Nanomaterial-Based Molecular Imaging in Cancer: Advances in Simulation and AI Integration
Abstract
1. Introduction
2. Nanomaterials in Cancer Imaging
2.1. Types of Nanomaterials and Their Applications
2.1.1. Quantum Dots
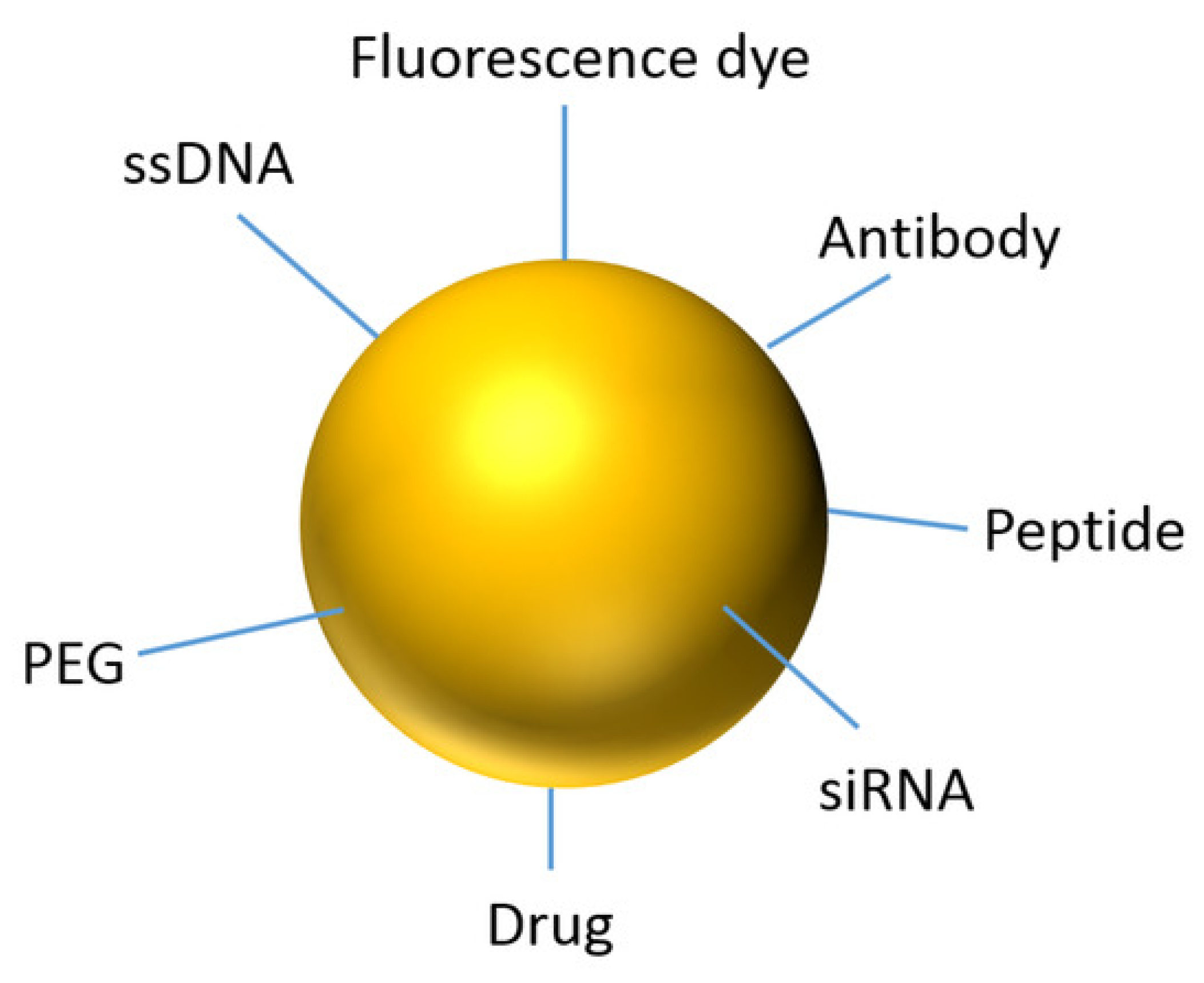
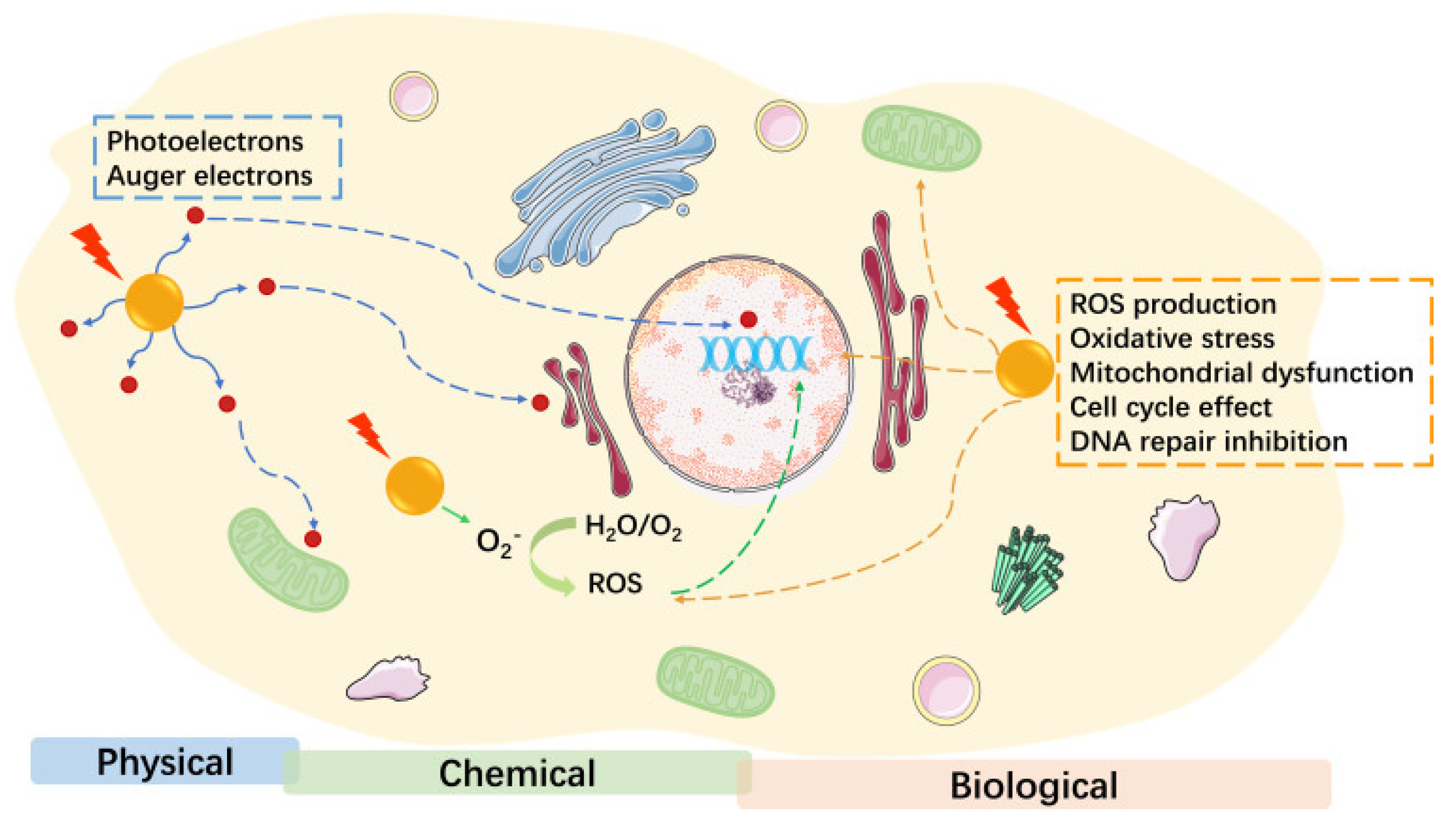
2.1.2. Gold Nanoparticles
2.1.3. Iron Oxide Nanoparticles
2.1.4. Silica-Based Nanoparticles
2.1.5. Polymeric and Liposomal Nanoparticles
2.2. Multimodal Imaging Approaches
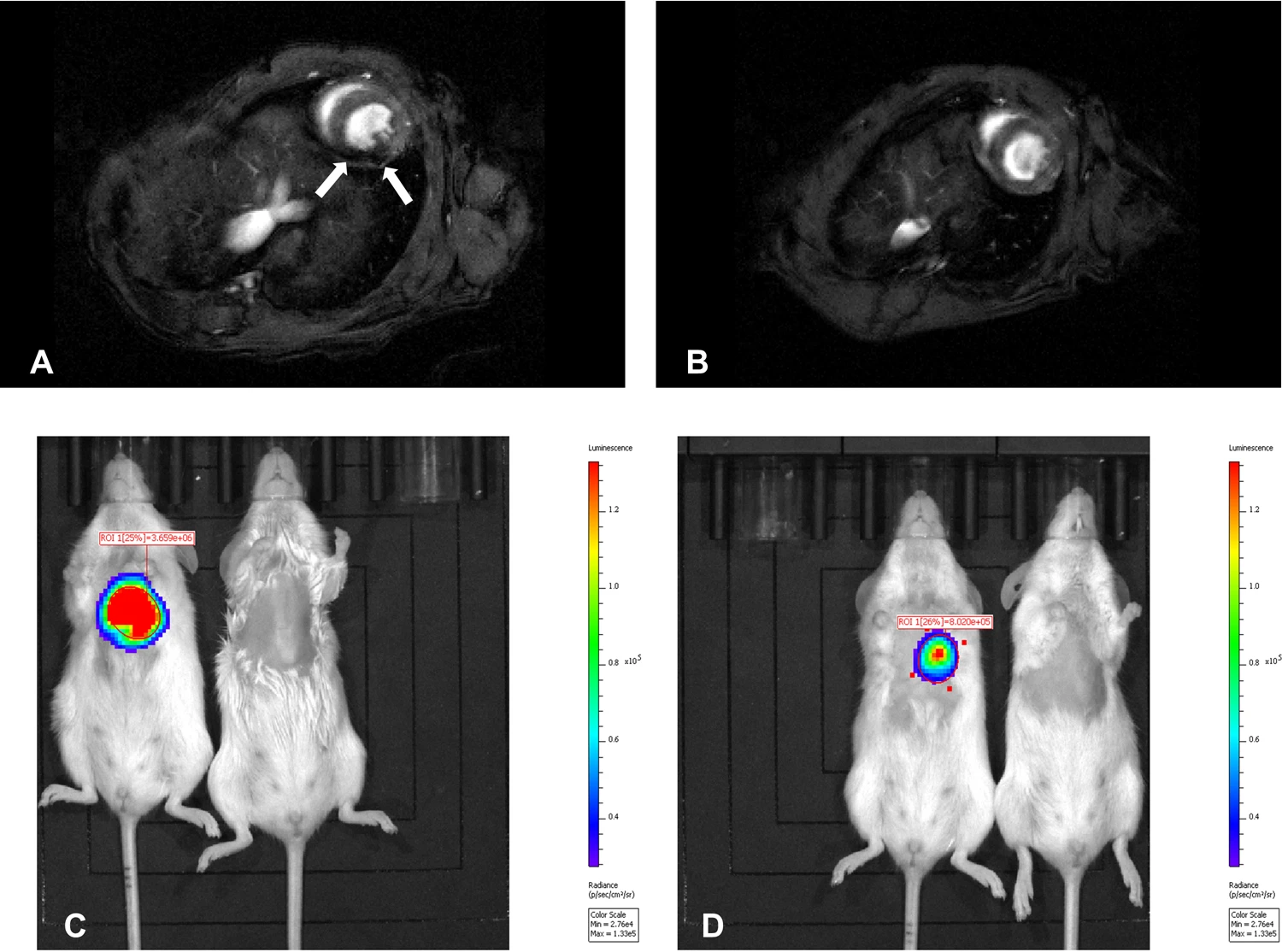
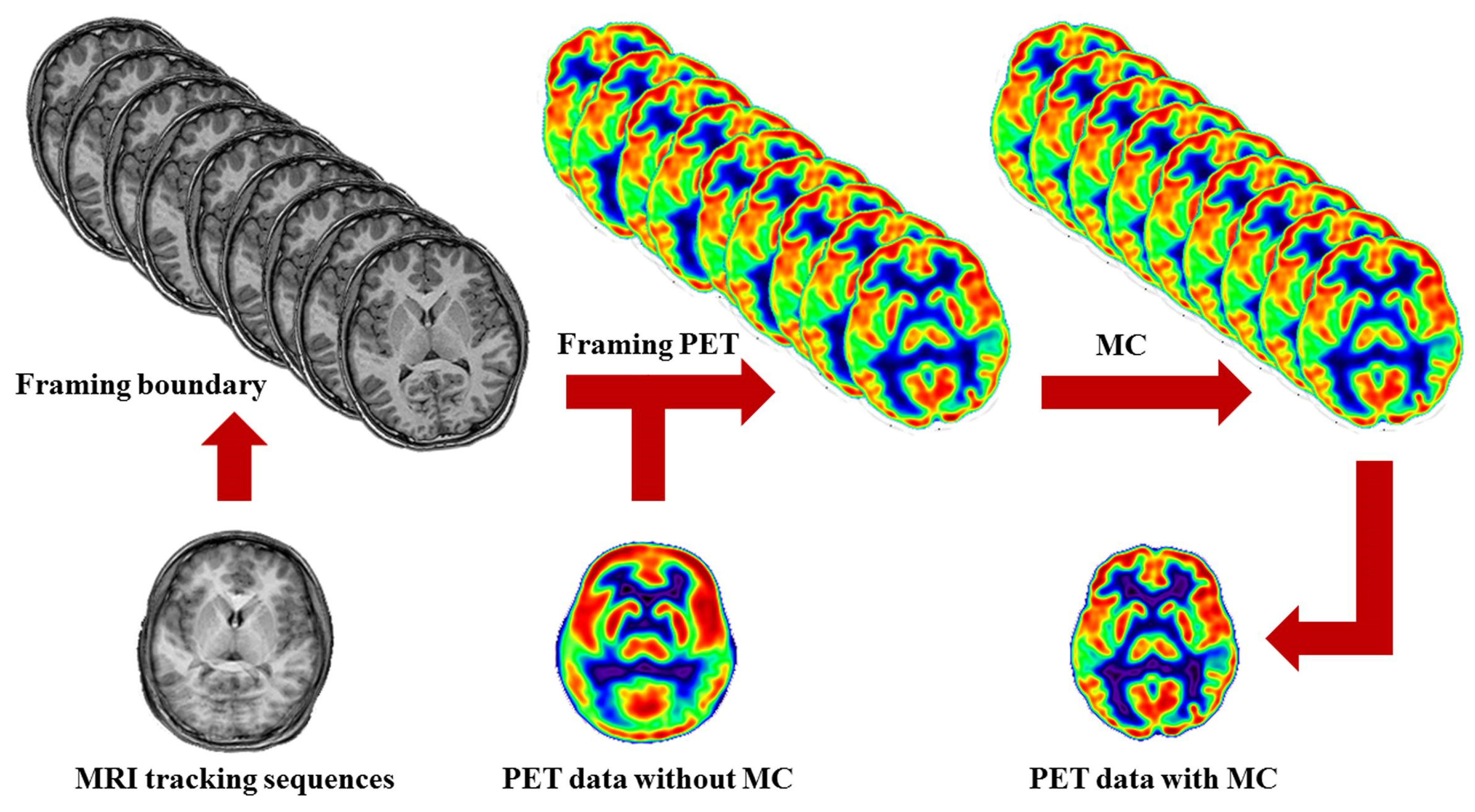
2.2.1. MRI-PET Hybrid Imaging with Nanoparticles
2.2.2. CT-MRI Dual-Modality Imaging Using Hybrid Nanoparticles
2.2.3. US-PAI with Nanoparticle Agents
2.2.4. Fluorescence-Magnetic Imaging for Guided Surgery
2.3. Clinical Impact and Future Perspectives
3. Monte Carlo Simulations for Nanoparticle-Based Imaging
3.1. Monte Carlo Simulation in Nanoparticle-Enhanced Imaging
3.1.1. Quantum Dot-Based Optical Imaging
3.1.2. Gold Nanoparticle-Enhanced CT Imaging
3.1.3. SPION-Labeled MRI Contrast Modeling
3.2. Optimization of Nanoparticle Parameters Using Simulations
3.2.1. Effect of Particle Size and Shape on Imaging Performance
3.2.2. Optimizing Concentration and Distribution in Tumor Tissues
3.2.3. Surface Functionalization and Targeting Efficiency
3.3. Case Studies and Practical Implementations
4. AI and ML in Nanomaterial-Based Imaging
4.1. AI in Medical Image Analysis
4.2. AI-Driven Nanoparticle-Enhanced Imaging Workflows
4.3. AI-Assisted Monte Carlo Simulations
4.4. Future AI Innovations in Cancer Imaging
5. Clinical Translation and Future Directions
5.1. Current Status of Nanomaterials in Clinical Imaging
5.2. Bridging the Gap Between Computational Models and Clinical Use
5.3. Regulatory and Safety Considerations
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| CT | Computed tomography |
| PET | Positron emission tomography |
| US | Ultrasound |
| MC | Monte Carlo |
| AI | Artificial intelligence |
| ML | Machine learning |
| QDs | Quantum dots |
| AuNPs | Gold nanoparticles |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| PAI | Photoacoustic imaging |
| NIR | Near-infrared |
| OCT | Optical coherence tomography |
| DL | Deep learning |
| RL | Reinforcement learning |
| CNNs | Convolutional neural networks |
| RNNs | Recurrent neural networks |
| GANs | Generative adversarial networks |
| GNNs | Graph neural networks |
| AR | Augmented reality |
| FDA | Food and drug administration |
| EMA | European medicines agency |
References
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.C.; Traughber, M.; Muzic, R.F., Jr. The role of imaging in radiation therapy planning: Past, present, and future. BioMed Res. Int. 2014, 2014, 231090. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.J.; Pomper, M.G. The evolution of imaging in cancer: Current state and future challenges. Semin. Oncol. 2011, 38, 3–15. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Bruns, O.T.; Bischof, T.S.; Harris, D.K.; Franke, D.; Shi, Y.; Riedemann, L.; Bawendi, M.G. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 2017, 1, 0056. [Google Scholar] [CrossRef]
- Chow, J.C.L. Characteristics of secondary electrons from irradiated gold nanoparticle in radiotherapy. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–18. ISBN 978-3-319-13188-7. [Google Scholar]
