, 43–48. [Google Scholar] [CrossRef]
- Hwang, K.-H.; Kim, J.-H.; Park, E.Y.; Cha, S.-K. An Effective Range of Polydeoxyribonucleotides Is Critical for Wound Healing Quality. Mol. Med. Rep. 2018, 18, 5166–5172. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Belletti, S.; Govoni, P.; Guizzardi, S.; Galli, C. A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes Wound Healing in a Primary Gingival Fibroblast Model. Appl. Sci. 2021, 11, 4405. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, S.-C.; Park, W.S.; Choi, I.-W.; Kim, H.-W.; Kang, H.W.; Kim, Y.-M.; Jung, W.-K. PCL/Gelatin Nanofibers Incorporated with Starfish Polydeoxyribonucleotides for Potential Wound Healing Applications. Mat. Design 2023, 229, 111912. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Babu, P.J. Design of 3D Smart Scaffolds Using Natural, Synthetic and Hybrid Derived Polymers for Skin Regenerative Applications. Smart Mat. Med. 2023, 4, 243–256. [Google Scholar] [CrossRef]
- Lee, D.; Kim, M.J.; Park, H.J.; Rah, G.C.; Choi, H.; Anh, S.-T.; Ji, G.H.; Kim, M.S.; Kim, G.; Shin, D.W.; et al. Current Practices and Perceived Effectiveness of Polynucleotides for Treatment of Facial Erythema by Cosmetic Physicians. Skin Res. Technol. 2023, 29, e13466. [Google Scholar] [CrossRef]
- Veronesi, F.; Dallari, D.; Sabbioni, G.; Carubbi, C.; Martini, L.; Fini, M. Polydeoxyribonucleotides (PDRNs) From Skin to Musculoskeletal Tissue Regeneration via Adenosine A2A Receptor Involvement. J. Cell. Physiol. 2017, 232, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Perez-Aso, M.; Mediero, A.; Cronstein, B.N. Adenosine A2A Receptor (A2AR) Is a Fine-Tune Regulator of the Collagen1:Collagen3 Balance. Purin. Signal. 2013, 9, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Baek, E.J.; Kim, K.H.; Kim, K.J.; Park, E.J. Polydeoxyribonucleotide Exerts Opposing Effects on ERK Activity in Human Skin Keratinocytes and Fibroblasts. Mol. Med. Rep. 2023, 28, 148. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Choi, M.S.; Kim, H.K.; Kim, W.S.; Bae, T.H.; Kim, M.K.; Chang, S.H. Polydeoxyribonucleotide Improves Tendon Healing Following Achilles Tendon Injury in Rats. J. Orthop. Res. 2018, 36, 1767–1776. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking Older: Fibroblast Collapse and Therapeutic Implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- McLafferty, E.; Hendry, C.; Farley, A. The Integumentary System: Anatomy, Physiology and Function of Skin. Nurs. Stand. 2012, 27, 35–42. [Google Scholar] [CrossRef]
- Raposio, E.; Guida, C.; Coradeghini, R.; Scanarotti, C.; Parodi, A.; Baldelli, I.; Fiocca, R.; Santi, P.L. In Vitro Polydeoxyribonucleotide Effects on Human Pre-Adipocytes. Cell Prolif. 2008, 41, 739–754. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, H.; Oh, S.M.; Yi, K. Polynucleotide Injection Treatment for Iatrogenic Fat Atrophy in Two Patients: Potential for Safe Volumization in Aesthetic Medicine. Skin Res. Technol. 2023, 29, e13439. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, M.-J.; Kweon, D.-K.; Lim, S.-T.; Lee, S.-J. Polydeoxyribonucleotide Activates Mitochondrial Biogenesis but Reduces MMP-1 Activity and Melanin Biosynthesis in Cultured Skin Cells. Appl. Biochem. Biotechnol. 2020, 191, 540–554. [Google Scholar] [CrossRef]
- Kim, T.-H.; Heo, S.-Y.; Han, J.S.; Jung, W.-K. Anti-Inflammatory Effect of Polydeoxyribonucleotides (PDRN) Extracted from Red Alga (Porphyra Sp.) (Ps-PDRN) in RAW 264.7 Macrophages Stimulated with Escherichia coli Lipopolysaccharides: A Comparative Study with Commercial PDRN. Cell Biochem. Funct. 2023, 41, 889–897. [Google Scholar] [CrossRef]
