Abstract
In the vascular system, pathological conditions that cause hypoxia are associated with increased platelet activity and thrombosis. Using a platelet spreading assay, we show that severe hypoxia (i.e., 1%), venous (i.e., 5%), and, surprisingly, arterial (i.e., 12%) oxygen concentrations cause a significant reduction in platelet surface area coverage on fibrinogen in comparison to atmospheric oxygen condition (i.e., 21% oxygen), whilst adhesion and spreading on collagen and CRP were not affected. Importantly, the addition of thrombin or zinc restored full platelet spreading on fibrinogen, indicating that the inhibition of platelet spreading on fibrinogen was due to defective integrin activation. Analysis of integrin activation with FACs via PAC-1 staining supported a significant reduction in integrin activation in hypoxic conditions. Interestingly, a fibrinogen matrix prepared at 1%, 5%, or 12% oxygen failed to induce full platelet spreading, even when the experiments were performed at atmospheric oxygen concentration, indicating that the structure and activity of the fibrinogen coating is affected by oxygen. The effect of oxygen on different matrix proteins is critical to understand, as these data clearly demonstrate that collagen and CRP can support platelet activation at all O2 concentrations, whilst fibrinogen mediated platelet activation and spreading is lost at physiological and pathological O2 concentrations. These data have clear implications for thrombus formation data and highlight the role of oxygen in regulating platelet function.
1. Introduction
Platelets are small (2–3 μM) anucleate blood cells derived from megakaryocytes that have an average lifespan of 8 to 10 days [1]. Platelets play an essential role in haemostasis, binding to a site of injury and forming a platelet plug to prevent excessive bleeding [2]. Upon vascular injury, the endothelial layer is disrupted and extracellular matrix (ECM) proteins, such as collagen, von Willebrand factor (vWF), and fibrinogen, are exposed to platelets [3]. Platelets bind to the ECM proteins via specific receptors, such as GPIb (vWF), GPVI, and α2β1 (collagen), and integrin αIIbβ3 (fibrinogen) [4,5,6,7]. The binding of agonists to these receptors activates multiple intracellular signalling cascades to initiate the secretion of dense and alpha granules. These granules store an abundance of platelet agonists, such as zinc, adenosine diphosphate (ADP), and serotonin, which are secreted into the bloodstream causing the activation of surrounding platelets, ultimately aiding thrombus formation [8,9,10].
As platelets move around the body via the bloodstream, they are exposed to different levels of oxygen (O2) [11]. The arteries transport oxygenated blood (approximately 12% O2) at high pressure and volume from the lungs to the heart, which then pumps it into the systemic circulation. The veins, on the other hand, carry slow-moving deoxygenated blood (approximately 5% O2) at low pressure back to the lungs [12,13]. However, in certain diseases, such as atherothrombosis, stroke, chronic obstructive pulmonary disease (COPD), and sleep apnoea, tissue hypoxia (approximately 1% O2) can occur [14,15,16,17]. The presence of hypoxia has been linked to hyperactive platelets and an increased thrombotic risk in both human and in vivo models [18,19,20,21]. However, the underlying mechanism of this increase in hyperactivity is unclear, with some studies suggesting that healthy platelets exposed to low O2 have upregulated expression of membrane P-selectin and activated integrin αIIbβ3 expression in response to agonist stimulation [22], whilst others suggest that αIIbβ3 activity is markedly reduced following platelet tissue exposure to hypoxic conditions [23]. Part of the issue is the variation in O2 concentration used and the lack of consistency within the experimental approach taken. We have previously shown how quickly platelets experience hypoxia is dependent on the volume and concentration of platelets present [24]. Control of these factors is critical to ensuring the correct O2 level and consistency of platelet response at different O2 levels [24].
Therefore, to fully investigate the effect of variable O2 concentrations on platelet spreading, we spread washed platelets on a range of different ECM proteins at 1% (hypoxic), 5% (venous), 12% (arterial), and 21% (normoxic) conditions and monitored how the platelets responded. This allowed us to establish how platelets behaved in a range of relevant O2 conditions and therefore to identify if any changes to the actin cytoskeleton induced in different O2 conditions could underpin why low O2 conditions encourage a prothrombotic phenotype.
2. Materials and Methods
2.1. Reagents
Chrono-Par collagen suspension (Chrono-Log Corporation, Havertown, PA, USA), fibrinogen (Enzyme Research, Swansea, UK), fibronectin (ThermoFisher Scientific, Loughborough, Leicestershire, UK), collagen-related peptide (CRP-A) (PPlus Medical, Letterkenny, Donegal, Ireland), alexa-fluor 647 labelled fibrinogen (ThermoFisher Scientific, Loughborough, Leicestershire, UK), and Eptifibatide (Tocris Bioscience, Abingdon, UK). ProLong diamond antifade mountant and paraformaldehyde (ThermoFisher Scientific, Loughborough, Leicestershire, UK). PPACK (D-Pehnylalanyl-L-prolyl-L-arginine chloromethyl ketone) and Fluoroescein Isothiocyanate (FITC) phalloidin (Enzo Life Sciences, Farmingdale, NY, USA). Phosphate-buffered solution (PBS) tablets (Gibco, Paisley, Scotland). CD42b Brilliant Violet and PAC1 FITC antibodies (BioLegend, San Diego, CA, USA). All other chemicals were from Sigma Ltd. (Poole, UK) unless otherwise stated.
2.2. Ethics and Donor Recruitment
Work was undertaken in accordance with NHS REC study 21/SC/0215 ‘Investigation of Blood cells for research into Cardiovascular disease’. Blood was obtained from drug-free, healthy volunteers who provided written informed consent.
2.3. Preparation of Blood
All experiments were conducted within 4 h of blood collection. For washed platelets (WP), whole blood was collected into acid citrate dextrose (ACD-A) and then centrifuged at 60× g for 10 min. Plasma was removed, and the blood was respun at 100× g for a further 10 min. The plasma was removed and added to the previously collected platelet-rich plasma (PRP). The PRP was treated with citric acid (0.3 mM) and centrifuged at 1000× g for 10 min. The platelet pellet was then resuspended in wash buffer (36 mM citric acid, 10 mM EDTA, 5 mM glucose, 5 mM KCl, and 9 mM NaCl) and centrifuged for a further 10 min at 1000× g. Platelets were counted using a Beckman Z1 Coulter Particle Counter (Beckman Coulter, High Wycombe, UK) and resuspended to 2.5 × 108 platelets/mL using modified Tyrode’s buffer (20 mM HEPES, 134 mM NaCl, 2 mM KCl, 0.34 mM Na2HPO4, 5.6 mM d-glucose anhydrase, 12 mM Na2CO3, and 1 mM MgCl2).
2.4. Deoxygenation of Platelets
The rate of platelet deoxygenation was established across a range of platelet concentrations using an OXY-4 ST oxygen meter attached to a Needle-Type Oxygen Microsensor (NTH-PSt7) (PreSens, Regensburg, Germany). WP at 2.5 × 108 platelets/mL were moved into a Whitley H35 Hypoxystation (Don Whitley Scientific, Bingley, UK) (set at either 1%, 5%, or 12% O2) and left for 2 h at 37 °C to reach equilibrium with the chamber. The corresponding normoxic control for each donor was also left in an incubator for 2 h at 37 °C under standard laboratory O2 levels/conditions. Following the 2-h incubations, platelets were diluted to the desired concentration using Tyrode’s buffer at the equivalent O2 concentration.
