Topical Delivery of Ceramide by Oil-in-Water Nanoemulsion to Retain Epidermal Moisture Content in Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
2.1.2. Animals
2.2. Development of O/W-NE
2.2.1. Preparation of O/W-NE
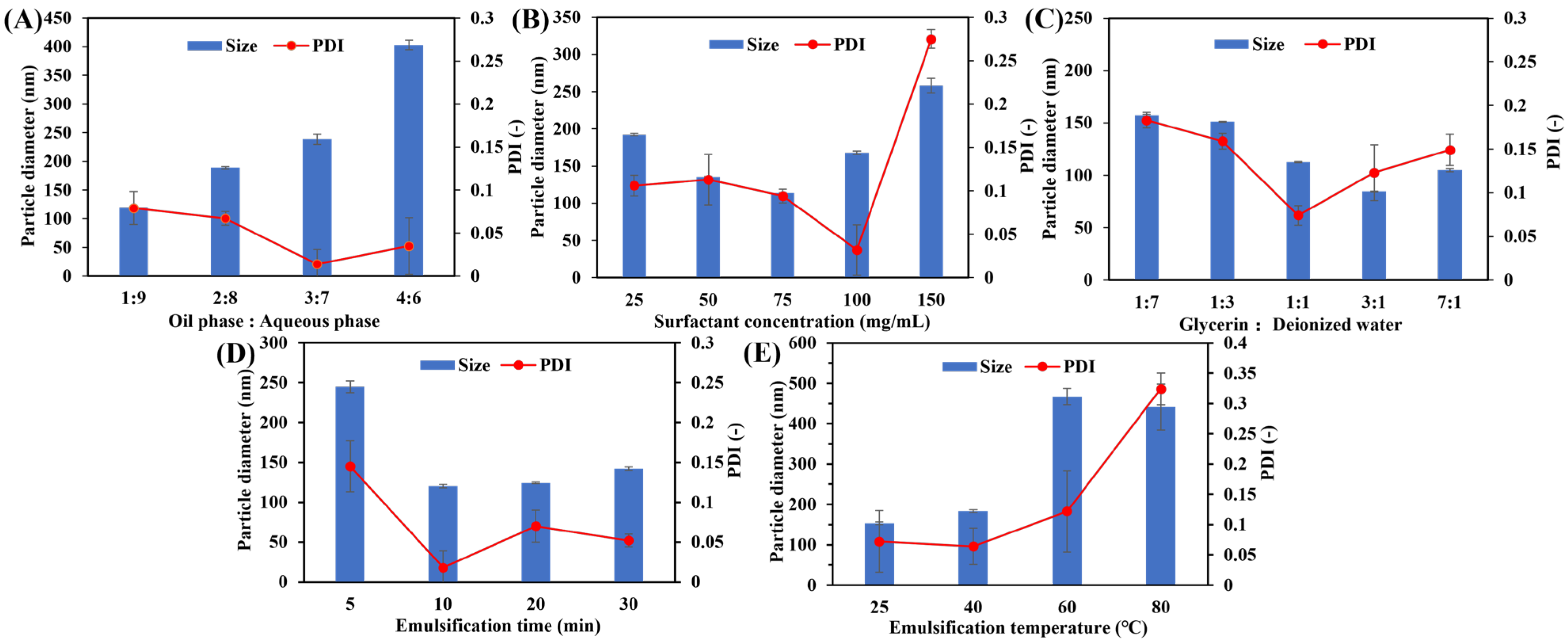
2.2.2. Optimization of Formulation
2.3. Characterization of O/W-NEs
2.3.1. Measurement of Particle Size and PDI
2.3.2. Solubility of Ceramide C2 in O/W-NE
2.3.3. Morphology of Ceramide C2-Loaded O/W-NE
2.4. Encapsulation Efficiency (EE) of Ceramide C2-Loaded O/W-NE
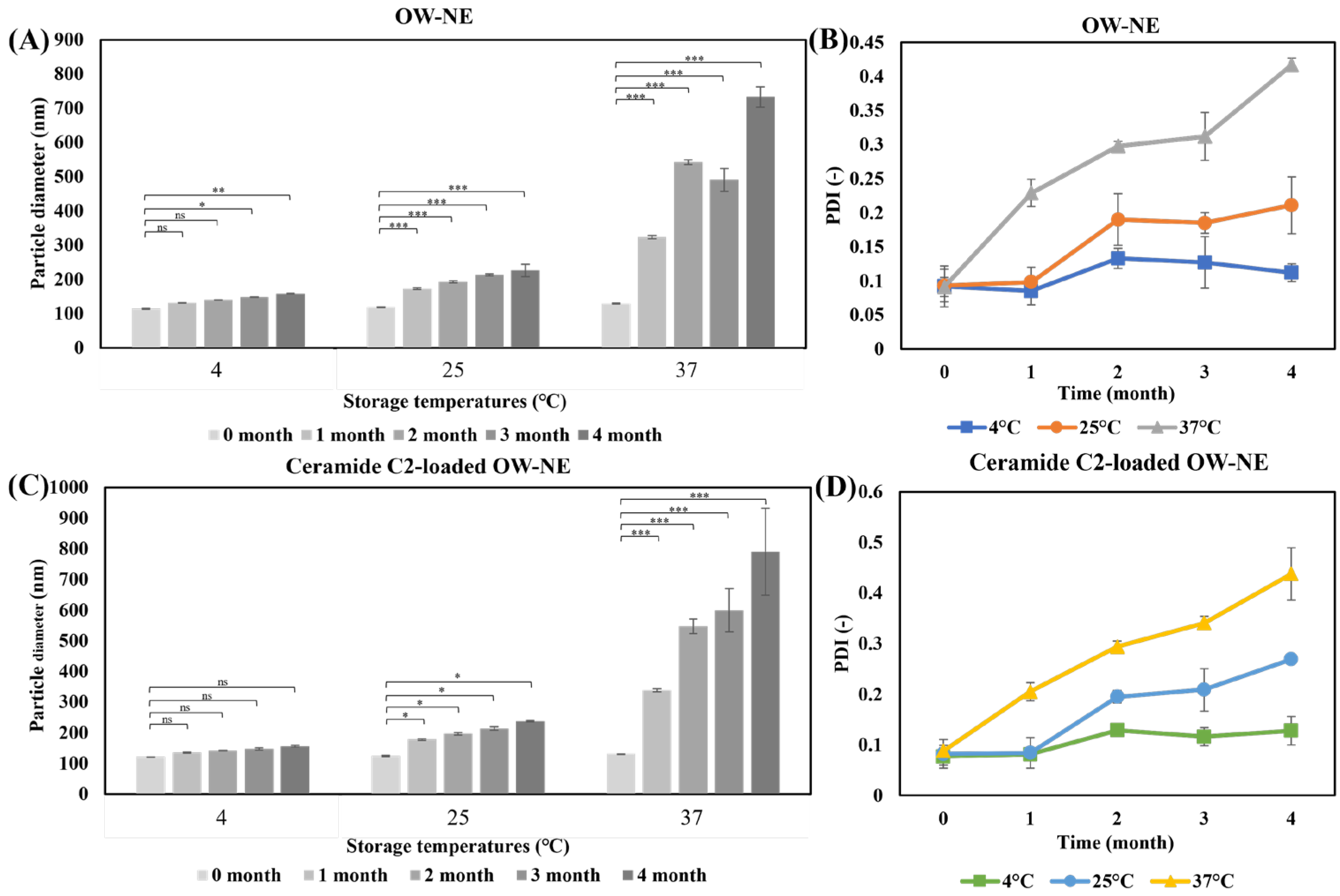
2.5. Stability of O/W-NE
2.6. In Vitro Drug Release from O/W-NE
2.7. Ex Vivo Permeability Study
2.7.1. Permeability Analysis of O/W-NEs
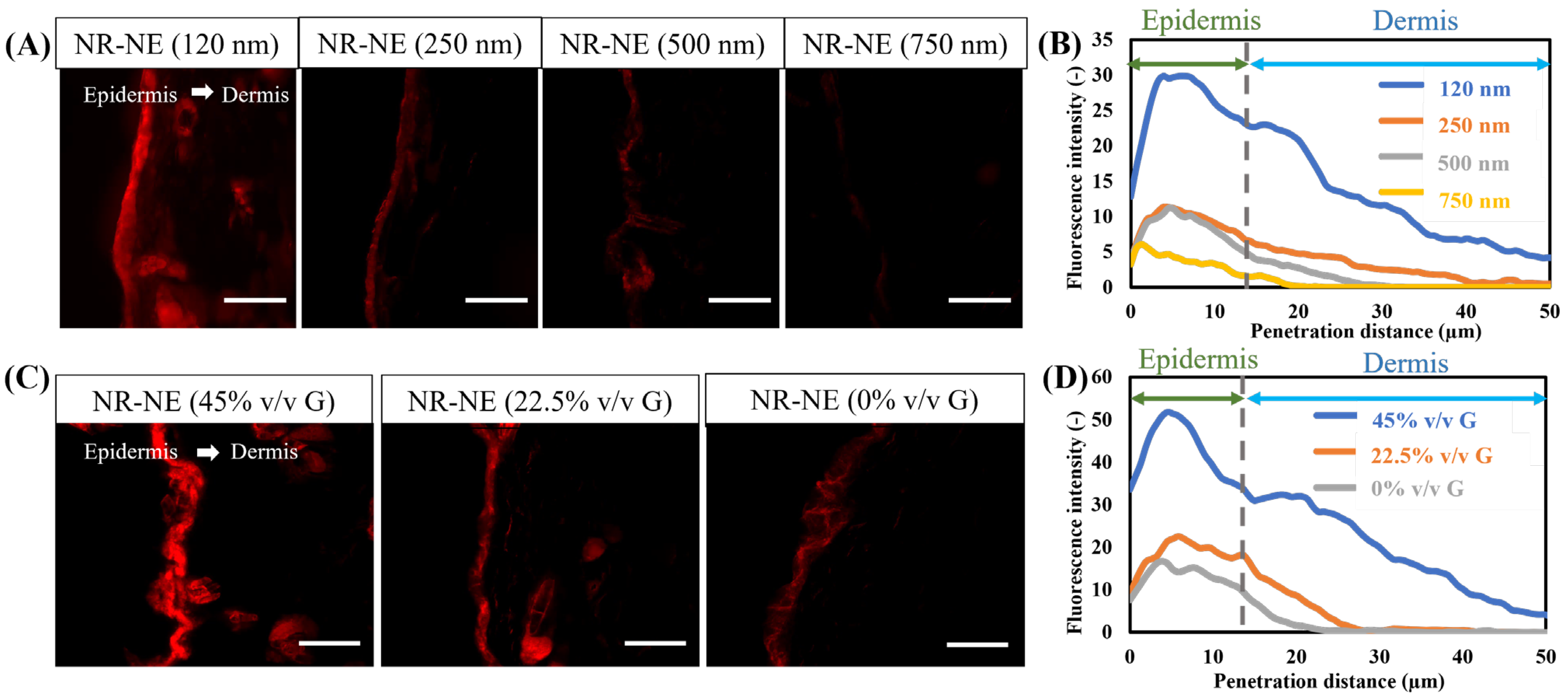
2.7.2. Penetration of NBD-Ceramide-Loaded O/W-NE
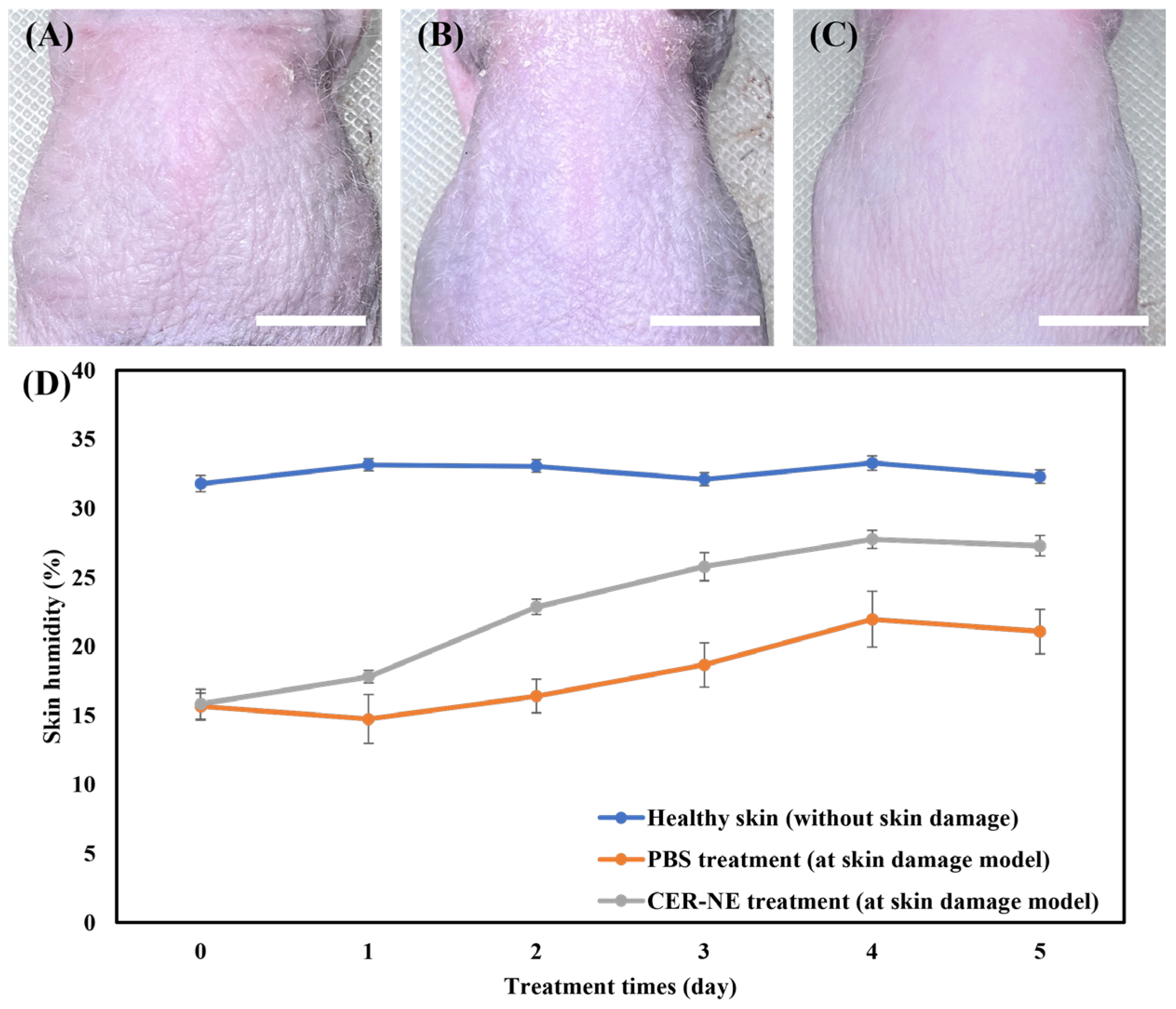
2.8. Skin Surface Irritation Test In Vivo
2.9. Efficacy Evaluation of Ceramide C2-Loaded O/W-NE In Vivo
2.10. Statistical Analysis
3. Results and Discussion
3.1. Development of Nanoemulsion Formulation
3.2. Characterization and Morphology of O/W-NE and Ceramide C2-Loaded O/W-NE
3.3. EE of Ceramide C2-Loaded O/W-NE
3.4. Long-Term Stability of O/W-NEs Under Different Temperature Conditions
3.5. In Vitro Drug Release
3.6. Permeability Analysis of O/W-NE
3.7. In Vitro Permeability Study of Ceramide Mediated-Loaded O/W-NE
3.8. Non-Irritating Skin Effect of C2-Loaded O/W-NE In Vivo
3.9. Efficacy Evaluation of Ceramide C2-Loaded O/W-NE in Enhancing Skin Hydration Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| O/W-NE | Oil-in-water nanoemulsion |
| TEWL | Trans-epidermal water loss |
| ICR | Institute of Cancer Research |
| HR-1 | Hairless-1 |
| PDI | Polydispersity index |
| TEM | Transmission electron microscopy |
| EE | Encapsulation efficiency |
| CLSM | Confocal laser scanning microscopy |
| H&E | Hematoxylin and eosin |
| S.D. | Standard deviation |
| PBS | Phosphate buffered saline |
References
