Epimaps of the SARS-CoV-2 Receptor-Binding Domain Mutational Landscape: Insights into Protein Stability, Epitope Prediction, and Antibody Binding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Homology Modelling
2.2. Crystal Structures of WT RBD–Antibody Complexes
2.3. Protein Preparation
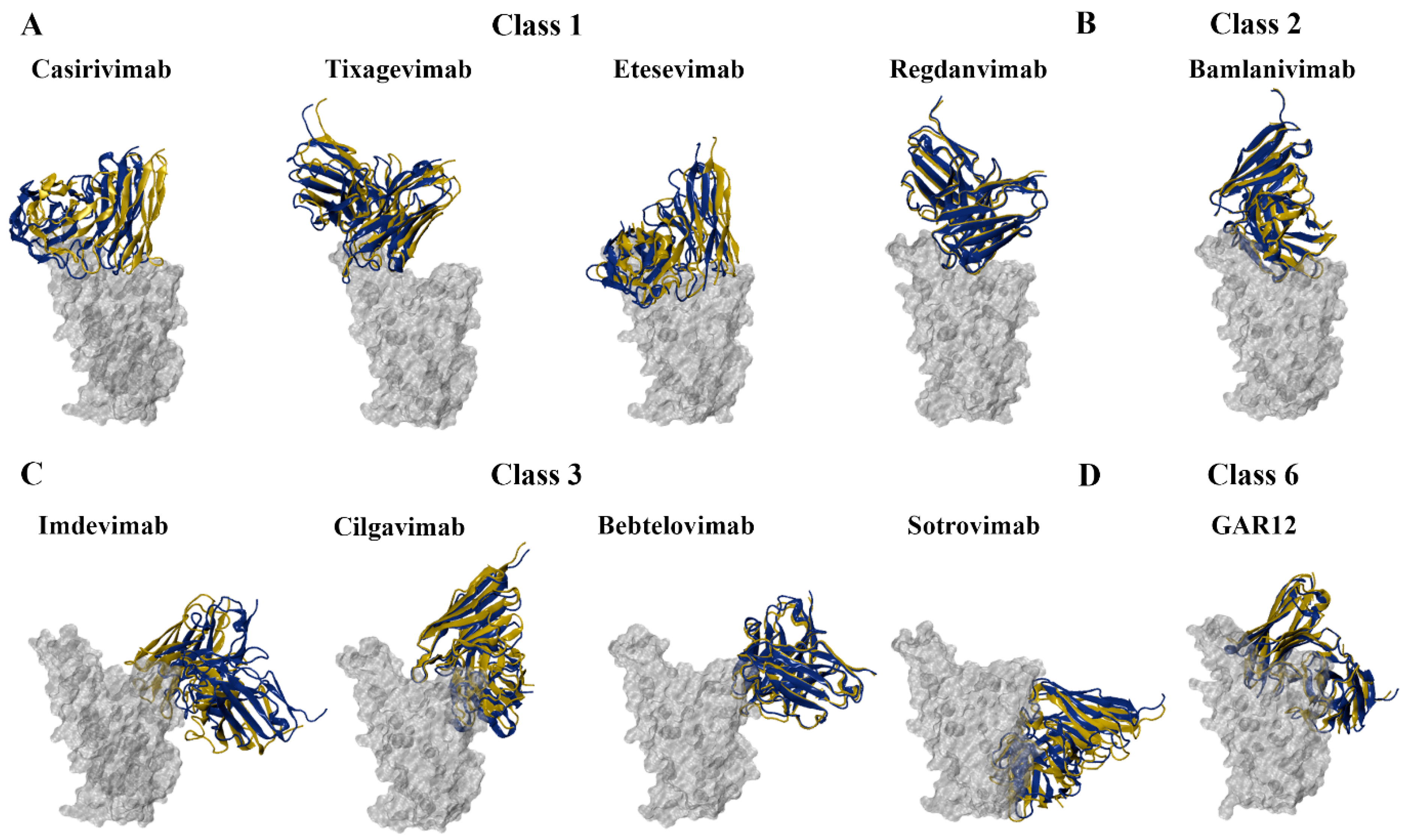
2.4. Protein–Antibody Modelling
2.5. Predicting the Effect of Single-Point Mutations on Protein Stability
2.6. Epitope Mapping
3. Results and Discussion
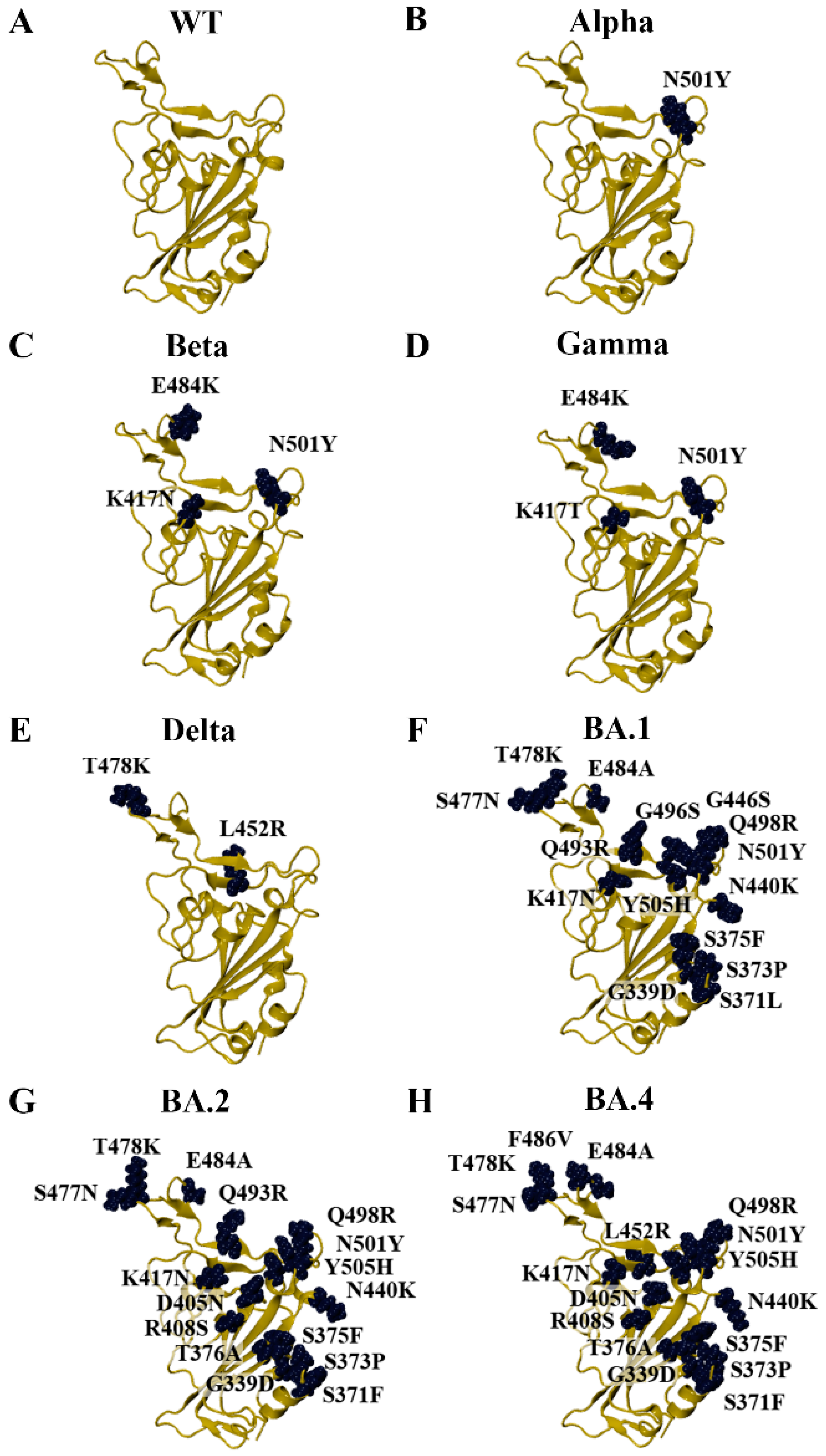
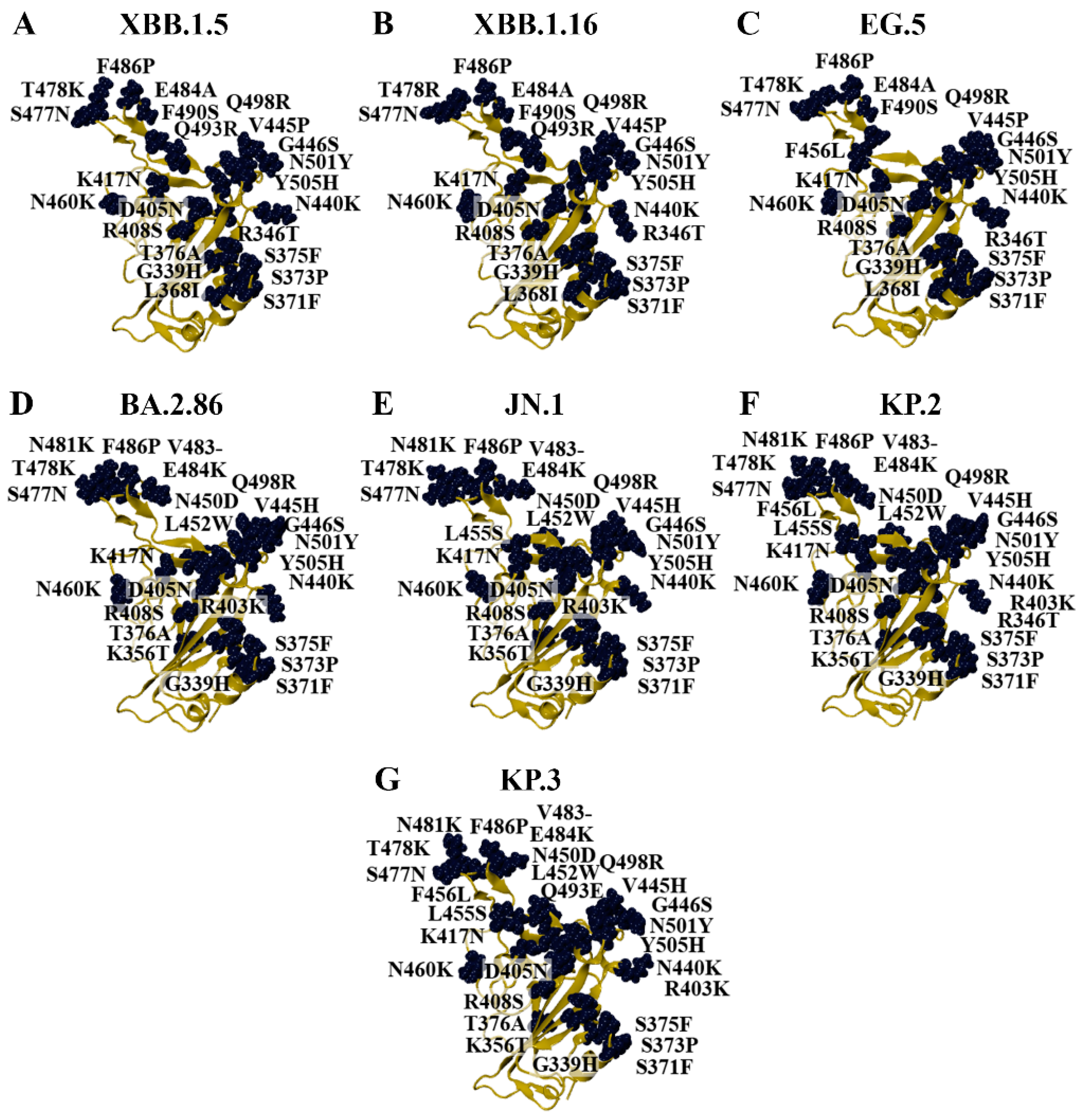
3.1. Modelling the RBD of SARS-CoV-2 Variants
3.2. Characterisation of Amino Acid Substitutions Affecting the Stability of the RBD
