The Role of Purinergic Mechanisms in the Excitability of Trigeminal Afferents of Rats with Prenatal Hyperhomocysteinemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals: The Model of Prenatal HHCY
2.2. Plasma Homocysteine and CGRP Concentration
2.3. Electrophysiological Recordings of the Electrical Activity of Trigeminal Afferents in Hemiskull Preparations
2.4. Ca2+ Imaging in the Culture of the Trigeminal Ganglion
2.5. Toluidine Blue Staining of Meningeal Mast Cells
2.6. Chemicals
2.7. Statistical Analysis
3. Results
3.1. Plasma Homocysteine and CGRP Levels
3.2. 4-Aminopyridine Increases the Frequency of Action Potentials of Trigeminal Afferents in Rats with pHHCY
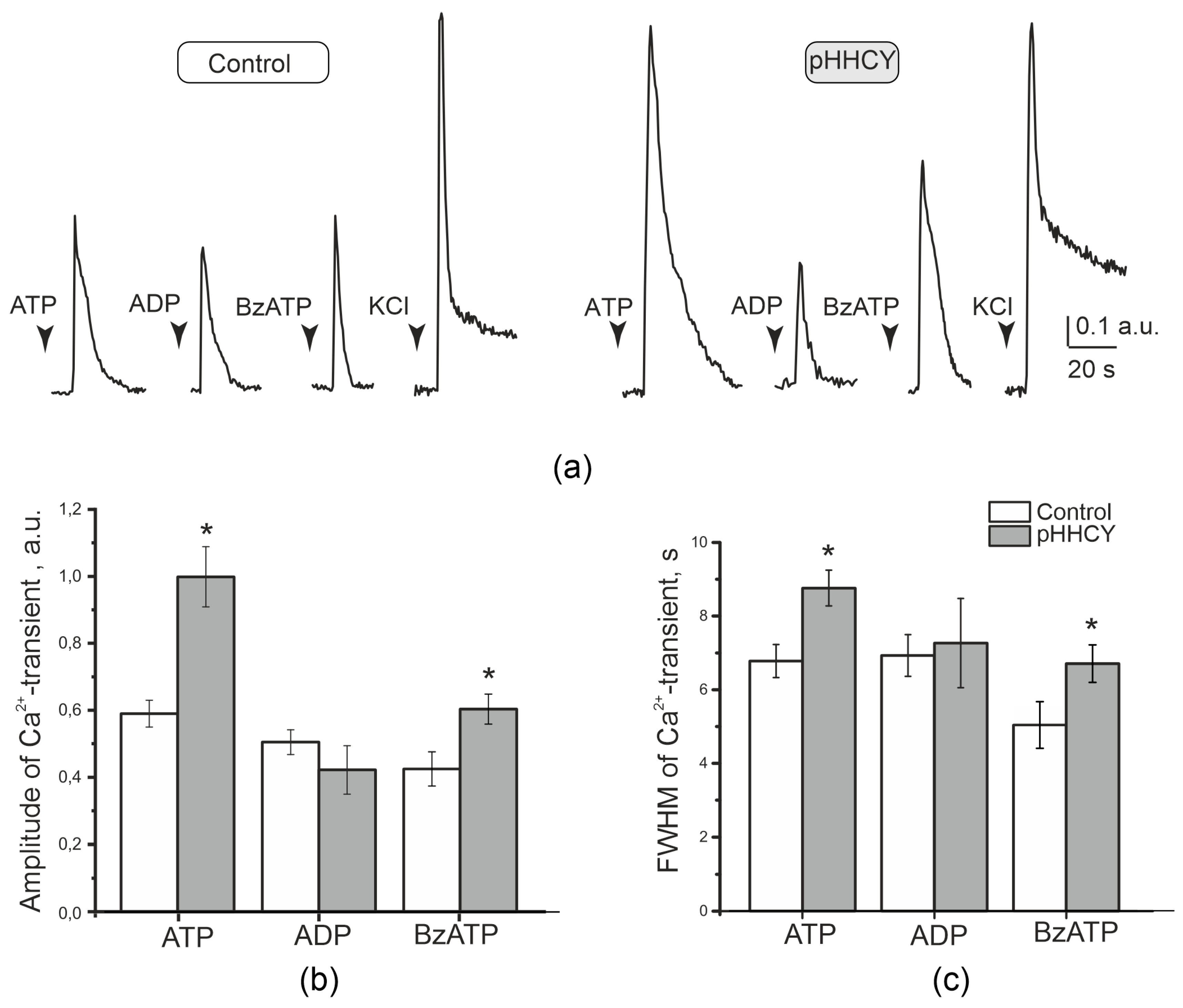
3.3. Effects of ATP on the Electrical Activity of the Trigeminal Nerve in Rats with pHHCY
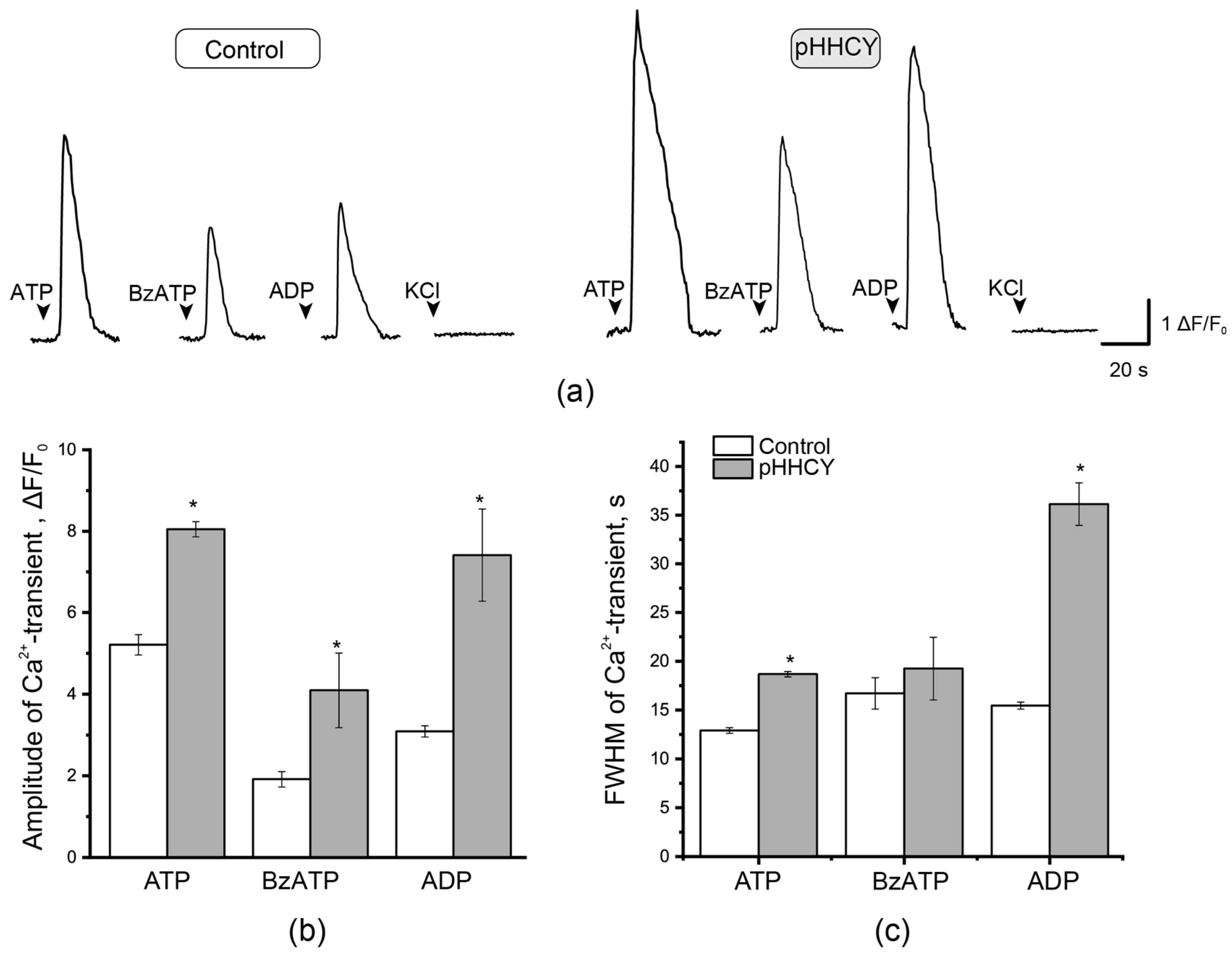
3.4. Ca2+ Transients in Isolated Cultured Cells of the TG of Rats with pHHCY
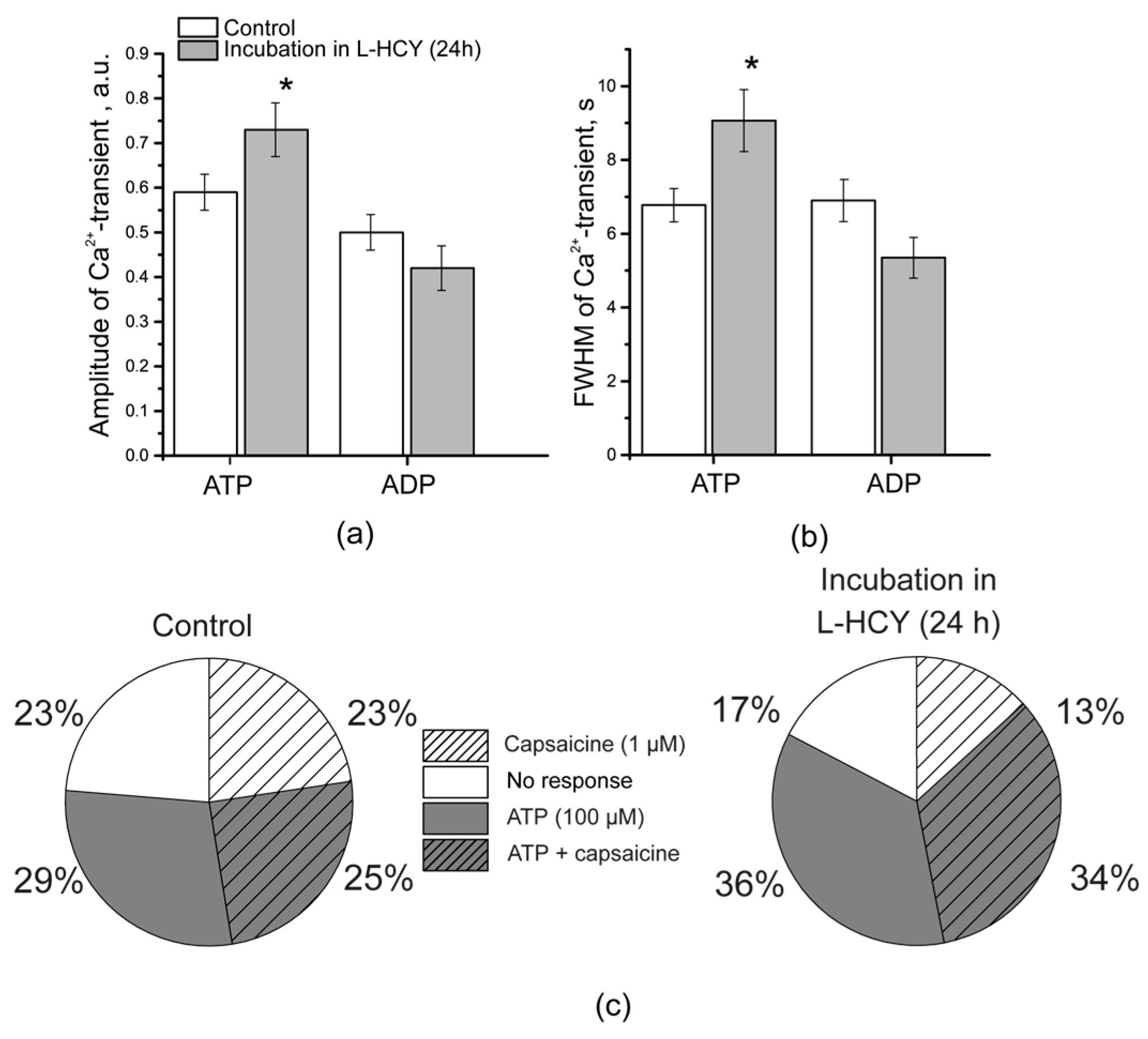
3.5. Ca2+ Transients in TG Neurons After Incubation in L-Homocysteine for 24 h
3.6. Mast Cell Degranulation in Response to BzATP in Rats with pHHCY
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HHCY | Hyperhomocysteinemia |
| pHHCY | Prenatal HHCY |
| ATP | Adenosine triphosphate |
| ADP | Adenosine diphosphate |
| BzATP | 2′(3′)-O-(4-Benzoylbenzoyl)adenosine-5′-triphosphate tri(triethylammonium) salt |
| TG | Trigeminal ganglion |
| CGRP | Calcitonin gene-related peptide |
| AP | Action potential |
| SGCs | Sattelite glial cells |
| 4-AP | 4-aminopiridine |
| CSD | Cortical spreading depression |
| MTHFR | 5,10-methylenetetrahydrofolate reductase |
| FWHM | Full width at half maximum |
References
- Castro, R.; Rivera, I.; Blom, H.J.; Jakobs, C.; de Almeida, I.T. Homocysteine Metabolism, Hyperhomocysteinaemia and Vascular Disease: An Overview. J. Inherit. Metab. Dis. 2006, 29, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kožich, V.; Stabler, S. Lessons Learned from Inherited Metabolic Disorders of Sulfur-Containing Amino Acids Metabolism. J. Nutr. 2020, 150, 2506S–2517S. [Google Scholar] [CrossRef]
- Nieraad, H.; Pannwitz, N.; Bruin, N.D.; Geisslinger, G.; Till, U. MetabolicRole and Animal Studies with a Focus on Cognitive Performance and Decline—A Review. Biomolecules 2021, 11, 1546. [Google Scholar] [CrossRef]
- Marroncini, G.; Martinelli, S.; Menchetti, S.; Bombardiere, F.; Martelli, F.S. Hyperhomocysteinemia and Disease-Is 10 Μmol/L a Suitable New Threshold Limit? Int. J. Mol. Sci. 2024, 25, 12295. [Google Scholar] [CrossRef] [PubMed]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef] [PubMed]
- van Meurs, J.B.J.; Pare, G.; Schwartz, S.M.; Hazra, A.; Tanaka, T.; Vermeulen, S.H.; Cotlarciuc, I.; Yuan, X.; Mälarstig, A.; Bandinelli, S.; et al. Common Genetic Loci Influencing Plasma Homocysteine Concentrations and Their Effect on Risk of Coronary Artery Disease. Am. J. Clin. Nutr. 2013, 98, 668–676. [Google Scholar] [CrossRef]
- Petras, M.; Tatarkova, Z.; Kovalska, M.; Mokra, D.; Dobrota, D.; Lehotsky, J.; Drgova, A. Hyperhomocysteinemia as a Risk Factor for the Neuronal System Disorders. J. Physiol. Pharmacol. 2014, 65, 15–23. [Google Scholar]
- Sharma, M.; Tiwari, M.; Tiwari, R.K. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic. Clin. Pharmacol. Toxicol. 2015, 117, 287–296. [Google Scholar] [CrossRef]
- Sitdikova, G.F.; Hermann, A.; Yakovlev, A.V. Neurotoxic and Neuroprotective Effects of Homocysteine and Hydrogen Sulfide. Uchenye Zap. Kazan. Univ. Seriya Estestv. Nauk. 2018, 160, 686–704. [Google Scholar]