- Elias, P.M.; Wakefield, J.S. Mechanisms of Abnormal Lamellar Body Secretion and the Dysfunctional Skin Barrier in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2014, 134, 781–791.e1. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Nădăban, A.; Bras, W.; MCabe, C.; Bunge, A.; Gooris, G.S. The Skin Barrier: An Extraordinary Interface with an Exceptional Lipid Organization. Prog. Lipid Res. 2023, 92, 101252. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, W.T.; Sawitri, S.; Astindari, A.; Utomo, B.; Listiawan, M.Y.; Ervianti, E.; Astari, L. The Efficacy of Moisturisers Containing Ceramide Compared with Other Moisturisers in the Management of Atopic Dermatitis: A Systematic Literature Review and Meta-Analysis. Indian J. Dermatol. 2023, 68, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yan, Y.; Li, J.; Cao, Z.; Shen, S.; Chang, Q.; Zhao, Y.; Wang, X.; Wang, P. Nuclear Receptors for Epidermal Lipid Barrier: Advances in Mechanisms and Applications. Exp. Dermatol. 2024, 33, e15107. [Google Scholar] [CrossRef]
- Sugiura, A.; Nomura, T.; Mizuno, A.; Imokawa, G. Reevaluation of the Non-Lesional Dry Skin in Atopic Dermatitis by Acute Barrier Disruption: An Abnormal Permeability Barrier Homeostasis with Defective Processing to Generate Ceramide. Arch. Dermatol. Res. 2014, 306, 427–440. [Google Scholar] [CrossRef]
- Mijaljica, D.; Townley, J.P.; Hondros, A.; Hewson, C.; Harrison, I.P.; Spada, F. Considering Phytosphingosine-Based Ceramide Formulations for Atopic Skin Care. Dermato 2024, 4, 5–22. [Google Scholar] [CrossRef]
- Fujii, M. The Pathogenic and Therapeutic Implications of Ceramide Abnormalities in Atopic Dermatitis. Cells 2021, 10, 2386. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Levin, J. The Clinical Relevance of Maintaining the Functional Integrity of the Stratum Corneum in Both Healthy and Disease-Affected Skin. J. Clin. Aesthet. Dermatol. 2011, 4, 22–42. [Google Scholar]
- Schild, J.; Kalvodová, A.; Zbytovská, J.; Farwick, M.; Pyko, C. The Role of Ceramides in Skin Barrier Function and the Importance of Their Correct Formulation for Skincare Applications. Int. J. Cosmet. Sci. 2024, 46, 526–543. [Google Scholar] [CrossRef]
- Norlén, L.; Lundborg, M.; Wennberg, C.; Narangifard, A.; Daneholt, B. The Skin’s Barrier: A Cryo-EM Based Overview of Its Architecture and Stepwise Formation. J. Investig. Dermatol. 2022, 142, 285–292. [Google Scholar] [CrossRef]