2.5. Platelet Spreading
Coverslips were coated with either collagen (100 μg/mL), CRP-A (100 μg/mL), fibrinogen (3 μg/mL or 100 μg/mL), or fibronectin (100 μg/mL). To allow enough time for the matrix to fully adhere/develop, the 3 ug/mL fibrinogen coverslips were coated overnight at 37 °C, while the 100 μg/mL fibrinogen, collagen, CRP-A, and fibronectin coverslips were coated for 1 h at 37 °C [25,26,27,28,29]. Fibrin coverslips were coated with fibrinogen (100 μg/mL) for 1 h before treatment with thrombin (1 U/mL) for 15 min and blocked with PPACK (40 μΜ) for 30 min [30]. The coverslips were washed twice with PBS between each step. Coverslips were then coated with denatured bovine serum albumin (BSA) (5 mg/mL) for 1 h at 37 °C. Coverslips were washed twice with PBS before the addition of WP (2 × 107 platelets/mL) to the coverslips for 25 min at 37 °C in the presence or absence of zinc sulfate (100 μM, zinc), thrombin (0.1 U/mL), or eptifibatide (9 mM). Coverslips were washed with PBS and fixed with 4% paraformaldehyde (PFA) for 10 min. Coverslips were then removed from the hypoxia chamber and permeabilized with 0.1% Triton X-100 for 5 min. Coverslips were stained with FITC-phalloidin for 1 h at room temperature before being washed with PBS and mounted with ProLong Diamond Antifade Mountant. Coverslips were imaged on a Zeiss Axio Imager fluorescence microscope with an ×63 oil immersion objective (1.4 NA) using Zen Pro software (blue edition) v2.0.0.0 (Zeiss, Cambridge, UK). Five images were captured per condition. Platelet adhesion and the platelet surface area were analyzed using ImageJ v1.54d (National Institutes of Health, Bethesda, MD, USA). Blinded imaging and analysis was carried out for Figures 3, S2 and S5.
2.6. Reoxygenation of Platelets
Following the 2-h deoxygenation in the hypoxia chamber, WPs (2.5 × 108 platelets/mL) were removed from the hypoxia chamber and left to reoxygenate under stirring conditions for 5 min before dilution to the desired concentration using normoxic Tyrode’s buffer.
2.7. Characterization of Fibrinogen Surface
Coverslips were coated with fluorescently labelled fibrinogen (100 μg/mL) for 1 h before being washed with PBS, blocked with denatured BSA (5 mg/mL) for a subsequent hour, and then fixed with 4% PFA for 15 min. Coverslips were then removed from the hypoxia chamber. Coverslips were mounted with ProLong Diamond Antifade Mountant (ThermoFisher Scientific, Loughborough, Leicestershire, UK). Coverslips were imaged on a Zeiss Axio Imager fluorescence microscope with an ×63 oil immersion objective (1.4 NA) using Zen Pro software (Zeiss, Cambridge, UK). Five images were captured per condition. All coverslips were kept in the dark throughout the experiment.
2.8. Flow Cytometry
WPs (1 × 107 platelets/mL) were stained with platelet-specific CD42b Brilliant Violet and PAC1 FITC antibody and treated with 0.1 U/mL thrombin for 20 min. Following stimulation, platelets were fixed with 0.5% PFA and analyzed using a BD LSR Fortessa cell analyzer (BD Bioscience, Franklin Lakes, NJ, USA), with a valid CS&T procedure completed each experimental day. For each sample, 10,000 CD42b specific events were read by the FACSDiva Software v9.7 (BD Biosciences, Franklin Lakes, NJ, USA) and then were analyzed using Floreada.io (https://floreada.io (accessed on 23 January 2025)).
2.9. Statistical Analysis
All statistical tests were performed using GraphPad Prism 8.0.1 software (GraphPad, San Diego, CA, USA). Data are presented as mean ± standard error of the mean (SEM), unless otherwise stated. Data were analyzed by paired t-tests and a one-way or two-way ANOVA, followed by a post-hoc test of Tukey’s multiple comparison. p < 0.05 was considered significant.
3. Results
3.1. Identification of the Correct O2 Concentration
The vast majority of in vitro experiments are performed at 21% O2 [24]. However, this O2 concentration is not found anywhere in the human body, so it is not physiologically relevant, and therefore it is crucial to identify how physiological O2 levels are relevant to platelet function [11]. To complete this, we first identified the correct procedure to incubate the platelets at the relevant O2 concentrations.
Initially, we confirmed the length of time taken for platelets to experience 1% O2 over a 3 h period. Concurrently, we monitored that platelets stored at 21% did not experience reduced O2 while incubated at 37 °C. When incubated at 1% O2, within 30 min, the O2 concentration in a solution of 2.5 × 108/mL platelets was at equilibrium with the chamber (Figure S1). Therefore, platelets were incubated for 2 h prior to use to ensure that they experienced a sustained period of the relevant O2 concentration before use within the spreading assay.
3.2. Platelet Spreading Is Impacted by O2 Concentration
After identifying the relevant incubation time for O2 equilibration, we next investigated if the various O2 concentrations induced changes in platelet spreading. Therefore, platelets were incubated at different O2 concentrations before being spread on either collagen, CRP-A, fibrin, fibronectin, low-density fibrinogen, or high-density fibrinogen for 25 min before fixation, staining, and imaging.
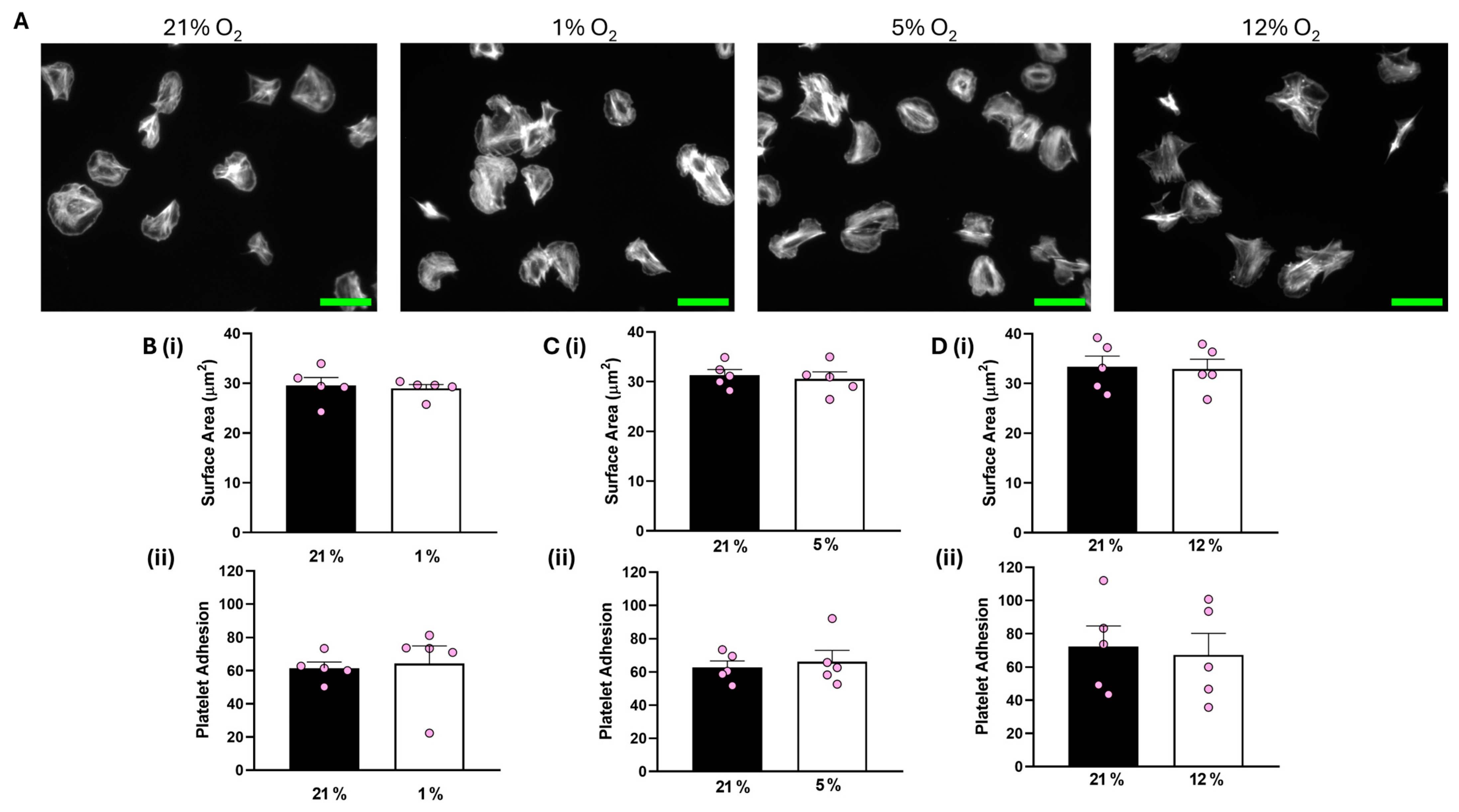
Analysis of our data showed that both platelet adhesion and the average surface area of platelets spread on collagen were unaffected at all O2 concentrations tested compared to the corresponding 21% O2 control (Figure 1). In addition, platelets spread on CRP-A at 1% O2 were also unchanged to those in a 21% O2 control (Figure S2).
