Unraveling the Molecular Mechanisms of Mosquito Salivary Proteins: New Frontiers in Disease Transmission and Control
Abstract
1. Introduction
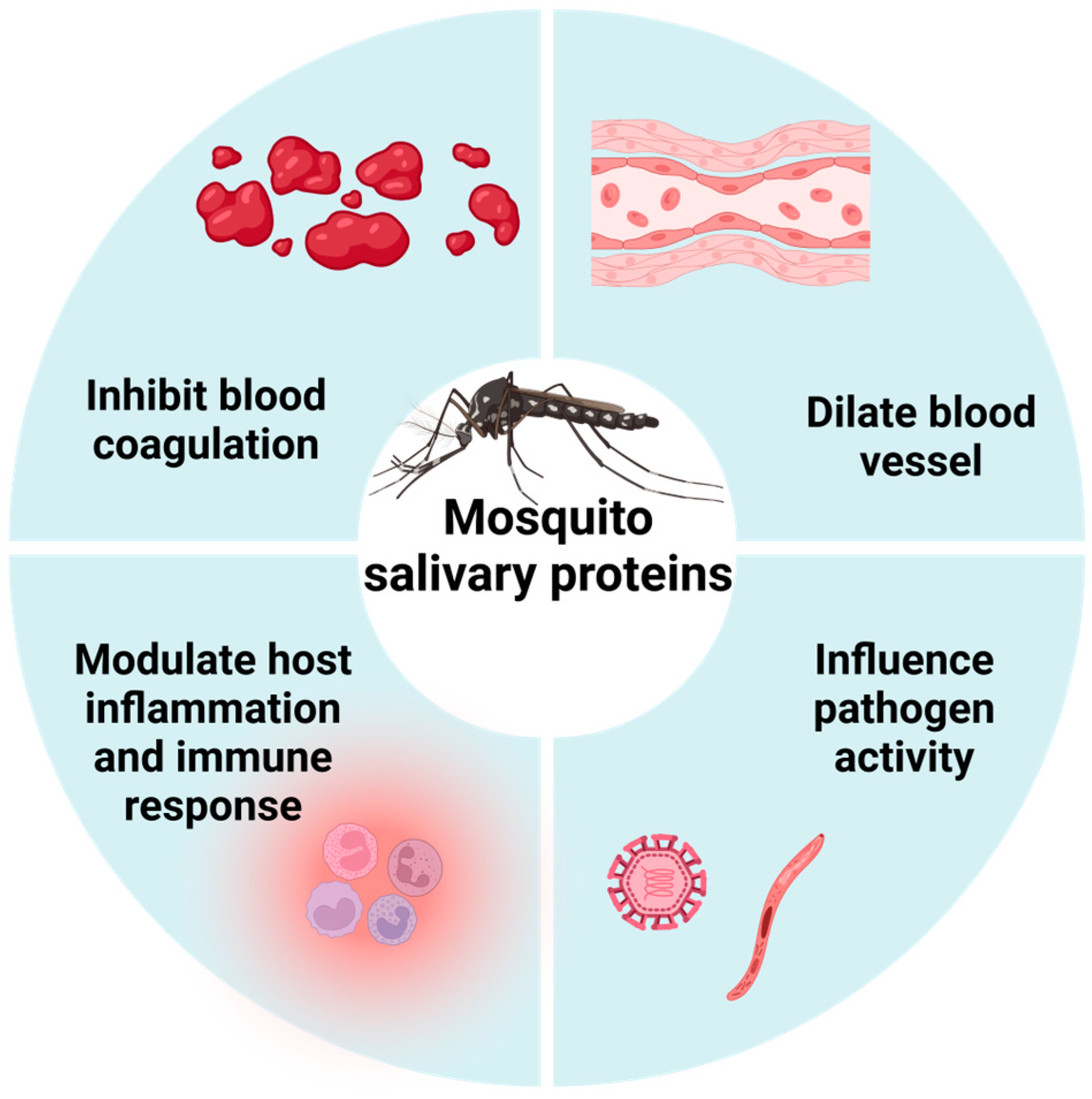
2. Types of MSPs and Their Physiological Functions
2.1. Anticoagulation
2.2. Vasodilation
2.3. Modulation of Inflammation and Immunity
2.4. Other Functions
2.5. Dynamic Changes in MSPs
| Category | Protein Name | Species | Function | Refs. |
|---|---|---|---|---|
| Anticoagulation | Aegyptin | Ae. aegypti | Binds collagen to block platelet aggregation | [21] |
| alALP | Ae. albopictus | Prolongs APTT, PT, TT, and BT | [22] | |
| AAPP | An. stephensi | Binds collagen to block platelet aggregation | [23] | |
| Apyrase | Ae. aegypti | Hydrolyzes ADP to inhibit platelet aggregation | [24] | |
| Hamadarin | An. stephensi | Anticoagulation by inhibiting FXII | [25] | |
| AngaD7L2 | An. gambiae | Interacts with FXII, FXIIa, and FXI to exert anticoagulant effect | [26] | |
| A serine protease inhibitor | Ae. aegypti | Anticoagulation by inhibiting FXa | [27] | |
| Anophelin | An. albimanus | Anticoagulation by inhibiting thrombin | [28,29] | |
| AaTI | Ae. aegypti | Prolongs PT, APTT, and TT | [30] | |
| Vasodilation | Sialokinin | Ae. aegypti | Induces vasodilation by activating NK-1R signaling pathway to release NO | [32,33,34] |
| AngaD7L1, AngaD7L3 | An. gambiae | Scavenges vasoconstrictors to inhibit vasoconstriction | [26] | |
| Peroxidase/catechol oxidase | An. albimanus | Scavenges biogenic amines to induce vasodilation | [35,36] | |
| Modulation of inflammation and immunity | SAAG-4 | Ae. aegypti | Programs CD4+ T cells to express IL-4 and reduce IFN-γ production | [55] |
| Other functions | An endonuclease | Cx. quinquefasciatus | May lower local viscosity to assist blood feeding | [58] |
| ADA | Cx. quinquefasciatus, Ae. aegypti | May reduce local pain and itching caused by adenosine | [60] | |
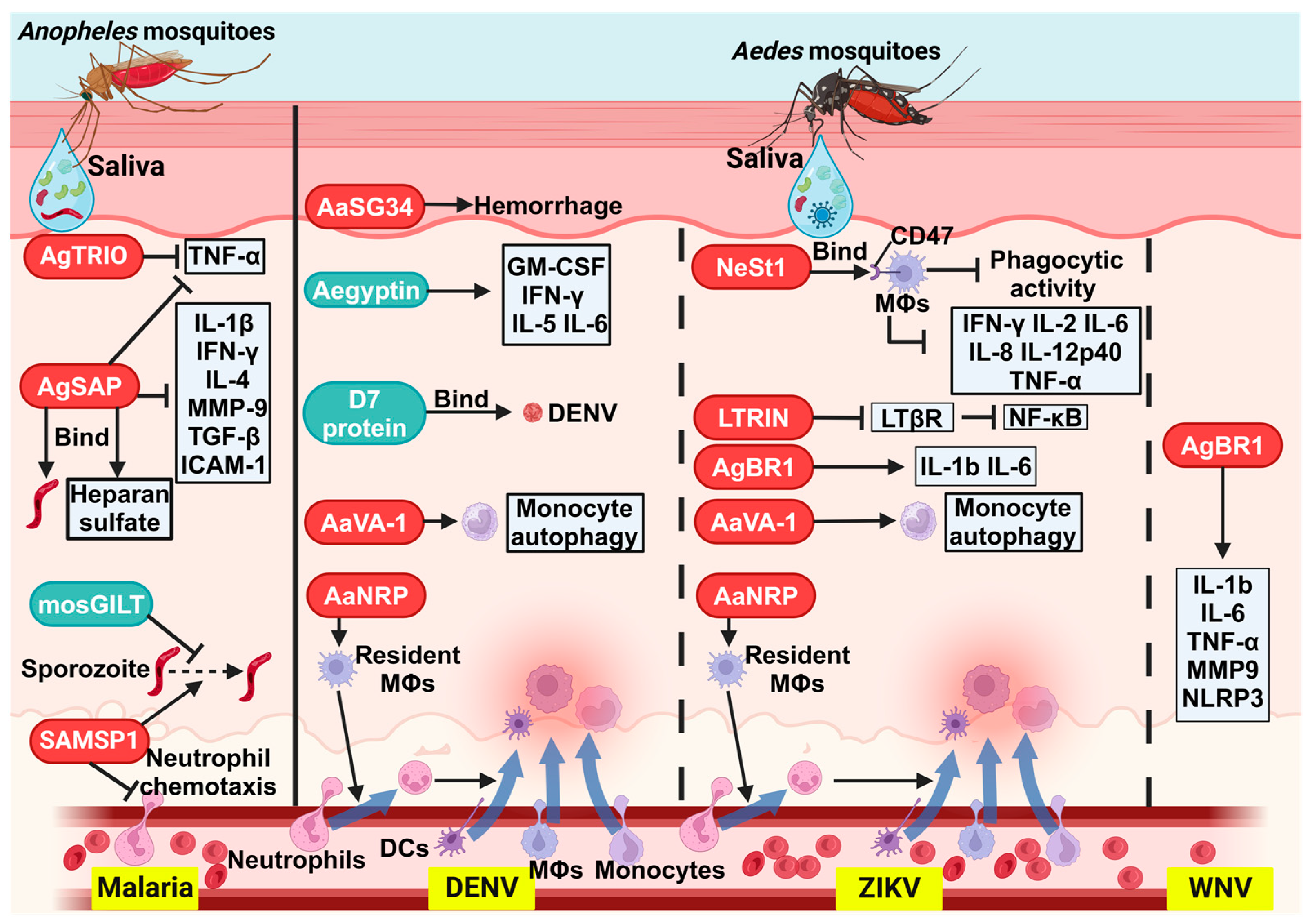
| Malaria | AgTRIO | An. gambiae | Inhibits TNF-α expression; facilitates Plasmodium infection; enhances mosquito host-seeking behavior | [67,68,69] |
| SAMSP-1 | An. gambiae | Enhances sporozoite gliding and traversal abilities; facilitates Plasmodium infection | [70] | |
| AgSAP | An. gambiae | Binds to sporozoites and heparan sulfate; inhibits local skin inflammatory responses; facilitates Plasmodium infection | [56] | |
| mosGILT | An. gambiae | Suppresses sporozoite speed and cell traversal activity, lowering the initial parasite burden in mice | [71] | |
| DENV | AT, ADA, 34-kDa protein, VA | Ae. aegypti | Promotes replication of DENV in human keratinocytes | [61] |
| AaSG34 | Ae. aegypti | Enhances DENV replication in mosquitoes and transmission in mice | [72] | |
| CLIPA3 | Ae. aegypti | Hydrolyzes extracellular matrix proteins, increasing virus binding to heparan sulfate proteoglycans; induces cell migration; enhances DENV infectivity | [57] | |
| AaVA-1 | Ae. aegypti | Activates autophagy in monocyte-derived cells, promoting dissemination of DENV in mice | [10] | |
| AaNRP | Ae. aegypti | Recruits neutrophils and other susceptible myeloid cells, promoting dissemination of DENV in mice | [73] | |
| A putative antibacterial cecropin-like peptide (AAEL000598) | Ae. aegypti | Inhibits DENV replication in C6/36 cells | [59] | |
| D7L1 | Ae. aegypti | Binds to DENV virions; inhibits DENV infection in U937 cells and mice | [74] | |
| Aegyptin | Ae. aegypti | Increases the expression of GM-CSF, IFN-γ, IL-5, and IL-6; inhibits DENV infection in mice | [75] | |
| WNV | AgBR1 | Ae. aegypti | Enhances WNV pathogenicity in mice | [76] |
| CHIKV | A putative antibacterial cecropin-like peptide (AAEL000598) | Ae. aegypti | Inhibits CHIKV infection in HEK-293T cells | [59] |
| ZIKV | LTRIN | Ae. aegypti | Interferes with LTβR, blocking NF-κB signaling and pro-inflammatory cytokine production, thereby enhancing ZIKV pathogenicity in mice | [77] |
| AgBR1 | Ae. aegypti | Enhances ZIKV pathogenicity in mice | [78] | |
| NeSt1 | Ae. aegypti | Suppresses local immune response, macrophage phagocytosis, and pro-inflammatory cytokine production, thereby enhancing ZIKV pathogenicity in mice | [54,79] | |
| AaVA-1 | Ae. aegypti | Activates autophagy in monocyte-derived cells, promoting dissemination of ZIKV in mice | [10] | |
| AaNRP | Ae. aegypti | Recruits neutrophils and other susceptible myeloid cells, promoting dissemination of ZIKV in mice | [73] |
3. Effects of MSPs on Pathogen Infection and Disease Transmission
3.1. Malaria
3.2. Dengue Fever
3.3. West Nile Fever
3.4. Chikungunya Fever
3.5. Zika Fever
3.6. Other Viral Infections
4. Surveillance Strategies for Mosquito-Borne Diseases by Targeting MSPs
5. Recent Advances in Vaccines Targeting MSPs
