The Human Thyroid-Derived CI-huThyrEC Cell Line Expresses the Thyrotropin (TSH) Receptor and Thyroglobulin but Lacks Other Essential Characteristics of Thyroid Follicular Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Three-Dimensional (3D) Culture
2.3. RNA Extraction, RT-PCR, and qPCR
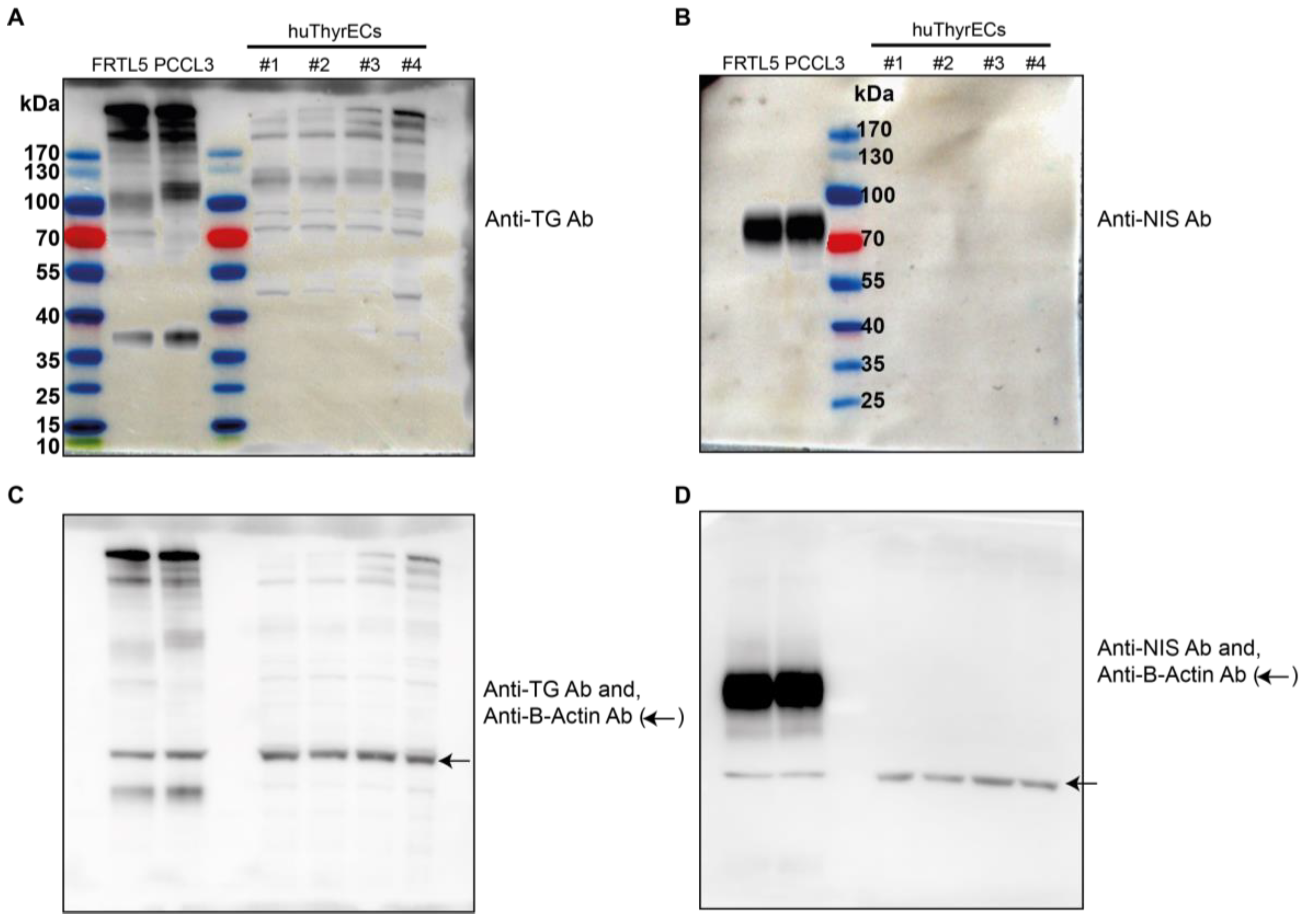
2.4. Western Blot
2.5. Iodide Uptake
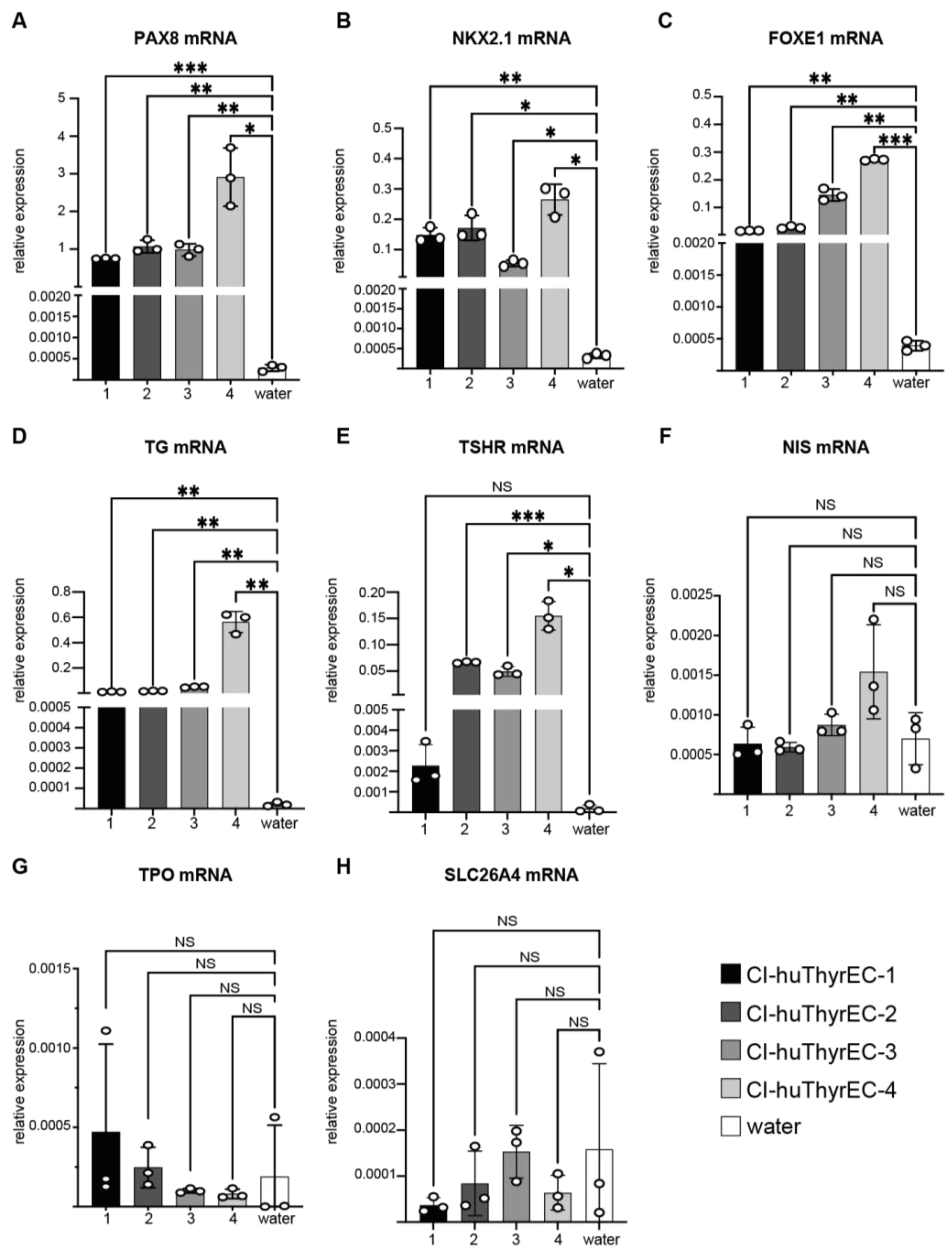
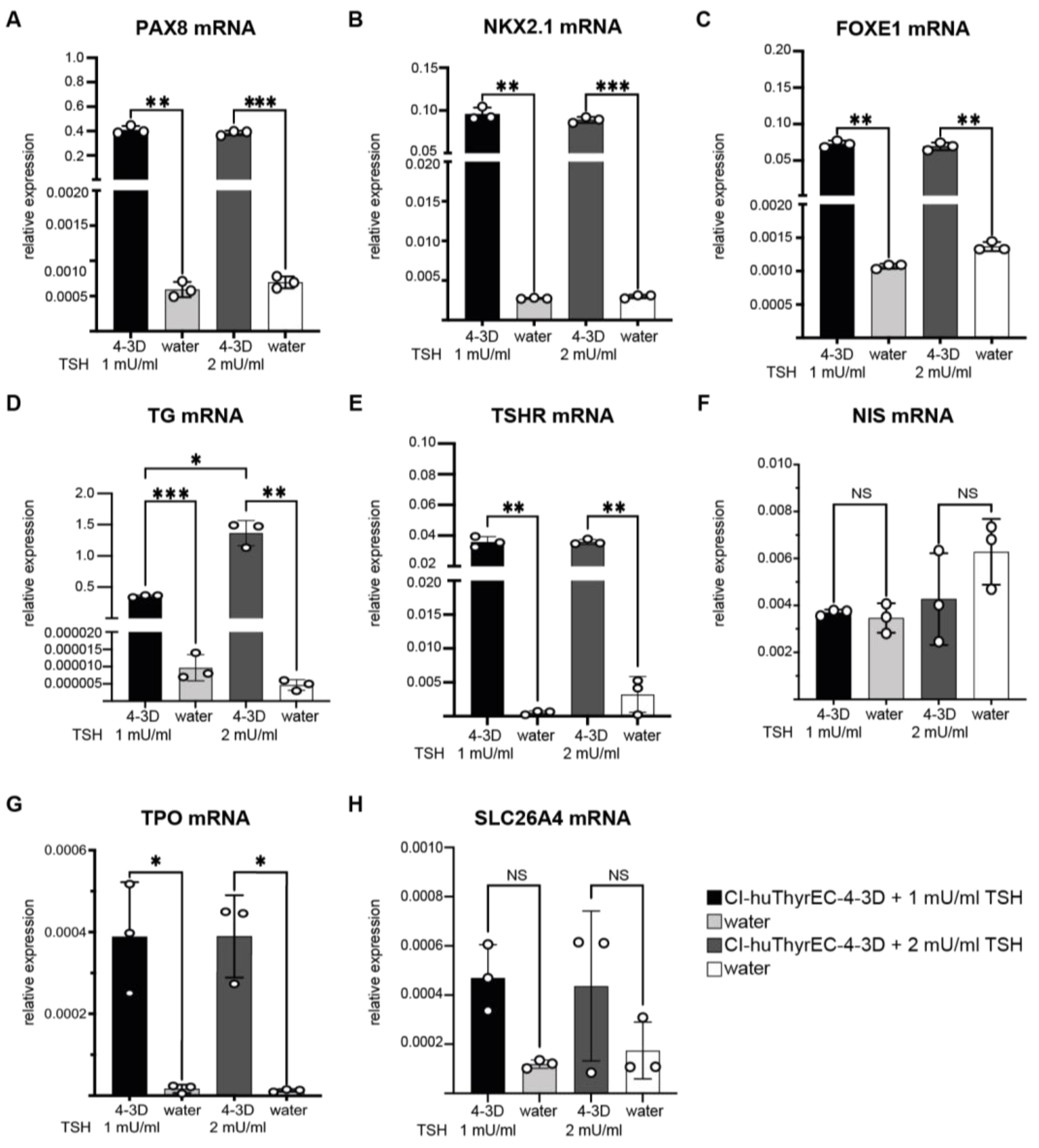
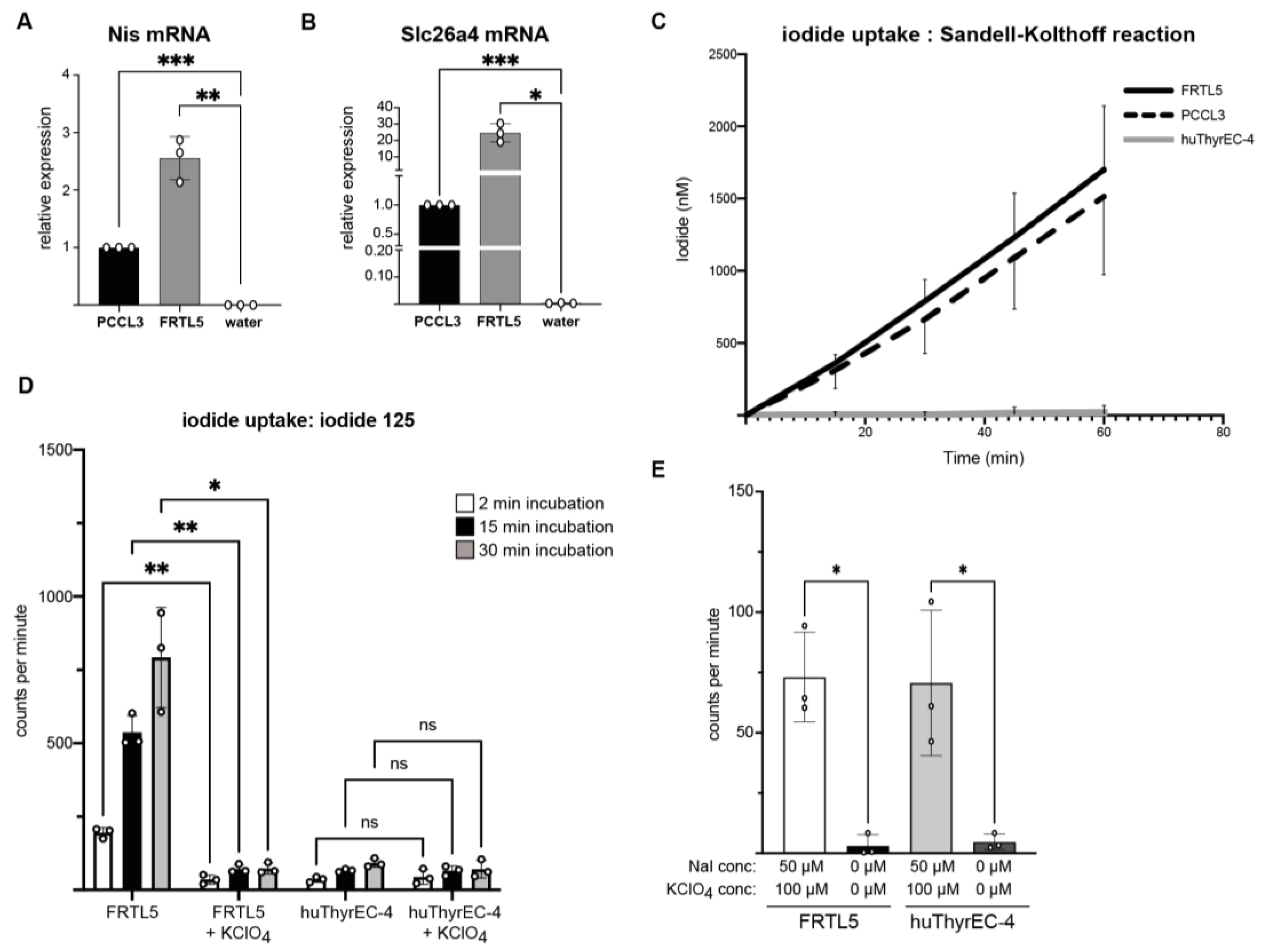
3. Results
3.1. Expression of Thyroid-Restricted Genes in Monolayer Culture
3.2. Expression of Thyroid-Restricted Genes in Three-Dimensional Culture
3.3. Intracellular Iodide Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopp, P. Thyroid hormone synthesis. In Werner and Ingbar’s the Thyroid: A Fundamental and Clinical Text; Braverman, L.E., Cooper, D.S., Kopp, P., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2021; pp. 59–85. [Google Scholar]
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel, L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Anderson, G.; Forrest, D.; Galton, V.A.; Gereben, B.; Kim, B.W.; Kopp, P.A.; Liao, X.H.; Obregon, M.J.; Peeters, R.P.; et