Evaluation of Lipid-Based Transfection in Primary Monocytes Within an Ex Vivo Whole-Blood Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Whole-Blood Transfection Using Three Different Transfection Reagents
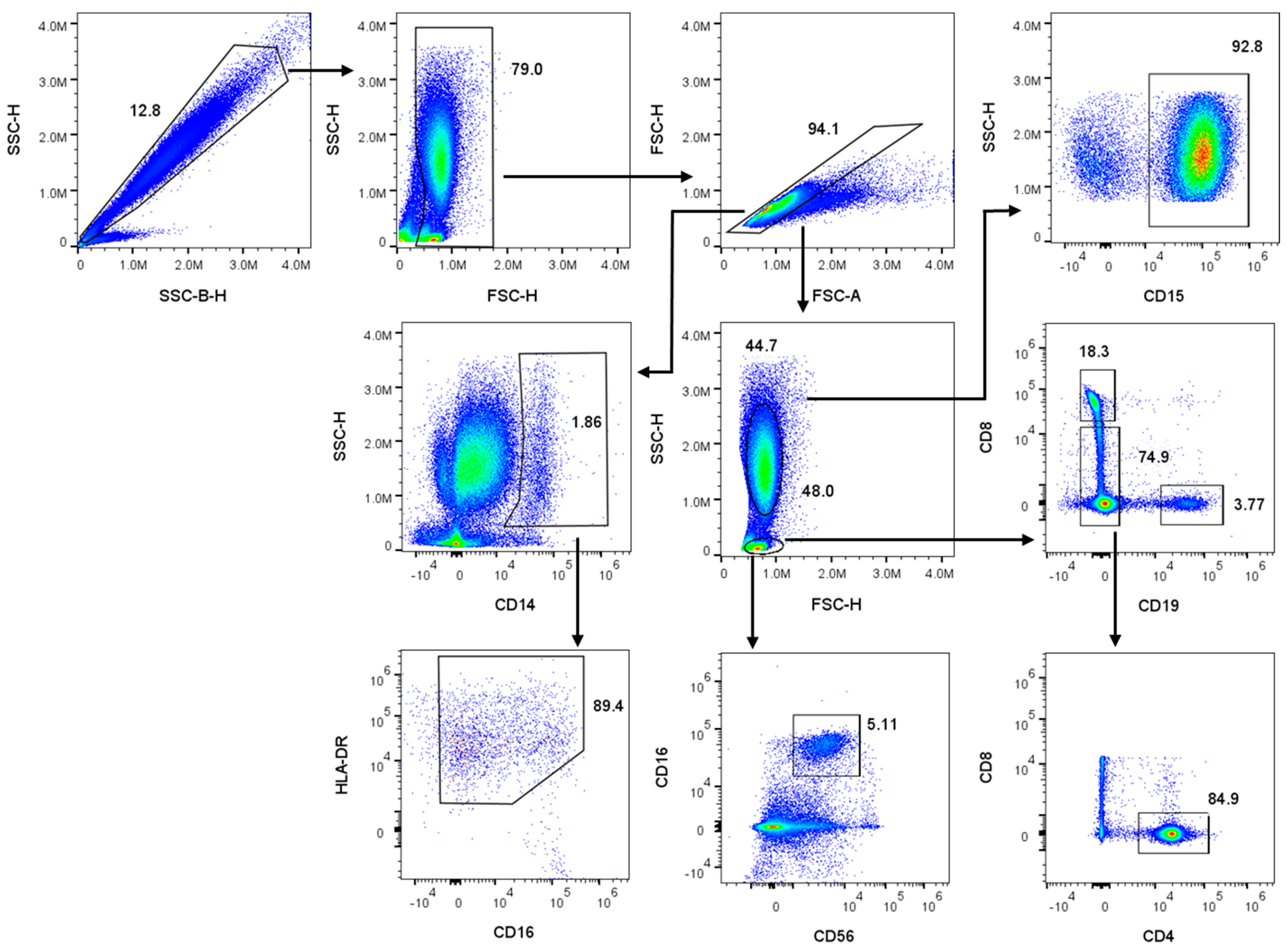
2.2. Two-Step Red Blood Cell Lysis and Sample Preparation for Flow Cytometry
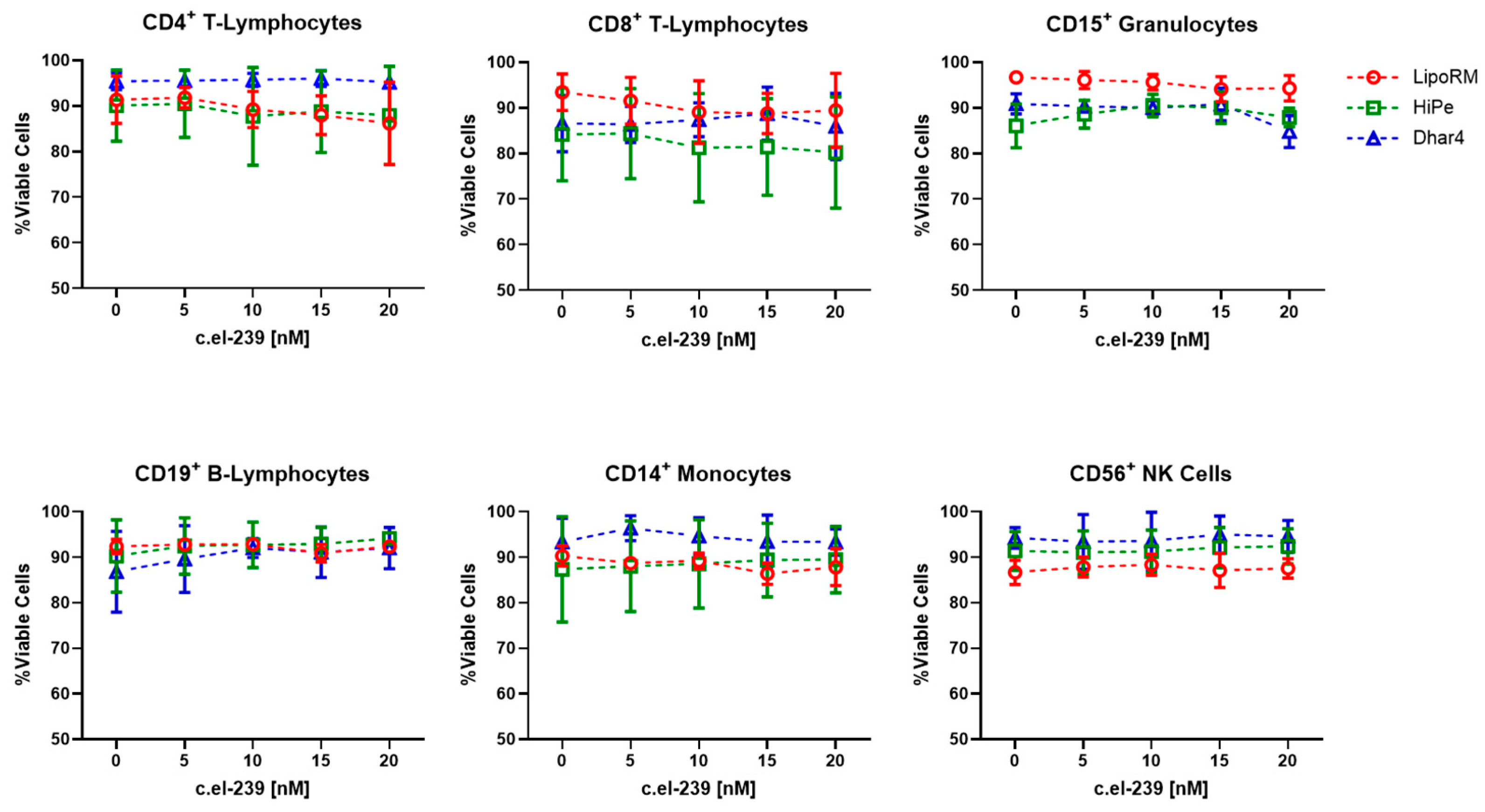
2.3. Cell Viability
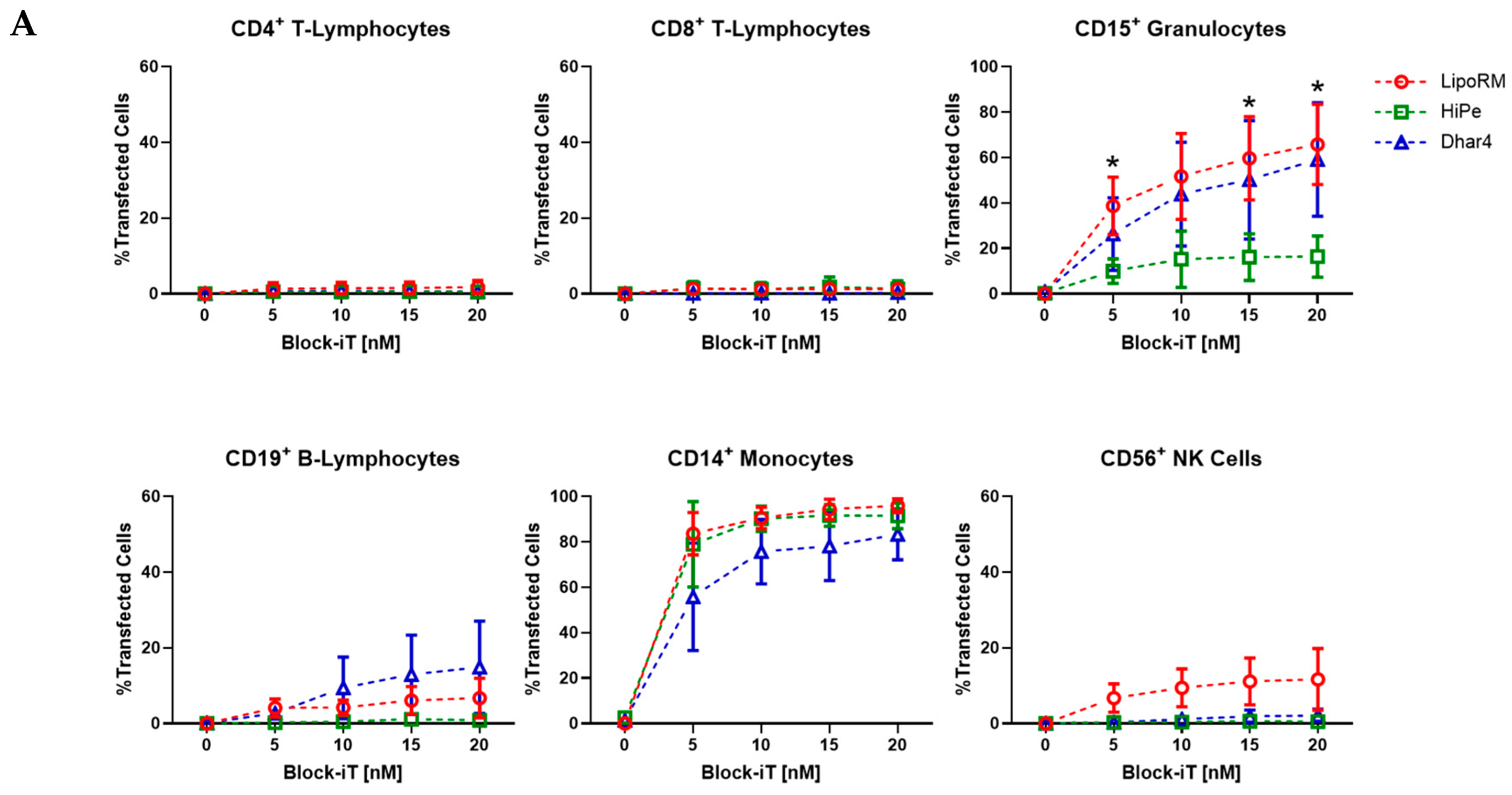
2.4. Transfection Efficiency
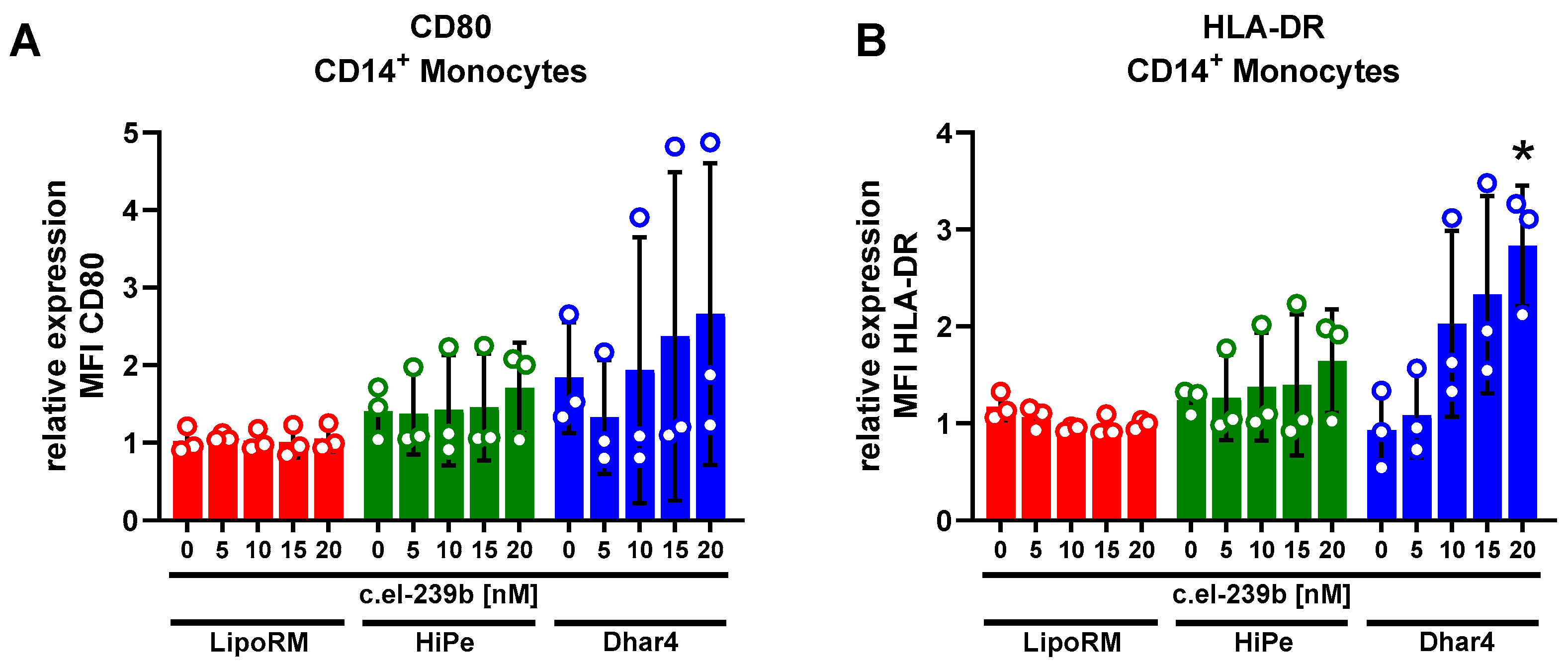
2.5. Immune Cell Activation Assay
2.6. Transfection Assay Ex Vivo Using siRNA and miRNA
2.7. Statistical Analysis
2.8. Antibodies
| PE—anti-CD4 | (Clone RPA-T4) |
| Pacific Blue—anti-CD4 | (Clone OKT4) |
| PE-Cy7—anti-CD8 | (Clone HIT8a) |
| PerCP—anti-CD14 | (Clone HCD14) |
| FITC—anti-CD15 | (Clone HI98) |
| FITC—anti-CD16 | (Clone 3G8) |
| Pacific Blue—anti-CD16 | (Clone 3G8 |
| APC—anti-CD19 | (Clone HIB19) |
| APC-Cy7—anti-CD56 | (Clone HCD56) |
| Alexa Fluor 488—anti-HLA-DR | (Clone L243) |
| PE—anti-HLA-DR | (Clone L243) |
| APC—anti-HLA-DR | (Clone L243) |
| APC—anti-CD80 | (Clone 2D10) |
3. Results
3.1. Viability of Whole-Blood Leukocyte Populations upon Ex Vivo Transfection
3.2. Transfection Efficiency Across Leukocyte Sub-Populations in Whole Blood
3.3. Immune Activation in Primary Monocytes upon Use of Transfection Reagents
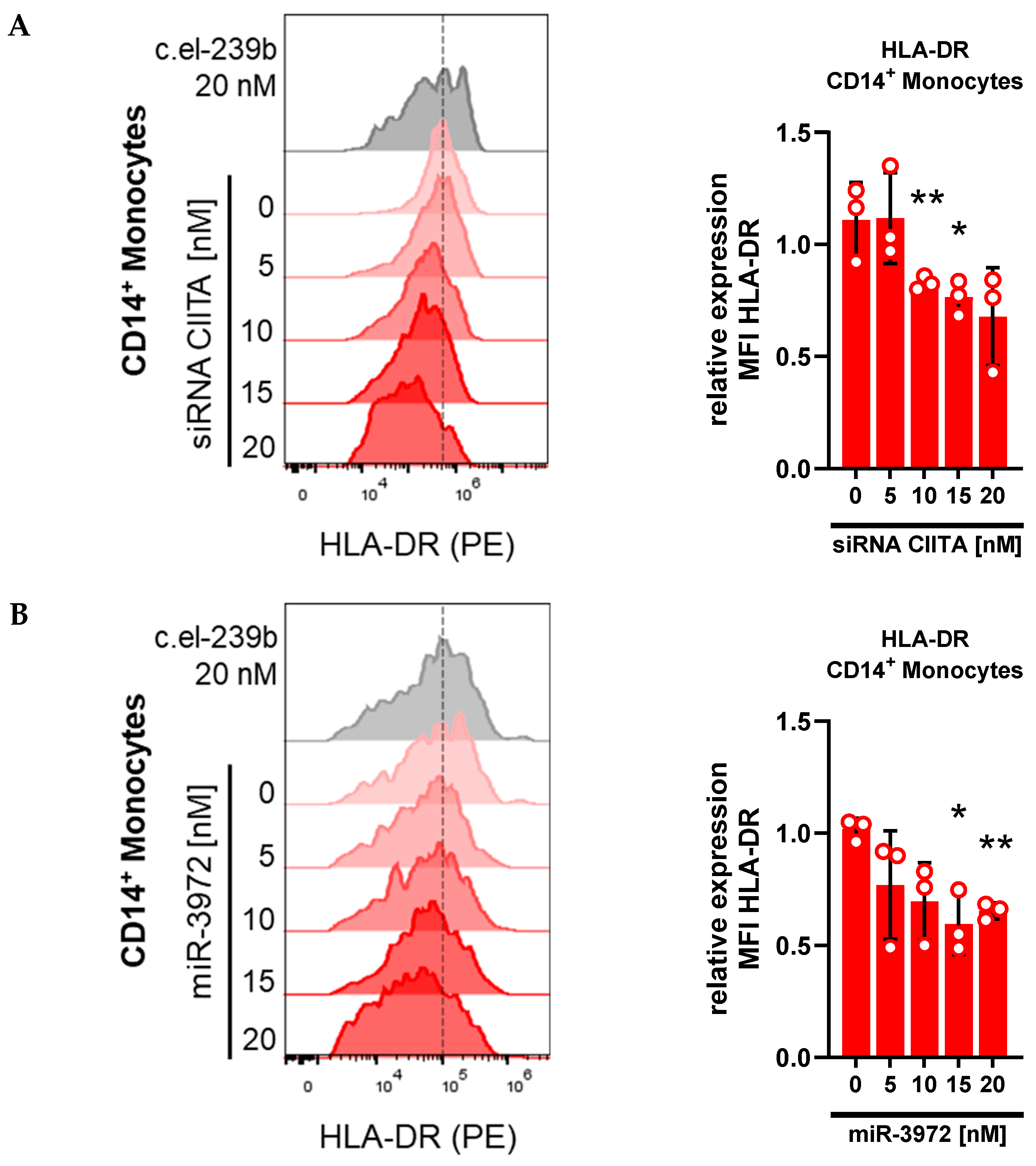