3.3. Epitope Mapping of the SARS-CoV-2 RBD
3.4. Predicting the Effects of RBD Mutations on the Binding of Monoclonal Antibodies
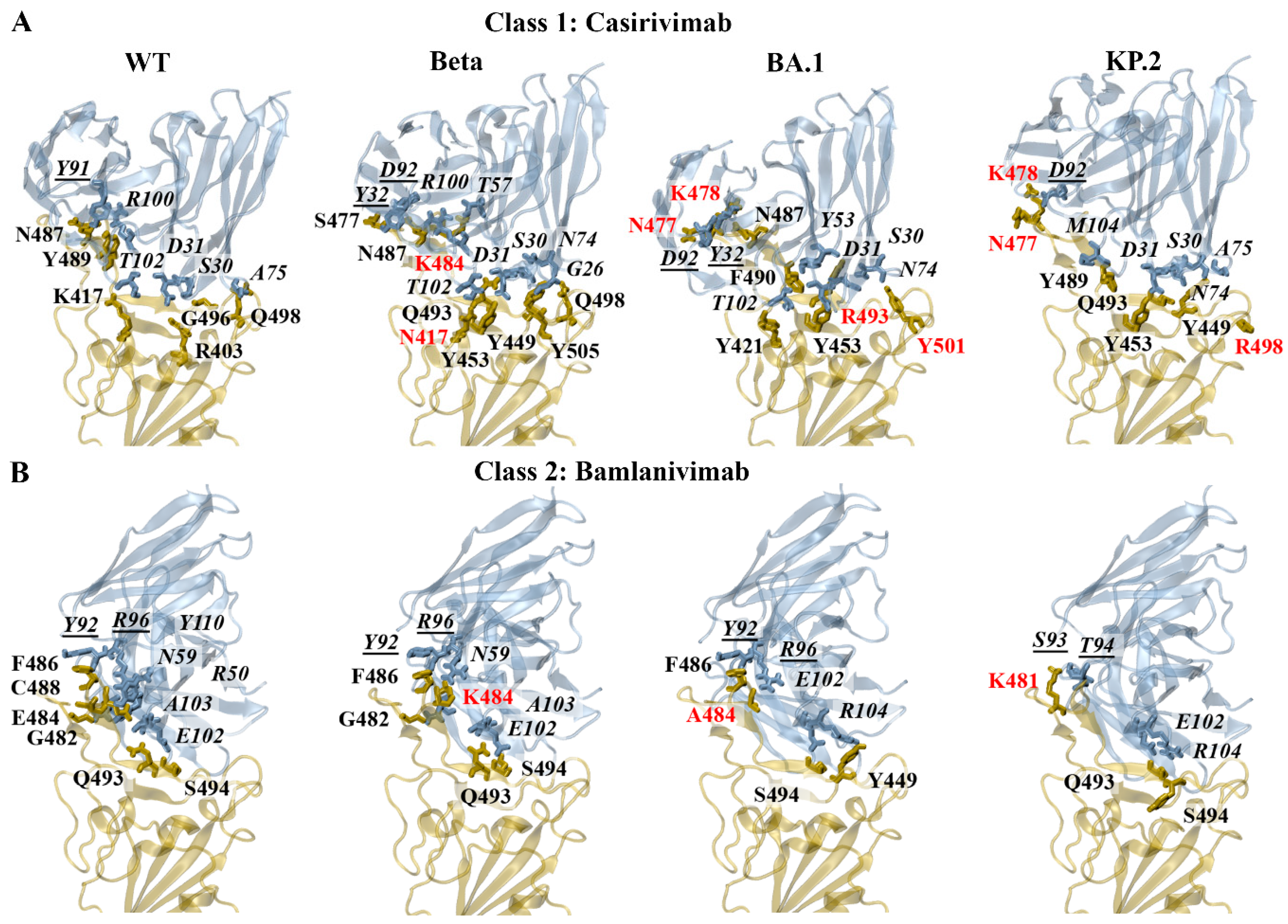
3.4.1. Class 1 and 2 Antibodies
Energy Contributions of Key Residues: F456, E484, F486, Q493
3.4.2. Class 3 and 6 Antibodies
Energy Contributions of Key Residues: R346, N440, L452, E484, F490, Q498
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tian, E.-K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Pustake, M.; Tambolkar, I.; Giri, P.; Gandhi, C. SARS, MERS and CoVID-19: An overview and comparison of clinical, laboratory and radiological features. J. Fam. Med. Prim. Care 2022, 11, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Bai, C.; Zhong, Q.; Gao, G.F. Overview of SARS-CoV-2 genome-encoded proteins. Sci. China Life Sci. 2022, 65, 280–294. [Google Scholar] [CrossRef]
- Wu, C.-r.; Yin, W.-c.; Jiang, Y.; Xu, H.E. Structure genomics of SARS-CoV-2 and its Omicron variant: Drug design templates for COVID-19. Acta Pharmacol. Sin. 2022, 43, 3021–3033. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.-f.; Xu, W.; Liu, S.-w. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Hodcroft, E.B. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. 2021. Available online: https://covariants.org/ (accessed on 2 January 2025).