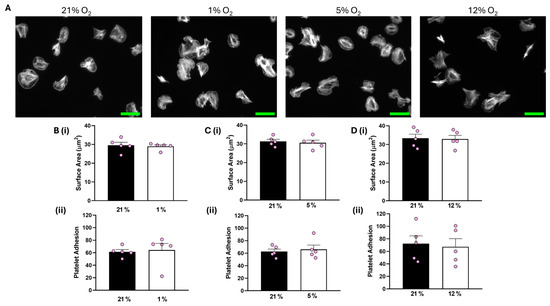
Figure 1.
Platelet spreading on collagen is unchanged by O2 concentration. Washed platelets (2 × 107 platelets/mL) were spread on a collagen matrix (100 μg/mL) for 25 min before being fixed with PFA, permeabilized with Triton X-100, and stained with FITC-phalloidin. (A) Representative images of platelet spreading on collagen at 21%, 1%, 5%, and 12% O2 with scale bar showing 10 μm. (B) Graphs showing (i) platelet surface area and (ii) average platelet adhesion at 1 % O2. (C) Graphs showing (i) platelet surface area and (ii) platelet adhesion at 5% O2 and (D) graphs showing (i) average surface area and (ii) platelet adhesion at 12% O2. Data are presented as mean ± SEM. n = 5. Statistical analysis was calculated using a paired t-test.
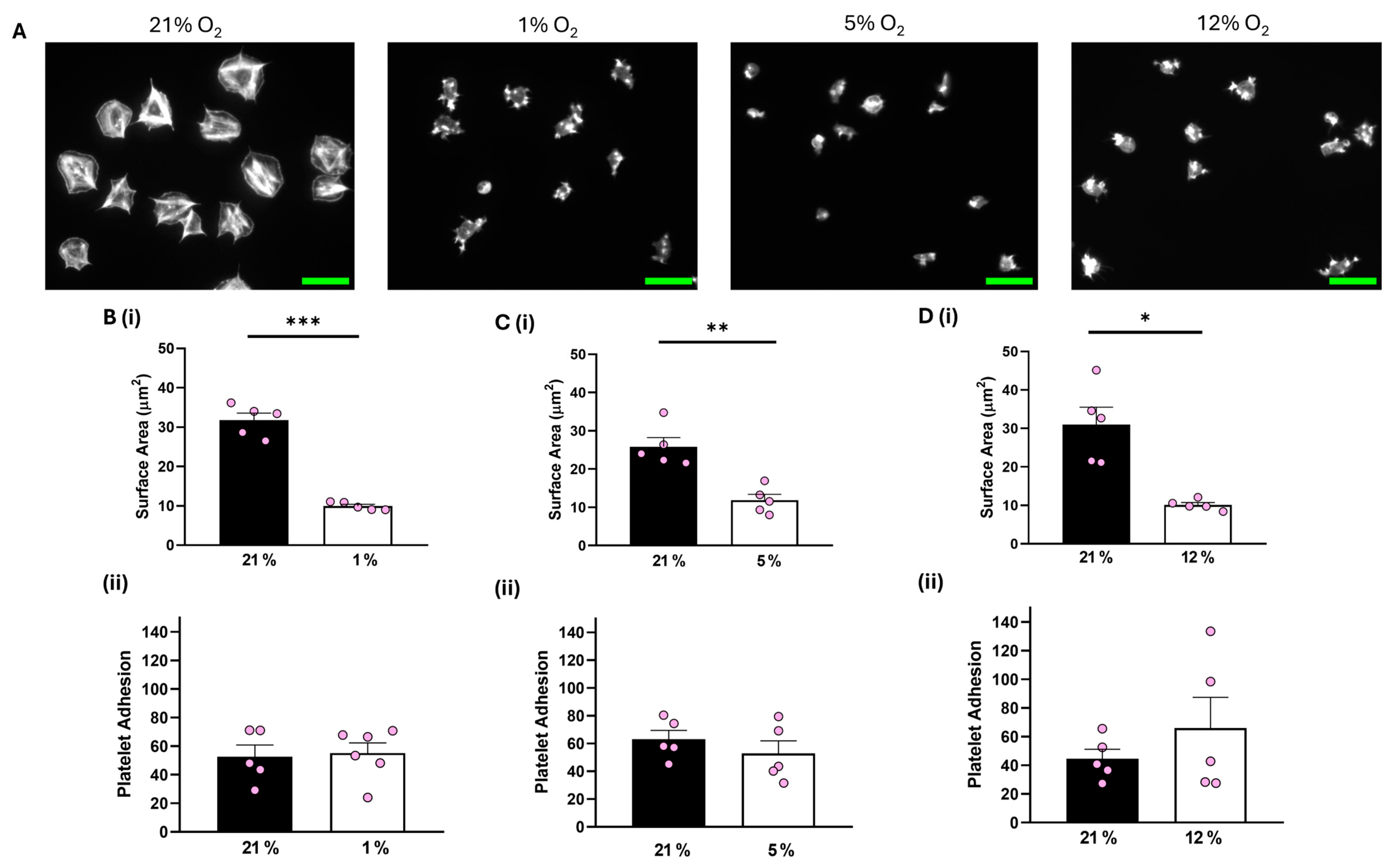
In contrast, although platelet adhesion was unaffected, platelets spread on 100 μg/mL fibrinogen at 1%, 5%, and 12% O2 showed a significant reduction of 16.34 µm2, 13.43 µm2, and 18.91 µm2, respectively, in the average surface area of platelets spread on fibrinogen in comparison to their normoxic control (Figure 2). Similar results were observed when platelets were spread on 3 μg/mL fibrinogen, whilst platelets did not spread well on fibronectin at any of the O2 concentrations tested (Figures S3 and S4). Moreover, we also showed no difference in the level of platelet spreading on fibrinogen (100 μg/mL) in the presence of the αIIbβ3 inhibitor, eptifibatide, at 1% O2 (Figure S5). Our findings suggest that while integrin αIIbβ3 can still interact with and mediate platelet adhesion to the fibrinogen matrix, it fails to promote platelet spreading and activation, while GPVI-mediated platelet spreading is unaffected by changes in O2 concentration.
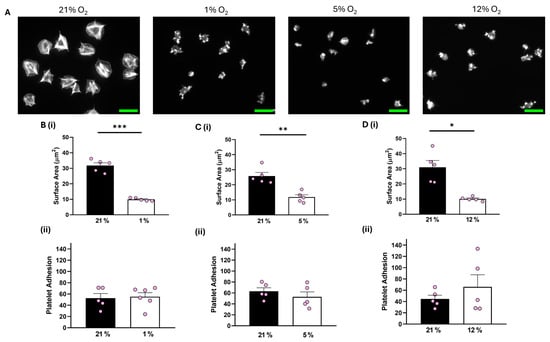
Figure 2.