6. Conclusions and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caraballo, H.; King, K. Emergency department management of mosquito-borne illness: Malaria, dengue, and West Nile virus. Emerg. Med. Pract. 2014, 16, 1–23, quiz 23–24. [Google Scholar] [PubMed]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.Y.; Shehzad, A.; Islam, S.U.; Al-Suhaimi, E.A.; Lee, Y.S. Mosquirix™ RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy. Vaccines 2022, 10, 713. [Google Scholar] [CrossRef]
- Tully, D.; Griffiths, C.L. Dengvaxia: The world’s first vaccine for prevention of secondary dengue. Ther. Adv. Vaccines Immunother. 2021, 9, 25151355211015839. [Google Scholar] [CrossRef] [PubMed]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.-B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Ramos Lopez, F.; Natama, H.M.; Weston, S.; et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Francischetti, I.M. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.A. Vector-transmitted disease vaccines: Targeting salivary proteins in transmission (SPIT). Trends Parasitol. 2015, 31, 363–372. [Google Scholar] [CrossRef]
- Calvo, E.; Mans, B.J.; Andersen, J.F.; Ribeiro, J.M. Function and evolution of a mosquito salivary protein family. J. Biol. Chem. 2006, 281, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Arca, B.; Lombardo, F.; Struchiner, C.J.; Ribeiro, J.M. Anopheline salivary protein genes and gene families: An evolutionary overview after the whole genome sequence of sixteen Anopheles species. BMC Genom. 2017, 18, 153. [Google Scholar] [CrossRef]
- Sun, P.; Nie, K.; Zhu, Y.; Liu, Y.; Wu, P.; Liu, Z.; Du, S.; Fan, H.; Chen, C.H.; Zhang, R.; et al. A mosquito salivary protein promotes flavivirus transmission by activation of autophagy. Nat. Commun. 2020, 11, 260. [Google Scholar] [CrossRef]
- King, J.G.; Vernick, K.D.; Hillyer, J.F. Members of the salivary gland surface protein (SGS) family are major immunogenic components of mosquito saliva. J. Biol. Chem. 2011, 286, 40824–40834. [Google Scholar] [CrossRef]
- Arca, B.; Ribeiro, J.M. Saliva of hematophagous insects: A multifaceted toolkit. Curr. Opin. Insect Sci. 2018, 29, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Oseno, B.; Marura, F.; Ogwang, R.; Muturi, M.; Njunge, J.; Nkumama, I.; Mwakesi, R.; Mwai, K.; Rono, M.K.; Mwakubambanya, R.; et al. Characterization of Anopheles gambiae D7 salivary proteins as markers of human-mosquito bite contact. Parasites Vectors 2022, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, S.; Goheen, M.M.; Sy, M.; Deme, A.B.; Ndiaye, I.M.; Diedhiou, Y.; Mbaye, A.M.; Hagadorn, K.A.; Sene, S.D.; Pouye, M.N.; et al. Two mosquito salivary antigens demonstrate promise as biomarkers of recent exposure to P. falciparum infected mosquito bites. J. Infect. Dis. 2024, jiae525. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.S.; Higgs, S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Titus, R.G.; Bishop, J.V.; Mejia, J.S. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 2006, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Champagne, D.E. Antihemostatic strategies of blood-feeding arthropods. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 375–396. [Google Scholar] [CrossRef]
- Arca, B.; Colantoni, A.; Fiorillo, C.; Severini, F.; Benes, V.; Di Luca, M.; Calogero, R.A.; Lombardo, F. MicroRNAs from saliva of anopheline mosquitoes mimic human endogenous miRNAs and may contribute to vector-host-pathogen interactions. Sci. Rep. 2019, 9, 2955. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Arcà, B. Chapter 2 From Sialomes to the Sialoverse: An Insight into Salivary Potion of Blood-Feeding Insects. In Advances in Insect Physiology; Academic Press: Cambridge, MA, USA, 2009; Volume 37, pp. 59–118. [Google Scholar]
- Ha, Y.-R.; Oh, S.-R.; Seo, E.-S.; Kim, B.-H.; Lee, D.-K.; Lee, S.-J. Detection of heparin in the salivary gland and midgut of Aedes togoi. Korean J. Parasitol. 2014, 52, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chagas, A.C.; Ramirez, J.L.; Jasinskiene, N.; James, A.A.; Ribeiro, J.M.; Marinotti, O.; Calvo, E. Collagen-binding protein, Aegyptin, regulates probing time and blood feeding success in the dengue vector mosquito, Aedes aegypti. Proc. Natl. Acad. Sci. USA 2014, 111, 6946–6951. [Google Scholar] [CrossRef]
- Li, X.P.; Lin, D.; Zhang, Y.; Chen, S.Q.; Bai, H.Q.; Zhang, S.N.; Liu, W.Q.; Liang, S.H. Expression and characterization of anticoagulant activity of salivary protein alALP from Asian tiger mosquito Aedes albopictus. Trop. Biomed. 2020, 37, 116–126. [Google Scholar]