- Kuppa, S.S.; Kim, H.-K.; Kang, J.-Y.; Lee, S.-C.; Yang, H.-Y.; Sankaranarayanan, J.; Seon, J.-K. Polynucleotides Suppress Inflammation and Stimulate Matrix Synthesis in an In Vitro Cell-Based Osteoarthritis Model. Int. J. Mol. Sci. 2023, 24, 12282. [Google Scholar] [CrossRef]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) Promotes Human Osteoblast Proliferation: A New Proposal for Bone Tissue Repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Mazzuco, R.; Evangelista, C.; Gobbato, D.O.; de Almeida, L.M. Clinical and Histological Comparative Outcomes after Injections of Poly-L-Lactic Acid and Calcium Hydroxyapatite in Arms: A Split Side Study. J. Cosmet. Dermatol. 2022, 21, 6727–6733. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Bass, L.M.; Goldberg, D.J.; Graivier, M.H.; Lorenc, Z.P. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthet. Surg. J. 2018, 38, S13–S17. [Google Scholar] [CrossRef]
- Goldberg, D.; Guana, A.; Volk, A.; Daro-Kaftan, E. Single-Arm Study for the Characterization of Human Tissue Response to Injectable Poly-L-Lactic Acid. Dermatol. Surg. 2013, 39, 915–922. [Google Scholar] [CrossRef]
- Kim, J.A.; Van Abel, D. Neocollagenesis in Human Tissue Injected with a Polycaprolactone-Based Dermal Filler. J. Cosmet. Laser Ther. 2015, 17, 99–101. [Google Scholar] [CrossRef]
- Christen, M.-O.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef]
- Bohnert, K.; Dorizas, A.; Lorenc, P.; Sadick, N.S. Randomized, Controlled, Multicentered, Double-Blind Investigation of Injectable Poly-l-Lactic Acid for Improving Skin Quality. Dermatol. Surg. 2019, 45, 718. [Google Scholar] [CrossRef]
- Kadouch, J.A. Calcium Hydroxylapatite: A Review on Safety and Complications. J. Cosmet. Dermatol. 2017, 16, 152–161. [Google Scholar] [CrossRef]
- Zago Sá Fortes, R.; Cassol Spanemberg, J.; Cherubini, K.; Salum, F.G. Adverse Events and Satisfaction Outcomes with Calcium Hydroxylapatite and Polycaprolactone Fillers in Facial Aesthetics: A Systematic Review. Cosmetics 2024, 11, 165. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Bertucci, V.; Sykes, J.M.; Duplechain, J.K. Adverse Reactions to Injectable Fillers. Facial Plast. Surg. 2016, 32, 532–555. [Google Scholar] [CrossRef]
- Palmieri, I.; Raichi, M. Biorevitalization of Postmenopausal Labia Majora, the Polynucleotide/Hyaluronic Acid Option. Obstet. Gynecol. Rep. 2019, 3. [Google Scholar] [CrossRef]
- Abdoli, R.; Zamani, P.; Ghasemi, M. Genetic Similarities and Phylogenetic Analysis of Human and Farm Animal Species Based on Mitogenomic Nucleotide Sequences. Meta Gene 2018, 15, 23–26. [Google Scholar] [CrossRef]
- Vanelli, R.; Costa, P.; Rossi, S.M.P.; Benazzo, F. Efficacy of Intra-Articular Polynucleotides in the Treatment of Knee Osteoarthritis: A Randomized, Double-Blind Clinical Trial. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 901–907. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, R.K.; In, Y. The Efficacy and Safety of Polydeoxyribonucleotide for the Treatment of Knee Osteoarthritis. Medicine 2019, 98, e17386. [Google Scholar] [CrossRef]
- Shu, Z.; Ji, Y.; Liu, F.; Jing, Y.; Jiao, C.; Li, Y.; Zhao, Y.; Wang, G.; Zhang, J. Proteomics Analysis of the Protective Effect of Polydeoxyribonucleotide Extracted from Sea Cucumber (Apostichopus japonicus) Sperm in a Hydrogen Peroxide-Induced RAW264.7 Cell Injury Model. Marine Drugs 2024, 22, 325. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lee, S.; Wang, H.; Lee, G.; Kim, S.; Ryu, Y.-H.; Chang, N.H.; Kang, Y.-W. Analysis of Skin Regeneration and Barrier-Improvement Efficacy of Polydeoxyribonucleotide Isolated from Panax Ginseng (C.A. Mey.) Adventitious Root. Molecules 2023, 28, 7240. [Google Scholar] [CrossRef]
- PharmaResearch, “Rejuran®” Accelerates Entry into Vietnam. Available online: https://pharmaresearch.co.kr/en/press/view.html?idx=262 (accessed on 18 November 2024).
