Oncobiomics: Leveraging Microbiome Translational Research in Immuno-Oncology for Clinical-Practice Changes
Abstract
1. Introduction
2. Impact of Microbes on Immunosurveillance
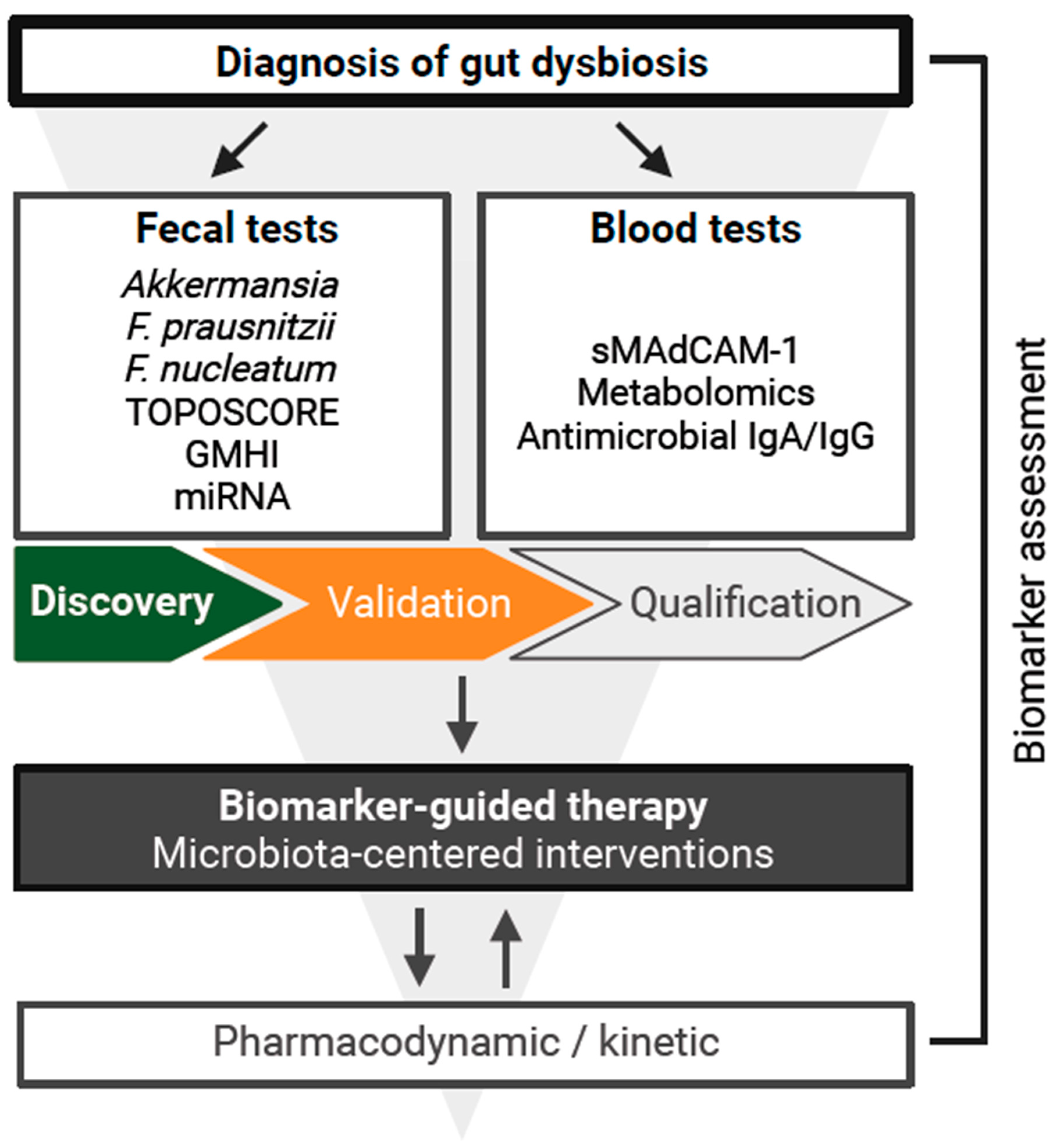
3. Development of Diagnostic Tools
4. Fecal Markers
5. Circulating Markers
6. Promising Microbiota-Centered Interventions (MCIs) in the Immuno-Oncology (I-O) Field
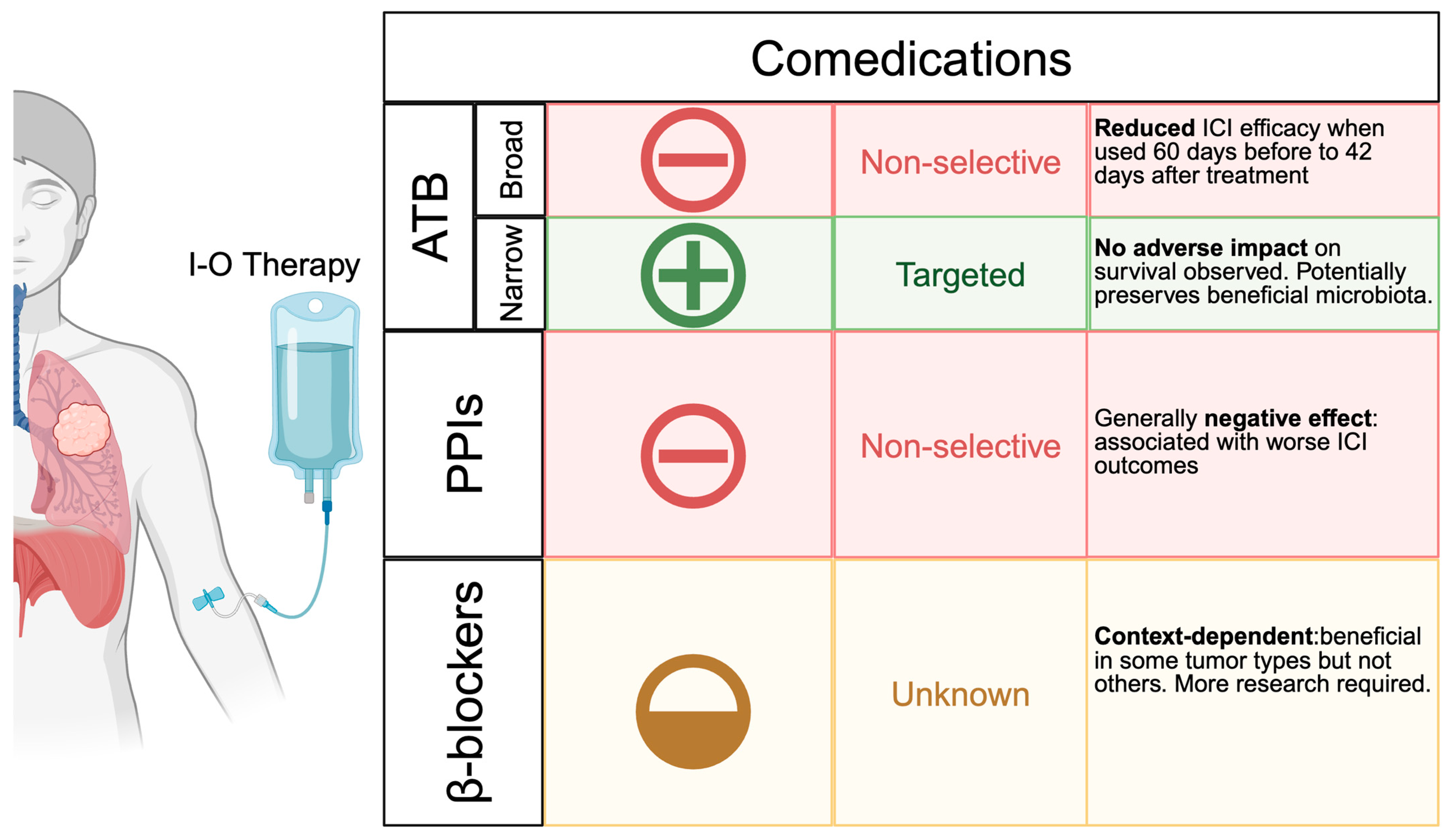
6.1. Comedications
6.2. Fecal Microbiota Transplantation (FMT)
| Study | Tumor | Setting | Protocol | N | Arms | Control Arm | Admin. Route | Donor (N) | Preparation Method | N FMT | Primary Endpoint | mFU | mPFS (mo) | mOS (mo) | ORR (%) | DCR (%) | DoR (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMC17-3956 [17] | Melanoma | Rescue | ICI | 10 | 1 | NA | Colonoscopy and oral capsules | Pts with CR for over a year (2) | ATB for 3 days + BCS | Colonoscopy on D0, oral capsules on D1 and D12 × 6 cycles | FMT/ICI-related AEs | 113 days | NR | NR | 30 | 30 | All FMT R had PFS > 6 mo |
| NCT03341143 [19] | Melanoma | Rescue | ICI | 15 | 1 | NA | Colonoscopy | PR > 24 mo or CR > 12 mo (7) | NR | 1 * | Whether FMT can convert NRs to Rs | 7 mo | 3 | 7 | 20 | 33 | Between 3 and 27 £ |
| NCT03772899 MIMic [22] | Melanoma | Upfront | Anti-PD-1 | 20 | 1 | NA | Oral capsules | HV (3) | BCS | 1 | Safety | 21 mo | NRe | NR (16 pts were still alive at FU) | 65 | 75 | 12 |
| NCT04951583 FMT-LUMINate [132,133] | Melanoma | Upfront | Anti-PD-1 + anti- CTLA-4 | 20 | 1 | NA | Oral capsules | HV (6) | BCS | 1 | ORR | 7 mo | NR | NR | 75 | 75 | Between 3 and 12 £ |
| NSCLC | Upfront | Anti-PD-1 | 20 | 1 | NA | Oral capsules | HV (6) | BCS | 1 | ORR | 7 mo | NR | NR | 75 | 90 | Between 3 and 9 £ | |
| NCT04264975 [129,134] | Solid cancers (GI tumors) | Rescue | Anti-PD-1 | 13 | 1 | NA | Colonoscopy | CR/PR ≥ 6 mo (6) | ATB for 5 days ° | 1 * | ORR | NR | NR | NR | 8 | 46 | NR |
| NCT04758507 TACITO [18,135] | RCC | Upfront | Anti-PD-1 + TKI | 50 | 2 (R 1:1) | Placebo (saline solution) | Colonoscopy and oral capsules | Long-term R > 12 mo to ICI (1) | NR | 3 | PFS rate at 1 year | 28 mo | 14 (vs. 9) | NRe (vs. 25) | 52 (vs. 28) | 90 (vs. 72) | NR |
6.3. Next-Generation Probiotics (NGP) and Live Biotherapeutic Products (LBPs)
6.4. Diet, Metabolites, and Prebiotics
7. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Routy, B.; Desilets, A.; Daillère, R.; Terrisse, S.; Kroemer, G.; Zitvogel, L. Microbiota-Centered Interventions: The Next Breakthrough in Immuno-Oncology? Cancer Discov. 2021, 11, 2396–2412. [Google Scholar] [CrossRef]
- Elkrief, A.; Méndez-Salazar, E.O.; Maillou, J.; Vanderbilt, C.M.; Gogia, P.; Desilets, A.; Messaoudene, M.; Kelly, D.; Ladanyi, M.; Hellmann, M.D.; et al. Antibiotics Are Associated with Worse Outcomes in Lung Cancer Patients Treated with Chemotherapy and Immunotherapy. Npj Precis. Onc. 2024, 8, 143. [Google Scholar] [CrossRef]