- Yoshida, S.; Sudo, T.; Niimi, M.; Tao, L.; Sun, B.; Kambayashi, J.; Watanabe, H.; Luo, E.; Matsuoka, H. Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito. Blood 2008, 111, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; McNicol, A.; James, A.A.; Peng, Z. Expression of functional recombinant mosquito salivary apyrase: A potential therapeutic platelet aggregation inhibitor. Platelets 2006, 17, 178–184. [Google Scholar] [CrossRef]
- Isawa, H.; Yuda, M.; Orito, Y.; Chinzei, Y. A mosquito salivary protein inhibits activation of the plasma contact system by binding to factor XII and high molecular weight kininogen. J. Biol. Chem. 2002, 277, 27651–27658. [Google Scholar] [CrossRef]
- Smith, L.B.; Duge, E.; Valenzuela-Leon, P.C.; Brooks, S.; Martin-Martin, I.; Ackerman, H.; Calvo, E. Novel salivary antihemostatic activities of long-form D7 proteins from the malaria vector Anopheles gambiae facilitate hematophagy. J. Biol. Chem. 2022, 298, 101971. [Google Scholar] [CrossRef]
- Stark, K.R.; James, A.A. A factor Xa-directed anticoagulant from the salivary glands of the yellow fever mosquito Aedes aegypti. Exp. Parasitol. 1995, 81, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.G.; Francischetti, I.M.; Ribeiro, J.M. Purification, cloning, and synthesis of a novel salivary anti-thrombin from the mosquito Anopheles albimanus. Biochemistry 1999, 38, 11209–11215. [Google Scholar] [CrossRef]
- Francischetti, I.M.; Valenzuela, J.G.; Ribeiro, J.M. Anophelin: Kinetics and mechanism of thrombin inhibition. Biochemistry 1999, 38, 16678–16685. [Google Scholar] [CrossRef]
- Watanabe, R.M.O.; Soares, T.S.; Morais-Zani, K.; Tanaka-Azevedo, A.M.; Maciel, C.; Capurro, M.L.; Torquato, R.J.S.; Tanaka, A.S. A novel trypsin Kazal-type inhibitor from Aedes aegypti with thrombin coagulant inhibitory activity. Biochimie 2010, 92, 933–939. [Google Scholar] [CrossRef]
- Ribeiro, J.M. Blood-feeding in mosquitoes: Probing time and salivary gland anti-haemostatic activities in representatives of three genera (Aedes, Anopheles, Culex). Med. Vet. Entomol. 2000, 14, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M. Characterization of a vasodilator from the salivary glands of the yellow fever mosquito Aedes aegypti. J. Exp. Biol. 1992, 165, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Champagne, D.E.; Ribeiro, J.M. Sialokinin I and II: Vasodilatory tachykinins from the yellow fever mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 1994, 91, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Martin-Martin, I.; Valenzuela Leon, P.C.; Amo, L.; Shrivastava, G.; Iniguez, E.; Aryan, A.; Brooks, S.; Kojin, B.B.; Williams, A.E.; Bolland, S.; et al. Aedes aegypti sialokinin facilitates mosquito blood feeding and modulates host immunity and vascular biology. Cell Rep. 2022, 39, 110648. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Nussenzveig, R.H. The salivary catechol oxidase/peroxidase activities of the mosquito Anopheles albimanus. J. Exp. Biol. 1993, 179, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Valenzuela, J.G. Purification and cloning of the salivary peroxidase/catechol oxidase of the mosquito Anopheles albimanus. J. Exp. Biol. 1999, 202, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.J. Type I hypersensitivity promotes Aedes aegypti blood feeding. Sci. Rep. 2021, 11, 14891. [Google Scholar] [CrossRef] [PubMed]
- Vander Does, A.; Labib, A.; Yosipovitch, G. Update on mosquito bite reaction: Itch and hypersensitivity, pathophysiology, prevention, and treatment. Front. Immunol. 2022, 13, 1024559. [Google Scholar] [CrossRef] [PubMed]
- Pingen, M.; Schmid, M.A.; Harris, E.; McKimmie, C.S. Mosquito Biting Modulates Skin Response to Virus Infection. Trends Parasitol. 2017, 33, 645–657. [Google Scholar] [CrossRef]
- Agarwal, A.; Joshi, G.; Nagar, D.P.; Sharma, A.K.; Sukumaran, D.; Pant, S.C.; Parida, M.M.; Dash, P.K. Mosquito saliva induced cutaneous events augment Chikungunya virus replication and disease progression. Infect. Genet. Evol. 2016, 40, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mota, J.; Sukupolvi-Petty, S.; Diamond, M.S.; Rico-Hesse, R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 2012, 86, 7637–7649. [Google Scholar] [CrossRef] [PubMed]
- McCracken, M.K.; Christofferson, R.C.; Chisenhall, D.M.; Mores, C.N. Analysis of Early Dengue Virus Infection in Mice as Modulated by Aedes aegypti Probing. J. Virol. 2014, 88, 1881–1889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demeure, C.E.; Brahimi, K.; Hacini, F.; Marchand, F.; Peronet, R.; Huerre, M.; St-Mezard, P.; Nicolas, J.F.; Brey, P.; Delespesse, G.; et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J. Immunol. 2005, 174, 3932–3940. [Google Scholar] [CrossRef]