- Gangavarapu, K.; Latif, A.A.; Mullen, J.L.; Alkuzweny, M.; Hufbauer, E.; Tsueng, G.; Haag, E.; Zeller, M.; Aceves, C.M.; Zaiets, K.; et al. Outbreak.info genomic reports: Scalable and dynamic surveillance of SARS-CoV-2 variants and mutations. Nat. Methods 2023, 20, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Woo, H.G. Omicron: A Heavily Mutated SARS-CoV-2 Variant Exhibits Stronger Binding to ACE2 and Potently Escapes Approved COVID-19 Therapeutic Antibodies. Front. Immunol. 2021, 12, 830527. [Google Scholar] [CrossRef] [PubMed]
- Andre, M.; Lau, L.S.; Pokharel, M.D.; Ramelow, J.; Owens, F.; Souchak, J.; Akkaoui, J.; Ales, E.; Brown, H.; Shil, R.; et al. From Alpha to Omicron: How Different Variants of Concern of the SARS-Coronavirus-2 Impacted the World. Biology 2023, 12, 1267. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Historical Working Definitions and Primary Actions for SARS-CoV-2 Variants. 2023. Available online: https://www.who.int/publications/m/item/historical-working-definitions-and-primary-actions-for-sars-cov-2-variants (accessed on 30 December 2024).
- Meng, B.; Abdullahi, A.; Ferreira, I.A.T.M.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef]
- Suzuki, R.; Yamasoba, D.; Kimura, I.; Wang, L.; Kishimoto, M.; Ito, J.; Morioka, Y.; Nao, N.; Nasser, H.; Uriu, K.; et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature 2022, 603, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Reuschl, A.-K.; Thorne, L.G.; Whelan, M.V.X.; Ragazzini, R.; Furnon, W.; Cowton, V.M.; De Lorenzo, G.; Mesner, D.; Turner, J.L.E.; Dowgier, G.; et al. Evolution of enhanced innate immune suppression by SARS-CoV-2 Omicron subvariants. Nat. Microbiol. 2024, 9, 451–463. [Google Scholar] [CrossRef]
- Cui, L.; Li, T.; Xue, W.; Zhang, S.; Wang, H.; Liu, H.; Gu, Y.; Xia, N.; Li, S. Comprehensive Overview of Broadly Neutralizing Antibodies against SARS-CoV-2 Variants. Viruses 2024, 16, 900. [Google Scholar] [CrossRef]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef]
- Singh, D.D.; Parveen, A.; Yadav, D.K. SARS-CoV-2: Emergence of New Variants and Effectiveness of Vaccines. Front. Cell Infect. Microbiol. 2021, 11, 777212. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Schrödinger LLC. The PyMOL Molecular Graphics System. Schrödinger LLC: New York, NY, USA, 2015; Version 1.8. [Google Scholar]
- Schrödinger LLC. Schrödinger Release 2022-2: Maestro; Schrödinger LLC: New York, NY, USA, 2022. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Ovchynnykova, O.; Kapusta, K.; Sizochenko, N.; Sukhyy, K.M.; Kolodziejczyk, W.; Hill, G.A.; Saloni, J. Homology Modeling and Molecular Dynamics-Driven Search for Natural Inhibitors That Universally Target Receptor-Binding Domain of Spike Glycoprotein in SARS-CoV-2 Variants. Molecules 2022, 27, 7336. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, M.; Li, J.; Xie, X.; Zhao, H.; Wang, Y.; Xu, X.; Zu, S.; Chen, C.; Wan, D.; et al. Identification of an immunogenic epitope and protective antibody against the furin cleavage site of SARS-CoV-2. EBioMedicine 2023, 87, 104401. [Google Scholar] [CrossRef]
- Samanta, A.; Alam, S.S.M.; Ali, S.; Hoque, M. Analyzing the interaction of human ACE2 and RBD of spike protein of SARS-CoV-2 in perspective of Omicron variant. Excli J. 2022, 21, 610–620. [Google Scholar] [CrossRef]
- Shen, M.Y.; Sali, A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006, 15, 2507–2524. [Google Scholar] [CrossRef]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- Dong, J.; Zost, S.J.; Greaney, A.J.; Starr, T.N.; Dingens, A.S.; Chen, E.C.; Chen, R.E.; Case, J.B.; Sutton, R.E.; Gilchuk, P.; et al. Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat. Microbiol. 2021, 6, 1233–1244. [Google Scholar] [CrossRef]
