Functional Investigation of a Novel PIWIL4 Mutation in Nonobstructive Azoospermia During the First Wave of Spermatogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. NOA Patient Population
2.3. Generation of the Piwil4R264W/R264W Knock-In Mice Model
2.4. Histological Analysis and Immunohistochemistry
2.5. Immunofluorescence and TUNEL Assay
2.6. Plasmids and Cell Transfection
2.7. Real-Time Quantitative PCR (RT–qPCR) Analyses and RNA-seq
2.8. Immunoblot Analysis
2.9. CASA
2.10. In Vitro Fertilization (IVF)
2.11. RIP-Seq
2.12. Bisulfite Conversion and PCR
2.13. Testicular Germ Cell Depletion and Regeneration
3. Results
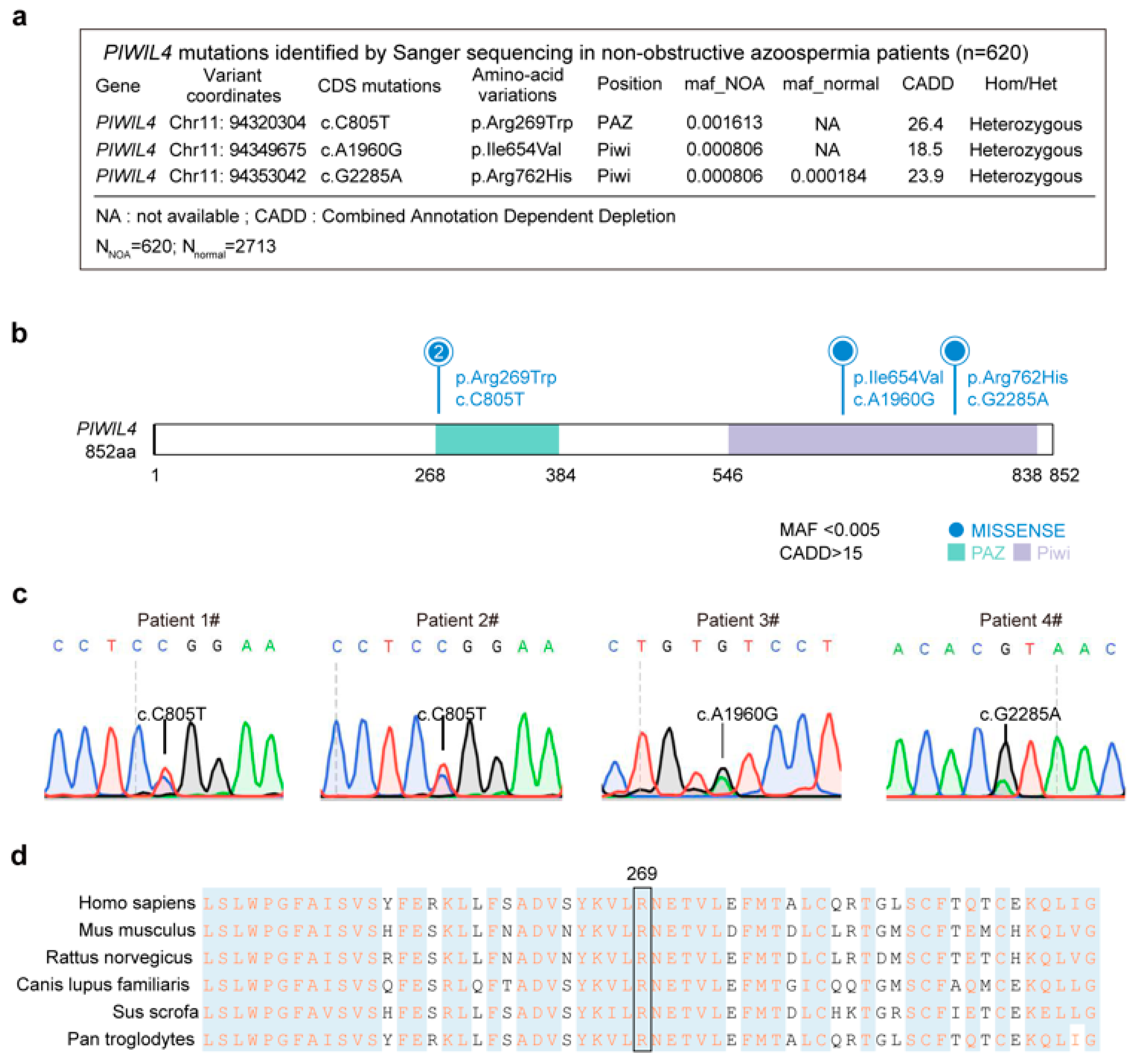
3.1. Identification of a Heterozygous Missense PIWIL4 Variant in Two Men from Unrelated Families with Nonobstructive Azoospermia
3.2. Altered Expression of the LINE-1 Transposon in Piwil4R264W/R264W Mutant Male Mice
3.3. Mutant PIWIL4 Does Not Affect Normal Spermatogenesis or Sperm Morphology
3.4. The First Wave of Spermatogenesis Is Damaged in Piwil4R264W/R264W Mutant Male Mice
3.5. Mutant PIWIL4 Shows Altered piRNA Loading Ability
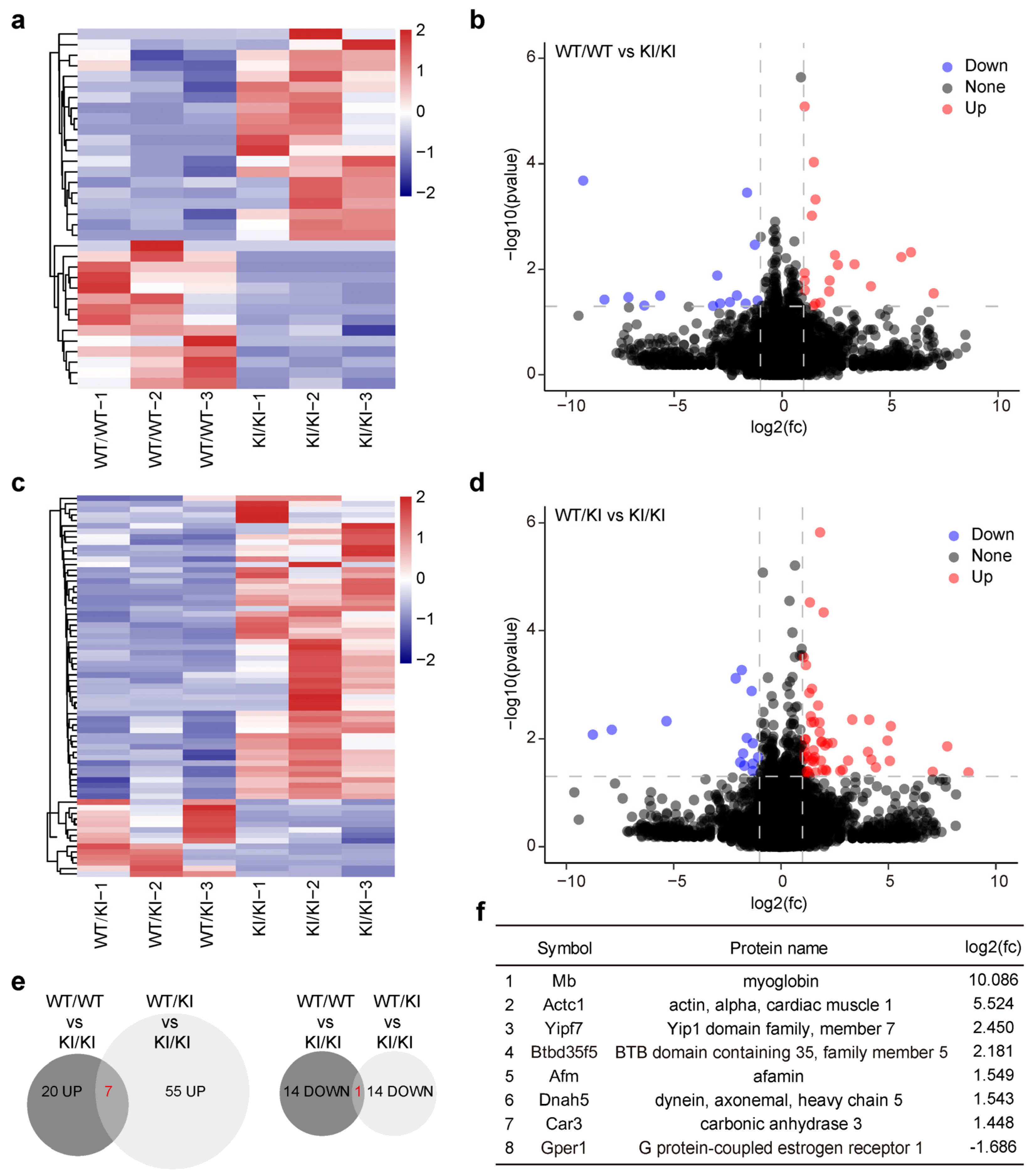
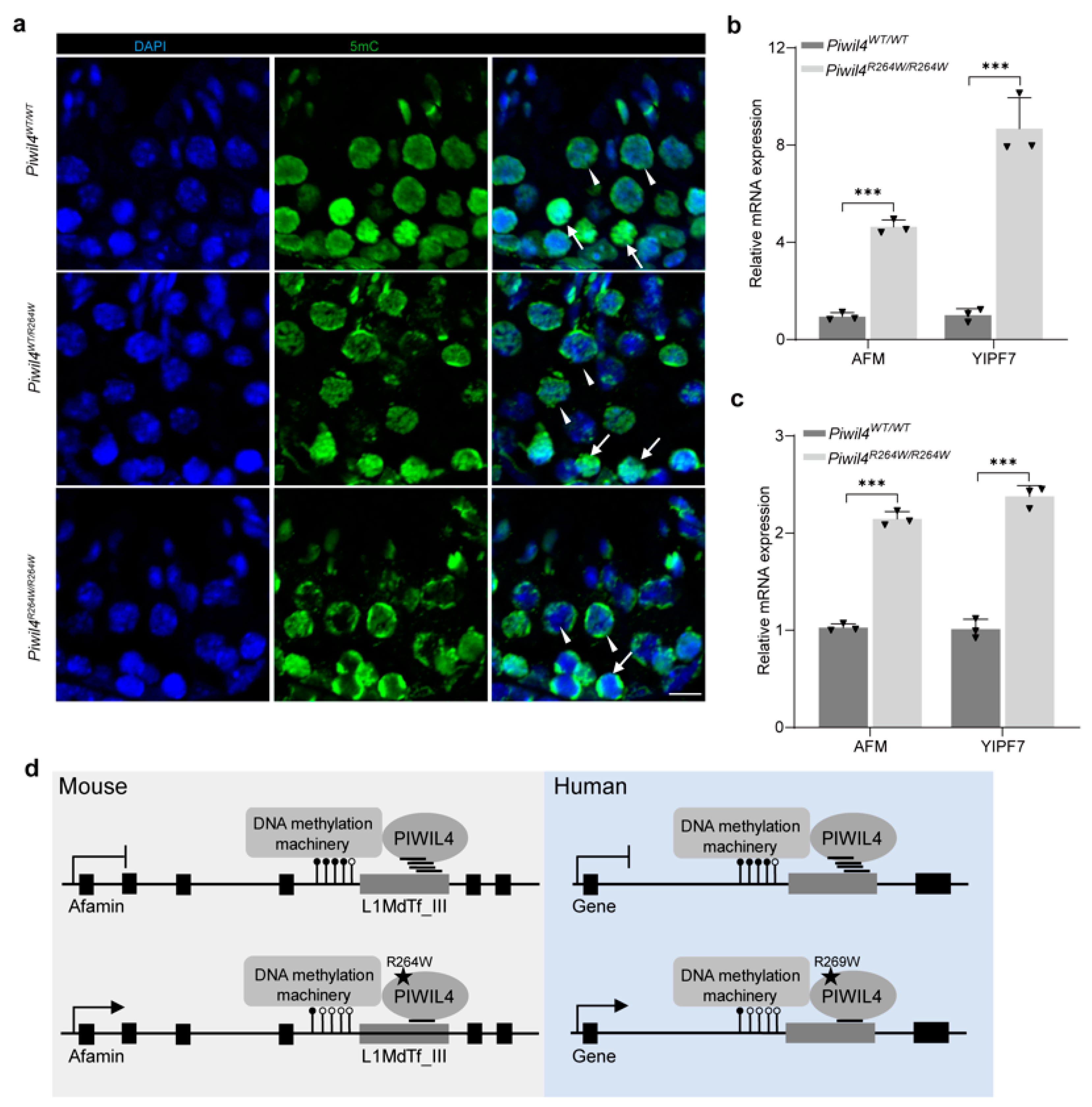
3.6. Genes Harboring Intact LINE-1 Sequences Are Overexpressed in Piwil4R264W/R264W Male Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.-S. Male infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.J.; Riera-Escamilla, A.; Wyrwoll, M.J.; Salas-Huetos, A.; Xavier, M.J.; Nagirnaja, L.; Friedrich, C.; Conrad, D.F.; Aston, K.I.; Krausz, C.; et al. A systematic review of the validated monogenic causes of human male infertility: 2020 update and a discussion of emerging gene-disease relationships. Hum. Reprod. Update 2021, 28, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.; Gaidatzis, D.; Pfeffer, S.; Lagos-Quintana, M.; Landgraf, P.; Iovino, N.; Morris, P.; Brownstein, M.J.; Kuramochi-Miyagawa, S.; Nakano, T.; et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 2006, 442, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Grivna, S.T.; Beyret, E.; Wang, Z.; Lin, H. A novel class of small RNAs in mouse spermatogenic cells. Genes. Dev. 2006, 20, 1709–1714. [Google Scholar] [CrossRef]