- Pingen, M.; Bryden, S.R.; Pondeville, E.; Schnettler, E.; Kohl, A.; Merits, A.; Fazakerley, J.K.; Graham, G.J.; McKimmie, C.S. Host Inflammatory Response to Mosquito Bites Enhances the Severity of Arbovirus Infection. Immunity 2016, 44, 1455–1469. [Google Scholar] [CrossRef]
- Thangamani, S.; Higgs, S.; Ziegler, S.; Vanlandingham, D.; Tesh, R.; Wikel, S. Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus. PLoS ONE 2010, 5, e12137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeidner, N.S.; Higgs, S.; Happ, C.M.; Beaty, B.J.; Miller, B.R. Mosquito feeding modulates Th1 and Th2 cytokines in flavivirus susceptible mice: An effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice. Parasite Immunol. 1999, 21, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Simons, F.E.; Peng, Z. A mouse model of mosquito allergy for study of antigen-specific IgE and IgG subclass responses, lymphocyte proliferation, and IL-4 and IFN-gamma production. Int. Arch. Allergy Immunol. 1998, 116, 269–277. [Google Scholar] [CrossRef]
- Schneider, B.S.; Soong, L.; Coffey, L.L.; Stevenson, H.L.; McGee, C.E.; Higgs, S. Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile virus infection. PLoS ONE 2010, 5, e11704. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Simons, F.E. Mosquito allergy: Immune mechanisms and recombinant salivary allergens. Int. Arch. Allergy Immunol. 2004, 133, 198–209. [Google Scholar] [CrossRef]
- Guerrero, D.; Cantaert, T.; Missé, D. Aedes Mosquito Salivary Components and Their Effect on the Immune Response to Arboviruses. Front. Cell. Infect. Microbiol. 2020, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Depinay, N.; Hacini, F.; Beghdadi, W.; Peronet, R.; Mecheri, S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J. Immunol. 2006, 176, 4141–4146. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Kneubehl, A.R.; Spencer Clinton, J.L.; Paust, S.; Rico-Hesse, R. Mosquito saliva alone has profound effects on the human immune system. PLoS Negl. Trop. Dis. 2018, 12, e0006439. [Google Scholar] [CrossRef] [PubMed]
- Owhashi, M.; Harada, M.; Suguri, S.; Ohmae, H.; Ishii, A. The role of saliva of Anopheles stephensi in inflammatory response: Identification of a high molecular weight neutrophil chemotactic factor. Parasitol. Res. 2001, 87, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Marin-Lopez, A.; Huck, J.D.; Esterly, A.T.; Azcutia, V.; Rosen, C.; Garcia-Milian, R.; Sefik, E.; Vidal-Pedrola, G.; Raduwan, H.; Chen, T.-Y.; et al. The human CD47 checkpoint is targeted by an immunosuppressive Aedes aegypti salivary factor to enhance arboviral skin infectivity. Sci. Immunol. 2024, 9, eadk9872. [Google Scholar] [CrossRef] [PubMed]
- Boppana, V.D.; Thangamani, S.; Adler, A.J.; Wikel, S.K. SAAG-4 is a novel mosquito salivary protein that programmes host CD4 T cells to express IL-4. Parasite Immunol. 2009, 31, 287–295. [Google Scholar] [CrossRef]
- Arora, G.; Sajid, A.; Chuang, Y.M.; Dong, Y.; Gupta, A.; Gambardella, K.; DePonte, K.; Almeras, L.; Dimopolous, G.; Fikrig, E. Immunomodulation by Mosquito Salivary Protein AgSAP Contributes to Early Host Infection by Plasmodium. mBio 2021, 12, e0309121. [Google Scholar] [CrossRef]
- Conway, M.J.; Watson, A.M.; Colpitts, T.M.; Dragovic, S.M.; Li, Z.; Wang, P.; Feitosa, F.; Shepherd, D.T.; Ryman, K.D.; Klimstra, W.B.; et al. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J. Virol. 2014, 88, 164–175. [Google Scholar] [CrossRef]
- Calvo, E.; Ribeiro, J.M.C. A novel secreted endonuclease from Culex quinquefasciatus salivary glands. J. Exp. Biol. 2006, 209, 2651–2659. [Google Scholar] [CrossRef][Green Version]
- Luplertlop, N.; Surasombatpattana, P.; Patramool, S.; Dumas, E.; Wasinpiyamongkol, L.; Saune, L.; Hamel, R.; Bernard, E.; Sereno, D.; Thomas, F.; et al. Induction of a peptide with activity against a broad spectrum of pathogens in the Aedes aegypti salivary gland, following Infection with Dengue Virus. PLoS Pathog. 2011, 7, e1001252. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Charlab, R.; Valenzuela, J.G. The salivary adenosine deaminase activity of the mosquitoes Culex quinquefasciatus and Aedes aegypti. J. Exp. Biol. 2001, 204, 2001–2010. [Google Scholar] [CrossRef]
- Surasombatpattana, P.; Ekchariyawat, P.; Hamel, R.; Patramool, S.; Thongrungkiat, S.; Denizot, M.; Delaunay, P.; Thomas, F.; Luplertlop, N.; Yssel, H.; et al. Aedes aegypti saliva contains a prominent 34-kDa protein that strongly enhances dengue virus replication in human keratinocytes. J. Investig. Dermatol. 2014, 134, 281–284. [Google Scholar] [CrossRef]
- Chowdhury, A.; Modahl, C.M.; Misse, D.; Kini, R.M.; Pompon, J. High resolution proteomics of Aedes aegypti salivary glands infected with either dengue, Zika or chikungunya viruses identify new virus specific and broad antiviral factors. Sci. Rep. 2021, 11, 23696. [Google Scholar] [CrossRef]