3.4. Whole-Blood Transfection with siRNA and miRNA Downregulates HLA-DR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setten, R.L.; Rossi, J.J.; Han, S. The Current State and Future Directions of RNAi-Based Therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Granot-Matok, Y.; Kon, E.; Dammes, N.; Mechtinger, G.; Peer, D. Therapeutic mRNA Delivery to Leukocytes. J. Control. Release 2019, 305, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Eberwine, J.H. Mammalian Cell Transfection: The Present and the Future. Anal. Bioanal. Chem. 2010, 397, 3173–3178. [Google Scholar] [CrossRef]
- Martin, B.; Sainlos, M.; Aissaoui, A.; Oudrhiri, N.; Hauchecorne, M.; Vigneron, J.-P.; Lehn, J.-M.; Lehn, P. The Design of Cationic Lipids for Gene Delivery. Curr. Pharm. Des. 2005, 11, 375–394. [Google Scholar] [CrossRef]
- Aljaberi, A.; Spelios, M.; Kearns, M.; Selvi, B.; Savva, M. Physicochemical Properties Affecting Lipofection Potency of a New Series of 1,2-Dialkoylamidopropane-Based Cationic Lipids. Colloids Surf. B Biointerfaces 2007, 57, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Resina, S.; Prevot, P.; Thierry, A.R. Physico-Chemical Characteristics of Lipoplexes Influence Cell Uptake Mechanisms and Transfection Efficacy. PLoS ONE 2009, 4, e6058. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.M. Cationic Lipid-Mediated Nucleic Acid Delivery: Beyond Being Cationic. Chem. Phys. Lipids 2010, 163, 245–252. [Google Scholar] [CrossRef]
- Zuhorn, I.S.; Bakowsky, U.; Polushkin, E.; Visser, W.H.; Stuart, M.C.A.; Engberts, J.B.F.N.; Hoekstra, D. Nonbilayer Phase of Lipoplex–Membrane Mixture Determines Endosomal Escape of Genetic Cargo and Transfection Efficiency. Mol. Ther. 2005, 11, 801–810. [Google Scholar] [CrossRef]