- Spada, F.; Barnes, T.M.; Greive, K.A. Skin Hydration Is Significantly Increased by a Cream Formulated to Mimic the Skin’s Own Natural Moisturizing Systems. Clin. Cosmet. Investig. Dermatol. 2018, 11, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Mao, G.; Pappas, A.; Mack, M.C.; Walters, R.M.; Southall, M.D. Topically Applied Ceramide Accumulates in Skin Glyphs. Clin. Cosmet. Investig. Dermatol. 2015, 8, 329–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kahraman, E.; Kaykın, M.; Şahin Bektay, H.; Güngör, S. Recent Advances on Topical Application of Ceramides to Restore Barrier Function of Skin. Cosmetics 2019, 6, 52. [Google Scholar] [CrossRef]
- Musielak, E.; Krajka-Kuźniak, V. Liposomes and Ethosomes: Comparative Potential in Enhancing Skin Permeability for Therapeutic and Cosmetic Applications. Cosmetics 2024, 11, 191. [Google Scholar] [CrossRef]
- Tessema, E.N.; Gebre-Mariam, T.; Paulos, G.; Wohlrab, J.; Neubert, R.H.H. Delivery of Oat-Derived Phytoceramides into the Stratum Corneum of the Skin Using Nanocarriers: Formulation, Characterization and in Vitro and Ex-Vivo Penetration Studies. Eur. J. Pharm. Biopharm. 2018, 127, 260–269. [Google Scholar] [CrossRef]
- Editor, P. Nanoemulsion: A Comprehensive Review of Formation, Properties, and Applications. PEXACY Int. J. Pharm. Sci. 2023, 2, 63–82. [Google Scholar]
- Gupta, A.; Burak Eral, H.; Alan Hatton, T.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent Insights into Nanoemulsions: Their Preparation, Properties and Applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef]
- Roy, A.; Nishchaya, K.; Rai, V.K. Nanoemulsion-Based Dosage Forms for the Transdermal Drug Delivery Applications: A Review of Recent Advances. Expert Opin. Drug Deliv. 2022, 19, 303–319. [Google Scholar] [CrossRef]
- Shakeel, F.; Ramadan, W. Transdermal Delivery of Anticancer Drug Caffeine from Water-in-Oil Nanoemulsions. Colloids Surf. B Biointerfaces 2010, 75, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Filipe, P.; Rosado, C.; Pereira-Leite, C. Nanodelivery Strategies for Skin Diseases with Barrier Impairment: Focusing on Ceramides and Glucocorticoids. Nanomaterials 2022, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Yafuji, M.; Hatto, S. Method for Producing a Dispersion of Ceramide Composition; WO2015136778A1; European Patent Office: Munich, Germany, 2009. [Google Scholar]