- Ma, X.; Gong, A.; Chen, B.; Zheng, J.; Chen, T.; Shen, Z.; Wu, A. Exploring a new SPION-based MRI contrast agent with excellent water-dispersibility, high specificity to cancer cells and strong MR imaging efficacy. Colloids Surf. B Biointerfaces 2015, 126, 44–49. [Google Scholar] [CrossRef]
- Chow, J.C. Monte Carlo Simulations in Nanomedicine: Advancing Cancer Imaging and Therapy. Nanomaterials 2025, 15, 117. [Google Scholar] [CrossRef]
- Castiglioni, I.; Rundo, L.; Codari, M.; Di Leo, G.; Salvatore, C.; Interlenghi, M.; Sardanelli, F. AI applications to medical images: From machine learning to deep learning. Phys. Med. 2021, 83, 9–24. [Google Scholar] [CrossRef]
- Yang, C.C. Explainable artificial intelligence for predictive modeling in healthcare. J. Healthc. Inform. Res. 2022, 6, 228–239. [Google Scholar] [CrossRef]
- Biju, V.; Itoh, T.; Anas, A.; Sujith, A.; Ishikawa, M. Semiconductor quantum dots and metal nanoparticles: Syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 2008, 391, 2469–2495. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Jing, L.; Behrens, A.M.; Jayawardena, S.; Tang, W.; Gao, M.; Jaklenec, A. Biocompatible semiconductor quantum dots as cancer imaging agents. Adv. Mater. 2018, 30, 1706356. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Hu, Y.; Gu, Z.; Liu, L.; Wu, H.C. Application of quantum dots in biological imaging. J. Nanomater. 2011, 2011, 834139. [Google Scholar] [CrossRef]
- Moore, J.A.; Chow, J.C. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express 2021, 2, 022001. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C. Recent advances in functionalized nanoparticles in cancer theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef]
- Vadhan-Raj, S.; Dahl, N.V.; Bernard, K.; Li, Z.; Strauss, W.E. Efficacy and safety of IV ferumoxytol for iron deficiency anemia in patients with cancer. J. Blood Med. 2017, 8, 199–209. [Google Scholar] [CrossRef]
- Shirshahi, V.; Soltani, M. Solid silica nanoparticles: Applications in molecular imaging. Contrast Media Mol. Imaging 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Chen, F.; Hableel, G.; Zhao, E.R.; Jokerst, J.V. Multifunctional nanomedicine with silica: Role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J. Colloid Interface Sci. 2018, 521, 261–279. [Google Scholar] [CrossRef]
- Petersen, A.L.; Henriksen, J.R.; Binderup, T.; Elema, D.R.; Rasmussen, P.H.; Hag, A.M.; Andresen, T.L. In vivo evaluation of PEGylated 64Cu-liposomes with theranostic and radiotherapeutic potential using micro PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 941–952. [Google Scholar] [CrossRef]
- Seo, J.W.; Zhang, H.; Kukis, D.L.; Meares, C.F.; Ferrara, K.W. A novel method to label preformed liposomes with 64Cu for positron emission tomography (PET) imaging. Bioconjug. Chem. 2008, 19, 2577–2584. [Google Scholar] [CrossRef]
- Martí-Bonmatí, L.; Sopena, R.; Bartumeus, P.; Sopena, P. Multimodality imaging techniques. Contrast Media Mol. Imaging 2010, 5, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ellison, P.A.; Lewis, C.M.; Hong, H.; Zhang, Y.; Shi, S.; Cai, W. Chelator-free synthesis of a dual-modality PET/MRI agent. Angew. Chem. Int. Ed. 2013, 52, 10–1002. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L. Magnetic nanoparticles as contrast agents in magnetic resonance imaging and radiosensitizers in radiotherapy. In Fundamentals and Industrial Applications of Magnetic Nanoparticles; Woodhead Publishing: Cambridge, UK, 2022; pp. 291–316. [Google Scholar]