- Wasungu, L.; Stuart, M.C.A.; Scarzello, M.; Engberts, J.B.F.N.; Hoekstra, D. Lipoplexes Formed from Sugar-Based Gemini Surfactants Undergo a Lamellar-to-Micellar Phase Transition at Acidic pH. Evidence for a Non-Inverted Membrane-Destabilizing Hexagonal Phase of Lipoplexes. Biochim. Biophys. Acta BBA-Biomembr. 2006, 1758, 1677–1684. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-Based Therapeutics—Developing a New Class of Drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Shirley, J.L.; De Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lu, Z.-R. Structure and Function of Cationic and Ionizable Lipids for Nucleic Acid Delivery. Pharm. Res. 2023, 40, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Scarfo, K.; Nantz, M.H.; Hecker, J.G. Lipid-Mediated Delivery of RNA Is More Efficient than Delivery of DNA in Non-Dividing Cells. Int. J. Pharm. 2010, 389, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Escalona-Rayo, O.; Zeng, Y.; Knol, R.A.; Kock, T.J.F.; Aschmann, D.; Slütter, B.; Kros, A. In Vitro and in Vivo Evaluation of Clinically-Approved Ionizable Cationic Lipids Shows Divergent Results between mRNA Transfection and Vaccine Efficacy. Biomed. Pharmacother. 2023, 165, 115065. [Google Scholar] [CrossRef]
- Coch, C.; Lück, C.; Schwickart, A.; Putschli, B.; Renn, M.; Höller, T.; Barchet, W.; Hartmann, G.; Schlee, M. A Human In Vitro Whole Blood Assay to Predict the Systemic Cytokine Response to Therapeutic Oligonucleotides Including siRNA. PLoS ONE 2013, 8, e71057. [Google Scholar] [CrossRef]