- Fardous, J.; Omoso, Y.; Joshi, A.; Yoshida, K.; Patwary, M.K.A.; Ono, F.; Ijima, H. Development and Characterization of Gel-in-Water Nanoemulsion as a Novel Drug Delivery System. Mater. Sci. Eng. C 2021, 124, 112076. [Google Scholar] [CrossRef] [PubMed]
- Bolourchian, N.; Bahjat, M. Design and In Vitro Evaluation of Eudragit-Based Extended Release Diltiazem Microspheres for Once- and Twice-Daily Administration: The Effect of Coating on Drug Release Behavior. Turk. J. Pharm. Sci. 2019, 16, 340–347. [Google Scholar] [CrossRef]
- Rodrigues, F.V.S.; Diniz, L.S.; Sousa, R.M.G.; Honorato, T.D.; Simao, D.O.; Araujo, C.R.M.; Goncalves, T.M.; Rolim, L.A.; Goto, P.L.; Tedesco, A.C.; et al. Preparation and Characterization of Nanoemulsion Containing a Natural Naphthoquinone. Quim. Nova 2018, 41, 756–761. [Google Scholar] [CrossRef]
- Fardous, J.; Omoso, Y.; Yoshida, K.; Ono, F.; Patwary, M.K.A.; Ijima, H. Gel-in-Water Nanodispersion for Potential Application in Intravenous Delivery of Anticancer Drugs. J. Biosci. Bioeng. 2022, 133, 174–180. [Google Scholar] [CrossRef]
- Kini, S.; Bahadur, D.; Panda, D. Mechanism of Anti-Cancer Activity of Benomyl Loaded Nanoparticles in Multidrug Resistant Cancer Cells. J. Biomed. Nanotechnol. 2015, 11, 877–889. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.; Peterchristoper, G.V. Release Kinetics—Concepts And Applications. Int. J. Pharm. Res. Technol. (IJPRT) 2018, 8, 12–20. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Varelas, C.; Dixon, D.; Steiner, C. Zero-Order Release from Biphasic Polymer Hydrogels. J. Control. Release 1995, 34, 185–192. [Google Scholar] [CrossRef]
- Mulye, N.V.; Turco, S.J. A Simple Model Based on First Order Kinetics to Explain Release of Highly Water Soluble Drugs from Porous Dicalcium Phosphate Dihydrate Matrices. Drug Dev. Ind. Pharm. 1995, 21, 943–953. [Google Scholar] [CrossRef]