- Lau, N.C.; Seto, A.G.; Kim, J.; Kuramochi-Miyagawa, S.; Nakano, T.; Bartel, D.P.; Kingston, R.E. Characterization of the piRNA complex from rat testes. Science 2006, 313, 363–367. [Google Scholar] [CrossRef]
- Wang, X.; Ramat, A.; Simonelig, M.; Liu, M.F. Emerging roles and functional mechanisms of PIWI-interacting RNAs. Nat. Rev. Mol. Cell Biol. 2023, 24, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Nagirnaja, L.; Mørup, N.; Nielsen, J.E.; Stakaitis, R.; Golubickaite, I.; Oud, M.S.; Winge, S.B.; Carvalho, F.; Aston, K.I.; Khani, F.; et al. Variant PNLDC1, Defective piRNA Processing, and Azoospermia. N. Engl. J. Med. 2021, 385, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, Y.Q.; Lu, L.Y. Defective piRNA Processing and Azoospermia. N. Engl. J. Med. 2022, 386, 1675–1676. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tan, Y.Q.; Liu, M.F. Defective piRNA Processing and Azoospermia. N. Engl. J. Med. 2022, 386, 1674–1675. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Li, L.; Yin, C. Defective piRNA Processing and Azoospermia. N. Engl. J. Med. 2022, 386, 1675. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.T.; Kang, J.Y.; Dai, P.; Wang, X.; Li, F.; Zhao, S.; Zhang, M.; Hua, M.M.; Lu, Y.; Zhu, Y.; et al. Ubiquitination-Deficient Mutations in Human Piwi Cause Male Infertility by Impairing Histone-to-Protamine Exchange during Spermiogenesis. Cell 2017, 169, 1090–1104.e1013. [Google Scholar] [CrossRef]
- Arafat, M.; Har-Vardi, I.; Harlev, A.; Levitas, E.; Zeadna, A.; Abofoul-Azab, M.; Dyomin, V.; Sheffield, V.C.; Lunenfeld, E.; Huleihel, M.; et al. Mutation in TDRD9 causes non-obstructive azoospermia in infertile men. J. Med. Genet. 