- Van Essen, M.F.; Schlagwein, N.; Van Gijlswijk-Janssen, D.J.; Anholts, J.D.H.; Eikmans, M.; Ruben, J.M.; Van Kooten, C. Culture Medium Used during Small Interfering RNA (siRNA) Transfection Determines the Maturation Status of Dendritic Cells. J. Immunol. Methods 2020, 479, 112748. [Google Scholar] [CrossRef]
- Prabhu, V.M.; Singh, A.K.; Padwal, V.; Nagar, V.; Patil, P.; Patel, V. Monocyte Based Correlates of Immune Activation and Viremia in HIV-Infected Long-Term Non-Progressors. Front. Immunol. 2019, 10, 2849. [Google Scholar] [CrossRef]
- Van Gool, S.W.; Vandenberghe, P.; Boer, M.D.; Ceuppens, J.L. CD80, CD86 and CD40 Provide Accessory Signals in a Multiple-Step T-Cell Activation Model. Immunol. Rev. 1996, 153, 47–83. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamasaki, K.; Tadagaki, K. Caspase Inhibition Improves Viability and Efficiency of Liposomal Transfection. Sci. Rep. 2023, 13, 21868. [Google Scholar] [CrossRef]
- Cui, S.; Wang, Y.; Gong, Y.; Lin, X.; Zhao, Y.; Zhi, D.; Zhou, Q.; Zhang, S. Correlation of the Cytotoxic Effects of Cationic Lipids with Their Headgroups. Toxicol. Res. 2018, 7, 473–479. [Google Scholar] [CrossRef]
- Jörgensen, A.M.; Wibel, R.; Bernkop-Schnürch, A. Biodegradable Cationic and Ionizable Cationic Lipids: A Roadmap for Safer Pharmaceutical Excipients. Small 2023, 19, 2206968. [Google Scholar] [CrossRef] [PubMed]
- Rico, L.G.; Salvia, R.; Ward, M.D.; Bradford, J.A.; Petriz, J. Flow-Cytometry-Based Protocols for Human Blood/Marrow Immunophenotyping with Minimal Sample Perturbation. STAR Protoc. 2021, 2, 100883. [Google Scholar] [CrossRef] [PubMed]