- Jones, B.E.; Brown-Augsburger, P.L.; Corbett, K.S.; Westendorf, K.; Davies, J.; Cujec, T.P.; Wiethoff, C.M.; Blackbourne, J.L.; Heinz, B.A.; Foster, D.; et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef]
- Westendorf, K.; Žentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022, 39, 110812. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.N.; Czudnochowski, N.; Liu, Z.; Zatta, F.; Park, Y.J.; Addetia, A.; Pinto, D.; Beltramello, M.; Hernandez, P.; Greaney, A.J.; et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature 2021, 597, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ryu, D.-K.; Lee, J.; Kim, Y.-I.; Seo, J.-M.; Kim, Y.-G.; Jeong, J.-H.; Kim, M.; Kim, J.-I.; Kim, P.; et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Rouet, R.; Henry, J.Y.; Johansen, M.D.; Sobti, M.; Balachandran, H.; Langley, D.B.; Walker, G.J.; Lenthall, H.; Jackson, J.; Ubiparipovic, S.; et al. Broadly neutralizing SARS-CoV-2 antibodies through epitope-based selection from convalescent patients. Nat. Commun. 2023, 14, 687. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Ambrosetti, F.; Olsen, T.H.; Olimpieri, P.P.; Jiménez-García, B.; Milanetti, E.; Marcatilli, P.; Bonvin, A.M.J.J. proABC-2: PRediction of AntiBody contacts v2 and its application to information-driven docking. Bioinformatics 2020, 36, 5107–5108. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.J.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Bjelkmar, P.; Larsson, P.; Cuendet, M.A.; Hess, B.; Lindahl, E. Implementation of the CHARMM Force Field in GROMACS: Analysis of Protein Stability Effects from Correction Maps, Virtual Interaction Sites, and Water Models. J. Chem. Theory Comput. 2010, 6, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Price, D.J.; Brooks, C.L., III. A modified TIP3P water potential for simulation with Ewald summation. J. Chem. Phys. 2004, 121, 10096–10103. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.J.; Kino, N.; Lockwood, J.L.; Hung, A.; El-Osta, A.; AbuMaziad, A.S.; Karagiannis, T.C. An In Silico Investigation of the Pathogenic G151R G Protein-Gated Inwardly Rectifying K+ Channel 4 Variant to Identify Small Molecule Modulators. Biology 2024, 13, 992. [Google Scholar] [CrossRef] [PubMed]
- Pitsillou, E.; Liang, J.J.; Beh, R.C.; Hung, A.; Karagiannis, T.C. Molecular dynamics simulations highlight the altered binding landscape at the spike-ACE2 interface between the Delta and Omicron variants compared to the SARS-CoV-2 original strain. Comput. Biol. Med. 2022, 149, 106035. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Pitsillou, E.; Karagiannis, C.; Darmawan, K.K.; Ng, K.; Hung, A.; Karagiannis, T.C. Interaction of the prototypical α-ketoamide inhibitor with the SARS-CoV-2 main protease active site in silico: Molecular dynamic simulations highlight the stability of the ligand-protein complex. Comput. Biol. Chem. 2020, 87, 107292. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Thornton, J.M. PROCHECK: Validation of protein-structure coordinates. In International Tables for Crystallography; Wiley: Hoboken, NJ, USA, 2012; pp. 684–687. [Google Scholar] [CrossRef]
- Studer, G.; Rempfer, C.; Waterhouse, A.M.; Gumienny, R.; Haas, J.; Schwede, T. QMEANDisCo—Distance constraints applied on model quality estimation. Bioinformatics 2020, 36, 1765–1771. [Google Scholar] [CrossRef]
- Honorato, R.V.; Trellet, M.E.; Jiménez-García, B.; Schaarschmidt, J.J.; Giulini, M.; Reys, V.; Koukos, P.I.; Rodrigues, J.P.G.L.M.; Karaca, E.; van Zundert, G.C.P.; et al. The HADDOCK2.4 web server for integrative modeling of biomolecular complexes. Nat. Protoc. 2024, 19, 3219–3241. [Google Scholar] [CrossRef]
- Bonvin Lab. HADDOCK2.4 Antibody—Antigen Tutorial Using PDB-Tools Webserver. 2024. Available online: https://www.bonvinlab.org/education/HADDOCK24/HADDOCK24-antibody-antigen/#scenario-2-a-loose-definition-of-the-epitope-is-known (accessed on 5 October 2024).