2017, 54, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Tu, C.; Meng, L.; Yuan, S.; Sjaarda, C.; Luo, A.; Du, J.; Li, W.; Gong, F.; Zhong, C.; et al. Loss-of-function mutations in TDRD7 lead to a rare novel syndrome combining congenital cataract and nonobstructive azoospermia in humans. Genet. Med. 2019, 21, 1209–1217. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, M.; Xiong, C.; Li, H. A homozygous PIWIL2 frameshift variant affects the formation and maintenance of human-induced pluripotent stem cell-derived spermatogonial stem cells and causes Sertoli cell-only syndrome. Stem Cell Res. Ther. 2022, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Wyrwoll, M.J.; Gaasbeek, C.M.; Golubickaite, I.; Stakaitis, R.; Oud, M.S.; Nagirnaja, L.; Dion, C.; Sindi, E.B.; Leitch, H.G.; Jayasena, C.N.; et al. The piRNA-pathway factor FKBP6 is essential for spermatogenesis but dispensable for control of meiotic LINE-1 expression in humans. Am. J. Hum. Genet. 2022, 109, 1850–1866. [Google Scholar] [CrossRef] [PubMed]
- Stallmeyer, B.; Bühlmann, C.; Stakaitis, R.; Dicke, A.-K.; Ghieh, F.; Meier, L.; Zoch, A.; MacKenzie MacLeod, D.; Steingröver, J.; Okutman, Ö.; et al. Inherited defects of piRNA biogenesis cause transposon de-repression, impaired spermatogenesis, and human male infertility. Nat. Commun. 2024, 15, 6637. [Google Scholar] [CrossRef] [PubMed]
- Carmell, M.A.; Girard, A.; van de Kant, H.J.; Bourc’his, D.; Bestor, T.H.; de Rooij, D.G.; Hannon, G.J. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell 2007, 12, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.A.; Sachidanandam, R.; Girard, A.; Fejes-Toth, K.; Hannon, G.J. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 2007, 316, 744–747. [Google Scholar] [CrossRef]
- Kuramochi-Miyagawa, S.; Watanabe, T.; Gotoh, K.; Totoki, Y.; Toyoda, A.; Ikawa, M.; Asada, N.; Kojima, K.; Yamaguchi, Y.; Ijiri, T.W.; et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes. Dev. 2008, 22, 908–917. [Google Scholar] [CrossRef]