- Smith, M.; Dai, A.; Ghilardi, G.; Amelsberg, K.V.; Devlin, S.M.; Pajarillo, R.; Slingerland, J.B.; Beghi, S.; Herrera, P.S.; Giardina, P.; et al. Gut Microbiome Correlates of Response and Toxicity Following Anti-CD19 CAR T Cell Therapy. Nat. Med. 2022, 28, 713–723. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Saini, N.Y.; Zamir, E.; Blumenberg, V.; Schubert, M.-L.; Mor, U.; Fante, M.A.; Schmidt, S.; Hayase, E.; Hayase, T.; et al. A Non-Antibiotic-Disrupted Gut Microbiome Is Associated with Clinical Responses to CD19-CAR-T Cell Cancer Immunotherapy. Nat. Med. 2023, 29, 906–916. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The Commensal Microbiome Is Associated with Anti–PD-1 Efficacy in Metastatic Melanoma Patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The Gut Microbiota Influences Anticancer Immunosurveillance and General Health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A Defined Commensal Consortium Elicits CD8 T Cells and Anti-Cancer Immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental Factors Shaping the Gut Microbiome in a Dutch Population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Fidelle, M.; Routy, B.; Kroemer, G.; Wargo, J.A.; Segata, N.; Zitvogel, L. Gut OncoMicrobiome Signatures (GOMS) as next-Generation Biomarkers for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 583–603. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Fidelle, M.; Iebba, V.; Alla, L.; Pasolli, E.; Segata, N.; Desnoyer, A.; Pietrantonio, F.; Ferrere, G.; et al. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur. Urol. 2020, 78, 195–206. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut Microbiome Modulates Response to Anti–PD-1 Immunotherapy in Melanoma Patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal Microbiota Transplant Promotes Response in Immunotherapy-Refractory Melanoma Patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Ciccarese, C.; Porcari, S.; Buti, S.; Fornarini, G.; Primi, F.; Giudice, G.C.; Damassi, A.; Giron Berrios, J.R.; Stumbo, L.; Arduini, D.; et al. LBA77 Fecal Microbiota Transplantation (FMT) versus Placebo in Patients Receiving Pembrolizumab plus Axitinib for Metastatic Renal Cell Carcinoma: Preliminary Results of the Randomized Phase II TACITO Trial. Ann. Oncol. 2024, 35, S1264. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal Microbiota Transplant Overcomes Resistance to Anti–PD-1 Therapy in Melanoma Patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus Ipilimumab with or without Live Bacterial Supplementation in Metastatic Renal Cell Carcinoma: A Randomized Phase 1 Trial. Nat. Med. 2022, 28, 704–712. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Meza, L.A.; Lee, K.; Malhotra, J.; Alcantara, M.; Zengin, Z.B.; Dizman, N.; Govindarajan, A.; Hsu, J.; Llamas-Quitiquit, M.; et al. Effect of CBM588 in Combination with Cabozantinib plus Nivolumab for Patients (Pts) with Metastatic Renal Cell Carcinoma (mRCC): A Randomized Clinical Trial. J. Clin. Oncol. 2023, 41, LBA104. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Punčochář, M.; et al. Fecal Microbiota Transplantation plus Anti-PD-1 Immunotherapy in Advanced Melanoma: A Phase I Trial. Nat. Med. 2023, 29, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Qian, W.; Sun, X.; Jiang, S. Small-Molecule Inhibitors, Immune Checkpoint Inhibitors, and More: FDA-Approved Novel Therapeutic Drugs for Solid Tumors from 1991 to 2021. J. Hematol. Oncol. 2022, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Baltussen, J.C.; Welters, M.J.P.; Verdegaal, E.M.E.; Kapiteijn, E.; Schrader, A.M.R.; Slingerland, M.; Liefers, G.-J.; Van Der Burg, S.H.; Portielje, J.E.A.; De Glas, N.A. Predictive Biomarkers for Outcomes of Immune Checkpoint Inhibitors (ICIs) in Melanoma: A Systematic Review. Cancers 2021, 13, 6366. [Google Scholar] [CrossRef]