- Tinelli, C.; Di Pino, A.; Ficulle, E.; Marcelli, S.; Feligioni, M. Hyperhomocysteinemia as a Risk Factor and Potential Nutraceutical Target for Certain Pathologies. Front. Nutr. 2019, 6, 49. [Google Scholar] [CrossRef]
- Sitdikova, G.; Hermann, A. Homocysteine: Biochemistry, Molecular Biology, and Role in Disease 2021. Biomolecules 2023, 13, 1111. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Osada, J.; Aratani, Y.; Kluckman, K.; Reddick, R.; Malinow, M.R.; Maeda, N. Mice Deficient in Cystathionine Beta-Synthase: Animal Models for Mild and Severe Homocyst(e)Inemia. Proc. Natl. Acad. Sci. USA 1995, 92, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Jadavji, N.M.; Deng, L.; Malysheva, O.; Caudill, M.A.; Rozen, R. MTHFR Deficiency or Reduced Intake of Folate or Choline in Pregnant Mice Results in Impaired Short-Term Memory and Increased Apoptosis in the Hippocampus of Wild-Type Offspring. Neuroscience 2015, 300, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, O.; Bogatova, K.; Mukhtarova, R.; Yakovlev, A.; Shakhmatova, V.; Gerasimova, E.; Ziyatdinova, G.; Hermann, A.; Sitdikova, G. Hydrogen Sulfide Alleviates Anxiety, Motor, and Cognitive Dysfunctions in Rats with Maternal Hyperhomocysteinemia via Mitigation of Oxidative Stress. Biomolecules 2020, 10, 995. [Google Scholar] [CrossRef]
- Shcherbitskaia; Vasilev, D.S.; Milyutina, Y.P.; Tumanova, N.L.; Zalozniaia, I.V.; Kerkeshko, G.O.; Arutjunyan, A.V. Maternal Hyperhomocysteinemia Induces Neuroinflammation and Neuronal Death in the Rat Offspring Cortex. Neurotox. Res. 2020, 38, 408–420. [Google Scholar] [CrossRef]
- Vasilev, D.S.; Shcherbitskaia, A.D.; Tumanova, N.L.; Mikhel, A.V.; Milyutina, Y.P.; Kovalenko, A.A.; Dubrovskaya, N.M.; Inozemtseva, D.B.; Zalozniaia, I.V.; Arutjunyan, A.V. Maternal Hyperhomocysteinemia Disturbs the Mechanisms of Embryonic Brain Development and Its Maturation in Early Postnatal Ontogenesis. Cells 2023, 12, 189. [Google Scholar] [CrossRef]
- Gerasimova, E.; Enikeev, D.; Yakovlev, A.; Zakharov, A.; Sitdikova, G. Chronic Hyperhomocysteinemia Impairs CSD Propagation and Induces Cortical Damage in a Rat Model of Migraine with Aura. Biomolecules 2024, 14, 1379. [Google Scholar] [CrossRef]
- Moschiano, F.; Amico, D.; Usai, S.; Grazzi, L.; Stefano, D.; Ciusani, M.; Erba, E.; Bussone, N. Homocysteine Plasma Levels in Patients with Migraine with Aura. Neurol. Sci. 2008, 29, 173–175. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C.; Meschi, T.; Cervellin, G.; Borghi, L. Homocysteine and Migraine. A Narrative Review. Clin. Chim. Acta 2014, 433, 5–11. [Google Scholar] [CrossRef]
- Cacciapuoti, F. Migraine Homocysteine-related: Old and New Mechanisms. Neurol. Clin. Neurosci. 2017, 5, 137–140. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; He, J.; Guo, L.; Li, H.; Teng, J. Effects of MTHFR C677T and A1298C Polymorphisms on Migraine Susceptibility: A Meta-Analysis of 26 Studies. Headache 2019, 59, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Siokas, V.; Mentis, A.-F.A.; Aloizou, A.-M.; Dastamani, M.; Tsouris, Z.; Aslanidou, P.; Brotis, A.; Dardiotis, E. Serum Homocysteine, Pyridoxine, Folate, and Vitamin B12 Levels in Migraine: Systematic Review and Meta-analysis. Headache 2020, 60, 1508–1534. [Google Scholar] [CrossRef] [PubMed]
- Gerasimova, E.; Burkhanova, G.; Chernova, K.; Zakharov, A.; Enikeev, D.; Khaertdinov, N.; Giniatullin, R.; Sitdikova, G. Hyperhomocysteinemia Increases Susceptibility to Cortical Spreading Depression Associated with Photophobia, Mechanical Allodynia, and Anxiety in Rats. Behav. Brain Res. 2021, 409, 113324. [Google Scholar] [CrossRef] [PubMed]