- Zoch, A.; Auchynnikava, T.; Berrens, R.V.; Kabayama, Y.; Schöpp, T.; Heep, M.; Vasiliauskaitė, L.; Pérez-Rico, Y.A.; Cook, A.G.; Shkumatava, A.; et al. SPOCD1 is an essential executor of piRNA-directed de novo DNA methylation. Nature 2020, 584, 635–639. [Google Scholar] [CrossRef]
- Zoch, A.; Konieczny, G.; Auchynnikava, T.; Stallmeyer, B.; Rotte, N.; Heep, M.; Berrens, R.V.; Schito, M.; Kabayama, Y.; Schöpp, T.; et al. C19ORF84 connects piRNA and DNA methylation machineries to defend the mammalian germ line. Mol. Cell 2024, 84, 1021–1035. [Google Scholar] [CrossRef]
- Dias Mirandela, M.; Zoch, A.; Leismann, J.; Webb, S.; Berrens, R.V.; Valsakumar, D.; Kabayama, Y.; Auchynnikava, T.; Schito, M.; Chowdhury, T.; et al. Two-factor authentication underpins the precision of the piRNA pathway. Nature 2024, 634, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Di Persio, S.; Tekath, T.; Siebert-Kuss, L.M.; Cremers, J.F.; Wistuba, J.; Li, X.; Meyer Zu Hörste, G.; Drexler, H.C.A.; Wyrwoll, M.J.; Tüttelmann, F.; et al. Single-cell RNA-seq unravels alterations of the human spermatogonial stem cell compartment in patients with impaired spermatogenesis. Cell Rep. Med. 2021, 2, 100395. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, C.; Comazzetto, S.; Grover, A.; Morgan, M.; Buness, A.; Nerlov, C.; O’Carroll, D. A transit-amplifying population underpins the efficient regenerative capacity of the testis. J. Exp. Med. 2017, 214, 1631–1641. [Google Scholar] [CrossRef]
- Kuroki, R.; Murata, Y.; Fuke, S.; Nakachi, Y.; Nakashima, J.; Kujoth, G.C.; Prolla, T.A.; Bundo, M.; Kato, T.; Iwamoto, K. Establishment of Quantitative PCR Assays for Active Long Interspersed Nuclear Element-1 Subfamilies in Mice and Applications to the Analysis of Aging-Associated Retrotransposition. Front. Genet. 2020, 11, 519206. [Google Scholar] [CrossRef] [PubMed]
- Kumaki, Y.; Oda, M.; Okano, M. QUMA: Quantification tool for methylation analysis. Nucleic Acids Res. 2008, 36, W170–W175. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Liu, J.; Tolia, N.H.; Schneiderman, J.; Smith, S.K.; Martienssen, R.A.; Hannon, G.J.; Joshua-Tor, L. The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nat. Struct. Biol. 2003, 10, 1026–1032. [Google Scholar] [CrossRef]