- Drzeniek, N.M.; Kahwaji, N.; Picht, S.; Dimitriou, I.M.; Schlickeiser, S.; Moradian, H.; Geissler, S.; Schmueck-Henneresse, M.; Gossen, M.; Volk, H. In Vitro Transcribed mRNA Immunogenicity Induces Chemokine-Mediated Lymphocyte Recruitment and Can Be Gradually Tailored by Uridine Modification. Adv. Sci. 2024, 11, 2308447. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class I and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting Effective microRNA Target Sites in Mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Vejnar, C.E.; Blum, M.; Zdobnov, E.M. miRmap Web: Comprehensive microRNA Target Prediction Online. Nucleic Acids Res. 2013, 41, W165–W168. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef]
- Moradian, H.; Roch, T.; Lendlein, A.; Gossen, M. mRNA Transfection-Induced Activation of Primary Human Monocytes and Macrophages: Dependence on Carrier System and Nucleotide Modification. Sci. Rep. 2020, 10, 4181. [Google Scholar] [CrossRef]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Transfection Types, Methods and Strategies: A Technical Review. PeerJ 2021, 9, e11165. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-Viral Vectors for Gene-Based Therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Altan-Bonnet, G.; Mukherjee, R. Cytokine-Mediated Communication: A Quantitative Appraisal of Immune Complexity. Nat. Rev. Immunol. 