- Gerasimova, E.; Yakovleva, O.; Enikeev, D.; Bogatova, K.; Hermann, A.; Giniatullin, R.; Sitdikova, G. Hyperhomocysteinemia Increases Cortical Excitability and Aggravates Mechanical Hyperalgesia and Anxiety in a Nitroglycerine-Induced Migraine Model in Rats. Biomolecules 2022, 12, 735. [Google Scholar] [CrossRef]
- Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Facchetti, S.; De Icco, R.; Martinelli, D.; Allena, M.; Greco, R.; Tassorelli, C. Biomarkers of Migraine: An Integrated Evaluation of Preclinical and Clinical Findings. Int. J. Mol. Sci. 2023, 24, 5334. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Vega, C.; Moy, J.; Dussor, G. Meningeal Afferent Signaling and the Pathophysiology of Migraine. Prog. Mol. Biol. Transl. Sci. 2015, 131, 537–564. [Google Scholar]
- Strassman, A.M.; Raymond, S.A.; Burstein, R. Sensitization of Meningeal Sensory Neurons and the Origin of Headaches. Nature 1996, 384, 560–564. [Google Scholar] [CrossRef]
- Bove, G.M.; Moskowitz, M.A. Primary Afferent Neurons Innervating Guinea Pig Dura. J. Neurophysiol. 1997, 77, 299–308. [Google Scholar] [CrossRef]
- Levy, D.; Strassman, A.M. Mechanical Response Properties of A and C Primary Afferent Neurons Innervating the Rat Intracranial Dura. J. Neurophysiol. 2002, 88, 3021–3031. [Google Scholar] [CrossRef]
- Strassman, A.M.; Levy, D. Response Properties of Dural Nociceptors in Relation to Headache. J. Neurophysiol. 2006, 95, 1298–1306. [Google Scholar] [CrossRef]
- Giniatullin, R.; Nistri, A. Role of ATP in Migraine Mechanisms: Focus on P2X3 Receptors. J. Headache Pain. 2023, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G.; Guerrero-Toro, C.; Kilinc, E. Nucleotide Homeo-Stasis and Purinergic Nociceptive Signaling in Rat Meninges. Purinergic Signal. 2016, 12, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, K.; Gafurov, O.; Guselnikova, V.; Nurkhametova, D.; Giniatullina, R.; Sitdikova, G.; Mattila, O.S.; Lindsberg, P.J.; Malm, T.M.; Giniatullin, R. Meningeal Mast Cells Contribute to ATP-Induced Nociceptive Firing in Trigeminal Nerve Terminals: Direct and Indirect Purinergic Mechanisms Triggering Migraine Pain. Front. Cell. Neurosci. 2019, 13, 195. [Google Scholar] [CrossRef]
- Kilinc, E.; Guerrero-Toro, C.; Zakharov, A.; Vitale, C.; Gubert-Olive, M.; Koroleva, K.; Timonina, A.; Luz, L.L.; Shelukhina, I.; Giniatullina, R.; et al. Serotonergic Mechanisms of Trigeminal Meningeal Nociception: Implications for Migraine Pain. Neuropharmacology 2017, 116, 160–173. [Google Scholar] [CrossRef]
- Vaughn, A.H.; Gold, M.S. Ionic Mechanisms Underlying Inflammatory Mediator-Induced Sensitization of Dural Afferents. J. Neurosci. 2010, 30, 7878–7888. [Google Scholar] [CrossRef]
- Fabbretti, E.; D’Arco, M.; Fabbro, A.; Simonetti, M.; Nistri, A.; Giniatullin, R. Delayed Upregulation of ATP P2X3 Receptors of Trigeminal Sensory Neurons by Calcitonin Gene-Related Peptide. J. Neurosci. 2006, 26, 6163–6171. [Google Scholar] [CrossRef] [PubMed]
- Cieślak, M.; Czarnecka, J.; Roszek, K.; Komoszyński, M. The Role of Purinergic Signaling in the Etiology of Migraine and Novel Antimigraine Treatment. Purinergic Signal. 2015, 11, 307–316. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K. Does Inflammation Have a Role in Migraine? Nat. Rev. Neurol. 2019, 15, 483–490. [Google Scholar] [CrossRef]
- Giniatullin, R.; Nistri, A.; Fabbretti, E. Molecular Mechanisms of Sensitization of Pain-Transducing P2X3 Receptors by the Migraine Mediators CGRP and NGF. Mol. Neurobiol. 2008, 37, 83–90. [Google Scholar] [CrossRef]
- da Cunha, A.A.; Ferreira, A.G.K.; Loureiro, S.O.; da Cunha, M.J.; Schmitz, F.; Netto, C.A.; Wyse, A.T.S. Chronic Hyperhomocysteinemia Increases Inflammatory Markers in Hippocampus and Serum of Rats. Neurochem. Res. 2012, 37, 1660–1669. [Google Scholar] [CrossRef]