- PharmaResearch Participates in “IMCAS PARIS 2024” with Rejuran®… Stepping into the European Market in Earnest. Available online: https://pharmaresearch.co.kr/en/press/view.html?idx=67&curpage=1 (accessed on 18 November 2024).
- Kim, J.H.; Jeong, J.J.; Lee, Y.I.; Lee, W.J.; Lee, C.; Chung, W.Y.; Nam, K.-H.; Lee, J.H. Preventive Effect of Polynucleotide on Post-Thyroidectomy Scars: A Randomized, Double-Blinded, Controlled Trial. Lasers Surg. Med. 2018, 50, 755–762. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.T.; Lee, Y.J.; Paik, S.H.; Moon, Y.S.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Jung, J.M.; et al. Comparison of the Effects of Polynucleotide and Hyaluronic Acid Fillers on Periocular Rejuvenation: A Randomized, Double-Blind, Split-Face Trial. J. Dermatol. Treat. 2022, 33, 254–260. [Google Scholar] [CrossRef]
- Olariu, L.; Dumitriu, B.G.; Gaidau, C.; Stanca, M.; Tanase, L.M.; Ene, M.D.; Stanculescu, I.-R.; Tablet, C. Bioactive Low Molecular Weight Keratin Hydrolysates for Improving Skin Wound Healing. Polymers 2022, 14, 1125. [Google Scholar] [CrossRef]
- Gruber, J.V.; Holtz, R.; Riemer, J. Hyaluronic Acid (HA) Stimulates the in Vitro Expression of CD44 Proteins but Not HAS1 Proteins in Normal Human Epidermal Keratinocytes (NHEKs) and Is HA Molecular Weight Dependent. J. Cosmet. Dermatol. 2022, 21, 1193–1198. [Google Scholar] [CrossRef]
- Farwick, M.; Lersch, P.; Strutz, G. Low Molecular Weight Hyaluronic Acid: Its Effects on Epidermal Gene Expression & Skin Ageing. SÖFW J. 2008, 134, 17. [Google Scholar]
- Maharjan, A.S.; Pilling, D.; Gomer, R.H. High and Low Molecular Weight Hyaluronic Acid Differentially Regulate Human Fibrocyte Differentiation. PLoS ONE 2011, 6, e26078. [Google Scholar] [CrossRef]
- Lee, K.W.A.; Chan, K.W.L.; Lee, A.; Lee, C.H.; Wan, J.; Wong, S.; Yi, K.H. Polynucleotides in Aesthetic Medicine: A Review of Current Practices and Perceived Effectiveness. Int. J. Mol. Sci. 2024, 25, 8224. [Google Scholar] [CrossRef]
- Scruggs, R.L.; Achter, E.K.; Ross, P.D. The Thermodynamic Effects of Exposing Nucleic Acid Bases to Water: Solubility Measurements in Water and Organic Solvents. Biopolymers 1972, 11, 1961–1972. [Google Scholar] [CrossRef]
- Singh, A.; Bhatia, D. Chapter 16—DNA Hydrogels: Principles, Synthesis, Characterization and Applications to Cell Biology. In Methods in Cell Biology; Shukla, A.K., Ed.; Biomolecular Interactions Part B; Academic Press: Cambridge, MA, USA, 2022; Volume 169, pp. 323–346. [Google Scholar] [CrossRef]
- Liu, D.; Wyttenbach, T.; Bowers, M.T. Hydration of Mononucleotides. J. Am. Chem. Soc. 2006, 128, 15155–15163. [Google Scholar] [CrossRef]
- Lee, S.L.; Debenedetti, P.G.; Errington, J.R.; Pethica, B.A.; Moore, D.J. A Calorimetric and Spectroscopic Study of DNA at Low Hydration. J. Phys. Chem. B 2004, 108, 3098–3106. [Google Scholar] [CrossRef]
- Khesbak, H.; Savchuk, O.; Tsushima, S.; Fahmy, K. The Role of Water H-Bond Imbalances in B-DNA Substate Transitions and Peptide Recognition Revealed by Time-Resolved FTIR Spectroscopy. J. Am. Chem. Soc. 2011, 133, 5834–5842. [Google Scholar] [CrossRef]
- Yatsunyk, L.A.; Neidle, S. On Water Arrangements in Right- and Left-Handed DNA Structures. Molecules 2024, 29, 505. [Google Scholar] [CrossRef]
- Falk, M. A Gravimetric Study of Hydration of Polynucleotides. Can. J. Chem. 1966, 44, 1107–1111. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, E.S.; Kim, S.W.; Hong, S.P.; Kim, J. Effects of Polynucleotide Dermal Filler in the Correction of Crow’s Feet Using an Antera Three-Dimensional Camera. Aesth. Plast. Surg. 2022, 46, 1902–1909. [Google Scholar] [CrossRef]
- Han, J.S.; Lee, W.S. Method for Extracting Polydeoxyribonucleotides and Polynucleotides Derived from Algae with the Effect of Neovascularization and Cell Regeneration. KR20230136092A, 26 September 2023. Available online: https://patents.google.com/patent/KR20230136092A/en?q=(PDRN+extraction)&oq=PDRN+extraction (accessed on 14 January 2025).