- Korsmeyer, R.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Solute and Penetrant Diffusion in Swellable Polymers. III. Drug Release from Glassy Poly(HEMA-Co-NVP) Copolymers. J. Control. Release 1984, 1, 89–98. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Langenbucher, F. Linearization of Dissolution Rate Curves by the Weibull Distribution. J. Pharm. Pharmacol. 1972, 24, 979–981. [Google Scholar] [CrossRef]
- Cohen, S.; Yoshioka, T.; Lucarelli, M.; Hwang, L.H.; Langer, R. Controlled Delivery Systems for Proteins Based on Poly(Lactic/Glycolic Acid) Microspheres. Pharm. Res. 1991, 8, 713–720. [Google Scholar] [CrossRef]
- Sibanda, W.; Pillay, V.; Danckwerts, M.P.; Viljoen, A.M.; van Vuuren, S.; Khan, R.A. Experimental Design for the Formulation and Optimization of Novel Cross-Linked Oilispheres Developed for in Vitro Site-Specific Release ofMentha Piperita Oil. AAPS PharmSciTech 2009, 5, 18. [Google Scholar] [CrossRef][Green Version]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Effect of Glycerol on Formation, Stability, and Properties of Vitamin-E Enriched Nanoemulsions Produced Using Spontaneous Emulsification. J. Colloid Interface Sci. 2013, 411, 105–113. [Google Scholar] [CrossRef]
- Sidhu, M.S.; Roy, M.M.; Elsanusi, O.A. Comparative Analysis of Water and Glycerin Emulsification: Particle Size, Stability, Engine Performance, and Emissions in Biodiesel Fuels. J. Renew. Energy 2024, 2024, 2357238. [Google Scholar] [CrossRef]
- Zafeiri, I.; Smith, P.; Norton, I.T.; Spyropoulos, F. Fabrication, Characterisation and Stability of Oil-in-Water Emulsions Stabilised by Solid Lipid Particles: The Role of Particle Characteristics and Emulsion Microstructure upon Pickering Functionality. Food Funct. 2017, 8, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.; Graves, S.; Wilking, J.; Lin, M. Extreme Emulsification: Formation and Structure of Nanoemulsions. Condens. Matter Phys. 2006, 9, 193–199. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Adsorption and Aggregation Properties of Some Polysorbates at Different Temperatures. J. Solut. Chem. 2018, 47, 1824–1840. [Google Scholar] [CrossRef]