- Scherer, E.B.; Loureiro, S.O.; Vuaden, F.C.; Da Cunha, A.A.; Schmitz, F.; Kolling, J.; Savio, L.E.; Bogo, M.R.; Bonan, C.D.; Netto, C.A.; et al. Mild Hyperhomocysteinemia Increases Brain Acetyl- Cholinesterase and Proinflflammatory Cytokine Levels in Different Tissues. Mol. Neurobiol. 2014, 50, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Sharma, I.; Kira, D.; Alhusban, S.; Samra, Y.A.; Jadeja, R.; Martin, P.; Al-Shabrawey, M.; Tawfik, A. Homocysteine Induces Inflammation in Retina and Brain. Biomolecules 2020, 10, 393. [Google Scholar] [CrossRef]
- Yakovlev, A.V.; Detterer, A.S.; Yakovleva, O.V.; Hermann, A.; Sitdikova, G.F. H2S Prevents the Disruption of the Blood-Brain Barrier in Rats with Prenatal Hyperhomocysteinemia. J. Pharmacol. Sci. 2024, 155, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Scherer, E.B.S.; da Cunha, A.A.; Kolling, J.; da Cunha, M.J.; Schmitz, F.; Sitta, A.; Lima, D.D.; Delwing, D.; Vargas, C.R.; Wyse, A.T.S. Development of an Animal Model for Chronic Mild Hyperhomocysteinemia and Its Response to Oxidative Damage. Int. J. Dev. Neurosci. 2011, 29, 693–699. [Google Scholar] [CrossRef]
- de S Moreira, D.; Figueiró, P.W.; Siebert, C.; Prezzi, C.A.; Rohden, F.; Guma, F.C.R.; Manfredini, V.; Wyse, A.T.S. Chronic Mild Hyperhomocysteinemia Alters Inflammatory and Oxidative/Nitrative Status and Causes Protein/DNA Damage, as Well as Ultrastructural Changes in Cerebral Cortex: Is Acetylsalicylic Acid Neuroprotective? Neurotox. Res. 2018, 33, 580–592. [Google Scholar] [CrossRef]
- Ramires Junior, O.V.; Dos Santos, T.M.; Silveira, J.S.; Leite-Aguiar, R.; Coutinho-Silva, R.; Savio, L.E.B.; Wyse, A.T.S. Rivastigmine Reverses the Decrease in Synapsin and Memory Caused by Homocysteine: Is There Relation to Inflammation? Mol. Neurobiol. 2022, 59, 4517–4534. [Google Scholar] [CrossRef]
- Familtseva, A.; Jeremic, N.; Kunkel, G.H.; Tyagi, S.C. Toll-like Receptor 4 Mediates Vascular Remodeling in Hyperhomocysteinemia. Mol. Cell. Biochem. 2017, 433, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Zanin, R.F.; Bergamin, L.S.; Morrone, F.B.; Coutinho-Silva, R.; de Souza Wyse, A.T.; Battastini, A.M.O. Pathological Concentrations of Homocysteine Increases IL-1β Production in Macrophages in a P2X7, NF-ĸB, and Erk-Dependent Manner. Purinergic Signal. 2015, 11, 463–470. [Google Scholar] [CrossRef]
- Han, S.; Wu, H.; Li, W.; Gao, P. Protective Effects of Genistein in Homocysteine-Induced Endothelial Cell Inflammatory Injury. Mol. Cell. Biochem. 2015, 403, 43–49. [Google Scholar] [CrossRef]
- Li, J.-J.; Li, Q.; Du, H.-P.; Wang, Y.-L.; You, S.-J.; Wang, F.; Xu, X.-S.; Cheng, J.; Cao, Y.-J.; Liu, C.-F.; et al. Homocysteine Triggers Inflammatory Responses in Macrophages through Inhibiting CSE-H2S Signaling via DNA Hypermethylation of CSE Promoter. Int. J. Mol. Sci. 2015, 16, 12560–12577. [Google Scholar] [CrossRef]
- Yakovleva, O.V.; Ziganshina, A.R.; Dmitrieva, S.A.; Arslanova, A.N.; Yakovlev, A.V.; Minibayeva, F.V.; Khaertdinov, N.N.; Ziyatdinova, G.K.; Giniatullin, R.A.; Sitdikova, G.F. Hydrogen Sulfide Ameliorates Developmental Impairments of Rat Offspring with Prenatal Hyperhomocysteinemia. Oxid. Med. Cell. Longev. 2018, 2018, 2746873. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Moskowitz, M.A. Meningeal Mechanisms and the Migraine Connection. Annu. Rev. Neurosci. 2023, 46, 39–58. [Google Scholar] [CrossRef]