- Méndez, R.; Leplae, R.; De Maria, L.; Wodak, S.J. Assessment of blind predictions of protein–protein interactions: Current status of docking methods. Proteins Struct. Funct. Bioinform. 2003, 52, 51–67. [Google Scholar] [CrossRef]
- Romero-Durana, M.; Jiménez-García, B.; Fernández-Recio, J. pyDockEneRes: Per-residue decomposition of protein–protein docking energy. Bioinformatics 2020, 36, 2284–2285. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, Q.; Pires, D.E.V.; Rodrigues, C.H.M.; Ascher, D.B. DDMut: Predicting effects of mutations on protein stability using deep learning. Nucleic Acids Res. 2023, 51, W122–W128. [Google Scholar] [CrossRef]
- Rodrigues, C.H.M.; Pires, D.E.V.; Ascher, D.B. DynaMut2: Assessing changes in stability and flexibility upon single and multiple point missense mutations. Protein Sci. 2021, 30, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ivanisenko, N.V.; Shashkova, T.I.; Shevtsov, A.; Sindeeva, M.; Umerenkov, D.; Kardymon, O. SEMA 2.0: Web-platform for B-cell conformational epitopes prediction using artificial intelligence. Nucleic Acids Res. 2024, 52, W533–W539. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- World Health Organization. Tracking SARS-CoV-2 Variants. 2025. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 2 January 2025).
- Han, P.; Li, L.; Liu, S.; Wang, Q.; Zhang, D.; Xu, Z.; Han, P.; Li, X.; Peng, Q.; Su, C.; et al. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell 2022, 185, 630–640.e610. [Google Scholar] [CrossRef]
- Li, L.; Liao, H.; Meng, Y.; Li, W.; Han, P.; Liu, K.; Wang, Q.; Li, D.; Zhang, Y.; Wang, L.; et al. Structural basis of human ACE2 higher binding affinity to currently circulating Omicron SARS-CoV-2 sub-variants BA.2 and BA.1.1. Cell 2022, 185, 2952–2960.e10. [Google Scholar] [CrossRef]
- Cao, Y.; Song, W.; Wang, L.; Liu, P.; Yue, C.; Jian, F.; Yu, Y.; Yisimayi, A.; Wang, P.; Wang, Y.; et al. Characterization of the enhanced infectivity and antibody evasion of Omicron BA.2.75. Cell Host Microbe 2022, 30, 1527–1539.e5. [Google Scholar] [CrossRef]
- Yue, C.; Song, W.; Wang, L.; Jian, F.; Chen, X.; Gao, F.; Shen, Z.; Wang, Y.; Wang, X.; Cao, Y. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect. Dis. 2023, 23, 278–280. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Y.; Jian, F.; Song, W.; Yisimayi, A.; Chen, X.; Xu, Y.; Wang, P.; Wang, J.; Yu, L.; et al. Antigenicity and infectivity characterisation of SARS-CoV-2 BA.2.86. Lancet Infect. Dis. 2023, 23, e457–e459. [Google Scholar] [CrossRef]
- Yamasoba, D.; Uriu, K.; Plianchaisuk, A.; Kosugi, Y.; Pan, L.; Zahradnik, J.; Ito, J.; Sato, K. Virological characteristics of the SARS-CoV-2 omicron XBB.1.16 variant. Lancet Infect. Dis. 2023, 23, 655–656. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Y.; Xu, Y.; Jian, F.; Song, W.; Yisimayi, A.; Wang, P.; Wang, J.; Liu, J.; Yu, L.; et al. Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. Lancet Infect. Dis. 2024, 24, e70–e72. [Google Scholar] [CrossRef]
- Wang, Q.; Mellis, I.A.; Ho, J.; Bowen, A.; Kowalski-Dobson, T.; Valdez, R.; Katsamba, P.S.; Wu, M.; Lee, C.; Shapiro, L.; et al. Recurrent SARS-CoV-2 spike mutations confer growth advantages to select JN.1 sublineages. Emerg. Microbes Infect. 2024, 13, 2402880. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.H.M.; Portelli, S.; Ascher, D.B. Exploring the effects of missense mutations on protein thermodynamics through structure-based approaches: Findings from the CAGI6 challenges. Hum. Genet. 2024. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.J.; Vasudevan, K.; Pragasam, A.K.; Gunasekaran, K.; Veeraraghavan, B.; Mutreja, A. Evolutionary Tracking of SARS-CoV-2 Genetic Variants Highlights an Intricate Balance of Stabilizing and Destabilizing Mutations. mBio 2021, 12, e0118821. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R.; Mallik, B. Omicron (B.1.1.529)—A new heavily mutated variant: Mapped location and probable properties of its mutations with an emphasis on S-glycoprotein. Int. J. Biol. Macromol. 2022, 219, 980–997. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, J.; Tian, M.; Huang, M.; Liu, S.; Xie, Y.; Han, P.; Bai, C.; Han, P.; Zheng, A.; et al. Omicron SARS-CoV-2 mutations stabilize spike up-RBD conformation and lead to a non-RBM-binding monoclonal antibody escape. Nat. Commun. 2022, 13, 4958. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Emergency Use Authorization—Archived Information. 2024. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization-archived-information#covid19 (accessed on 23 December 2024).
- Therapeutic Goods Administration. Update on Effectiveness of Monoclonal Antibodies Against COVID Variants. 2023. Available online: https://www.tga.gov.au/news/news/update-effectiveness-monoclonal-antibodies-against-covid-variants#:~:text=Monoclonal%20antibodies%20targeting%20the%20SARS,spike%20protein%20on%20its%20surface (accessed on 23 December 2024).