- Luz, A.M.; Barbosa, G.; Manske, C.; Tavares, F.W. Tween-80 on Water/Oil Interface: Structure and Interfacial Tension by Molecular Dynamics Simulations. Langmuir 2023, 39, 3255–3265. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Shah, J.; Nair, A.B. Innovations in Nanoemulsion Technology: Enhancing Drug Delivery for Oral, Parenteral, and Ophthalmic Applications. Pharmaceutics 2024, 16, 1333. [Google Scholar] [CrossRef]
- Yilmaz, E.; Borchert, H.-H. Design of a Phytosphingosine-Containing, Positively-Charged Nanoemulsion as a Colloidal Carrier System for Dermal Application of Ceramides. Eur. J. Pharm. Biopharm. 2005, 60, 91–98. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as Vehicles for Transdermal Delivery of Aceclofenac. AAPS PharmSciTech 2007, 8, 104. [Google Scholar] [CrossRef]
- Hosmer, J.; Reed, R.; Bentley, M.V.L.B.; Nornoo, A.; Lopes, L.B. Microemulsions Containing Medium-Chain Glycerides as Transdermal Delivery Systems for Hydrophilic and Hydrophobic Drugs. AAPS PharmSciTech 2009, 10, 589–596. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic Light Scattering and Transmission Electron Microscopy in Drug Delivery: A Roadmap for Correct Characterization of Nanoparticles and Interpretation of Results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Zhang, S.; Li, J.; Zheng, H.; Jin, H.; Xu, J. Comparison of Different Protein Emulsifiers on Physicochemical Properties of β-Carotene-Loaded Nanoemulsion: Effect on Formation, Stability, and In Vitro Digestion. Nanomaterials 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Walia, N.; Dasgupta, N.; Ranjan, S.; Ramalingam, C.; Gandhi, M. Methods for Nanoemulsion and Nanoencapsulation of Food Bioactives. Environ. Chem. Lett. 2019, 17, 1471–1483. [Google Scholar] [CrossRef]
- Gaur, P.K.; Mishra, S.; Verma, A.; Verma, N. Ceramide–Palmitic Acid Complex Based Curcumin Solid Lipid Nanoparticles for Transdermal Delivery: Pharmacokinetic and Pharmacodynamic Study. J. Exp. Nanosci. 2016, 11, 38–53. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of Nanoemulsions Stabilized by Model Food-Grade Emulsifiers Using High-Pressure Homogenization: Factors Affecting Particle Size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering Emulsions: Versatility of Colloidal Particles and Recent Applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Zhou, Y. Research Progress in Preparation, Stability and Application of Nanoemulsion. J. Phys. Conf. Ser. 2022, 2152, 012044. [Google Scholar] [CrossRef]