al. American Thyroid Association Guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid 2014, 24, 88–168. [Google Scholar] [CrossRef] [PubMed]
- Ambesi-Impiombato, F.S.; Villone, G. The FRTL-5 thyroid cell strain as a model for studies on thyroid cell growth. Acta Endocrinol. Suppl. 1987, 281, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Bidey, S.P.; Lambert, A.; Robertson, W.R. Thyroid cell growth, differentiation and function in the FRTL-5 cell line: A survey. J. Endocrinol. 1988, 119, 365–376. [Google Scholar] [CrossRef]
- Leoni, S.; Galante, P.; Ricarte-Filho, J.; Kimura, E. Differential gene expression analysis of iodide-treated rat thyroid follicular cell line PCCl3. Genomics 2008, 91, 356–366. [Google Scholar] [CrossRef]
- Medina, D.L.; Santisteban, P. Thyrotropin-dependent proliferation of in vitro rat thyroid cell systems. Eur. J. Endocrinol. 2000, 143, 161–178. [Google Scholar] [CrossRef]
- Lemoine, N.R.; Mayall, E.; Jones, T.; Sheer, D.; McDermid, S.; Kendall-Taylor, P.; Wynford-Thomas, D. Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br. J. Cancer 1989, 60, 897–903. [Google Scholar] [CrossRef]