- Madru, R.; Budassi, M.; Benveniste, H.; Lee, H.; Smith, S.D.; Schlyer, D.J.; Strand, S.E. Simultaneous preclinical positron emission tomography-magnetic resonance imaging study of lymphatic drainage of chelator-free 64Cu-labeled nanoparticles. Cancer Biother. Radiopharm. 2018, 33, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wierzbinski, K.R.; Szymanski, T.; Rozwadowska, N.; Rybka, J.D.; Zimna, A.; Zalewski, T.; Kurpisz, M.K. Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts. Sci. Rep. 2018, 8, 3682. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, X. MRI-driven PET image optimization for neurological applications. Front. Neurosci. 2019, 13, 782. [Google Scholar] [CrossRef]
- Chow, J.C.; Santiago, C.A. DNA damage of iron-gold nanoparticle heterojunction irradiated by kV photon beams: A monte carlo study. Appl. Sci. 2023, 13, 8942. [Google Scholar] [CrossRef]
- Koothradan, S.; Nayeem, S.; Elyas, K.K. PEGylated iron oxide-gold core–shell nanoparticles for tumor-targeted delivery of Rapamycin. 3 Biotech 2025, 15, 23. [Google Scholar] [CrossRef]
- Azhdarzadeh, M.; Atyabi, F.; Saei, A.A.; Varnamkhasti, B.S.; Omidi, Y.; Fateh, M.; Dinarvand, R. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surf. B Biointerfaces 2016, 143, 224–232. [Google Scholar] [CrossRef]
- Tarkistani, M.A.M.; Komalla, V.; Kayser, V. Recent advances in the use of iron–gold hybrid nanoparticles for biomedical applications. Nanomaterials 2021, 11, 1227. [Google Scholar] [CrossRef]
- Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast agents for photoacoustic and thermoacoustic imaging: A review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, T.; Qiu, H.; Gu, Y.; Chen, Q.; Zuo, C.; Ma, H. Simultaneous photoacoustic and ultrasound imaging: A review. Ultrasonics 2024, 139, 107277. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, S.; Fan, Z.; Huang, G.; Tang, J.; Nie, L. Current strategies of photoacoustic imaging assisted cancer theragnostics toward clinical studies. ACS Photonics 2022, 9, 2555–2578. [Google Scholar] [CrossRef]
- Chi, C.; Du, Y.; Ye, J.; Kou, D.; Qiu, J.; Wang, J.; Chen, X. Intraoperative imaging-guided cancer surgery: From current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics 2014, 4, 1072. [Google Scholar] [CrossRef]
- Zamboni, C.G.; Farahani, K.; Green, J.J. Image-Guided Drug Delivery. In Nanotheranostics for Cancer Applications; Springer: Cham, Switzerland, 2019; pp. 345–393. [Google Scholar]
- Akakuru, O.U.; Zhang, Z.; Iqbal, M.Z.; Zhu, C.; Zhang, Y.; Wu, A. Chemotherapeutic nanomaterials in tumor boundary delineation: Prospects for effective tumor treatment. Acta Pharm. Sin. B 2022, 12, 2640–2657. [Google Scholar] [CrossRef]
- Lim, E.K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef]
- Cui, M.; Zhu, M.; Tang, D.; Cao, Z.; Xiao, H.; Shang, K. Nanotheranostics with Radionuclides for Cancer Diagnosis and Therapy. Adv. Funct. Mater. 2025, 2423445. [Google Scholar] [CrossRef]
- Satheeskumar, R. AI-driven diagnostics and personalized treatment planning in oral oncology: Innovations and future directions. Oral Oncol. Rep. 2025, 13, 100704. [Google Scholar] [CrossRef]