Platelet spreading on fibrinogen is reduced at physiologically relevant O2 levels. Washed platelets (2 × 107 platelets/mL) were spread on a fibrinogen matrix (100 μg/mL) for 25 min before being fixed with PFA, permeabilized with Triton X-100, and stained with FITC-phalloidin. (A) Representative images of platelet spreading on fibrinogen at 21%, 1%, 5%, and 12% O2 with scale bar showing 10 μm. (B) Graphs showing (i) platelet surface area and (ii) average platelet adhesion at 1% O2, (C) graphs showing (i) platelet surface area and (ii) platelet adhesion at 5% O2, and (D) graphs showing (i) average surface area and (ii) platelet adhesion at 12% O2. Data are presented as mean ± SEM. n = 5. Statistical analysis was calculated using a paired t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
After identifying the influence of O2 concentration on a fibrinogen matrix compared to collagen, we sought to spread platelets on fibrin (100 μg/mL) (Figure 3). While adhesion to the matrix was unaffected by O2 concentration, platelets kept at 1% O2 showed a clear reduction (13.37 μm2) in surface area when compared to their normoxic counterpart. Moreover, in the presence of eptifibatide, the platelet spreading was further reduced. These data suggest that fibrin is at least partially able to induce spreading, although the impact of reduced O2 on integrin αIIbβ3 function still impedes the level of spreading.
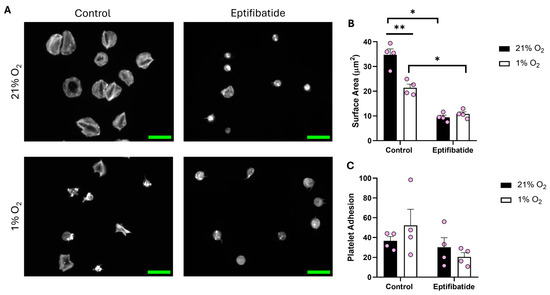
Figure 3.
Platelet spreading on fibrin is reduced in hypoxic O2 levels. Washed platelets (2 × 107 platelets/mL) were preincubated with eptifibatide (9 μM) for 2 min before spreading on fibrin (100 µg/mL) for 25 min before being fixed with PFA, permeabilized with Triton X-100, and stained with FITC-phalloidin. (A) Representative images of platelets treated with zinc, thrombin, and the control at 21% and 1% O2, with scale bar showing 10 μm. Graphs showing (B) average platelet surface area and (C) platelet adhesion. Data are presented as mean ± SEM. n = 4. Statistical analysis was calculated using repeated measures two-way ANOVA with a Tukey’s post hoc test. * p < 0.05, ** p < 0.01.
3.3. Platelet Spreading Is Restored by Addition of Thrombin or Zinc at All O2 Concentrations
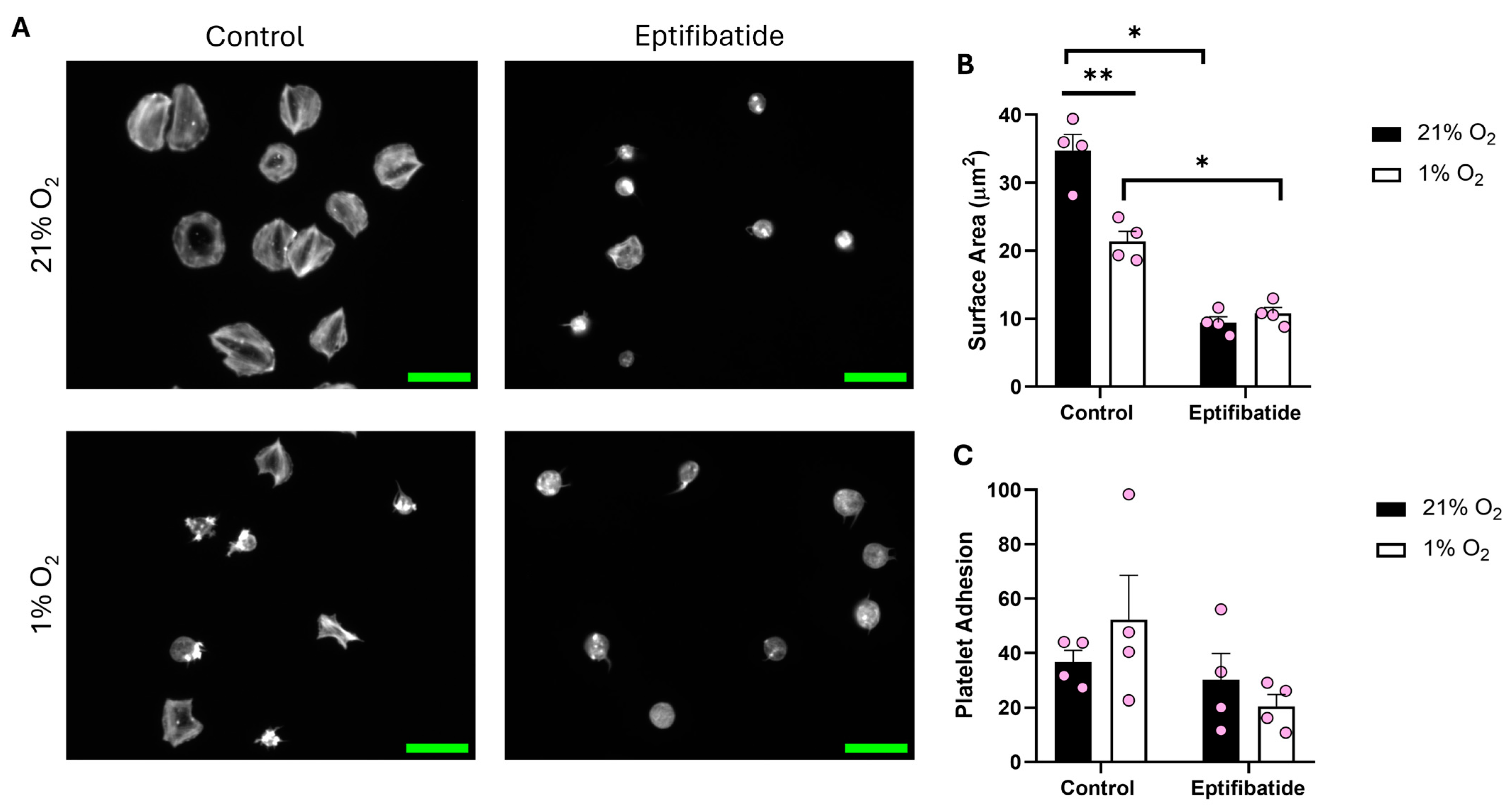
We then sought to establish whether the lack of spreading on a fibrinogen matrix at lower O2 concentrations was due to a lack of overall integrin αIIbβ3 activity or a lack of response to the fibrinogen. To assess this, we treated platelets with either zinc or thrombin before spreading on fibrinogen. Zinc and thrombin are both prime examples of direct platelet agonists and have previously been shown to induce full platelet spreading on a fibrinogen matrix [25,31].
Treating platelets with either zinc or thrombin before spreading re-established platelet spreading across all of the O2 concentrations tested (including normoxia). We observed a significant increase of 20.15 µm2 and 17.37 µm2 in the platelet surface area following zinc and thrombin treatment, respectively, when compared to the control in hypoxia (Figure 4). Platelets responded to zinc and thrombin in a similar manner when spread on 3 μg/mL fibrinogen and 100 μg/mL fibronectin (Figures S3 and S4) in a hypoxic environment, as well as at arterial and venous O2 levels (Figures S6–S9). Our data suggest that platelet function per se is not altered by low O2 concentrations, but platelet spreading on fibrinogen and fibrin is specifically reduced.
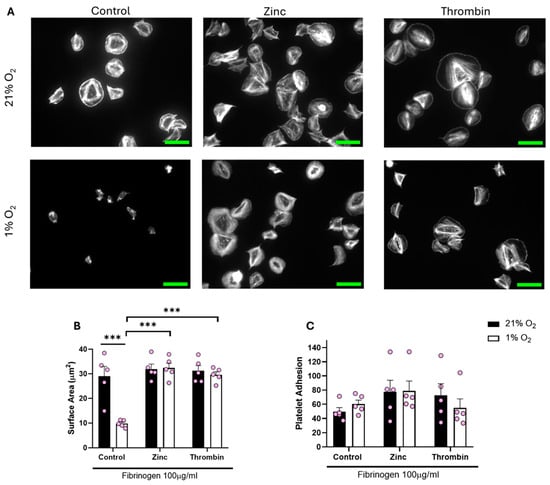
Figure 4.