- Manfredini, M.; Poli, P.P.; Beretta, M.; Pellegrini, M.; Salina, F.E.; Maiorana, C. Polydeoxyribonucleotides Pre-Clinical Findings in Bone Healing: A Scoping Review. Dentist. J. 2023, 11, 280. [Google Scholar] [CrossRef]
- Walia, S.; Morya, V.; Gangrade, A.; Naskar, S.; Guduru Teja, A.; Dalvi, S.; Maiti, P.K.; Ghoroi, C.; Bhatia, D. Designer DNA Hydrogels Stimulate 3D Cell Invasion by Enhanced Receptor Expression and Membrane Endocytosis. ACS Biomater. Sci. Eng. 2021, 7, 5933–5942. [Google Scholar] [CrossRef]
- Zhang, H.; Vandesompele, J.; Braeckmans, K.; Smedt, S.C.D.; Remaut, K. Nucleic Acid Degradation as Barrier to Gene Delivery: A Guide to Understand and Overcome Nuclease Activity. Chem. Soc. Rev. 2024, 53, 317–360. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, H.; Cao, T.; Liu, D. Supramolecular Hydrogels Based on DNA Self-Assembly. Acc. Chem. Res. 2017, 50, 659–668. [Google Scholar] [CrossRef]
- Burdick, J.A.; Murphy, W.L. Moving from Static to Dynamic Complexity in Hydrogel Design. Nat. Commun. 2012, 3, 1269. [Google Scholar] [CrossRef]
- Quan, T.; Wang, F.; Shao, Y.; Rittié, L.; Xia, W.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J. Enhancing Structural Support of the Dermal Microenvironment Activates Fibroblasts, Endothelial Cells and Keratinocytes in Aged Human Skin in Vivo. J. Investig. Dermatol. 2013, 133, 658–667. [Google Scholar] [CrossRef]
- Ayatollahi, A.; Firooz, A.; Samadi, A. Evaluation of Safety and Efficacy of Booster Injections of Hyaluronic Acid in Improving the Facial Skin Quality. J. Cosmet. Dermatol. 2020, 19, 2267–2272. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermato-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.; Boreham, A.; Brodwolf, R.; Vávrová, K.; Alexiev, U.; Friess, W.; Hedtrich, S. Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules. Mol. Pharm. 2015, 12, 1391–1401. [Google Scholar] [CrossRef] [PubMed]


| Trademarked Process | Resulting Product | Registered Owner |
|---|---|---|
| DOTTM (DNA Fragment Optimizing Technology) [65,66] | DOT TM PDRN/DOT TM PN | PharmaResearch Co., Ltd. (Gangneung, Republic of Korea) |
| HPT TM (Highly Purified Technology) [24,25] | Polynucleotide-HPT TM, PN-HPT TM | MASTELLI S.R.L. (Sanremo, Italy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, C.; Porcello, A.; Cerrano, M.; Hadjab, F.; Chemali, M.; Lourenço, K.; Hadjab, B.; Raffoul, W.; Applegate, L.A.; Laurent, A.E. From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules. Biomolecules 2025, 15, 148. https://doi.org/10.3390/biom15010148
Marques C, Porcello A, Cerrano M, Hadjab F, Chemali M, Lourenço K, Hadjab B, Raffoul W, Applegate LA, Laurent AE. From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules. Biomolecules. 2025; 15(1):148. https://doi.org/10.3390/biom15010148
Chicago/Turabian StyleMarques, Cíntia, Alexandre Porcello, Marco Cerrano, Farid Hadjab, Michèle Chemali, Kelly Lourenço, Basste Hadjab, Wassim Raffoul, Lee Ann Applegate, and Alexis E. Laurent. 2025. "From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules" Biomolecules 15, no. 1: 148. https://doi.org/10.3390/biom15010148
APA StyleMarques, C., Porcello, A., Cerrano, M., Hadjab, F., Chemali, M., Lourenço, K., Hadjab, B., Raffoul, W., Applegate, L. A., & Laurent, A. E. (2025). From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules. Biomolecules, 15(1), 148. https://doi.org/10.3390/biom15010148