- Fidelle, M.; Rauber, C.; Alves Costa Silva, C.; Tian, A.-L.; Lahmar, I.; De La Varende, A.-L.M.; Zhao, L.; Thelemaque, C.; Lebhar, I.; Messaoudene, M.; et al. A Microbiota-Modulated Checkpoint Directs Immunosuppressive Intestinal T Cells into Cancers. Science 2023, 380, eabo2296. [Google Scholar] [CrossRef]
- Bonato, A.; Belluomini, L.; Vitali, G.; Almonte, A.; Menu, E.; Gattazzo, F.; Birebent, R.; Parisi, C.; Planchard, D.; Naoun, N.; et al. 156P Microbiota-Related Multiomics to Assess the Clinical Relevance of Antibiotics (ATB) in Immunotherapy (ICI). Ann. Oncol. 2024, 35, S277. [Google Scholar] [CrossRef]
- Messaoudene, M.; Ferreira, S.; Saint-Lu, N.; Ponce, M.; Truntzer, C.; Boidot, R.; Le Bescop, C.; Loppinet, T.; Corbel, T.; Féger, C.; et al. The DAV132 Colon-Targeted Adsorbent Does Not Interfere with Plasma Concentrations of Antibiotics but Prevents Antibiotic-Related Dysbiosis: A Randomized Phase I Trial in Healthy Volunteers. Nat. Commun. 2024, 15, 8083. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia Muciniphila Predicts Clinical Response to PD-1 Blockade in Patients with Advanced Non-Small-Cell Lung Cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef]
- de La Varende, A.L.M.; Tian, A.L.; Sehl, C.; Lahmar, I.; Ly, P.; Durand, S.; Kroemer, G.; Schoonjans, K.; Zitvogel, L.; Fidelle, M. 562 Bile acids circumvent gut dysbiosis-induced resistance to anti-PD-1. J. Immunother. Cancer 2024, 12, A642. [Google Scholar] [CrossRef]
- Gleeson, P.J.; Benech, N.; Chemouny, J.; Metallinou, E.; Berthelot, L.; da Silva, J.; Bex-Coudrat, J.; Boedec, E.; Canesi, F.; Bounaix, C.; et al. The Gut Microbiota Posttranslationally Modifies IgA1 in Autoimmune Glomerulonephritis. Sci. Transl. Med. 2024, 16, eadl6149. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, A.; Daillère, R.; Sassi, M.; Sixt, B.S.; Liu, P.; Loos, F.; Richard, C.; Rabu, C.; Alou, M.T.; Goubet, A.-G.; et al. Cross-Reactivity between Tumor MHC Class I–Restricted Antigens and an Enterococcal Bacteriophage. Science 2020, 369, 936–942. [Google Scholar] [CrossRef]
- Zitvogel, L.; Kroemer, G. Cross-Reactivity between Microbial and Tumor Antigens. Curr. Opin. Immunol. 2022, 75, 102171. [Google Scholar] [CrossRef]
- Naghavian, R.; Faigle, W.; Oldrati, P.; Wang, J.; Toussaint, N.C.; Qiu, Y.; Medici, G.; Wacker, M.; Freudenmann, L.K.; Bonté, P.-E.; et al. Microbial Peptides Activate Tumour-Infiltrating Lymphocytes in Glioblastoma. Nature 2023, 617, 807–817. [Google Scholar] [CrossRef]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the Gut and Tumor Microbiota in Cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Stewart, L.; Edgar, J.D.M.; Blakely, G.; Patrick, S. Antigenic Mimicry of Ubiquitin by the Gut Bacterium Bacteroides Fragilis: A Potential Link with Autoimmune Disease. Clin. Exp. Immunol. 2018, 194, 153–165. [Google Scholar] [CrossRef]
- Hofer, S.J.; Simon, A.K.; Bergmann, M.; Eisenberg, T.; Kroemer, G.; Madeo, F. Mechanisms of Spermidine-Induced Autophagy and Geroprotection. Nat. Aging 2022, 2, 1112–1129. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-Derived Inosine Modulates Response to Checkpoint Inhibitor Immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Elmarsafawi, A.G.; Hesterberg, R.S.; Fernandez, M.R.; Yang, C.; Darville, L.N.; Liu, M.; Koomen, J.M.; Phanstiel, O.; Atkins, R.; Mullinax, J.E.; et al. Modulating the Polyamine/Hypusine Axis Controls Generation of CD8+ Tissue-Resident Memory T Cells. JCI Insight 2023, 8, e169308. [Google Scholar] [CrossRef] [PubMed]