2019, 19, 205–217. [Google Scholar] [CrossRef]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T Cell Receptor (TCR) Signaling in Health and Disease. Signal Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of Cationic Lipids and Cationic Polymers in Gene Delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Bexborn, F.; Engberg, A.E.; Sandholm, K.; Mollnes, T.E.; Hong, J.; Nilsson Ekdahl, K. Hirudin versus Heparin for Use in Whole Blood in Vitro Biocompatibility Models. J. Biomed. Mater. Res. A 2009, 89A, 951–959. [Google Scholar] [CrossRef]
- Scherer, O.; Maeß, M.B.; Lindner, S.; Garscha, U.; Weinigel, C.; Rummler, S.; Werz, O.; Lorkowski, S. A Procedure for Efficient Non-Viral siRNA Transfection of Primary Human Monocytes Using Nucleofection. J. Immunol. Methods 2015, 422, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.K.; Zhou, L.H.; Tam, K.C.; Too, H.P. Enhanced Non-Viral Gene Delivery by Coordinated Endosomal Release and Inhibition of β-Tubulin Deactylase. Nucleic Acids Res. 2017, 45, e38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.R.K.; Shou, Y.; Chan, B.; Krishaa, L.; Tay, A. Materials for Improving Immune Cell Transfection. Adv. Mater. 2021, 33, 2007421. [Google Scholar] [CrossRef]
- Friend, D.S.; Papahadjopoulos, D.; Debs, R.J. Endocytosis and Intracellular Processing Accompanying Transfection Mediated by Cationic Liposomes. Biochim. Biophys. Acta BBA-Biomembr. 1996, 1278, 41–50. [Google Scholar] [CrossRef]
- Gresch, O.; Altrogge, L. Transfection of Difficult-to-Transfect Primary Mammalian Cells. In Protein Expression in Mammalian Cells; Hartley, J.L., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 801, pp. 65–74. ISBN 978-1-61779-351-6. [Google Scholar]