Platelet spreading on fibrinogen at hypoxic O2 levels is restored following pre-treatment with zinc and thrombin. Washed platelets (2 × 107 platelets/mL) were preincubated with zinc (100 μM) and thrombin (0.1 U/mL) for 2 min before spreading on fibrinogen (100 µg/mL) for 25 min before being fixed with PFA, permeabilized with Triton X-100, and stained with FITC-phalloidin. (A) Representative images of platelets treated with zinc, thrombin, and the control at 21% and 1% O2, with scale bar showing 10 μm. Graphs showing (B) average platelet surface area and (C) platelet adhesion. Data are presented as mean ± SEM. n = 5 Statistical analysis was calculated using repeated measures two-way ANOVA with a Tukey’s post hoc test. *** p < 0.001.
To further examine the level of integrin αIIbβ3 activity, we incubated washed platelets at 1% and 21% O2 before stimulation with thrombin. We then analyzed the level of integrin activation using a PAC-1 antibody via flow cytometry. This data identified that whilst thrombin induced a strong activation of integrin αIIbβ3 in normoxic conditions, at lower O2 concentrations, the integrin was poorly activated (Figure S10).
3.4. Preparation of Fibrinogen in Different O2 Concentrations Alters Platelet Spreading
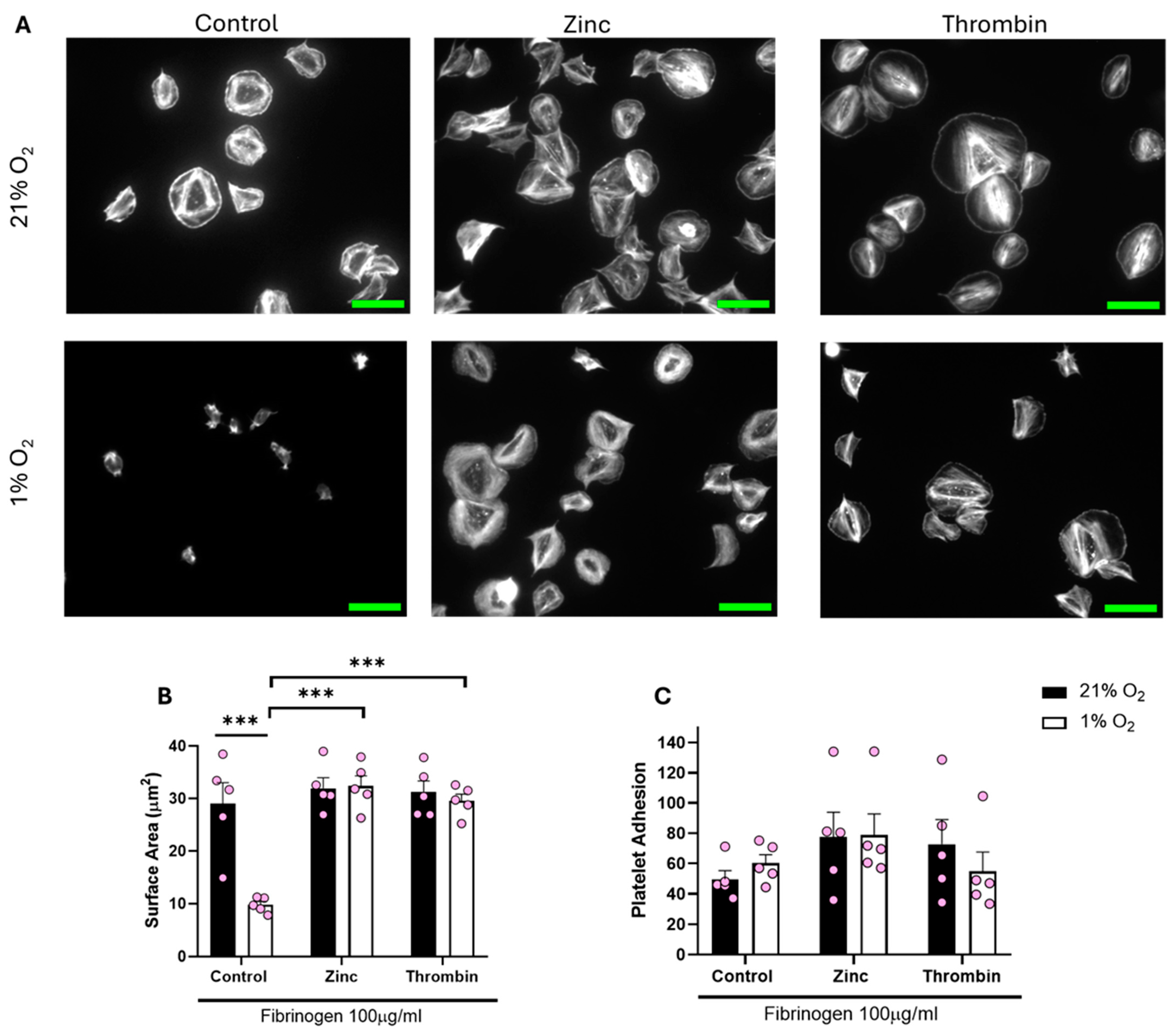
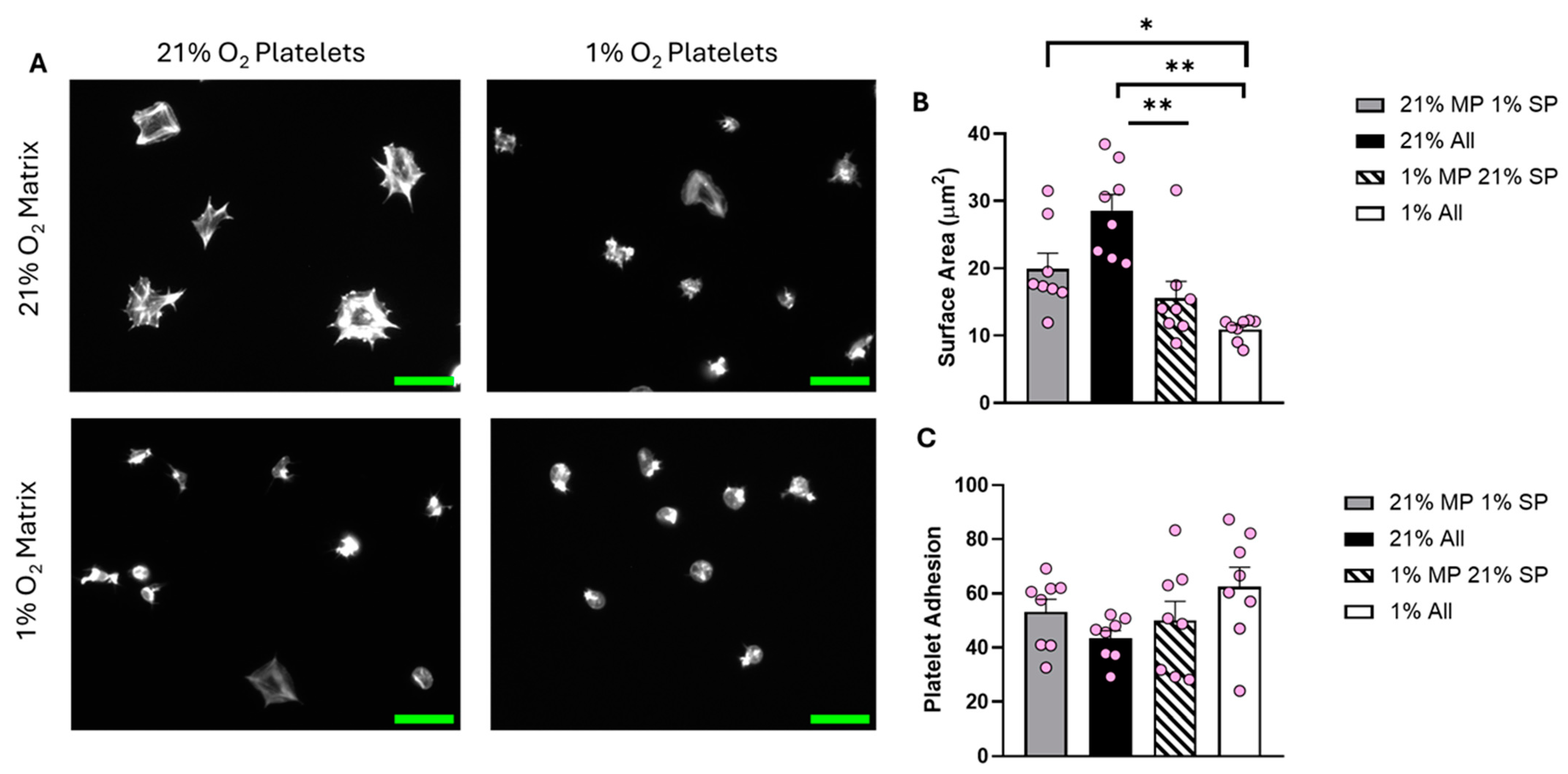
Although the lack of spreading on fibrinogen is potentially due to a lack of integrin activation, we also sought to investigate if the reduction in fibrinogen-mediated platelet spreading observed at physiologically relevant O2 levels could be attributed to the fibrinogen matrix itself. Therefore, fibrinogen was coated on coverslips in 1%, 5%, 12%, and 21% O2 conditions before the addition of platelets either at 1%, 5%, 12%, or 21% O2.