- Al-Habsi, M.; Chamoto, K.; Matsumoto, K.; Nomura, N.; Zhang, B.; Sugiura, Y.; Sonomura, K.; Maharani, A.; Nakajima, Y.; Wu, Y.; et al. Spermidine Activates Mitochondrial Trifunctional Protein and Improves Antitumor Immunity in Mice. Science 2022, 378, eabj3510. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.; Sedej, S.; Kroemer, G. NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation 2021, 144, 1795–1817. [Google Scholar] [CrossRef] [PubMed]
- St Paul, M.; Saibil, S.D.; Han, S.; Israni-Winger, K.; Lien, S.C.; Laister, R.C.; Sayad, A.; Penny, S.; Amaria, R.N.; Haydu, L.E.; et al. Coenzyme A Fuels T Cell Anti-Tumor Immunity. Cell Metab. 2021, 33, 2415–2427.e6. [Google Scholar] [CrossRef]
- Kovatcheva, M.; Melendez, E.; Chondronasiou, D.; Pietrocola, F.; Bernad, R.; Caballe, A.; Junza, A.; Capellades, J.; Holguín-Horcajo, A.; Prats, N.; et al. Vitamin B12 Is a Limiting Factor for Induced Cellular Plasticity and Tissue Repair. Nat. Metab. 2023, 5, 1911–1930. [Google Scholar] [CrossRef]
- Gamrath, L.; Pedersen, T.B.; Møller, M.V.; Volmer, L.M.; Holst-Christensen, L.; Vestermark, L.W.; Donskov, F. Role of the Microbiome and Diet for Response to Cancer Checkpoint Immunotherapy: A Narrative Review of Clinical Trials. Curr. Oncol. Rep. 2025, 27, 45–58. [Google Scholar] [CrossRef]
- Almonte, A.A.; Rangarajan, H.; Yip, D.; Fahrer, A.M. How Does the Gut Microbiome Influence Immune Checkpoint Blockade Therapy? Immunol. Cell Biol. 2021, 99, 361–372. [Google Scholar] [CrossRef]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; De Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8+ T Cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- Roberti, M.P.; Yonekura, S.; Duong, C.P.M.; Picard, M.; Ferrere, G.; Tidjani Alou, M.; Rauber, C.; Iebba, V.; Lehmann, C.H.K.; Amon, L.; et al. Chemotherapy-Induced Ileal Crypt Apoptosis and the Ileal Microbiome Shape Immunosurveillance and Prognosis of Proximal Colon Cancer. Nat. Med. 2020, 26, 919–931. [Google Scholar] [CrossRef]
- Fidelle, M.; Yonekura, S.; Picard, M.; Cogdill, A.; Hollebecque, A.; Roberti, M.P.; Zitvogel, L. Resolving the Paradox of Colon Cancer Through the Integration of Genetics, Immunology, and the Microbiota. Front. Immunol. 2020, 11, 600886. [Google Scholar] [CrossRef]
- Roberti, M.P.; Rauber, C.; Kroemer, G.; Zitvogel, L. Impact of the Ileal Microbiota on Colon Cancer. Semin. Cancer Biol. 2022, 86, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Yonekura, S.; Slowicka, K.; Petta, I.; Rauber, C.; Routy, B.; Richard, C.; Iebba, V.; Tidjani Alou, M.; Becharef, S.; et al. Ileal Immune Tonus Is a Prognosis Marker of Proximal Colon Cancer in Mice and Patients. Cell Death Differ. 2021, 28, 1532–1547. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, S.; Terrisse, S.; Alves Costa Silva, C.; Lafarge, A.; Iebba, V.; Ferrere, G.; Goubet, A.-G.; Fahrner, J.-E.; Lahmar, I.; Ueda, K.; et al. Cancer Induces a Stress Ileopathy Depending on β-Adrenergic Receptors and Promoting Dysbiosis That Contributes to Carcinogenesis. Cancer Discov. 2022, 12, 1128–1151. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Verschoor, Y.L.; Tan, P.B.; Balduzzi, S.; Van Lent, A.U.; Grootscholten, C.; Dokter, S.; Büller, N.V.; Grotenhuis, B.A.; Kuhlmann, K.; et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair–Deficient Colon Cancer. N. Engl. J. Med. 2024, 390, 1949–1958. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van Den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in MMR-Proficient and MMR-Deficient Early-Stage Colon Cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Roberti, M.P.; Picard, M.; Yonekura, S.; Zitvogel, L. Turning Tolerogenic into Immunogenic Ileal Cell Death through Ileal Microbiota: The Key to Unlock the Mystery of Colon Cancer Immunoscore? Oncoimmunology 2020, 9, 1778834. [Google Scholar] [CrossRef]
- Battaglia, T.W.; Mimpen, I.L.; Traets, J.J.H.; van Hoeck, A.; Zeverijn, L.J.; Geurts, B.S.; de Wit, G.F.; Noë, M.; Hofland, I.; Vos, J.L.; et al. A Pan-Cancer Analysis of the Microbiome in Metastatic Cancer. Cell 2024, 187, 2324–2335.e19. [Google Scholar] [CrossRef]
- Ghaddar, B.; Biswas, A.; Harris, C.; Omary, M.B.; Carpizo, D.R.; Blaser, M.J.; De, S. Tumor Microbiome Links Cellular Programs and Immunity in Pancreatic Cancer. Cancer Cell 2022, 40, 1240–1253.e5. [Google Scholar] [CrossRef]
- Kalaora, S.; Nagler, A.; Nejman, D.; Alon, M.; Barbolin, C.; Barnea, E.; Ketelaars, S.L.C.; Cheng, K.; Vervier, K.; Shental, N.; et al. Identification of Bacteria-Derived HLA-Bound Peptides in Melanoma. Nature 2021, 592, 138–143. [Google Scholar] [CrossRef]
- Narunsky-Haziza, L.; Sepich-Poore, G.D.; Livyatan, I.; Asraf, O.; Martino, C.; Nejman, D.; Gavert, N.; Stajich, J.E.; Amit, G.; González, A.; et al. Pan-Cancer Analyses Reveal Cancer-Type-Specific Fungal Ecologies and Bacteriome Interactions. Cell 2022, 185, 3789–3806.e17. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type-Specific Intracellular Bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef] [PubMed]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37.e6. [Google Scholar] [CrossRef]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal Microbiota Enhances Pancreatic Carcinogenesis in Preclinical Models. Carcinogenesis 2018, 39, 1068–1078. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, H.; Tan, X.; Shi, C.; Ma, Y.; Meng, D.; Zhou, M.; Lv, Z.; Wang, S.; Jin, Y. Intratumoural Microbiota: A New Frontier in Cancer Development and Therapy. Sig. Transduct. Target. Ther. 2024, 9, 15. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral Microbiota: Roles in Cancer Initiation, Development and Therapeutic Efficacy. Sig. Transduct. Target. Ther. 2023, 8, 35. [Google Scholar] [CrossRef]
- Choi, Y.; Lichterman, J.N.; Coughlin, L.A.; Poulides, N.; Li, W.; Del Valle, P.; Palmer, S.N.; Gan, S.; Kim, J.; Zhan, X.; et al. Immune Checkpoint Blockade Induces Gut Microbiota Translocation That Augments Extraintestinal Antitumor Immunity. Sci. Immunol. 2023, 8, eabo2003. [Google Scholar] [CrossRef]