- Loo, J.; Sicher, I.; Goff, A.; Kim, O.; Clary, N.; Alexeev, A.; Sulchek, T.; Zamarayeva, A.; Han, S.; Calero-Garcia, M. Microfluidic Transfection of mRNA into Human Primary Lymphocytes and Hematopoietic Stem and Progenitor Cells Using Ultra-Fast Physical Deformations. Sci. Rep. 2021, 11, 21407. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Das, B.; Mishra, A.; Sahay, P.; Upadhyay, P. Monocytes Isolated by Positive and Negative Magnetic Sorting Techniques Show Different Molecular Characteristics and Immunophenotypic Behaviour. F1000Research 2018, 6, 2045. [Google Scholar] [CrossRef] [PubMed]
- Elkord, E.; Williams, P.E.; Kynaston, H.; Rowbottom, A.W. Human Monocyte Isolation Methods Influence Cytokine Production from in Vitro Generated Dendritic Cells. Immunology 2005, 114, 204–212. [Google Scholar] [CrossRef]
- Garratt, L.W. Current Understanding of the Neutrophil Transcriptome in Health and Disease. Cells 2021, 10, 2406. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Ellis, B.A.; Munafo, D.B.; Brzezinska, A.A.; Catz, S.D. Gene Transfer and Expression in Human Neutrophils. The Phox Homology Domain of P47 Phox Translocates to the Plasma Membrane but Not to the Membrane of Mature Phagosomes. BMC Immunol. 2006, 7, 28. [Google Scholar] [CrossRef]
- Nakamura, T.; Kuroi, M.; Fujiwara, Y.; Warashina, S.; Sato, Y.; Harashima, H. Small-Sized, Stable Lipid Nanoparticle for the Efficient Delivery of siRNA to Human Immune Cell Lines. Sci. Rep. 2016, 6, 37849. [Google Scholar] [CrossRef]