- Kurashige, T.; Shimamura, M.; Nagayama, Y. Reevaluation of the Effect of Iodine on Thyroid Cell Survival and Function Using PCCL3 and Nthy-ori 3-1 Cells. J. Endocr. Soc. 2020, 4, bvaa146. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Phay, J.E.; Shen, R.; Pellegata, N.S.; Saji, M.; Ringel, M.D.; de la Chapelle, A.; He, H. Primary Cell Culture Systems for Human Thyroid Studies. Thyroid 2016, 26, 1131–1140. [Google Scholar] [CrossRef]
- van der Vaart, J.; Bosmans, L.; Sijbesma, S.F.; Knoops, K.; van de Wetering, W.J.; Otten, H.G.; Begthel, H.; Borel Rinkes, I.H.; Korving, J.; Lentjes, E.G.; et al. Adult mouse and human organoids derived from thyroid follicular cells and modeling of Graves’ hyperthyroidism. Proc. Natl. Acad. Sci. USA 2021, 118, e2117017118. [Google Scholar] [CrossRef]
- Deisenroth, C.; Soldatow, V.Y.; Ford, J.; Stewart, W.; Brinkman, C.; LeCluyse, E.L.; MacMillan, D.K.; Thomas, R.S. Development of an In Vitro Human Thyroid Microtissue Model for Chemical Screening. Toxicol. Sci. 