Analysis of the data showed a clear difference in the level of platelet spreading between the four conditions investigated (Figure 5). Platelets spread solely in the normoxic environment had the largest average surface area (30.76 µm2). As expected, fibrinogen prepared at 21% O2 did not support full spreading if the platelets were incubated at 1% O2. Interestingly, normoxic platelets spread on fibrinogen prepared in 1% O2 conditions were also significantly inhibited, suggesting that the fibrinogen matrix itself was no longer supporting effective platelet spreading. These results were replicated at 5% O2 (Figure S11). At 12% O2, although the trend was still present, the results were no longer significantly different (Figure S12).
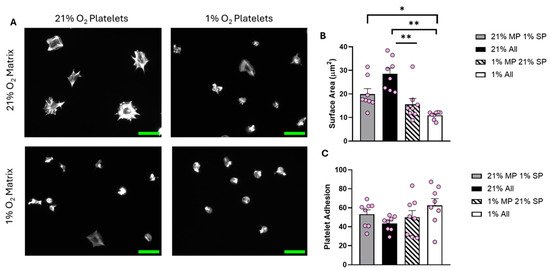
Figure 5.
The O2 concentration at which the fibrinogen matrix is prepared plays an important role in determining platelet spreading. Fibrinogen matrices were prepared at both 21% and 1% O2, platelet spreading was then conducted using washed platelets kept at 21% and 1% O2. Washed platelets (2 × 107 platelets/mL) were spread on 100 μg/mL fibrinogen for 25 min before being fixed with PFA, permeabilized with Triton X-100, and stained with FITC-phalloidin. (A) Representative images of platelet spreading at each condition, with a scale bar showing 10 μm. Graphs show (B) average platelet surface area and (C) platelet adhesion. Data are presented as mean ± SEM. n = 8. Statistical analysis was calculated using repeated-measures one-way ANOVA with a Tukey’s post hoc test. * p < 0.05, ** p < 0.01. Matrix prep (MP), spread platelets (SP).
To understand if platelet spreading on fibrinogen could be recovered, we reoxygenated washed platelets stored at 1% O2 by stirring them for 5 min using a light transmission aggregometer in an open cuvette at 21% O2 before spreading them on fibrinogen prepared both at 21% and 1% O2. Analysis of this data identified that the reoxygenated platelets responded as expected and completed a similar level of spreading to that seen with platelets at 21% O2. However, they were still unable to spread on fibrinogen prepared at 1% O2, indicating that the fibrinogen matrix was still unable to induce platelet activation and spreading (Figure S13). To ensure it was not due to an alteration in fibrinogen coverage in hypoxic conditions, alexa-fluor 647-fibrinogen was coated onto coverslips and then imaged. This identified a similar level of fibrinogen coverage at both 21% and 1% O2 (Figure S14).
Our data indicate that both platelets themselves and the fibrinogen matrix are impacted by a reduction in O2 level to a hypoxic level.
4. Discussion
Globally, cardiovascular disease (CVD) is the leading cause of mortality, with an estimated 17.9 million deaths in 2019 (Cardiovascular Diseases (CVD), available online https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed 25 September 2024)) [32]. With an aging population and increasing numbers of obese and diabetic patients, this figure is predicted to grow further [33]. Key to this disease is the role of platelet hyperreactivity, but also periods of reduced O2 levels within the vascular system, both of which have been noted to provide a prothrombotic environment [14,34,35,36]. Thus, it is important to understand the influence O2 concentration might have on platelet activation.
Here, we used the platelet spreading assay to present evidence that: (1) Only washed platelets kept in a normoxic environment have sustained integrin αIIbβ3-mediated platelet spreading. (2) Collagen and CRP spreading is unaffected by O2 concentration. (3) The inhibition of platelet spreading at 1%, 5%, and 12% O2 is removed through the addition of thrombin and zinc. (4) Fibrinogen itself is less reactive if prepared in low O2 conditions, indicating that O2 can also influence the matrix protein fibrinogen’s function. (5) Reoxygenation of platelets restores platelet spreading, but these platelets still cannot spread on fibrinogen prepared at 1% O2. Our data therefore indicates that O2 can affect platelet activation by modulating integrin activation in washed platelet conditions, but also critically by reducing the ability of fibrinogen itself to activate platelets directly. These data underscore the importance of using physiologically relevant O2 conditions within experimental conditions.
The platelet spreading assay has been used to show how platelets respond to different environmental conditions, including O2 concentrations [23,24,37,38]. Here, we showed that platelets can adhere successfully to all matrices tested at each O2 concentration but spread significantly less at 1%, 5%, and 12% O2 in comparison to the normoxic control on fibrin, fibronectin, and fibrinogen. This work agrees with previous publications but importantly deepens our understanding of O2’s role in platelet spreading by utilizing a wider range of O2 concentrations and matrix proteins than other publications. Importantly, we demonstrated that the reduction in platelet surface area and spreading on fibrinogen observed by Kiouptsi et al. [23] is not a phenomenon observed only in hypoxia but is also present at physiologically relevant 5% and 12% O2. This indicates that the normoxic condition is the outlier, as it allows full spreading in comparison to the more physiologically relevant 1%, 5%, and 12% O2 concentrations. However, the absence of a response to the fibrinogen matrix at 1%, 5%, and 12% O2 is not due to a lack of the ability of the platelets to activate, as the addition of thrombin or zinc induced full platelet spreading. Thrombin and zinc are both known platelet agonists, with zinc stored within alpha granules and secreted by platelets potentiating platelet activation [39]. Platelet incubation with zinc and thrombin can induce platelet activation through secondary pathways to integrin αIIbβ3 [6,39,40]. Whilst our data show that platelets are able to spread on the fibrinogen matrix at low O2 concentrations in the presence of an agonist, this is likely due to inside-out signalling induced by zinc or thrombin, rather than outside-in signalling by fibrinogen. Therefore, the activation of the platelet by the fibrinogen itself is circumvented, allowing the platelet to fully spread.
Interestingly, this reduction in spreading seems to be a combination of two effects. Firstly, the integrin is activated to a lesser extent in lower O2 concentrations compared to 21%. Although this is as previously published for 1%, the 12% O2 data is surprising [23]. The integrin activation seems to be much more sensitive to O2 concentration than expected. In addition to the reduction in integrin activation, we also established that the ability of fibrinogen to activate platelets was affected by the level of O2, as platelets at 21% O2 can fully activate αIIbβ3 and yet were unable to spread on fibrinogen prepared at 1%, 5%, or 12% O2. Furthermore, reoxygenated platelets recovered their integrin function, in agreement with Kusanto et al. [24], and Kiouptsi et al. [23], and showed full spreading on fibrinogen at 21% O2, but were still unable to spread on the fibrinogen matrix prepared at 1% O2. Although it is unclear why there is a reduction due to the preparation of fibrinogen at 1%, we were able to show that fluorescently labelled fibrinogen covered the coverslip in similar manners at both 21 and 1%, indicating this aspect is not affected.