- Rashid, M.; Sharma, M. AI-Assisted Diagnosis and Treatment Planning—A Discussion of How AI Can Assist Healthcare Professionals in Making More Accurate Diagnoses and Treatment Plans for Diseases. In AI in Disease Detection: Advancements and Applications; Wiley Online Library: Hoboken, NJ, USA, 2025; pp. 313–336. [Google Scholar]
- Puttasiddaiah, R.; Basavegowda, N.; Lakshmanagowda, N.K.; Raghavendra, V.B.; Sagar, N.; Sridhar, K.; Sharma, M. Emerging Nanoparticle-Based Diagnostics and Therapeutics for Cancer: Innovations and Challenges. Pharmaceutics 2025, 17, 70. [Google Scholar] [CrossRef]
- Carter, L.L.; Cashwell, E.D. Particle-Transport Simulation with the Monte Carlo Method; TID-26607; Los Alamos National Lab. (LANL): Los Alamos, NM, USA, 1975. [Google Scholar]
- Rogers, D.W.O. Fifty years of Monte Carlo simulations for medical physics. Phys. Med. Biol. 2006, 51, R287. [Google Scholar] [CrossRef]
- Chow, J.C. Recent progress in Monte Carlo simulation on gold nanoparticle radiosensitization. AIMS Biophys. 2018, 5, 231–244. [Google Scholar] [CrossRef]
- Chow, J.C.L. Recent Progress of Gold Nanomaterials in Cancer Therapy. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 1–30. ISBN 978-3-030-11155-7. [Google Scholar] [CrossRef]
- Nguyen, D.; Nguyen, H.A.; Lyding, J.W.; Gruebele, M. Imaging and manipulating energy transfer among quantum dots at individual dot resolution. ACS Nano 2017, 11, 6328–6335. [Google Scholar] [CrossRef] [PubMed]
- Kuno, M.; Fromm, D.P.; Johnson, S.T.; Gallagher, A.; Nesbitt, D.J. Modeling distributed kinetics in isolated semiconductor quantum dots. Phys. Rev. B 2003, 67, 125304. [Google Scholar] [CrossRef]
- Mirzaei, M.R.; Rostami, A.; Matloub, S.; Nazari, M. Design and optimization of graphene quantum dot-based luminescent solar concentrator using Monte-Carlo simulation. Energy Built Environ. 2023, 4, 140–147. [Google Scholar] [CrossRef]
- Jones, B.L.; Cho, S.H. The feasibility of polychromatic cone-beam x-ray fluorescence computed tomography (XFCT) imaging of gold nanoparticle-loaded objects: A Monte Carlo study. Phys. Med. Biol. 2011, 56, 3719. [Google Scholar] [CrossRef]
- Albayedh, F.; Chow, J.C. Monte Carlo simulation on the imaging contrast enhancement in nanoparticle-enhanced radiotherapy. J. Med. Phys. 2018, 43, 195–199. [Google Scholar]
- Abdulle, A.; Chow, J.C. Contrast enhancement for portal imaging in nanoparticle-enhanced radiotherapy: A Monte Carlo phantom evaluation using flattening-filter-free photon beams. Nanomaterials 2019, 9, 920. [Google Scholar] [CrossRef]
- Ali, Z.; Zhang, Y.; Kaul, M.G.; Truong, B.; Bhanot, D.; Adam, G.; Wei, H. Structural control of magnetic nanoparticles for positive nuclear magnetic resonance imaging. Nucl. Sci. Technol. 2024, 35, 168. [Google Scholar] [CrossRef]
- Teeman, E.; Shasha, C.; Evans, J.E.; Krishnan, K.M. Intracellular dynamics of superparamagnetic iron oxide nanoparticles for magnetic particle imaging. Nanoscale 2019, 11, 7771–7780. [Google Scholar] [CrossRef]
- Parishan, M.; Safari, R.; Bordbar, M.; Rakeb, Z.; Faghihi, R. Optimization of the gold layer in multifunctional theranostic core-shell magnetic nanoparticles (MNP@ Au) for radiation dose enhancement in an MRI-guided proton therapy system: A Monte Carlo simulation. Radiat. Phys. Chem. 2025, 229, 112477. [Google Scholar] [CrossRef]