- Li, P.-H.; Lu, W.-C. Effects of Storage Conditions on the Physical Stability of D-Limonene Nanoemulsion. Food Hydrocoll. 2016, 53, 218–224. [Google Scholar] [CrossRef]
- Fard, G.H.; Moinipoor, Z.; Anastasova-Ivanova, S.; Iqbal, H.M.N.; Dwek, M.V.; Getting, S.; Keshavarz, T. Development of Chitosan, Pullulan, and Alginate Based Drug-Loaded Nano-Emulsions as a Potential Malignant Melanoma Delivery Platform. Carbohydr. Polym. Technol. Appl. 2022, 4, 100250. [Google Scholar] [CrossRef]
- Varma, M.V.S.; Kaushal, A.M.; Garg, A.; Garg, S. Factors Affecting Mechanism and Kinetics of Drug Release from Matrix-Based Oral Controlled Drug Delivery Systems. Am. J. Drug Deliv. 2004, 2, 43–57. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.-W.; Huang, M.-T.; Ho, C.-T.; Huang, Q. Enhancing Anti-Inflammation Activity of Curcumin through O/W Nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.S.; Weisspapir, M.R.; Friedman, D.I. Enhanced Transdermal Delivery of Diazepam by Submicron Emulsion (SME) Creams. Pharm. Res. 1995, 12, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-Formulations for Transdermal Drug Delivery: A Review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Roussel, L.; Atrux-Tallau, N.; Pirot, F. Glycerol as a Skin Barrier Influencing Humectant. In Treatment of Dry Skin Syndrome: The Art and Science of Moisturizers; Lodén, M., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 473–480. ISBN 978-3-642-27606-4. [Google Scholar]
- Yanagiya, S.; Takahashi, A.; Goto, N. Mechanical Properties of Stratum Corneum in Glycerin Solution by Atomic Force Microscopy. e-J. Surf. Sci. Nanotechnol. 2015, 13, 461–464. [Google Scholar] [CrossRef][Green Version]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielinska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, S.; Wang, G.; Yan, Z.; Wu, G.; Tang, L.; Wang, W. Nanocarrier-Based Transdermal Drug Delivery Systems for Dermatological Therapy. Pharmaceutics 2024, 16, 1384. [Google Scholar] [CrossRef]