- Lingel, A.; Simon, B.; Izaurralde, E.; Sattler, M. Nucleic acid 3′-end recognition by the Argonaute2 PAZ domain. Nat. Struct. Mol. Biol. 2004, 11, 576–577. [Google Scholar] [CrossRef]
- Manakov, S.A.; Pezic, D.; Marinov, G.K.; Pastor, W.A.; Sachidanandam, R.; Aravin, A.A. MIWI2 and MILI Have Differential Effects on piRNA Biogenesis and DNA Methylation. Cell Rep. 2015, 12, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, P.J. Multiple LINEs of retrotransposon silencing mechanisms in the mammalian germline. Semin. Cell Dev. Biol. 2016, 59, 118–125. [Google Scholar] [CrossRef]
- Yoshida, S.; Sukeno, M.; Nakagawa, T.; Ohbo, K.; Nagamatsu, G.; Suda, T.; Nabeshima, Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 2006, 133, 1495–1505. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, Y.; Schuster, A.S.; Ortogero, N.; Nilsson, E.E.; Skinner, M.K.; Yan, W. Conditional inactivation of Miwi2 reveals that MIWI2 is only essential for prospermatogonial development in mice. Cell Death Differ. 2014, 21, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lan, Y.; Pandey, R.R.; Homolka, D.; Berger, S.L.; Pillai, R.S.; Bartolomei, M.S.; Wang, P.J. TEX15 associates with MILI and silences transposable elements in male germ cells. Genes. Dev. 2020, 34, 745–750. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lee, C.H.; Swigut, T.; Grow, E.; Gu, B.; Bassik, M.C.; Wysocka, J. Selective silencing of euchromatic L1s revealed by genome-wide screens for L1 regulators. Nature 2018, 553, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, M.; Comazzetto, S.; Saini, H.; De Fazio, S.; Carrieri, C.; Morgan, M.; Vasiliauskaite, L.; Benes, V.; Enright, A.J.; O’Carroll, D. Multiple epigenetic mechanisms and the piRNA pathway enforce LINE1 silencing during adult spermatogenesis. Mol. Cell 2013, 50, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, N.; Barau, J.; Teissandier, A.; Walter, M.; Borsos, M.; Servant, N.; Bourc’his, D. DNA methylation restrains transposons from adopting a chromatin signature permissive for meiotic recombination. Genes. Dev. 2015, 29, 1256–1270. [Google Scholar] [CrossRef]