- Zambianchi, P.; Hermógenes, G.; Zambianchi, J.K. Quantification of gold nanoparticles using total reflection X-ray fluorescence by Monte Carlo simulation (MCNP code) applied to cancer cell research. Radiat. Phys. Chem. 2022, 193, 109937. [Google Scholar] [CrossRef]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Choi, H.S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, X.; Wang, C.; Sun, X.; Liu, H.; Wang, F.; Huang, C. Effects of nanoparticle sizes, shapes, and permittivity on plasmonic imaging. Opt. Express 2022, 30, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Q.; Chun, J.; Fichthorn, K.; De Yoreo, J.; Zheng, H. Nanoparticle assembly and oriented attachment: Correlating controlling factors to the resulting structures. Chem. Rev. 2023, 123, 3127–3159. [Google Scholar] [CrossRef]
- Chow, J.C.L. Evaluation of the risk and benefit of using functionalized nanomaterials as contrast agents in image-guided radiotherapy: A Monte Carlo study on the imaging dose and contrast enhancement. In Handbook of Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 281–308. [Google Scholar]
- Pérez-López, C.E.; Garnica-Garza, H.M. Monte Carlo modeling and optimization of contrast-enhanced radiotherapy of brain tumors. Phys. Med. Biol. 2011, 56, 4059. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Pan, D.; Gong, Q.; Gu, Z.; Luo, K. Enhancing drug penetration in solid tumors via nanomedicine: Evaluation models, strategies and perspectives. Bioact. Mater. 2024, 32, 445–472. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Deng, H.; Dutta, P.; Liu, J. Stochastic simulations of nanoparticle internalization through transferrin receptor dependent clathrin-mediated endocytosis. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2104–2111. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold nanoparticles as radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Dou, Y.; Guo, Y.; Li, X.; Li, X.; Wang, S.; Wang, L.; Chang, J. Size-tuning ionization to optimize gold nanoparticles for simultaneous enhanced CT imaging and radiotherapy. ACS Nano 2016, 10, 2536–2548. [Google Scholar] [CrossRef]
- Chow, J.C. Dose enhancement effect in radiotherapy: Adding gold nanoparticles to tumor in cancer treatment. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 383–403. [Google Scholar]
- Chow, J.C. Photon and electron interactions with gold nanoparticles: A Monte Carlo study on gold nanoparticle-enhanced radiotherapy. In Nanobiomaterials in Medical Imaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 45–70. [Google Scholar]
- Tabish, T.A.; Dey, P.; Mosca, S.; Salimi, M.; Palombo, F.; Matousek, P.; Stone, N. Smart gold nanostructures for light mediated cancer theranostics: Combining optical diagnostics with photothermal therapy. Adv. Sci. 2020, 7, 1903441. [Google Scholar] [CrossRef] [PubMed]
- Zagaynova, E.V.; Shirmanova, M.V.; Kirillin, M.Y.; Khlebtsov, B.N.; Orlova, A.G.; Balalaeva, I.V.; Kamensky, V.A. Contrasting properties of gold nanoparticles for optical coherence tomography: Phantom, in vivo studies and Monte Carlo simulation. Phys. Med. Biol. 2008, 53, 4995. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Shen, B.; Cheng, Z. Fluorescent imaging of cancerous tissues for targeted surgery. Adv. Drug Deliv. Rev. 2014, 76, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, V.; Pramanik, M. Monte Carlo simulation of light transport in tissue for optimizing light delivery in photoacoustic imaging of the sentinel lymph node. J. Biomed. Opt. 2013, 18, 106008. [Google Scholar] [CrossRef]