- Bramson, J.L.; Bodner, C.A.; Graham, R.W. Activation of Host Antitumoral Responses by Cationic Lipid/DNA Complexes. Cancer Gene Ther. 2000, 7, 353–359. [Google Scholar] [CrossRef]
- Blasius, A.L.; Beutler, B. Intracellular Toll-like Receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and Immune System Interplay: Challenges and Potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef]
- Forsbach, A.; Müller, C.; Montino, C.; Kritzler, A.; Curdt, R.; Benahmed, A.; Jurk, M.; Vollmer, J. Impact of Delivery Systems on siRNA Immune Activation and RNA Interference. Immunol. Lett. 2012, 141, 169–180. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moolan-Vadackumchery, R.; Zhang, L.; Stüber, F. Evaluation of Lipid-Based Transfection in Primary Monocytes Within an Ex Vivo Whole-Blood Model. Biomolecules 2025, 15, 391. https://doi.org/10.3390/biom15030391
Moolan-Vadackumchery R, Zhang L, Stüber F. Evaluation of Lipid-Based Transfection in Primary Monocytes Within an Ex Vivo Whole-Blood Model. Biomolecules. 2025; 15(3):391. https://doi.org/10.3390/biom15030391
Chicago/Turabian StyleMoolan-Vadackumchery, Robin, Lan Zhang, and Frank Stüber. 2025. "Evaluation of Lipid-Based Transfection in Primary Monocytes Within an Ex Vivo Whole-Blood Model" Biomolecules 15, no. 3: 391. https://doi.org/10.3390/biom15030391
APA StyleMoolan-Vadackumchery, R., Zhang, L., & Stüber, F. (2025). Evaluation of Lipid-Based Transfection in Primary Monocytes Within an Ex Vivo Whole-Blood Model. Biomolecules, 15(3), 391. https://doi.org/10.3390/biom15030391