- Nuñez-Calonge, R.; Cortes, S.; Caballero Peregrín, P.; Gutierrez Gonzalez, L.M.; Kireev, R. Seminal Plasma and Serum Afamin Levels Are Associated with Infertility in Men with Oligoasthenoteratozoospermia. Reprod. Sci. 2021, 28, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Nistal, M. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J. Anat. 1984, 139 Pt 3, 535–552. [Google Scholar]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, N.; Tachibana, K.; Ogasawara, S.; Matsuzaki, H.; Hagiuda, J.; Ishikawa, H.; Mochida, K.; Inoue, K.; Ogonuki, N.; Ogura, A.; et al. A heterozygous mutation of GALNTL5 affects male infertility with impairment of sperm motility. Proc. Natl. Acad. Sci. USA 2014, 111, 1120–1125. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Q.; Guo, W.; Song, S.; Wang, N.; Chen, X.; Sun, A.; Yan, L.; Qiao, J. Loss of CEP70 function affects acrosome biogenesis and flagella formation during spermiogenesis. Cell Death Dis. 2021, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Mou, L.; Zhang, Q.; Diao, R.; Cai, Z.; Gui, Y. A functional variant in the UBE2B gene promoter is associated with idiopathic azoospermia. Reprod. Biol. Endocrinol. 2015, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ni, M.; Tian, S.; Guo, W.; Cai, S.; Sondergaard, M.T.; Chen, Y.; Mu, Y.; Estillore, J.P.; Wang, R.; et al. A gain-of-function mutation in the ITPR1 gating domain causes male infertility in mice. J. Cell Physiol. 2022, 237, 3305–3316. [Google Scholar] [CrossRef]