- Lukács, M.; Haanes, K.A.; Majláth, Z.; Tajti, J.; Vécsei, L.; Warfvinge, K.; Edvinsson, L. Dural Administration of Inflammatory Soup or Complete Freund’s Adjuvant Induces Activation and Inflammatory Response in the Rat Trigeminal Ganglion. J. Headache Pain. 2015, 16, 564. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Ermakova, E.; Shaidullova, K.; Gafurov, O.; Kabirova, A.; Nurmieva, D.; Sitdikova, G. Implications of High Homocysteine Levels in Migraine Pain: An Experimental Study of the Excitability of Peripheral Meningeal Afferents in Rats with Hyperhomocysteinemia. Headache 2024, 64, 533–546. [Google Scholar] [CrossRef]
- Gerasimova, E.; Yakovleva, O.; Burkhanova, G.; Ziyatdinova, G.; Khaertdinov, N.; Sitdikova, G. Effects of Maternal Hyperhomocysteinemia on the Early Physical Development and Neurobehavioral Maturation of Rat Offspring. BioNanoScience 2016, 7, 155–158. [Google Scholar] [CrossRef]
- Koroleva, K.; Mustafina, A.; Yakovlev, A.; Hermann, A.; Giniatullin, R.; Sitdikova, G. Receptor Mechanisms Mediating the Pro-Nociceptive Action of Hydrogen Sulfide in Rat Trigeminal Neurons and Meningeal Afferents. Front. Cell. Neurosci. 2017, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, K.; Ermakova, E.; Mustafina, A.; Giniatullina, R.; Giniatullin, R.; Sitdikova, G. Protective Effects of Hydrogen Sulfide against the ATP-Induced Meningeal Nociception. Front. Cell. Neurosci. 2020, 14, 266. [Google Scholar] [CrossRef]
- Gusel’nikova, V.V.; Sukhorukova, E.G.; Fedorova, E.A.; Polevshchikov, A.V.; Korzhevskiĭ, D.É. Method for simultaneous visualization of mast cells and nerve terminals in the rodent thymus. Morfologiia 2014, 145, 70–73. [Google Scholar]
- Mikhailov, N.; Shelukhina; Koroleva, K.; Vitale, C.; Giniatullin, R. Parasympathetic Cholinergic and Peptidergic Mechanisms of Trigeminal Pain. Anesth. Pain Med. 2017, 7, e42210. [Google Scholar]
- Nurkhametova, D.F.; Koroleva, K.S.; Gafurov, O.S.; Giniatullina, R.R.; Sitdikova, G.F.; Giniatullin, R.A. Mast Cell Mediators as Pain Triggers in Migraine: Comparison of Histamine and Serotonin in the Activation of Primary Afferents in the Meninges in Rats. Neurosci. Behav. Physiol. 2020, 50, 900–906. [Google Scholar] [CrossRef]
- Hall, D.A.; Langmead, C.J. Matching Models to Data: A Receptor Pharmacologist’s Guide. Br. J. Pharmacol. 2010, 161, 1276–1290. [Google Scholar] [CrossRef]
- Cupini, L.M.; Stipa, E. Migraine Aura Status and Hyperhomocysteinaemia. Cephalalgia 2007, 27, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Isobe, C.; Terayama, Y. A Remarkable Increase in Total Homocysteine Concentrations in the CSF of Migraine Patients with Aura. Headache 2010, 50, 1561–1569. [Google Scholar] [CrossRef]
- Lipton, S.A.; Kim, W.-K.; Choi, Y.-B.; Kumar, S.; D’Emilia, D.M.; Rayudu, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity Associated with Dual Actions of Homocysteine at the N-Methyl-d-Aspartate Receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine Elicits a DNA Damage Response in Neurons That Promotes Apoptosis and Hypersensitivity to Excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [CrossRef]
- Zhou, N.; Rungta, R.L.; Malik, A.; Han, H.; Wu, D.C.; MacVicar, B.A. Regenerative Glutamate Release by Presynaptic NMDA Receptors Contributes to Spreading Depression. J. Cereb. Blood Flow. Metab. 2013, 33, 1582–1594. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Giniatullin, R.; Antonov, S.M. High Sensitivity of Cerebellar Neurons to Homocysteine Is Determined by Expression of GluN2C and GluN2D Subunits of NMDA Receptors. Biochem. Biophys. Res. Commun. 2018, 506, 648–652. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kyles, P.; Kalani, A.; Tyagi, N. Hydrogen Sulfide Ameliorates Homocysteine-Induced Alzheimer’s Disease-like Pathology, Blood-Brain Barrier Disruption, and Synaptic Disorder. Mol. Neurobiol. 2016, 53, 2451–2467. [Google Scholar] [CrossRef] [PubMed]