- Goubet, A.-G. Could the Tumor-Associated Microbiota Be the New Multi-Faceted Player in the Tumor Microenvironment? Front. Oncol. 2023, 13, 1185163. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Jiang, S.-S.; Xie, Y.-L.; Xiao, X.-Y.; Kang, Z.-R.; Lin, X.-L.; Zhang, L.; Li, C.-S.; Qian, Y.; Xu, P.-P.; Leng, X.-X.; et al. Fusobacterium Nucleatum-Derived Succinic Acid Induces Tumor Resistance to Immunotherapy in Colorectal Cancer. Cell Host Microbe 2023, 31, 781–797.e9. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Míguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A Distinct Fusobacterium Nucleatum Clade Dominates the Colorectal Cancer Niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Cheng, Z.; Yin, Z.; Xu, J.; Wu, F.; Jin, Y.; Yang, G. Airway Fusobacterium Is Associated with Poor Response to Immunotherapy in Lung Cancer. Onco Targets Ther. 2022, 15, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, M.; Pu, F.; Ren, J.; Qu, X. Transforming Intratumor Bacteria into Immunopotentiators to Reverse Cold Tumors for Enhanced Immuno-Chemodynamic Therapy of Triple-Negative Breast Cancer. J. Am. Chem. Soc. 2023, 145, 26296–26307. [Google Scholar] [CrossRef]
- Chagneau, C.V.; Payros, D.; Tang-Fichaux, M.; Auvray, F.; Nougayrède, J.-P.; Oswald, E. The Pks Island: A Bacterial Swiss Army Knife? Colibactin: Beyond DNA Damage and Cancer. Trends Microbiol. 2022, 30, 1146–1159. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; Van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational Signature in Colorectal Cancer Caused by Genotoxic Pks+ E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef]
- Zitvogel, L.; Fidelle, M.; Kroemer, G. Long-Distance Microbial Mechanisms Impacting Cancer Immunosurveillance. Immunity 2024, 57, 2013–2029. [Google Scholar] [CrossRef]
- Wang, M.; Rousseau, B.; Qiu, K.; Huang, G.; Zhang, Y.; Su, H.; Le Bihan-Benjamin, C.; Khati, I.; Artz, O.; Foote, M.B.; et al. Killing Tumor-Associated Bacteria with a Liposomal Antibiotic Generates Neoantigens That Induce Anti-Tumor Immune Responses. Nat. Biotechnol. 2023, 42, 1263–1274. [Google Scholar] [CrossRef]
- Goubet, A.-G.; Lordello, L.; Alves Costa Silva, C.; Peguillet, I.; Gazzano, M.; Mbogning-Fonkou, M.D.; Thelemaque, C.; Lebacle, C.; Thibault, C.; Audenet, F.; et al. Escherichia Coli-Specific CXCL13-Producing TFH Are Associated with Clinical Efficacy of Neoadjuvant PD-1 Blockade against Muscle-Invasive Bladder Cancer. Cancer Discov. 2022, 12, 2280–2307. [Google Scholar] [CrossRef]
- Elkrief, A.; Montesion, M.; Sivakumar, S.; Hale, C.; Bowman, A.S.; Begüm Bektaş, A.; Bradic, M.; Kang, W.; Chan, E.; Gogia, P.; et al. Intratumoral Escherichia Is Associated with Improved Survival to Single-Agent Immune Checkpoint Inhibition in Patients With Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 3339–3349. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Cisalpino, D.; Varadarajan, S.; Hellman, J.; Warren, H.S.; Cascalho, M.; Inohara, N.; Núñez, G. Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens. Immunity 2016, 44, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; Birebent, R.; Lebhar, I.; Xiberras, M.; Marques, M.; Reni, A.; Flament, C.; Bonato, A.; Belluomini, L.; Chanfreau-Paris, H.; et al. Impact of Microbiota Specific Circulating Memory T Cells in Response to Immunotherapy. J. Clin. Oncol. 2024, 42, 2572. [Google Scholar] [CrossRef]
- Belluomini, L.; Bonato, A.; Almonte, A.; Gattazzo, F.; Lebhar, I.; Birebent, R.; Flament, C.; Xiberras, M.; Marques, M.; Ly, P.; et al. 1172O Akkermansia Muciniphila-Based Multi-Omic Profiling in Advanced Non-Small Cell Lung Cancer. Ann. Oncol. 2024, 35, S762. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer–Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Swanton, C.; Bernard, E.; Abbosh, C.; André, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing Cancer Complexity: Hallmarks of Systemic Disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef]