- Yatsenko, A.N.; Roy, A.; Chen, R.; Ma, L.; Murthy, L.J.; Yan, W.; Lamb, D.J.; Matzuk, M.M. Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum. Mol. Genet. 2006, 15, 3411–3419. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.A.; McDermott, D.A.; Wilkes, D.; Poulsen, M.N.; Nolan, M.A.; Goldstein, M.; Basson, C.T.; McKnight, G.S. Haploinsufficiency at the protein kinase A RI alpha gene locus leads to fertility defects in male mice and men. Mol. Endocrinol. 2006, 20, 2504–2513. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lin, Y.-H.; Chen, H.-I.; Wang, Y.-Y.; Chiou, Y.-W.; Lin, H.-H.; Pan, H.-A.; Wu, C.-M.; Su, S.-M.; Hsu, C.-C.; et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum. Mutat. 2012, 33, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-J.; Wang, Y.-Z.; Wang, X.-B.; Yao, C.-C.; Zhao, L.-Y.; Zhang, Z.-B.; Wu, Y.; Chen, W.; Li, Z. Novel mutation in ODF2 causes multiple morphological abnormalities of the sperm flagella in an infertile male. Asian J. Androl. 2022, 24, 463–472. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Du, Q.; Li, W.; Zou, Z.; Wang, C.; Zhou, Y.; Hu, Z.; Gu, Y.; Li, F. Functional Investigation of a Novel PIWIL4 Mutation in Nonobstructive Azoospermia During the First Wave of Spermatogenesis. Biomolecules 2025, 15, 297. https://doi.org/10.3390/biom15020297
Wang X, Du Q, Li W, Zou Z, Wang C, Zhou Y, Hu Z, Gu Y, Li F. Functional Investigation of a Novel PIWIL4 Mutation in Nonobstructive Azoospermia During the First Wave of Spermatogenesis. Biomolecules. 2025; 15(2):297. https://doi.org/10.3390/biom15020297
Chicago/Turabian StyleWang, Xiayu, Qian Du, Wanqian Li, Zhongyu Zou, Chikun Wang, Yan Zhou, Zhibin Hu, Yayun Gu, and Feng Li. 2025. "Functional Investigation of a Novel PIWIL4 Mutation in Nonobstructive Azoospermia During the First Wave of Spermatogenesis" Biomolecules 15, no. 2: 297. https://doi.org/10.3390/biom15020297
APA StyleWang, X., Du, Q., Li, W., Zou, Z., Wang, C., Zhou, Y., Hu, Z., Gu, Y., & Li, F. (2025). Functional Investigation of a Novel PIWIL4 Mutation in Nonobstructive Azoospermia During the First Wave of Spermatogenesis. Biomolecules, 15(2), 297. https://doi.org/10.3390/biom15020297