- Zemel, B.M.; Ritter, D.M.; Covarrubias, M.; Muqeem, T. A-Type KV Channels in Dorsal Root Ganglion Neurons: Diversity, Function, and Dysfunction. Front. Mol. Neurosci. 2018, 11, 253. [Google Scholar] [CrossRef]
- Viatchenko-Karpinski, V.; Ling, J.; Gu, J.G. Down-Regulation of Kv4.3 Channels and a-Type K+ Currents in V2 Trigeminal Ganglion Neurons of Rats Following Oxaliplatin Treatment. Mol. Pain 2018, 14, 1744806917750995. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Yoshida, S.; Takahashi, M.; Saiki, C.; Takeda, M. The Roles of ID, IA and IK in the Electrophysiological Functions of Small-Diameter Rat Trigeminal Ganglion Neurons. Curr. Mol. Pharmacol. 2010, 3, 30–36. [Google Scholar] [CrossRef]
- Chien, L.-Y.; Cheng, J.-K.; Chu, D.; Cheng, C.-F.; Tsaur, M.-L. Reduced Expression of A-Type Potassium Channels in Primary Sensory Neurons Induces Mechanical Hypersensitivity. J. Neurosci. 2007, 27, 9855–9865. [Google Scholar] [CrossRef]
- Hara, N.; Takeda, M.; Takahashi, M.; Matsumoto, S. Iontophoretic Application of an A-Type Potassium Channel Blocker to the Trigeminal Ganglion Neurons Enhances the Excitability of Aδ- and C-Neurons Innervating the Temporomandibular Joint in Rats. Neurosci. Res. 2012, 74, 216–222. [Google Scholar] [CrossRef]
- Takeda, M.; Tanimoto, T.; Nasu, M.; Matsumoto, S. Temporomandibular Joint Inflammation Decreases the Voltage-Gated K+ Channel Subtype 1.4-Immunoreactivity of Trigeminal Ganglion Neurons in Rats. Eur. J. Pain. 2008, 12, 189–195. [Google Scholar] [CrossRef]
- Dong, X.-D.; Mann, M.K.; Kumar, U.; Svensson, P.; Arendt-Nielsen, L.; Hu, J.W.; Sessle, B.J.; Cairns, B.E. Sex-Related Differences in NMDA-Evoked Rat Masseter Muscle Afferent Discharge Result from Estrogen-Mediated Modulation of Peripheral NMDA Receptor Activity. Neuroscience 2007, 146, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Ivanusicl, J.; Beainil, D.; Hatchl, R.; Staikopoulosl, V.; Sesslel, B.; Jenningsl, E.; Ivanusic, J.J.; Beaini, D.; Hatch, R.J.; Staikopoulos, V. Peripheral N-Methyl-d-Aspartate Receptors Contribute to Mechanical Hypersensitivity in a Rat Model of Inflflammatorytemporomandibular Joint Pain. Eur. J. Pain. 2011, 15, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bolton, A.D.; Phillips, M.A.; Constantine-Paton, M. Homocysteine Reduces NMDAR Desensitization and Differentially Modulates Peak Amplitude of NMDAR Currents, Depending on GluN2 Subunit Composition. J. Neurophysiol. 2013, 110, 1567–1582. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Abushik, P.A.; Giniatullin, R.; Antonov, S.M. GluN2A Subunit-Containing NMDA Receptors Are the Preferential Neuronal Targets of Homocysteine. Front. Cell. Neurosci. 2016, 10, 246. [Google Scholar] [CrossRef]
- Guerrero-Toro, C.; Koroleva, K.; Ermakova, E.; Gafurov, O.; Abushik, P.; Tavi, P.; Sitdikova, G.; Giniatullin, R. Testing the Role of Glutamate NMDA Receptors in Peripheral Trigeminal Nociception Implicated in Migraine Pain. Int. J. Mol. Sci. 2022, 23, 1529. [Google Scholar] [CrossRef]
- Giniatullin, R.; Nistri, A. Desensitization Properties of P2X3 Receptors Shaping Pain Signaling. Front. Cell. Neurosci. 2013, 7, 245. [Google Scholar] [CrossRef]
- North, R.A. P2X3 Receptors and Peripheral Pain Mechanisms. J. Physiol. 2004, 554, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.-P.; Ase, A.R.; Séguéla, P. P2X Receptor Channels in Chronic Pain Pathways. Br. J. Pharmacol. 2018, 175, 2219–2230. [Google Scholar] [CrossRef]