This reduction in the reactivity of the fibrinogen matrix is therefore critical to understand, as it asks key questions about the role of fibrinogen within thrombi, as in vivo thrombi will form at between 5–12% O2 depending on the location of the thrombus. Firstly, is fibrinogen in the blood similarly affected? Secondly, how relevant is platelet spreading on fibrinogen, or is fibrinogen only required for platelet adhesion? These two questions are key, as the answers would alter our understanding of the role of fibrinogen within a thrombus. There are hints at the answers, as Schurr et al. identified, for example, that lamellipodia are not required for thrombus formation in vivo [41], indicating that the adhesion we see here on fibrinogen at 1% O2 could still support thrombus formation. It is also important to note that the platelet response to collagen is unaffected by O2 conditions and therefore can still act as a matrix protein capable of both adhering and activating platelets, demonstrating its critical prothrombotic nature. Overall, the effect of O2 on fibrinogen (and other matrix proteins) is certainly an area for further research.
It is important to acknowledge, however, that although the hypoxia chamber is an effective mechanism to induce a constant O2 environment, it still has some limitations when replicating O2 conditions experienced in vivo. Most importantly, O2 gradients that are found in vivo are not possible to replicate within the chamber [42]. Secondly, the time frame for blood cells to experience different O2 concentrations may not accurately replicate that which happens in the body.
5. Conclusions
Overall, our data identify that the ability of platelets to spread on fibrinogen in washed platelet conditions is defined by two key factors: the activity of the integrin αIIbβ3 and the preparation of the fibrinogen. This is key to acknowledge, as it indicates that it is not just the cells themselves that can be affected by changes to O2 concentrations, but also key components of an assay, such as matrix proteins, and thus experimental data from these experiments need to appreciate all factors to be fully understood.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom15040501/s1, Figure S1: Establishing experimental parameters; Figure S2: Platelet spreading on CRP-A is unaffected by hypoxic conditions; Figure S3: Platelet spreading on fibrinogen (3 μg/mL) at 1% O2 levels is restored following pretreatment with zinc and thrombin; Figure S4: Platelet spreading on fibronectin is increased/promoted when platelets are treated with zinc or thrombin; Figure S5: Platelet spreading on fibrinogen following eptifibatide treatment is unaffected at 1% O2; Figure S6: Platelet spreading on high density fibrinogen at venous O2 levels is restored following pretreatment with zinc and thrombin; Figure S7: Platelet spreading on low density fibrinogen at venous O2 levels is restored following pretreatment with zinc and thrombin; Figure S8: Platelet spreading on high density fibrinogen at arterial O2 levels is restored following pretreatment with zinc and thrombin; Figure S9: Platelet spreading on low density fibrinogen at arterial O2 levels is restored following pretreatment with zinc and thrombin; Figure S10: Integrin αIIbβ3 function is markedly reduced in hypoxic platelets; Figure S11: Location of fibrinogen matrix preparation plays an important factor in platelet spreading; Figure S12: Location of fibrinogen matrix preparation plays an important factor in platelet spreading; Figure S13: Platelet spreading on fibrinogen is restored following reoxygenation of platelets; Figure S14: Formation of a fibrinogen monolayer is unaffected by oxygen concentration.
Author Contributions
Conceptualization, S.D.J.C. and M.A.; methodology, S.V.L.L., K.S.W., Z.B. and L.N.-A.; formal analysis, S.V.L.L. and L.B.; data curation, S.V.L.L. and S.D.J.C.; writing—original draft preparation, S.V.L.L., Z.B., L.N.-A., G.P. and S.D.J.C.; writing—review and editing, S.V.L.L., Z.B., L.N.-A., K.S.W., G.P. and S.D.J.C.; supervision, S.D.J.C. and G.P.; project administration, S.D.J.C.; funding acquisition, M.A. and S.D.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the British Heart Foundation, grant number FS/PhD/22/29265.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by NHS REC study 21/SC/0215 ‘Investigation of Blood cells for research into Cardiovascular disease’ on 2 August 2021.
Informed Consent Statement
Blood was obtained from drug-free healthy volunteers who provided written informed consent.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Italiano, J.E., Jr.; Lecine, P.; Shivdasani, R.A.; Hartwig, J.H. Blood Platelets Are Assembled Principally at the Ends of Proplatelet Processes Produced by Differentiated Megakaryocytes. J. Cell Biol. 1999, 147, 1299–1312. [Google Scholar] [PubMed]
- Storey, R.F.; Thomas, M.R. The Role of Platelets in Inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef]
- Clemetson, K.J. Platelets and Primary Haemostasis. Thromb. Res. 2012, 129, 220–224. [Google Scholar] [CrossRef]
- Rayes, J.; Watson, S.P.; Nieswandt, B. Functional Significance of the Platelet Immune Receptors GPVI and CLEC-2. J. Clin. Investig. 2019, 129, 12–23. [Google Scholar] [PubMed]
- Rivera, J.; Lozano, M.L.; Navarro-Núñez, L.; Vicente García, V. Platelet Receptors and Signaling in the Dynamics of Thrombus Formation. Haematologica 2009, 94, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet Integrin AiIbβ3: Signal Transduction, Regulation, and Its Therapeutic Targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar]
- Kroll, M.H.; Harris, T.S.; Moake, J.L.; Handin, R.I.; Schafer, A.I. Von Willebrand Factor Binding to Platelet Gplb Initiates Signals for Platelet Activation. J. Clin. Investig. 1991, 88, 1568–1573. [Google Scholar] [CrossRef]
- Italiano, J.E.; Richardson, J.L.; Patel-Hett, S.; Battinelli, E.; Zaslavsky, A.; Short, S.; Ryeom, S.; Folkman, J.; Klement, G.L. Angiogenesis Is Regulated by a Novel Mechanism: Pro- and Antiangiogenic Proteins Are Organized into Separate Platelet α Granules and Differentially Released. Blood 2008, 111, 1227–1233. [Google Scholar] [CrossRef]
- Ambrosio, A.L.; Di Pietro, S.M. Storage Pool Diseases Illuminate Platelet Dense Granule Biogenesis. Platelets 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Woulfe, D.; Yang, J.; Brass, L. ADP and Platelets: The End of the Beginning. J. Clin. Investig. 2001, 107, 1503–1505. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining Normoxia, Physoxia and Hypoxia in Tumours—Implications for Treatment Response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.H.; Anderson, S.E.; Iaizzo, P.A. Human Coronary Venous Anatomy: Implications for Interventions. J. Cardiovasc. Transl. Res. 2013, 6, 208–217. [Google Scholar] [CrossRef]
- Gavaghan, M. Vascular Hemodynamics. AORN J. 1998, 68, 211–226. [Google Scholar] [CrossRef]
- Abe, H.; Semba, H.; Takeda, N. The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J. Atheroscler. Thromb. 2017, 24, 884–894. [Google Scholar] [CrossRef]