- Mukhlif, B.A.; Ullah, M.I.; Uthirapathy, S.; Menon, S.V.; Sharma, R.S.K.; Kadhim, A.J.; Kadhum, W.R. Multifunctional nanoparticles for image-guided drug delivery in nuclear medicine: Advancements and applications. J. Radioanal. Nucl. Chem. 2025, 334, 1107–1121. [Google Scholar] [CrossRef]
- Kim, M.; Yun, J.; Cho, Y.; Shin, K.; Jang, R.; Bae, H.J.; Kim, N. Deep learning in medical imaging. Neurospine 2019, 16, 657. [Google Scholar] [CrossRef]
- You, C.; Yang, L.; Zhang, Y.; Wang, G. Low-dose CT via deep CNN with skip connection and network-in-network. In Developments in X-Ray Tomography XII; SPIE: Bellingham, WA, USA, 2019; Volume 11113, pp. 429–434. [Google Scholar]
- Chattopadhyay, A.; Maitra, M. MRI-based brain tumour image detection using CNN based deep learning method. Neurosci. Inform. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Ma, J.; Zheng, M.; Lyu, Q.; Rajora, M.A.; Ma, S.; Zheng, G. Deep learning for automatic organ and tumor segmentation in nanomedicine pharmacokinetics. Theranostics 2024, 14, 973. [Google Scholar] [CrossRef]
- Nigam, S.; Gjelaj, E.; Wang, R.; Wei, G.W.; Wang, P. Machine learning and deep learning applications in magnetic particle imaging. J. Magn. Reson. Imaging 2025, 61, 42–51. [Google Scholar] [CrossRef]
- Shafi, S.M.; Chinnappan, S.K. Hybrid transformer-CNN and LSTM model for lung disease segmentation and classification. PeerJ Comput. Sci. 2024, 10, e2444. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.; Ren, Z.; Zhu, J.; Lee, C. Machine learning-augmented surface-enhanced spectroscopy toward next-generation molecular diagnostics. Nanoscale Adv. 2023, 5, 538–570. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; He, P.; Lv, X.; Ren, X.; Li, Y.; Liu, Y.; Shan, H. Material decomposition of spectral CT images via attention-based global convolutional generative adversarial network. Nucl. Sci. Technol. 2023, 34, 45. [Google Scholar] [CrossRef]
- Mi, K.; Chou, W.C.; Chen, Q.; Yuan, L.; Kamineni, V.N.; Kuchimanchi, Y.; Lin, Z. Predicting tissue distribution and tumor delivery of nanoparticles in mice using machine learning models. J. Control. Release 2024, 374, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Nakamaru, Y.; Yamashita, F. Application of machine learning techniques in population pharmacokinetics/pharmacodynamics modeling. Drug Metab. Pharmacokinet. 2024, 56, 101004. [Google Scholar] [CrossRef]
- Anas, E.M.A.; Zhang, H.K.; Kang, J.; Boctor, E. Enabling fast and high quality LED photoacoustic imaging: A recurrent neural networks based approach. Biomed. Opt. Express 2018, 9, 3852–3866. [Google Scholar] [CrossRef]
- Carne, D.; Peoples, J.; Feng, D.; Ruan, X. Accelerated prediction of photon transport in nanoparticle media using machine learning trained with monte carlo simulations. ASME J. Heat Mass Transf. 2023, 145, 052502. [Google Scholar] [CrossRef]
- Singh, A.V.; Maharjan, R.S.; Kanase, A.; Siewert, K.; Rosenkranz, D.; Singh, R.; Luch, A. Machine-learning-based approach to decode the influence of nanomaterial properties on their interaction with cells. ACS Appl. Mater. Interfaces 2020, 13, 1943–1955. [Google Scholar] [CrossRef]
- Gupta, D.; Wal, P.; Wal, A.; KR, S.; Kumar, M.; Panda, K.C.; Sharma, M.C. AI in Clinical Trials and Drug Development: Challenges and Potential and Advancements. Curr. Drug Discov. Technol. 2024, 22, 1–13. [Google Scholar] [CrossRef]