- Sato, M.; Sato, T.; Yajima, T.; Shimazaki, K.; Ichikawa, H. The Transient Receptor Potential Cation Channel Subfamily V Members 1 and 2, P2X Purinoceptor 3 and Calcitonin Gene-Related Peptide in Sensory Neurons of the Rat Trigeminal Ganglion, Innervating the Periosteum, Masseter Muscle and Facial Skin. Arch. Oral Biol. 2018, 96, 66–73. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, A.A.; Ferreira, A.G.K.; Wyse, A.T.S. Increased Inflammatory Markers in Brain and Blood of Rats Subjected to Acute Homocysteine Administration. Metab. Brain Dis. 2010, 25, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ivanov, S.; Lagunin, A.; Goel, R.K. Attenuation of Hyperhomocysteinemia Induced Vascular Dementia by Sodium Orthovanadate Perhaps via PTP1B: Pertinent Downstream Outcomes. Behav. Brain Res. 2019, 364, 29–40. [Google Scholar] [CrossRef]
- Yakovlev, A.V.; Dmitrieva, S.A.; Krasnova, A.N.; Yakovleva, O.V.; Sitdikova, G.F. Levels of Protein Carbonylation and Activity of Proteases in the Brain of Newborn Rats with Prenatal Hyperhomocysteinemia. Neurochem. J. 2022, 16, 263–270. [Google Scholar] [CrossRef]
- Kamath, A.F.; Chauhan, A.K.; Kisucka, J.; Dole, V.S.; Loscalzo, J.; Handy, D.E.; Wagner, D.D. Elevated Levels of Homocysteine Compromise Blood-Brain Barrier Integrity in Mice. Blood 2006, 107, 591–593. [Google Scholar] [CrossRef]
- Beard, R.S., Jr.; Reynolds, J.J.; Bearden, S.E. Hyperhomocysteinemia Increases Permeability of the Blood-Brain Barrier by NMDA Receptor-Dependent Regulation of Adherens and Tight Junctions. Blood 2011, 118, 2007–2014. [Google Scholar] [CrossRef]
- Kumar, M.; Sandhir, R. Hydrogen Sulfide Attenuates Hyperhomocysteinemia-Induced Blood-Brain Barrier Permeability by Inhibiting MMP-9. Int. J. Neurosci. 2022, 132, 1061–1071. [Google Scholar] [CrossRef]
- Wirkner, K.; Sperlagh, B.; Illes, P. P2X3 Receptor Involvement in Pain States. Mol. Neurobiol. 2007, 36, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Staikopoulos, V.; Sessle, B.J.; Furness, J.B.; Jennings, E.A. Localization of P2X2 and P2X3 Receptors in Rat Trigeminal Ganglion Neurons. Neuroscience 2007, 144, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Messlinger, K.; Balcziak, L.K.; Russo, A.F. Cross-Talk Signaling in the Trigeminal Ganglion: Role of Neuropeptides and Other Mediators. J. Neural Transm. 2020, 127, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L.; et al. Disruption of the P2X7 Purinoceptor Gene Abolishes Chronic Inflammatory and Neuropathic Pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef]
- Nurkhametova, D.; Siniavin, A.; Streltsova, M.; Kudryavtsev, D.; Kudryavtsev, I.; Giniatullina, R.; Tsetlin, V.; Malm, T.; Giniatullin, R. Does Cholinergic Stimulation Affect the P2X7 Receptor-Mediated Dye Uptake in Mast Cells and Macrophages? Front. Cell. Neurosci. 2020, 14, 548376. [Google Scholar] [CrossRef]
- Ceruti, S.; Villa, G.; Fumagalli, M.; Colombo, L.; Magni, G.; Zanardelli, M.; Fabbretti, E.; Verderio, C.; van den Maagdenberg, A.M.J.M.; Nistri, A.; et al. Calcitonin Gene-Related Peptide-Mediated Enhancement of Purinergic Neuron/Glia Communication by the Algogenic Factor Bradykinin in Mouse Trigeminal Ganglia from Wild-Type and R192Q Cav2.1 Knock-in Mice: Implications for Basic Mechanisms of Migraine Pain. J. Neurosci. 2011, 31, 3638–3649. [Google Scholar] [CrossRef]
- Dux, M.; Rosta, J.; Messlinger, K. TRP Channels in the Focus of Trigeminal Nociceptor Sensitization Contributing to Primary Headaches. Int. J. Mol. Sci. 2020, 21, 342. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Mechanisms and Pain—An Update. Eur. J. Pharmacol. 2013, 716, 24–40. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G. P2Y Receptors in Neuropathic Pain. Pharmacol. Biochem. Behav. 2019, 186, 172788. [Google Scholar] [CrossRef]