- Gabryelska, A.; Łukasik, Z.M.; Makowska, J.S.; Białasiewicz, P. Obstructive Sleep Apnea: From Intermittent Hypoxia to Cardiovascular Complications via Blood Platelets. Front. Neurol. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.D.; Mitchell, P.D.; Mcnicholas, W.T. Hypoxemia in Patients with COPD: Cause, Effects, and Disease Progression. Int. J. Copd 2011, 6, 199–208. [Google Scholar]
- Tarbell, J.; Mahmoud, M.; Corti, A.; Cardoso, L.; Caro, C. The Role of Oxygen Transport in Atherosclerosis and Vascular Disease. J. R. Soc. Interface 2020, 17, 20190732. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, T.; Ahmad, S.; Gupta, N.; Sahu, A.; Ahmad, Y.; Nair, V.; Chatterjee, T.; Bajaj, N.; Sengupta, S.; Ganju, L.; et al. Altered Expression of Platelet Proteins and Calpain Activity Mediate Hypoxia-Induced Prothrombotic Phenotype. Blood 2014, 123, 1250–1260. [Google Scholar] [CrossRef]
- Delaney, C.; Davizon-Castillo, P.; Allawzi, A.; Posey, J.; Gandjeva, A.; Neeves, K.; Tuder, R.M.; Di Paola, J.; Stenmark, K.R.; Nozik, E.S. Platelet Activation Contributes to Hypoxia-Induced Inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L413–L421. [Google Scholar] [CrossRef]
- Oga, T.; Chin, K.; Tabuchi, A.; Sumi, K.; Takahashi, K.; Handa, T.; Takahashi, K.; Taniguchi, R.; Kondo, H.; Kawato, M.; et al. Effects of Obstructive Sleep Apnea with Intermittent Hypoxia on Platelet Aggregability. J. Atheroscler. Thromb. 2009. [Google Scholar] [CrossRef]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-Term Cardiovascular Outcomes in Men with Obstructive Sleep Apnoea-Hypopnoea with or without Treatment with Continuous Positive Airway Pressure: An Observational Study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Mix, D.S.; Ture, S.K.; Schmidt, R.A.; Mohan, A.; Pariser, D.; Stoner, M.C.; Shah, P.; Chen, L.; Zhang, H.; et al. Hypoxia and Ischemia Promote a Maladaptive Platelet Phenotype. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1594–1606. [Google Scholar] [CrossRef]
- Kiouptsi, K.; Gambaryan, S.; Walter, E.; Walter, U.; Jurk, K.; Reinhardt, C. Hypoxia Impairs Agonist-Induced Integrin AIIbβ3 Activation and Platelet Aggregation. Sci. Rep. 2017, 7, 7621. [Google Scholar] [CrossRef]
- Kusanto, B.; Gordon, A.; Naylor-Adamson, L.; Atkinson, L.; Coupland, C.; Booth, Z.; Ahmed, Y.; Pires, I.M.; Stasiuk, G.J.; Sturmey, R.; et al. Practical Considerations of Dissolved Oxygen Levels for Platelet Function under Hypoxia. Int. J. Mol. Sci. 2021, 22, 13223. [Google Scholar] [CrossRef]
- Coupland, C.A.; Naylor-Adamson, L.; Booth, Z.; Price, T.W.; Gil, H.M.; Firth, G.; Avery, M.; Ahmed, Y.; Stasiuk, G.J.; Calaminus, S.D.J. Platelet Zinc Status Regulates Prostaglandin-Induced Signaling, Altering Thrombus Formation. J. Thromb. Haemost. 2023, 21, 2545–2558. [Google Scholar] [CrossRef]
- McCarty, O.J.T.; Zhao, Y.; Andrew, N.; Machesky, L.M.; Staunton, D.; Frampton, J.; Watson, S.P. Evaluation of the Role of Platelet Integrins in Fibronectin-Dependent Spreading and Adhesion. J. Thromb. Haemost. 2004, 2, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Thornber, K.; McCarty, O.J.T.; Watson, S.P.; Pears, C.J. Distinct but Critical Roles for Integrin AIIbβ 3 in Platelet Lamellipodia Formation on Fibrinogen, Collagen-Related Peptide and Thrombin. FEBS J. 2006, 273, 5032–5043. [Google Scholar] [CrossRef] [PubMed]
- Jiroušková, M.; Jaiswal, J.K.; Coller, B.S. Ligand Density Dramatically Affects Integrin IIb3-Mediated Platelet Signaling and Spreading. Blood 2007, 109, 5260–5269. [Google Scholar] [CrossRef]
- Qiu, Y.; Brown, A.C.; Myers, D.R.; Sakurai, Y.; Mannino, R.G.; Tran, R.; Ahn, B.; Hardy, E.T.; Kee, M.F.; Kumar, S.; et al. Platelet Mechanosensing of Substrate Stiffness during Clot Formation Mediates Adhesion, Spreading, and Activation. Proc. Natl. Acad. Sci. USA 2014, 111, 14430–14435. [Google Scholar] [CrossRef]
- Alshehri, O.M.; Hughes, C.E.; Montague, S.; Watson, S.K.; Frampton, J.; Bender, M.; Watson, S.P. Fibrin Activates GPVI in Human and Mouse Platelets. Blood 2015, 126, 1601–1608. [Google Scholar] [CrossRef]
- Mazharian, A.; Roger, S.; Berrou, E.; Adam, F.; Kauskot, A.; Nurden, P.; Jandrot-Perrus, M.; Bryckaert, M. Protease-Activating Receptor-4 Induces Full Platelet Spreading on a Fibrinogen Matrix: Involvement of ERK2 and P38 and Ca2+ Mobilization. J. Biol. Chem. 2007, 282, 5478–5487. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Cardiovascular diseases (CVDs). 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 25 September 2024).
- Roth, G.A.; Nguyen, G.; Forouzanfar, M.H.; Mokdad, A.H.; Naghavi, M.; Murray, C.J.L. Estimates of Global and Regional Premature Cardiovascular Mortality in 2025. Circulation 2015, 132, 1270–1271. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, Y.; Li, P.; Lu, H.; Li, H.; Ge, J. Hypoxia and Panvascular Diseases: Exploring the Role of Hypoxia-Inducible Factors in Vascular Smooth Muscle Cells under Panvascular Pathologies. Sci. Bull. 2023, 68, 1954–1974. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, W.; Li, C.; Zhao, R.; Lu, H.; Song, S.; Zhou, Y.; Hu, Y.; Shi, B.; Ge, J. Hypoxia-Induced Signaling in the Cardiovascular System: Pathogenesis and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Maclay, J.D.; McAllister, D.A.; Johnston, S.; Raftis, J.; McGuinnes, C.; Deans, A.; Newby, D.E.; Mills, N.L.; MacNee, W. Increased Platelet Activation in Patients with Stable and Acute Exacerbation of COPD. Thorax 2011, 66, 769–774. [Google Scholar] [CrossRef]
- Tyagi, T.; Jain, K.; Gu, S.X.; Qiu, M.; Gu, V.W.; Melchinger, H.; Rinder, H.; Martin, K.A.; Gardiner, E.E.; Lee, A.I.; et al. A Guide to Molecular and Functional Investigations of Platelets to Bridge Basic and Clinical Sciences. Nat. Cardiovasc. Res. 2022, 1, 223–237. [Google Scholar] [CrossRef]
- Lickert, S.; Sorrentino, S.; Studt, J.D.; Medalia, O.; Vogel, V.; Schoen, I. Morphometric Analysis of Spread Platelets Identifies Integrin AiIbβ3-Specific Contractile Phenotype. Sci. Rep. 2018, 8, 5428. [Google Scholar] [CrossRef]
- Watson, B.R.; White, N.A.; Taylor, K.A.; Howes, J.M.; Malcor, J.D.M.; Bihan, D.; Sage, S.O.; Farndale, R.W.; Pugh, N. Zinc Is a Transmembrane Agonist That Induces Platelet Activation in a Tyrosine Phosphorylation-Dependent Manner. Metallomics 2016, 8, 91–100. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of Platelet Activation and Coagulation and Its Role in Vascular Injury and Arterial Thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Schurr, Y.; Sperr, A.; Volz, J.; Beck, S.; Reil, L.; Kusch, C.; Eiring, P.; Bryson, S.; Sauer, M.; Nieswandt, B.; et al. Platelet Lamellipodium Formation Is Not Required for Thrombus Formation and Stability. Blood 2019, 134, 2318–2329. [Google Scholar] [CrossRef]
- Brennan, M.D.; Rexius-Hall, M.L.; Elgass, L.J.; Eddington, D.T. Oxygen Control with Microfluidics. Lab Chip 2014, 14, 4305–4318. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).