- Mu, X.; Lin, Z.; Sun, Y.; Chen, L.; Lv, Q.; Ji, C.; Kuang, X.; Li, W.; Shang, Z.; Cheng, J.; et al. Aedes albopictus salivary adenosine deaminase is an immunomodulatory factor facilitating dengue virus replication. Sci. Rep. 2023, 13, 16660. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Wikel, S.K. Differential expression of Aedes aegypti salivary transcriptome upon blood feeding. Parasites Vectors 2009, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.A. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef] [PubMed]
- Wasinpiyamongkol, L.; Patramool, S.; Luplertlop, N.; Surasombatpattana, P.; Doucoure, S.; Mouchet, F.; Seveno, M.; Remoue, F.; Demettre, E.; Brizard, J.P.; et al. Blood-feeding and immunogenic Aedes aegypti saliva proteins. Proteomics 2010, 10, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.M.; Freudzon, M.; Yang, J.; Dong, Y.; Dimopoulos, G.; Fikrig, E. Anopheles gambiae Lacking AgTRIO Inefficiently Transmits Plasmodium berghei to Mice. Infect. Immun. 2019, 87, e00326-19. [Google Scholar] [CrossRef]
- Chuang, Y.-M.; Dong, Y.; Stone, H.; Abouneameh, S.; Tang, X.-D.; Raduwan, H.; Dimopoulos, G.; Fikrig, E. Anopheles gambiae lacking AgTRIO probe inefficiently on a mammalian host. Cell Rep. 2024, 43, 114600. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, S.M.; Agunbiade, T.A.; Freudzon, M.; Yang, J.; Hastings, A.K.; Schleicher, T.R.; Zhou, X.; Craft, S.; Chuang, Y.M.; Gonzalez, F.; et al. Immunization with AgTRIO, a Protein in Anopheles Saliva, Contributes to Protection against Plasmodium Infection in Mice. Cell Host Microbe 2018, 23, 523–535 e525. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.M.; Agunbiade, T.A.; Tang, X.D.; Freudzon, M.; Almeras, L.; Fikrig, E. The Effects of A Mosquito Salivary Protein on Sporozoite Traversal of Host Cells. J. Infect. Dis. 2021, 224, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, T.R.; Yang, J.; Freudzon, M.; Rembisz, A.; Craft, S.; Hamilton, M.; Graham, M.; Mlambo, G.; Tripathi, A.K.; Li, Y.; et al. A mosquito salivary gland protein partially inhibits Plasmodium sporozoite cell traversal and transmission. Nat. Commun. 2018, 9, 2908. [Google Scholar] [CrossRef]
- Sri-In, C.; Weng, S.C.; Chen, W.Y.; Wu-Hsieh, B.A.; Tu, W.C.; Shiao, S.H. A salivary protein of Aedes aegypti promotes dengue-2 virus replication and transmission. Insect Biochem. Mol. Biol. 2019, 111, 103181. [Google Scholar] [CrossRef]
- Wang, Z.; Nie, K.; Liang, Y.; Niu, J.; Yu, X.; Zhang, O.; Liu, L.; Shi, X.; Wang, Y.; Feng, X.; et al. A mosquito salivary protein-driven influx of myeloid cells facilitates flavivirus transmission. EMBO J. 2024, 43, 1690–1721. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.J.; Londono-Renteria, B.; Troupin, A.; Watson, A.M.; Klimstra, W.B.; Fikrig, E.; Colpitts, T.M. Aedes aegypti D7 Saliva Protein Inhibits Dengue Virus Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004941. [Google Scholar] [CrossRef] [PubMed]
- McCracken, M.K.; Christofferson, R.C.; Grasperge, B.J.; Calvo, E.; Chisenhall, D.M.; Mores, C.N. Aedes aegypti salivary protein “aegyptin” co-inoculation modulates dengue virus infection in the vertebrate host. Virology 2014, 468–470, 133–139. [Google Scholar] [CrossRef]
- Uraki, R.; Hastings, A.K.; Brackney, D.E.; Armstrong, P.M.; Fikrig, E. AgBR1 antibodies delay lethal Aedes aegypti-borne West Nile virus infection in mice. NPJ Vaccines 2019, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Guo, X.; Shen, C.; Hao, X.; Sun, P.; Li, P.; Xu, T.; Hu, C.; Rose, O.; Zhou, H.; et al. Salivary factor LTRIN from Aedes aegypti facilitates the transmission of Zika virus by interfering with the lymphotoxin-β receptor. Nat. Immunol. 2018, 19, 342–353. [Google Scholar] [CrossRef]
- Uraki, R.; Hastings, A.K.; Marin-Lopez, A.; Sumida, T.; Takahashi, T.; Grover, J.R.; Iwasaki, A.; Hafler, D.A.; Montgomery, R.R.; Fikrig, E. Aedes aegypti AgBR1 antibodies modulate early Zika virus infection of mice. Nat. Microbiol. 2019, 4, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Hastings, A.K.; Uraki, R.; Gaitsch, H.; Dhaliwal, K.; Stanley, S.; Sproch, H.; Williamson, E.; MacNeil, T.; Marin-Lopez, A.; Hwang, J.; et al. Aedes aegypti NeSt1 Protein Enhances Zika Virus Pathogenesis by Activating Neutrophils. J. Virol. 2019, 93, e00395-19. [Google Scholar] [CrossRef]
- Crompton, P.D.; Moebius, J.; Portugal, S.; Waisberg, M.; Hart, G.; Garver, L.S.; Miller, L.H.; Barillas-Mury, C.; Pierce, S.K. Malaria immunity in man and mosquito: Insights into unsolved mysteries of a deadly infectious disease. Annu. Rev. Immunol. 2014, 32, 157–187. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Malaria. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 30 November 2024).