2020, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Ogundipe, V.M.; Groen, A.H.; Hosper, N.; Nagle, P.W.; Hess, J.; Faber, H.; Jellema, A.L.; Baanstra, M.; Links, T.P.; Unger, K.; et al. Generation and Differentiation of Adult Tissue-Derived Human Thyroid Organoids. Stem Cell Rep. 2021, 16, 913–925. [Google Scholar] [CrossRef]
- Kühnlenz, J. A microfluidic thyroid-liver platform to assess chemical safety in humans. Altex 2023, 40, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.J.; Kip, A.M.; Romitti, M.; Nazzari, M.; Tegel, A.; Stich, M.; Krause, C.; Caiment, F.; Costagliola, S.; Moroni, L.; et al. Thyroid-on-a-Chip: An Organoid Platform for In Vitro Assessment of Endocrine Disruption. Adv. Healthc. Mater. 2023, 12, e2201555. [Google Scholar] [CrossRef] [PubMed]
- Romitti, M.; Tourneur, A.; Fonseca, B.d.F.d.; Doumont, G.; Gillotay, P.; Liao, X.-H.; Eski, S.E.; Van Simaeys, G.; Chomette, L.; Lasolle, H.; et al. Transplantable human thyroid organoids generated from embryonic stem cells to rescue hypothyroidism. Nat. Commun. 2022, 13, 7057. [Google Scholar] [CrossRef]