- Terrisse, S.; Goubet, A.-G.; Ueda, K.; Thomas, A.M.; Quiniou, V.; Thelemaque, C.; Dunsmore, G.; Clave, E.; Gamat-Huber, M.; Yonekura, S.; et al. Immune System and Intestinal Microbiota Determine Efficacy of Androgen Deprivation Therapy against Prostate Cancer. J. Immunother. Cancer 2022, 10, e004191. [Google Scholar] [CrossRef]
- Derosa, L.; Iebba, V.; Silva, C.A.C.; Piccinno, G.; Wu, G.; Lordello, L.; Routy, B.; Zhao, N.; Thelemaque, C.; Birebent, R.; et al. Custom Scoring Based on Ecological Topology of Gut Microbiota Associated with Cancer Immunotherapy Outcome. Cell 2024, 187, 3373–3389.e16. [Google Scholar] [CrossRef]
- Terrisse, S.; Derosa, L.; Iebba, V.; Ghiringhelli, F.; Vaz-Luis, I.; Kroemer, G.; Fidelle, M.; Christodoulidis, S.; Segata, N.; Thomas, A.M.; et al. Intestinal Microbiota Influences Clinical Outcome and Side Effects of Early Breast Cancer Treatment. Cell Death Differ. 2021, 28, 2778–2796. [Google Scholar] [CrossRef]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.-S.; Derosa, L.; Khan, M.A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C.; et al. Gut Microbiota Signatures Are Associated with Toxicity to Combined CTLA-4 and PD-1 Blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- Alves Costa Silva, C.; Piccinno, G.; Suissa, D.; Bourgin, M.; Schreibelt, G.; Durand, S.; Birebent, R.; Fidelle, M.; Sow, C.; Aprahamian, F.; et al. Influence of Microbiota-Associated Metabolic Reprogramming on Clinical Outcome in Patients with Melanoma from the Randomized Adjuvant Dendritic Cell-Based MIND-DC Trial. Nat. Commun. 2024, 15, 1633. [Google Scholar] [CrossRef] [PubMed]
- Bol, K.F.; Schreibelt, G.; Bloemendal, M.; Van Willigen, W.W.; Hins-de Bree, S.; De Goede, A.L.; De Boer, A.J.; Bos, K.J.H.; Duiveman-de Boer, T.; Olde Nordkamp, M.A.M.; et al. Adjuvant Dendritic Cell Therapy in Stage IIIB/C Melanoma: The MIND-DC Randomized Phase III Trial. Nat. Commun. 2024, 15, 1632. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Hsu, J.; Bergerot, P.G.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; Pal, S.K. Randomized Trial Assessing Impact of Probiotic Supplementation on Gut Microbiome and Clinical Outcome from Targeted Therapy in Metastatic Renal Cell Carcinoma. Cancer Med. 2021, 10, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bredon, M.; Danne, C.; Pham, H.P.; Ruffié, P.; Bessede, A.; Rolhion, N.; Creusot, L.; Brot, L.; Alonso, I.; Langella, P.; et al. Faecalibacterium Prausnitzii Strain EXL01 Boosts Efficacy of Immune Checkpoint Inhibitors. OncoImmunology 2024, 13, 2374954. [Google Scholar] [CrossRef]