- White, N.J.; Pukrittayakamee, S.; Hien, T.T.; Faiz, M.A.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet 2014, 383, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.S.; Mathieu, C.; Peronet, R.; Mecheri, S. Anopheles stephensi saliva enhances progression of cerebral malaria in a murine model. Vector Borne Zoonotic Dis. 2011, 11, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Billman, Z.P.; Seilie, A.M.; Murphy, S.C. Purification of Plasmodium Sporozoites Enhances Parasite-Specific CD8+ T Cell Responses. Infect. Immun. 2016, 84, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Alger, N.E.; Harant, J.A.; Willis, L.C.; Jorgensen, G.M. Sporozoite and normal salivary gland induced immunity in malaria. Nature 1972, 238, 341. [Google Scholar] [CrossRef]
- Kebaier, C.; Voza, T.; Vanderberg, J. Neither Mosquito Saliva nor Immunity to Saliva Has a Detectable Effect on the Infectivity of Plasmodium Sporozoites Injected into Mice. Infect. Immun. 2010, 78, 545–551. [Google Scholar] [CrossRef]
- Pollock, T.; Leitao, R.; Galan-Rodriguez, C.; Wong, K.A.; Rodriguez, A. Daily Plasmodium yoelii infective mosquito bites do not generate protection or suppress previous immunity against the liver stage. Malaria J. 2011, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Messmore, A.S.; Scrafford, D.A.; Sacks, D.L.; Kamhawi, S.; McDowell, M.A. Uninfected mosquito bites confer protection against infection with malaria parasites. Infect. Immun. 2007, 75, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Schleicher, T.R.; Dong, Y.; Park, H.B.; Lan, J.; Cresswell, P.; Crawford, J.; Dimopoulos, G.; Fikrig, E. Disruption of mosGILT in Anopheles gambiae impairs ovarian development and Plasmodium infection. J. Exp. Med. 2020, 217, e20190682. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Simoes, M.L.; Marois, E.; Dimopoulos, G. CRISPR/Cas9 -mediated gene knockout of Anopheles gambiae FREP1 suppresses malaria parasite infection. PLoS Pathog. 2018, 14, e1006898. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, S.A.M. Dengue fever. BMJ 2015, 351, h4661. [Google Scholar] [CrossRef] [PubMed]
- Dengue and Severe Dengue. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 26 November 2024).
- Normile, D. Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts. Science 2013, 342, 415. [Google Scholar] [CrossRef]
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell Mol. Life Sci. 2010, 67, 2773–2786. [Google Scholar] [CrossRef]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef]
- Schmid, M.A.; Glasner, D.R.; Shah, S.; Michlmayr, D.; Kramer, L.D.; Harris, E. Mosquito Saliva Increases Endothelial Permeability in the Skin, Immune Cell Migration, and Dengue Pathogenesis during Antibody-Dependent Enhancement. PLoS Pathog. 2016, 12, e1005676. [Google Scholar] [CrossRef]
- McCracken, M.K.; Gromowski, G.D.; Garver, L.S.; Goupil, B.A.; Walker, K.D.; Friberg, H.; Currier, J.R.; Rutvisuttinunt, W.; Hinton, K.L.; Christofferson, R.C.; et al. Route of inoculation and mosquito vector exposure modulate dengue virus replication kinetics and immune responses in rhesus macaques. PLoS Negl. Trop. Dis. 2020, 14, e0008191. [Google Scholar] [CrossRef]
- Ader, D.B.; Celluzzi, C.; Bisbing, J.; Gilmore, L.; Gunther, V.; Peachman, K.K.; Rao, M.; Barvir, D.; Sun, W.; Palmer, D.R. Modulation of dengue virus infection of dendritic cells by Aedes aegypti saliva. Viral Immunol. 2004, 17, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Vogels, C.B.; Goertz, G.P.; Pijlman, G.P.; Koenraadt, C.J. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes Infect. 2017, 6, e96. [Google Scholar] [CrossRef]
- Gould, E.A.; Solomon, T. Pathogenic flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of West Nile virus. Biomed. Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.S.; Soong, L.; Girard, Y.A.; Campbell, G.; Mason, P.; Higgs, S. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 2006, 19, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Styer, L.M.; Lim, P.Y.; Louie, K.L.; Albright, R.G.; Kramer, L.D.; Bernard, K.A. Mosquito saliva causes enhancement of West Nile virus infection in mice. J. Virol. 2011, 85, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Moser, L.A.; Lim, P.Y.; Styer, L.M.; Kramer, L.D.; Bernard, K.A. Parameters of Mosquito-Enhanced West Nile Virus Infection. J. Virol. 2016, 90, 292–299. [Google Scholar] [CrossRef]
- Schneider, B.S.; McGee, C.E.; Jordan, J.M.; Stevenson, H.L.; Soong, L.; Higgs, S. Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted West Nile virus infection. PLoS ONE 2007, 2, e1171. [Google Scholar] [CrossRef] [PubMed]
- Machain-Williams, C.; Reagan, K.; Wang, T.; Zeidner, N.S.; Blair, C.D. Immunization with Culex tarsalis mosquito salivary gland extract modulates West Nile virus infection and disease in mice. Viral Immunol. 2013, 26, 84–92. [Google Scholar] [CrossRef]
- Garcia, M.; Alout, H.; Diop, F.; Damour, A.; Bengue, M.; Weill, M.; Misse, D.; Leveque, N.; Bodet, C. Innate Immune Response of Primary Human Keratinocytes to West Nile Virus Infection and Its Modulation by Mosquito Saliva. Front. Cell Infect. Microbiol. 2018, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.W. The Newala epidemic. III. The virus: Isolation, pathogenic properties and relationship to the epidemic. J. Hyg. 1956, 54, 177–191. [Google Scholar] [CrossRef]
- Higgs, S.; Vanlandingham, D. Chikungunya virus and its mosquito vectors. Vector Borne Zoonotic Dis. 2015, 15, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- de Souza, W.M.; Ribeiro, G.S.; de Lima, S.T.S.; de Jesus, R.; Moreira, F.R.R.; Whittaker, C.; Sallum, M.A.M.; Carrington, C.V.F.; Sabino, E.C.; Kitron, U.; et al. Chikungunya: A decade of burden in the Americas. Lancet Reg. Health Am. 2024, 30, 100673. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.J.; Haddow, A.J. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53; an additional note on Chikungunya virus isolations and serum antibodies. Trans. R. Soc. Trop. Med. Hyg. 1957, 51, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.; Ryoo, S.G.; Vasileva, D.; Yaghoubi, S. Prevalence of chronic comorbidities in chikungunya: A systematic review and meta-analysis. Int. J. Infect. Dis. 2018, 67, 107–113. [Google Scholar] [CrossRef]