- Ma, R.; Morshed, S.A.; Latif, R.; Davies, T.F. Thyroid cell differentiation from murine induced pluripotent stem cells. Front. Endocrinol. 2015, 6, 56. [Google Scholar] [CrossRef]
- Kurmann, A.A.; Serra, M.; Hawkins, F.; Rankin, S.A.; Mori, M.; Astapova, I.; Ullas, S.; Lin, S.; Bilodeau, M.; Rossant, J.; et al. Regeneration of Thyroid Function by Transplantation of Differentiated Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 527–542. [Google Scholar] [CrossRef]
- Antonica, F.; Kasprzyk, D.F.; Opitz, R.; Iacovino, M.; Liao, X.-H.; Dumitrescu, A.M.; Refetoff, S.; Peremans, K.; Manto, M.; Kyba, M.; et al. Generation of functional thyroid from embryonic stem cells. Nature 2012, 491, 66–71. [Google Scholar] [CrossRef]
- Hopperstad, K.; Truschel, T.; Wahlicht, T.; Stewart, W.; Eicher, A.; May, T.; Deisenroth, C. Characterization of Novel Human Immortalized Thyroid Follicular Epithelial Cell Lines. Appl. In Vitro Toxicol. 2021, 7, 39–49. [Google Scholar] [CrossRef]
- InSCREENex. Thyroid Epithelial Cells. 2022. Available online: https://www.inscreenex.de/products/human-immortalized-cell-lines/thyroid-epithelial-cells/ (accessed on 8 January 2023).