- Wang, C.; Fan, W.; Zhang, Z.; Wen, Y.; Xiong, L.; Chen, X. Advanced Nanotechnology Leading the Way to Multimodal Imaging-Guided Precision Surgical Therapy. Adv Mater. 2019, 31, e1904329. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, L.; Rich, J.M.; Gupta, R.; Pedraza, A.; Ucpinar, B.; Okhawere, K.E.; Badani, K.K. AI-powered real-time annotations during urologic surgery: The future of training and quality metrics. Urol. Oncol. Semin. Orig. Investig. 2024, 42, 57–66. [Google Scholar] [CrossRef]
- Gokcekuyu, Y.; Ekinci, F.; Guzel, M.S.; Acici, K.; Aydin, S.; Asuroglu, T. Artificial Intelligence in Biomaterials: A Comprehensive Review. Appl. Sci. 2024, 14, 6590. [Google Scholar] [CrossRef]
- Tao, H.; Wu, T.; Aldeghi, M.; Wu, T.C.; Aspuru-Guzik, A.; Kumacheva, E. Nanoparticle synthesis assisted by machine learning. Nat. Rev. Mater. 2021, 6, 701–716. [Google Scholar] [CrossRef]
- Ho, D.; Wang, P.; Kee, T. Artificial intelligence in nanomedicine. Nanoscale Horiz. 2019, 4, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.B.; Varallyay, C.G.; Horvath, A.; Bashir, M.R.; Choyke, P.L.; Daldrup-Link, H.E.; Neuwelt, E.A. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017, 92, 47–66. [Google Scholar] [CrossRef]
- Nguyen, K.L.; Yoshida, T.; Han, F.; Ayad, I.; Reemtsen, B.L.; Salusky, I.B.; Finn, J.P. MRI with ferumoxytol: A single center experience of safety across the age spectrum. J. Magn. Reson. Imaging 2017, 45, 804–812. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Zhang, R.; Thoröe-Boveleth, S.; Moosavifar, M.; Abergel, R.J.; Kiessling, F.; Pallares, R.M. Gold Nanoparticle-Enabled Fluorescence Sensing of Gadolinium-Based Contrast Agents in Urine. ACS Appl. Nano Mater. 2025, 8, 2013–2021. [Google Scholar]
- Hu, X.; Zhong, Y.; Jia, X.; Yang, K. Optimizing energy threshold selection for low-concentration contrast agent quantification in small animal photon-counting CT. Phys. Med. Biol. 2025, 70, 045004. [Google Scholar] [CrossRef]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How nanomaterials are transforming drug delivery, bio-imaging, and diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Huang, J.; Yang, L.; Wang, F.; Wu, Y.; Nan, Y.; Wu, W.; Yang, G. Enhancing global sensitivity and uncertainty quantification in medical image reconstruction with Monte Carlo arbitrary-masked mamba. Med. Image Anal. 2025, 99, 103334. [Google Scholar] [CrossRef]
- Lin, G.; Deng, S.; Wang, X.; Zhao, X. Scatter correction based on quasi-Monte Carlo for CT reconstruction. arXiv 2025, arXiv:2501.05039. [Google Scholar]
- Albergueiro, R.; Antunes, V.; Santos, J. Effect of OSEM Reconstruction Iteration Number and Monte Carlo Collimator Modeling on 166Ho Activity Quantification in SPECT/CT. Appl. Sci. 2025, 15, 1589. [Google Scholar] [CrossRef]
- Caie, P.D.; Dimitriou, N.; Arandjelović, O. Precision medicine in digital pathology via image analysis and machine learning. In Artificial Intelligence in Pathology; Elsevier: Amsterdam, The Netherlands, 2025; pp. 233–257. [Google Scholar]
- Kaul, S.; Tank, S.; Patel, M. Regulation of nanomaterials and nanomedicines for clinical applications. In Targeted Therapy for the Central Nervous System; Academic Press: Cambridge, MA, USA, 2025; pp. 423–440. [Google Scholar]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Frangioni, J.V. Nanoparticles for biomedical imaging: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Fu, X.; Pu, Y.; Fu, S.; Liang, H.; Yang, L.; Ai, H. Clinical translational barriers against nanoparticle-based imaging agents. Adv. Drug Deliv. Rev. 2022, 191, 114587. [Google Scholar] [CrossRef]