- Puiprom, O.; Morales Vargas, R.E.; Potiwat, R.; Chaichana, P.; Ikuta, K.; Ramasoota, P.; Okabayashi, T. Characterization of chikungunya virus infection of a human keratinocyte cell line: Role of mosquito salivary gland protein in suppressing the host immune response. Infect. Genet. Evol. 2013, 17, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Wichit, S.; Diop, F.; Hamel, R.; Talignani, L.; Ferraris, P.; Cornelie, S.; Liegeois, F.; Thomas, F.; Yssel, H.; Misse, D. Aedes Aegypti saliva enhances chikungunya virus replication in human skin fibroblasts via inhibition of the type I interferon signaling pathway. Infect. Genet. Evol. 2017, 55, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Epelboin, Y.; Talaga, S.; Epelboin, L.; Dusfour, I. Zika virus: An updated review of competent or naturally infected mosquitoes. PLoS Negl. Trop. Dis. 2017, 11, e0005933. [Google Scholar] [CrossRef] [PubMed]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.O.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonca, M.C.L.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- de Laval, F.; Matheus, S.; Labrousse, T.; Enfissi, A.; Rousset, D.; Briolant, S. Kinetics of Zika Viral Load in Semen. N. Engl. J. Med. 2017, 377, 697–699. [Google Scholar] [CrossRef]
- Masmejan, S.; Musso, D.; Vouga, M.; Pomar, L.; Dashraath, P.; Stojanov, M.; Panchaud, A.; Baud, D. Zika Virus. Pathogens 2020, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, L.; Zhang, Z.; Marin-Lopez, A. Current Advances in Zika Vaccine Development. Vaccines 2022, 10, 1816. [Google Scholar] [CrossRef] [PubMed]
- Christian, K.M.; Song, H.; Ming, G.L. Pathophysiology and Mechanisms of Zika Virus Infection in the Nervous System. Annu. Rev. Neurosci. 2019, 42, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, G.; Valenzuela-Leon, P.C.; Botello, K.; Calvo, E. Aedes aegypti saliva modulates inflammasome activation and facilitates flavivirus infection in vitro. iScience 2024, 27, 108620. [Google Scholar] [CrossRef]
- Valenzuela-Leon, P.C.; Shrivastava, G.; Martin-Martin, I.; Cardenas, J.C.; Londono-Renteria, B.; Calvo, E. Multiple Salivary Proteins from Aedes aegypti Mosquito Bind to the Zika Virus Envelope Protein. Viruses 2022, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Le Coupanec, A.; Babin, D.; Fiette, L.; Jouvion, G.; Ave, P.; Misse, D.; Bouloy, M.; Choumet, V. Aedes mosquito saliva modulates Rift Valley fever virus pathogenicity. PLoS Negl. Trop. Dis. 2013, 7, e2237. [Google Scholar] [CrossRef]
- Park, S.L.; Huang, Y.-J.S.; Lyons, A.C.; Ayers, V.B.; Hettenbach, S.M.; McVey, D.S.; Noronha, L.E.; Burton, K.R.; Hsu, W.-W.; Higgs, S.; et al. Mosquito Saliva Modulates Japanese Encephalitis Virus Infection in Domestic Pigs. Front. Virol. 2021, 1, 724016. [Google Scholar] [CrossRef]
- Lefteri, D.A.; Bryden, S.R.; Pingen, M.; Terry, S.; McCafferty, A.; Beswick, E.F.; Georgiev, G.; Van der Laan, M.; Mastrullo, V.; Campagnolo, P.; et al. Mosquito saliva enhances virus infection through sialokinin-dependent vascular leakage. Proc. Natl. Acad. Sci. USA 2022, 119, e2114309119. [Google Scholar] [CrossRef]
- Beier, J.C.; Killeen, G.F.; Githure, J.I. Short report: Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 1999, 61, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Gimnig, J.E.; Walker, E.D.; Otieno, P.; Kosgei, J.; Olang, G.; Ombok, M.; Williamson, J.; Marwanga, D.; Abong’o, D.; Desai, M.; et al. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am. J. Trop. Med. Hyg. 2013, 88, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Briët, O.J.T.; Huho, B.J.; Gimnig, J.E.; Bayoh, N.; Seyoum, A.; Sikaala, C.H.; Govella, N.; Diallo, D.A.; Abdullah, S.; Smith, T.A.; et al. Applications and limitations of Centers for Disease Control and Prevention miniature light traps for measuring biting densities of African malaria vector populations: A pooled-analysis of 13 comparisons with human landing catches. Malar. J. 2015, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Monroe, A.; Moore, S.; Okumu, F.; Kiware, S.; Lobo, N.F.; Koenker, H.; Sherrard-Smith, E.; Gimnig, J.; Killeen, G.F. Methods and indicators for measuring patterns of human exposure to malaria vectors. Malar. J. 2020, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Sagna, A.B.; Yobo, M.C.; Elanga Ndille, E.; Remoue, F. New Immuno-Epidemiological Biomarker of Human Exposure to Aedes Vector Bites: From Concept to Applications. Trop. Med. Infect. Dis. 2018, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Hemme, R.R.; Poole-Smith, B.K.; Hunsperger, E.A.; Felix, G.E.; Horiuchi, K.; Biggerstaff, B.J.; Lopez-Ortiz, R.; Barrera, R. Non-human primate antibody response to mosquito salivary proteins: Implications for dengue virus transmission in Puerto Rico. Acta Trop. 2016, 164, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Buezo Montero, S.; Gabrieli, P.; Montarsi, F.; Borean, A.; Capelli, S.; De Silvestro, G.; Forneris, F.; Pombi, M.; Breda, A.; Capelli, G.; et al. IgG Antibody Responses to the Aedes albopictus 34k2 Salivary Protein as Novel Candidate Marker of Human Exposure to the Tiger Mosquito. Front. Cell. Infect. Microbiol. 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Kearney, E.A.; Agius, P.A.; Chaumeau, V.; Cutts, J.C.; Simpson, J.A.; Fowkes, F.J.I. Anopheles salivary antigens as serological biomarkers of vector exposure and malaria transmission: A systematic review with multilevel modelling. elife 2021, 10, e73080. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.E.; Chea, S.; Parker, D.M.; Bohl, J.A.; Lay, S.; Mateja, A.; Man, S.; Nhek, S.; Ponce, A.; Sreng, S.; et al. Development of Inapparent Dengue Associated With Increased Antibody Levels to Aedes aegypti Salivary Proteins: A Longitudinal Dengue Cohort in Cambodia. J. Infect. Dis. 2022, 226, 1327–1337. [Google Scholar] [CrossRef]
- Doucoure, S.; Mouchet, F.; Cournil, A.; Le Goff, G.; Cornelie, S.; Roca, Y.; Giraldez, M.G.; Simon, Z.B.; Loayza, R.; Misse, D.; et al. Human antibody response to Aedes aegypti saliva in an urban population in Bolivia: A new biomarker of exposure to Dengue vector bites. Am. J. Trop. Med. Hyg. 2012, 87, 504–510. [Google Scholar] [CrossRef]
- Machain-Williams, C.; Mammen, M.P., Jr.; Zeidner, N.S.; Beaty, B.J.; Prenni, J.E.; Nisalak, A.; Blair, C.D. Association of human immune response to Aedes aegypti salivary proteins with dengue disease severity. Parasite Immunol. 2012, 34, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.M.I.; Bakli, M.; Fontaine, A.; Bakkali, N.; Vu Hai, V.; Audebert, S.; Boublik, Y.; Pagès, F.; Remoué, F.; Rogier, C.; et al. Assessment of Anopheles salivary antigens as individual exposure biomarkers to species-specific malaria vector bites. Malar. J. 2012, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Lombardo, F.; Ronca, R.; Mangano, V.; Sirima, S.B.; Nebie, I.; Fiorentino, G.; Modiano, D.; Arca, B. Differential antibody response to the Anopheles gambiae gSG6 and cE5 salivary proteins in individuals naturally exposed to bites of malaria vectors. Parasit. Vectors 2014, 7, 549. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.L.; Shakeri, H.; Rozo-Lopez, P.; Conway, M.J.; Duggan, N.; Jaberi-Douraki, M.; Colpitts, T.M. Serosurvey of Human Antibodies Recognizing Aedes aegypti D7 Salivary Proteins in Colombia. Front. Public Health 2018, 6, 111. [Google Scholar] [CrossRef]