- Levy, O.; Dai, G.; Riedel, C.; Ginter, C.S.; Paul, E.M.; Lebowitz, A.N.; Carrasco, N. Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc. Natl. Acad. Sci. USA 1997, 94, 5568–5573. [Google Scholar] [CrossRef]
- Waltz, F.; Pillette, L.; Ambroise, Y. A nonradioactive iodide uptake assay for sodium iodide symporter function. Anal. Biochem. 2010, 396, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Pesce, L.; Bizhanova, A.; Caraballo, J.C.; Westphal, W.; Butti, M.L.; Comellas, A.; Kopp, P. TSH regulates pendrin membrane abundance and enhances iodide efflux in thyroid cells. Endocrinology 2012, 153, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Galietta, L.J.V. Chloride channels as drug targets. Nat. Rev. Drug Discov. 2009, 8, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Undeutsch, H.J.; Posabella, A.; Alber, A.B.; Bawa, P.S.; Villacorta-Martin, C.; Wang, F.; Ikonomou, L.; Kotton, D.N.; Hollenberg, A.N. Derivation of transplantable human thyroid follicular epithelial cells from induced pluripotent stem cells. Stem Cell Rep. 2024, 19, 1690–1705. [Google Scholar] [CrossRef]
- Szinnai, G.; Lacroix, L.; Carré, A.; Guimiot, F.; Talbot, M.; Martinovic, J.; Delezoide, A.-L.; Vekemans, M.; Michiels, S.; Caillou, B.; et al. Sodium/iodide symporter (NIS) gene expression is the limiting step for the onset of thyroid function in the human fetus. J. Clin. Endocrinol. Metab. 2007, 92, 70–76. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halbout, M.; Kopp, P.A. The Human Thyroid-Derived CI-huThyrEC Cell Line Expresses the Thyrotropin (TSH) Receptor and Thyroglobulin but Lacks Other Essential Characteristics of Thyroid Follicular Cells. Biomolecules 2025, 15, 375. https://doi.org/10.3390/biom15030375
Halbout M, Kopp PA. The Human Thyroid-Derived CI-huThyrEC Cell Line Expresses the Thyrotropin (TSH) Receptor and Thyroglobulin but Lacks Other Essential Characteristics of Thyroid Follicular Cells. Biomolecules. 2025; 15(3):375. https://doi.org/10.3390/biom15030375
Chicago/Turabian StyleHalbout, Mathias, and Peter A. Kopp. 2025. "The Human Thyroid-Derived CI-huThyrEC Cell Line Expresses the Thyrotropin (TSH) Receptor and Thyroglobulin but Lacks Other Essential Characteristics of Thyroid Follicular Cells" Biomolecules 15, no. 3: 375. https://doi.org/10.3390/biom15030375
APA StyleHalbout, M., & Kopp, P. A. (2025). The Human Thyroid-Derived CI-huThyrEC Cell Line Expresses the Thyrotropin (TSH) Receptor and Thyroglobulin but Lacks Other Essential Characteristics of Thyroid Follicular Cells. Biomolecules, 15(3), 375. https://doi.org/10.3390/biom15030375