- Simonetti, M.; Giniatullin, R.; Fabbretti, E. Mechanisms Mediating the Enhanced Gene Transcription of P2X3 Receptor by Calcitonin Gene-Related Peptide in Trigeminal Sensory Neurons. J. Biol. Chem. 2008, 283, 18743–18752. [Google Scholar] [CrossRef]
- Souslova, V.; Cesare, P.; Ding, Y.; Akopian, A.N.; Stanfa, L.; Suzuki, R.; Carpenter, K.; Dickenson, A.; Boyce, S.; Hill, R.; et al. Warm-Coding Deficits and Aberrant Inflammatory Pain in Mice Lacking P2X3 Receptors. Nature 2000, 407, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Edvinsson, L. The Trigeminovascular System and Migraine: Studies Characterizing Cerebrovascular and Neuropeptide Changes Seen in Humans and Cats. Ann. Neurol. 1993, 33, 48–56. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Larrosa, D.; Ramón, C.; Vega, J.; Martínez-Camblor, P.; Pascual, J. Interictal Increase of CGRP Levels in Peripheral Blood as a Biomarker for Chronic Migraine. Neurology 2013, 81, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache 2019, 59, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu Irmak, D.; Kilinc, E.; Tore, F. Shared Fate of Meningeal Mast Cells and Sensory Neurons in Migraine. Front. Cell. Neurosci. 2019, 13, 136. [Google Scholar] [CrossRef]
- Guan, L.C.; Dong, X.; Green, D.P. Roles of Mast Cells and Their Interactions with the Trigeminal Nerve in Migraine Headache. Mol. Pain 2023, 19, 174480692311813. [Google Scholar] [CrossRef]
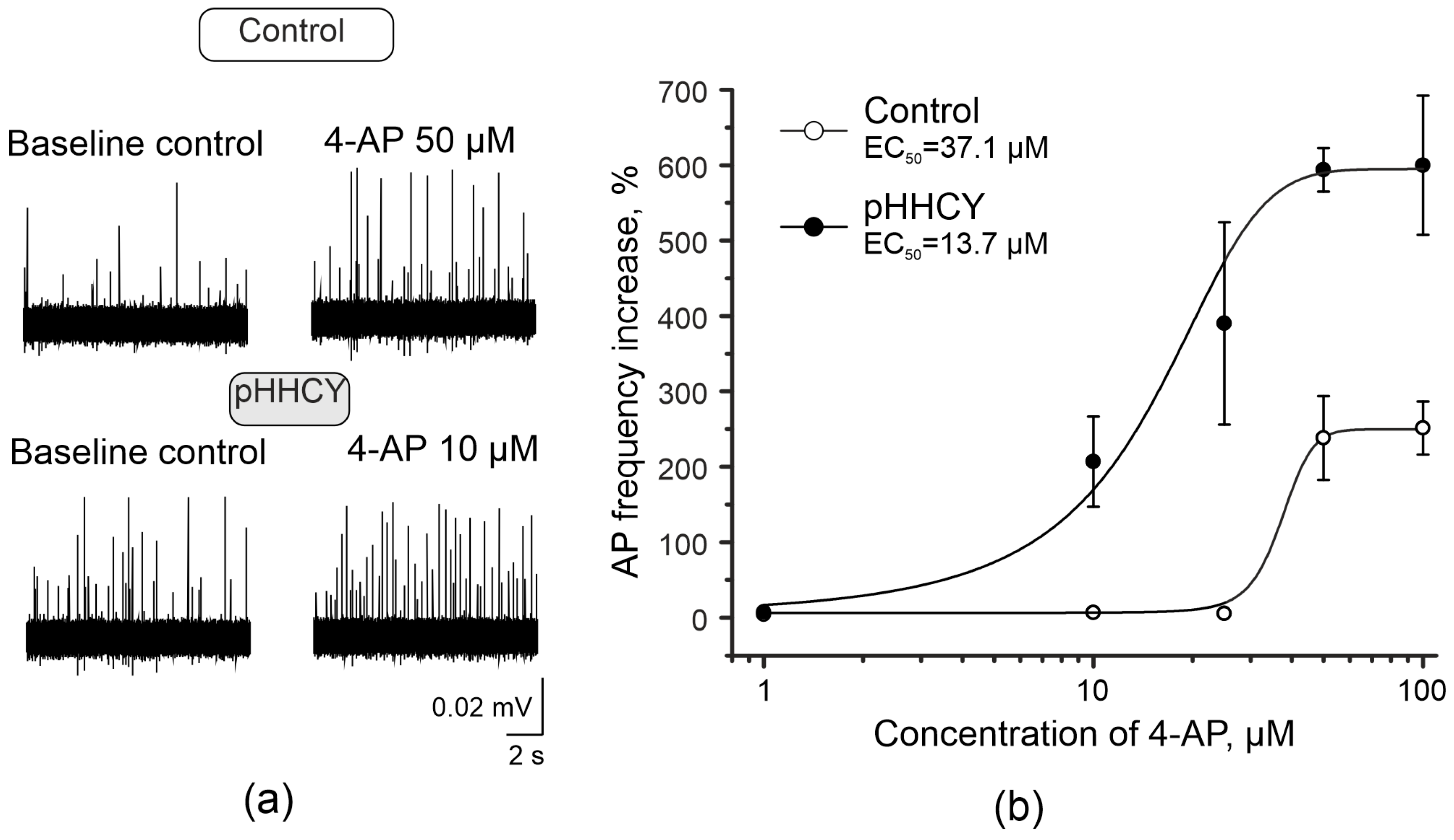


| 4-AP Concentration | Mean AP Frequency Per 20 min in the Control Group | Mean AP Frequency Per 20 min in the pHHCY Group | ||
| Baseline Values | 4-AP | Baseline Values | 4-AP | |
| 10 μM | 959.2 ± 207.6 | 1006.5 ± 189.3 (p = 0.58) n = 4 | 1282.5 ± 75.7 | 3813.1 ± 586.3 * (* p = 0.036) n = 6 |
| 25 μM | 1445.1 ± 53.5 | 1526.2 ± 112.7 (p = 0.41) n = 5 | 1842.6 ± 134.9 | 5131.1 ± 715.9 * (* p = 0.04) n = 6 |
| 50 μM | 939.6 ± 105.2 | 3469.1 ± 820.1 * (* p = 0.01) n = 8 | 775.8 ± 45.2 | 5412.5 ± 461.4 * (* p = 0.04) n = 5 |
| 100 μM | 611.2 ± 123.9 | 2460.6 ± 771.5 * (* p = 0.036) n = 6 | 943.6 ± 41.8 | 6600.8 ± 886.8 * (* p = 0.036) n = 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermakova, E.; Svitko, S.; Kabirova, A.; Nevsky, E.; Yakovleva, O.; Gilizhdinova, K.; Shaidullova, K.; Hermann, A.; Sitdikova, G. The Role of Purinergic Mechanisms in the Excitability of Trigeminal Afferents of Rats with Prenatal Hyperhomocysteinemia. Biomolecules 2025, 15, 419. https://doi.org/10.3390/biom15030419
Ermakova E, Svitko S, Kabirova A, Nevsky E, Yakovleva O, Gilizhdinova K, Shaidullova K, Hermann A, Sitdikova G. The Role of Purinergic Mechanisms in the Excitability of Trigeminal Afferents of Rats with Prenatal Hyperhomocysteinemia. Biomolecules. 2025; 15(3):419. https://doi.org/10.3390/biom15030419
Chicago/Turabian StyleErmakova, Elizaveta, Svetlana Svitko, Alsu Kabirova, Egor Nevsky, Olga Yakovleva, Karina Gilizhdinova, Kseniia Shaidullova, Anton Hermann, and Guzel Sitdikova. 2025. "The Role of Purinergic Mechanisms in the Excitability of Trigeminal Afferents of Rats with Prenatal Hyperhomocysteinemia" Biomolecules 15, no. 3: 419. https://doi.org/10.3390/biom15030419
APA StyleErmakova, E., Svitko, S., Kabirova, A., Nevsky, E., Yakovleva, O., Gilizhdinova, K., Shaidullova, K., Hermann, A., & Sitdikova, G. (2025). The Role of Purinergic Mechanisms in the Excitability of Trigeminal Afferents of Rats with Prenatal Hyperhomocysteinemia. Biomolecules, 15(3), 419. https://doi.org/10.3390/biom15030419