- Chea, S.; Willen, L.; Nhek, S.; Ly, P.; Tang, K.; Oristian, J.; Salas-Carrillo, R.; Ponce, A.; Leon, P.C.V.; Kong, D.; et al. Antibodies to Aedes aegypti D7L salivary proteins as a new serological tool to estimate human exposure to Aedes mosquitoes. Front. Immunol. 2024, 15, 1368066. [Google Scholar] [CrossRef]
- Olajiga, O.M.; Marin-Lopez, A.; Cardenas, J.C.; Gutierrez-Silva, L.Y.; Gonzales-Pabon, M.U.; Maldonado-Ruiz, L.P.; Worges, M.; Fikrig, E.; Park, Y.; Londono-Renteria, B. Aedes aegypti anti-salivary proteins IgG levels in a cohort of DENV-like symptoms subjects from a dengue-endemic region in Colombia. Front. Epidemiol. 2022, 2, 1002857. [Google Scholar] [CrossRef]
- Buezo Montero, S.; Gabrieli, P.; Severini, F.; Picci, L.; Di Luca, M.; Forneris, F.; Facchinelli, L.; Ponzi, M.; Lombardo, F.; Arcà, B. Analysis in a murine model points to IgG responses against the 34k2 salivary proteins from Aedes albopictus and Aedes aegypti as novel promising candidate markers of host exposure to Aedes mosquitoes. PLoS Negl. Trop. Dis. 2019, 13, e0007806. [Google Scholar] [CrossRef] [PubMed]
- Fustec, B.; Phanitchat, T.; Aromseree, S.; Pientong, C.; Thaewnongiew, K.; Ekalaksananan, T.; Cerqueira, D.; Poinsignon, A.; Elguero, E.; Bangs, M.J.; et al. Serological biomarker for assessing human exposure to Aedes mosquito bites during a randomized vector control intervention trial in northeastern Thailand. PLoS Negl. Trop. Dis. 2021, 15, e0009440. [Google Scholar] [CrossRef]
- Doucoure, S.; Drame, P.M. Salivary Biomarkers in the Control of Mosquito-Borne Diseases. Insects 2015, 6, 961–976. [Google Scholar] [CrossRef]
- Gotuzzo, E.; Yactayo, S.; Cordova, E. Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013, 89, 434–444. [Google Scholar] [CrossRef] [PubMed]
- WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk. World Health Organization. Available online: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk (accessed on 26 November 2024).
- R21/Matrix-M Malaria Vaccine: Evidence to Recommendations Framework. World Health Organization. Available online: https://www.who.int/publications/m/item/r21-matrix-m-malaria-vaccine--evidence-to-recommendations-framework--2023 (accessed on 26 November 2024).
- Ly, H. Ixchiq (VLA1553): The first FDA-approved vaccine to prevent disease caused by Chikungunya virus infection. Virulence 2024, 15, 2301573. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.A. Immunologic Basis of Vaccine Vectors. Immunity 2010, 33, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.E.; Morens, D.M.; Kamhawi, S.; Valenzuela, J.G.; Memoli, M. Mosquito Saliva: The Hope for a Universal Arbovirus Vaccine? J. Infect. Dis. 2018, 218, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.E.; Oliveira, F.; Coutinho-Abreu, I.V.; Herbert, S.; Meneses, C.; Kamhawi, S.; Baus, H.A.; Han, A.; Czajkowski, L.; Rosas, L.A.; et al. Safety and immunogenicity of a mosquito saliva peptide-based vaccine: A randomised, placebo-controlled, double-blind, phase 1 trial. Lancet 2020, 395, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Friedman-Klabanoff, D.J.; Birkhold, M.; Short, M.T.; Wilson, T.R.; Meneses, C.R.; Lacsina, J.R.; Oliveira, F.; Kamhawi, S.; Valenzuela, J.G.; Hunsberger, S.; et al. Safety and immunogenicity of AGS-v PLUS, a mosquito saliva peptide vaccine against arboviral diseases: A randomized, double-blind, placebo-controlled Phase 1 trial. EBioMedicine 2022, 86, 104375. [Google Scholar] [CrossRef]
- Wang, Y.; Marin-Lopez, A.; Jiang, J.; Ledizet, M.; Fikrig, E. Vaccination with Aedes aegypti AgBR1 Delays Lethal Mosquito-Borne Zika Virus Infection in Mice. Vaccines 2020, 8, 145. [Google Scholar] [CrossRef]
- Marin-Lopez, A.; Wang, Y.; Jiang, J.; Ledizet, M.; Fikrig, E. AgBR1 and NeSt1 antisera protect mice from Aedes aegypti-borne Zika infection. Vaccine 2021, 39, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Reagan, K.L.; Machain-Williams, C.; Wang, T.; Blair, C.D. Immunization of mice with recombinant mosquito salivary protein D7 enhances mortality from subsequent West Nile virus infection via mosquito bite. PLoS Negl. Trop. Dis. 2012, 6, e1935. [Google Scholar] [CrossRef]
- Pandey, R.K.; Dahiya, S.; Mahita, J.; Sowdhamini, R.; Prajapati, V.K. Vaccination and immunization strategies to design Aedes aegypti salivary protein based subunit vaccine tackling Flavivirus infection. Int. J. Biol. Macromol. 2019, 122, 1203–1211. [Google Scholar] [CrossRef]
- Pandey, R.K.; Bhatt, T.K.; Prajapati, V.K. Novel Immunoinformatics Approaches to Design Multi-epitope Subunit Vaccine for Malaria by Investigating Anopheles Salivary Protein. Sci. Rep. 2018, 8, 1125. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S. In silico design of a multi-epitope Chimera from Aedes aegypti salivary proteins OBP 22 and OBP 10: A promising candidate vaccine. J. Vector Borne Dis. 2022, 59, 327–336. [Google Scholar] [CrossRef]
- Kim, K.-S. Current Challenges in the Development of Vaccines and Drugs Against Emerging Vector-borne Diseases. Curr. Med. Chem. 2019, 26, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; He, X.; Tao, J.; Sun, H.; Yang, J. Unraveling the Molecular Mechanisms of Mosquito Salivary Proteins: New Frontiers in Disease Transmission and Control. Biomolecules 2025, 15, 82. https://doi.org/10.3390/biom15010082
Guo J, He X, Tao J, Sun H, Yang J. Unraveling the Molecular Mechanisms of Mosquito Salivary Proteins: New Frontiers in Disease Transmission and Control. Biomolecules. 2025; 15(1):82. https://doi.org/10.3390/biom15010082
Chicago/Turabian StyleGuo, Jiayin, Xiaoe He, Jianli Tao, Hui Sun, and Jing Yang. 2025. "Unraveling the Molecular Mechanisms of Mosquito Salivary Proteins: New Frontiers in Disease Transmission and Control" Biomolecules 15, no. 1: 82. https://doi.org/10.3390/biom15010082
APA StyleGuo, J., He, X., Tao, J., Sun, H., & Yang, J. (2025). Unraveling the Molecular Mechanisms of Mosquito Salivary Proteins: New Frontiers in Disease Transmission and Control. Biomolecules, 15(1), 82. https://doi.org/10.3390/biom15010082