- Greaney, A.J.; Starr, T.N.; Barnes, C.O.; Weisblum, Y.; Schmidt, F.; Caskey, M.; Gaebler, C.; Cho, A.; Agudelo, M.; Finkin, S.; et al. Mapping mutations to the SARS-CoV-2 RBD that escape binding by different classes of antibodies. Nat. Commun. 2021, 12, 4196. [Google Scholar] [CrossRef]
- Nguyen, H.; Lan, P.D.; Nissley, D.A.; O’Brien, E.P.; Li, M.S. Cocktail of REGN Antibodies Binds More Strongly to SARS-CoV-2 Than Its Components, but the Omicron Variant Reduces Its Neutralizing Ability. J. Phys. Chem. B 2022, 126, 2812–2823. [Google Scholar] [CrossRef]
- Fung, K.M.; Lai, S.J.; Lin, T.L.; Tseng, T.S. Antigen-Antibody Complex-Guided Exploration of the Hotspots Conferring the Immune-Escaping Ability of the SARS-CoV-2 RBD. Front. Mol. Biosci. 2022, 9, 797132. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Niu, T.; Xie, Y.; Zhao, Z.; Li, D.; He, Q.; Sun, W.; Shi, K.; Guo, W.; et al. Key mechanistic features of the trade-off between antibody escape and host cell binding in the SARS-CoV-2 Omicron variant spike proteins. EMBO J. 2024, 43, 1484–1498. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.-J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef]
- Focosi, D.; Casadevall, A.; Franchini, M.; Maggi, F. Sotrovimab: A Review of Its Efficacy against SARS-CoV-2 Variants. Viruses 2024, 16, 217. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wu, L.; Xu, Z.; Wang, X.; Xie, Y.; Chai, Y.; Zheng, A.; Zhou, J.; Qiao, S.; Huang, M.; et al. An updated atlas of antibody evasion by SARS-CoV-2 Omicron sub-variants including BQ.1.1 and XBB. Cell Rep. Med. 2023, 4, 100991. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, K.; Gu, Y.; Xu, Z.; Shu, C.; Li, D.; Sun, J.; Cong, M.; Li, X.; Zhao, X.; et al. Spike structures, receptor binding, and immune escape of recently circulating SARS-CoV-2 Omicron BA.2.86, JN.1, EG.5, EG.5.1, and HV.1 sub-variants. Structure 2024, 32, 1055–1067.e1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e278. [Google Scholar] [CrossRef]
- Deng, X.; Garcia-Knight, M.A.; Khalid, M.M.; Servellita, V.; Wang, C.; Morris, M.K.; Sotomayor-González, A.; Glasner, D.R.; Reyes, K.R.; Gliwa, A.S.; et al. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell 2021, 184, 3426–3437.e8. [Google Scholar] [CrossRef]
- Wilhelm, A.; Toptan, T.; Pallas, C.; Wolf, T.; Goetsch, U.; Gottschalk, R.; Vehreschild, M.; Ciesek, S.; Widera, M. Antibody-Mediated Neutralization of Authentic SARS-CoV-2 B.1.617 Variants Harboring L452R and T478K/E484Q. Viruses 2021, 13, 1693. [Google Scholar] [CrossRef]


| Conserved | Non-Conserved | |
|---|---|---|
| SEMA-1D | 333–339, 341–347, 356–357, 359–362, 375, 377–389, 405, 409–417, 424, 426–431, 440–449, 456–458, 460, 471–499, 501–503, 505, 526 | 340, 355, 358, 369, 370, 371–374, 376, 403–404, 406, 408, 425, 432, 450–455, 459, 462, 500, 504, and 523 |
| SEMA-3D | 333–347, 355–360, 370, 373, 383, 385–386, 405, 414, 436–451, 472–494, 496, 516–521 | 348, 349, 354, 361, 367, 369, 371, 374–378, 380–382, 389, 393, 396, 408–409, 413, 415–420, 427–428, 430, 452, 454, 456–458, 470–471, 495, 497–498, 500–505, 509, 522–523 |
| Antibody | Heavy Chain | Light Chain |
|---|---|---|
| Casirivimab | R403, E406, K417, Y449, F456, Y473, A475, E484, G485, F486, N487, C488, Y489, F490, L492, Q493, S494, Y495, G496, Q498, N501 | A475, G476, S477, T478, F486, N487 |
| Imdevimab | R346, N439, N440, L441, S443, K444, V445, G446, G447, N448, Y449, N450, Q498, P499 | N439, V445, P499, T500, N501 |
| Tixagevimab | K417, F456, K458, Y473, A475, G476, S477, T478, E484, G485, F486, N487, Y489, Q493 | T478, P479, C480, V483, E484, G485, F486, C488 |
| Bebtelovimab | T345, R346, N439, N440, L441, S443, K444, V445, N450, P499 | V445, G446, G447, Y449, N450, L452, E484, F490, L492, Q493, S494 |
| Regdanvimab | R403, K417, G446, Y449, N450, L452, F456, E484, G485, F486, Y489, F490, L492, Q493, S494, Y495, G496, Q498, N501, Y505 | T478, V483, E484, G485, F486 |