- Klang, V.; Schwarz, J.C.; Valenta, C. Nanoemulsions in Dermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 255–266. ISBN 978-3-662-45013-0. [Google Scholar]
- Zhou, H.; Yue, Y.; Liu, G.; Li, Y.; Zhang, J.; Gong, Q.; Yan, Z.; Duan, M. Preparation and Characterization of a Lecithin Nanoemulsion as a Topical Delivery System. Nanoscale Res. Lett. 2009, 5, 224–230. [Google Scholar] [CrossRef]
- Takagi, Y.; Nakagawa, H.; Higuchi, K.; Imokawa, G. Characterization of Surfactant-Induced Skin Damage through Barrier Recovery Induced by Pseudoacylceramides. Dermatology 2005, 211, 128–134. [Google Scholar] [CrossRef]




| Items | Content |
|---|---|
| Oil and aqueous phases at volume ratios | 1:9, 2:8, 3:7, 4:6 |
| Surfactant concentration | 25, 50, 75, 100, 150 mg/mL |
| Glycerin and deionized water at volume ratios | 1:7, 1:3, 1:1, 3:1, 7:1 |
| Ultrasonic temperature | 25, 40, 60, 80 °C |
| Ultrasonic time | 5, 10, 15, 20 min |
| Sample | Particle Diameter | PDI | Zeta Potential |
|---|---|---|---|
| O/W-NE | 112.5 ± 1.5 nm | 0.094 ± 0.013 | −17.0 ± 1.8 mV |
| Ceramide C2-loaded O/W-NE | 117.3 ± 0.6 nm | 0.098 ± 0.015 | −17.6 ± 1.2 mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wu, L.; Zhang, Y.; Hu, J.; Fardous, J.; Ikegami, Y.; Ijima, H. Topical Delivery of Ceramide by Oil-in-Water Nanoemulsion to Retain Epidermal Moisture Content in Dermatitis. Biomolecules 2025, 15, 608. https://doi.org/10.3390/biom15050608
Zhou Y, Wu L, Zhang Y, Hu J, Fardous J, Ikegami Y, Ijima H. Topical Delivery of Ceramide by Oil-in-Water Nanoemulsion to Retain Epidermal Moisture Content in Dermatitis. Biomolecules. 2025; 15(5):608. https://doi.org/10.3390/biom15050608
Chicago/Turabian StyleZhou, Yu, Lichun Wu, Yi Zhang, Jia Hu, Jannatul Fardous, Yasuhiro Ikegami, and Hiroyuki Ijima. 2025. "Topical Delivery of Ceramide by Oil-in-Water Nanoemulsion to Retain Epidermal Moisture Content in Dermatitis" Biomolecules 15, no. 5: 608. https://doi.org/10.3390/biom15050608
APA StyleZhou, Y., Wu, L., Zhang, Y., Hu, J., Fardous, J., Ikegami, Y., & Ijima, H. (2025). Topical Delivery of Ceramide by Oil-in-Water Nanoemulsion to Retain Epidermal Moisture Content in Dermatitis. Biomolecules, 15(5), 608. https://doi.org/10.3390/biom15050608