| Type of Nanomaterial | Applications |
|---|---|
| Quantum Dots (QDs) [12,13,14] |
|
| Gold Nanoparticles (AuNPs) [15,16] |
|
| Iron Oxide Nanoparticles [17] |
|
| Silica-Based Nanoparticles [18,19] |
|
| Polymeric and Liposomal NPs [20,21] |
|
| Category | Key Aspects | Current Status |
|---|---|---|
| Nanomaterials in Clinical Imaging [94,95,96,97,98] | Ferumoxytol (iron oxide NPs) for MRI | Approved for off-label use |
| Au nanoparticles for X-ray contrast in CT and multi-modal imaging | Preclinical and early clinical trials | |
| Bridging Computational Models and Clinical Use [99,100,101,102] | MC simulation integration | Incorporating high-fidelity simulation outputs into clinical imaging platforms |
| AI-driven personalized imaging protocols | Using ML to tailor imaging protocols based on patient-specific parameters | |
| Regulatory and Safety Considerations [103,104,105,106] | FDA and EMA guidelines | Evaluation criteria include pharmacokinetics, long-term retention, and immunogenicity |
| Toxicity mitigation strategies | Development of biodegradable nanoparticles, functionalized coatings, and controlled release mechanisms | |
| Long-term monitoring | Implementation of longitudinal studies and post-market surveillance |
| Application Area | Current Clinical Applications | Potential Developing Applications | References |
|---|---|---|---|
| Medical Imaging | AI-assisted image interpretation in radiology (e.g., detecting lung nodules in CT, breast cancer in mammography) | AI-driven real-time image enhancement, AI-automated image segmentation for nanoparticle tracking | [94,95,96,97,98] |
| Radiotherapy Planning | AI-based auto-contouring of organs-at-risk, dose prediction models | Quantum computing-enhanced AI for Monte Carlo-based treatment planning | [99,100,101] |
| Nanoparticle Imaging | AI-driven segmentation of nanoparticle contrast agents in MRI and CT | AI-predictive modeling of nanoparticle biodistribution and pharmacokinetics | [102,103] |
| Personalized Medicine | AI-assisted patient stratification for targeted therapies | AI-integrated multi-modal imaging and omics data fusion for individualized treatment planning | [102,103,104] |
| Regulatory and Safety | AI-supported quality control in nanoparticle manufacturing | AI-guided risk assessment and regulatory decision-making automation | [105,106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, J.C.L. Nanomaterial-Based Molecular Imaging in Cancer: Advances in Simulation and AI Integration. Biomolecules 2025, 15, 444. https://doi.org/10.3390/biom15030444
Chow JCL. Nanomaterial-Based Molecular Imaging in Cancer: Advances in Simulation and AI Integration. Biomolecules. 2025; 15(3):444. https://doi.org/10.3390/biom15030444
Chicago/Turabian StyleChow, James C. L. 2025. "Nanomaterial-Based Molecular Imaging in Cancer: Advances in Simulation and AI Integration" Biomolecules 15, no. 3: 444. https://doi.org/10.3390/biom15030444
APA StyleChow, J. C. L. (2025). Nanomaterial-Based Molecular Imaging in Cancer: Advances in Simulation and AI Integration. Biomolecules, 15(3), 444. https://doi.org/10.3390/biom15030444