| Bamlanivimab | Y449, L452, F456, I472, N481, G482, V483, E484, G485, Y489, F490, L492, Q493, S494 | N481, V483, E484, G485, F486, Y489 |
| Etesevimab | R408, T415, G416, K417, F456, R457, K458, S459, N460, Y473, Q474, A475, G476, S477, F486, N487, Y489, F490, Q493 | R403, D405, E406, R408, Q409, K417, Y449, S494, Y495, Q498, T500, N501, G502, G504, Y505 |
| Bebtelovimab | T345, R346, N439, N440, L441, D442, S443, K444, V445, G446, G447, N448, Y449, N450, P499, R509 | N439, N440, V445, G446, Q498, P499, T500, N501, G502, V503, Q506 |
| S309 | T333, N334, L335, P337, G339, E340, V341, F342, N343, A344, T345, R346, N354, K356, R357, I358, S359, N360, C361, L441, R509 | T345, N440, L441, K444, V445, R509 |
| GAR12 | R346, F347, A348, K444, G446, G447, N448, Y449, N450, L452, T470, I472, N481, G482, V483, E484 F490, L492, S494 | T345, R346, N440, L441, D442, S443, K444, V445, G446, N448, Y451, R509 |
| Class 1 | Class 2 | ||||
|---|---|---|---|---|---|
| Casirivimab | Tixagevimab | Regdanvimab | Etesevimab | Bamlanivimab | |
| WT | −32.2 | −50.9 | −40.5 | −28.0 | −44.2 |
| Alpha | −31.9 | −52.4 | −36.2 | −40.5 | −41.6 |
| Beta | −30.5 | −39.9 | −29.9 | −45.7 | −19.7 |
| Gamma | −31.1 | −51.6 | −33.1 | −44.4 | −29.5 |
| Delta | −41.1 | −30.9 | −30.1 | −32.7 | −35.5 |
| BA.1 | −33.1 | −42.3 | −31.7 | −26.1 | −26.0 |
| BA.2 | −35.8 | −36.0 | −32.7 | −25.7 | −23.2 |
| BA.4/5 | −24.0 | −27.4 | −24.1 | −24.1 | −31.4 |
| XBB.1.5 | −20.2 | −29.4 | −23.4 | −26.9 | −7.4 |
| XBB.1.16 | −33.6 | −16.8 | −21.1 | −33.8 | −20.0 |
| EG.5 | −20.9 | −17.5 | −19.0 | −26.1 | −26.6 |
| BA.2.86 | −28.8 | −10.1 | −29.8 | −30.9 | −19.0 |
| JN.1 | −23.9 | −22.5 | −36.8 | −34.3 | −12.0 |
| KP.2 | −23.2 | −15.8 | −17.6 | −16.9 | −27.5 |
| KP.3 | −12.7 | −24.7 | −35.7 | −20.8 | −23.4 |
| Class 3 | Class 6 | ||||
|---|---|---|---|---|---|
| Imdevimab | Cilgavimab | Bebtelovimab | S309 | GAR12 | |
| WT | −10.9 | −19.7 | −27.6 | −20.0 | −23.6 |
| Alpha | −20.8 | −16.6 | −28.8 | −24.0 | −35.4 |
| Beta | −22.3 | −23.1 | −25.9 | −16.0 | −21.9 |
| Gamma | −20.3 | −21.7 | −28.2 | −16.7 | −28.4 |
| Delta | −9.4 | −10.6 | −22.6 | −18.7 | −11.0 |
| BA.1 | −16.2 | −20.4 | −20.1 | −22.5 | −24.0 |
| BA.2 | −20.1 | −18.1 | −17.3 | −6.1 | −19.1 |
| BA.4/5 | −27.5 | −14.1 | −19.1 | −31.4 | −25.2 |
| XBB.1.5 | −17.1 | −22.4 | −14.1 | −17.0 | −21.9 |
| XBB.1.16 | −21.3 | −16.1 | −10.6 | −38.2 | −17.7 |
| EG.5 | −9.3 | −11.9 | −18.0 | −14.7 | −17.0 |
| BA.2.86 | −21.5 | −25.8 | −22.6 | −24.3 | −21.0 |
| JN.1 | −9.7 | −23.2 | −20.9 | −34.2 | −21.6 |
| KP.2 | −22.2 | −26.7 | −16.9 | −21.5 | −29.6 |
| KP.3 | −7.4 | −10.3 | −13.3 | −20.0 | −20.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitsillou, E.; El-Osta, A.; Hung, A.; Karagiannis, T.C. Epimaps of the SARS-CoV-2 Receptor-Binding Domain Mutational Landscape: Insights into Protein Stability, Epitope Prediction, and Antibody Binding. Biomolecules 2025, 15, 301. https://doi.org/10.3390/biom15020301
Pitsillou E, El-Osta A, Hung A, Karagiannis TC. Epimaps of the SARS-CoV-2 Receptor-Binding Domain Mutational Landscape: Insights into Protein Stability, Epitope Prediction, and Antibody Binding. Biomolecules. 2025; 15(2):301. https://doi.org/10.3390/biom15020301
Chicago/Turabian StylePitsillou, Eleni, Assam El-Osta, Andrew Hung, and Tom C. Karagiannis. 2025. "Epimaps of the SARS-CoV-2 Receptor-Binding Domain Mutational Landscape: Insights into Protein Stability, Epitope Prediction, and Antibody Binding" Biomolecules 15, no. 2: 301. https://doi.org/10.3390/biom15020301
APA StylePitsillou, E., El-Osta, A., Hung, A., & Karagiannis, T. C. (2025). Epimaps of the SARS-CoV-2 Receptor-Binding Domain Mutational Landscape: Insights into Protein Stability, Epitope Prediction, and Antibody Binding. Biomolecules, 15(2), 301. https://doi.org/10.3390/biom15020301