- Martín, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermúdez-Humarán, L.G.; Sokol, H.; Chatel, J.-M.; Langella, P. Faecalibacterium: A Bacterial Genus with Promising Human Health Applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; Ghensi, P.; et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019, 176, 649–662.e20. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kim, M.; Bakshi, U.; Cunningham, K.Y.; Davis, J.M.; Lazaridis, K.N.; Nelson, H.; Chia, N.; Sung, J. A Predictive Index for Health Status Using Species-Level Gut Microbiome Profiling. Nat. Commun. 2020, 11, 4635. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, N.; Zhang, C.; Lam, Y.Y.; Zhao, L. Guild-Based Analysis for Understanding Gut Microbiome in Human Health and Diseases. Genome Med. 2021, 13, 22. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, G.; Zhao, N. Guild-Based Approach for Mitigating Information Loss and Distortion Issues in Microbiome Analysis. J. Clin. Investig. 2024, 134, e185395. [Google Scholar] [CrossRef]
- Iebba, V.; Albiges, L.; Alla, L.; Colomba, E.; Silva, C.A.C.; Pons, N.; Baciarello, G.; Le Chatelier, E.; Fizazi, K.; Routy, B.; et al. Prior Tyrosine Kinase Inhibitors (TKI) and Antibiotics (ATB) Use Are Associated with Distinct Gut Microbiota “guilds” in Renal Cell Carcinoma (RCC). Ann. Oncol. 2019, 30 (Suppl. S5), v356–v402. [Google Scholar] [CrossRef]
- Cascetta, P.; Reni, A.; Facchinetti, F.; Meyer, M.-L.; Riudavetz, M.; Aldea, M.; Dall’Olio, F.G.; Marinello, A.; Tagliamento, M.; Remon Masip, J.; et al. Discontinuation of Immunotherapy over 2 Years for Patients with Non–Small Cell Lung Cancer (NSCLC): A Search for Predictors. J. Clin. Oncol. 2023, 41, 2661. [Google Scholar] [CrossRef]
- Thomas, A.M.; Manghi, P.; Asnicar, F.; Pasolli, E.; Armanini, F.; Zolfo, M.; Beghini, F.; Manara, S.; Karcher, N.; Pozzi, C.; et al. Metagenomic Analysis of Colorectal Cancer Datasets Identifies Cross-Cohort Microbial Diagnostic Signatures and a Link with Choline Degradation. Nat. Med. 2019, 25, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, G. Cancer Microbiome Characterization: Tumor Stage, Location, Genomic Instability and Patient Outcome in CRC and Melanoma 2024. Ph.D. Thesis, Università degli studi di Trento, Trento, Italy, 2024. [Google Scholar]
- Naccarati, A.; Dragomir, M.P.; Tarallo, S.; Gagliardi, A.; Alberini, V.; Buchler, T.; Liska, V.; Gallo, G.; Vymetalkova, V.; Vodickova, L.; et al. Fecal miRNA Profiles in Colorectal Cancers with Mucinous Morphology. Mutagenesis 2024, 40, geae015. [Google Scholar] [CrossRef]
- Pardini, B.; Ferrero, G.; Tarallo, S.; Gallo, G.; Francavilla, A.; Licheri, N.; Trompetto, M.; Clerico, G.; Senore, C.; Peyre, S.; et al. A Fecal MicroRNA Signature by Small RNA Sequencing Accurately Distinguishes Colorectal Cancers: Results From a Multicenter Study. Gastroenterology 2023, 165, 582–599.e8. [Google Scholar] [CrossRef]
- Illescas, O.; Ferrero, G.; Belfiore, A.; Pardini, B.; Tarallo, S.; Ciniselli, C.M.; Noci, S.; Daveri, E.; Signoroni, S.; Cattaneo, L.; et al. Modulation of Faecal miRNAs Highlights the Preventive Effects of a Mediterranean Low-Inflammatory Dietary Intervention. Clin. Nutr. 2024, 43, 951–959. [Google Scholar] [CrossRef]
- Tarallo, S.; Ferrero, G.; Gallo, G.; Francavilla, A.; Clerico, G.; Realis Luc, A.; Manghi, P.; Thomas, A.M.; Vineis, P.; Segata, N.; et al. Correction for Tarallo et al., “Altered Fecal Small RNA Profiles in Colorectal Cancer Reflect Gut Microbiome Composition in Stool Samples”. mSystems 2020, 5, e00072-20. [Google Scholar] [CrossRef]
- Tarallo, S.; Ferrero, G.; Gallo, G.; Francavilla, A.; Clerico, G.; Realis Luc, A.; Manghi, P.; Thomas, A.M.; Vineis, P.; Segata, N.; et al. Altered Fecal Small RNA Profiles in Colorectal Cancer Reflect Gut Microbiome Composition in Stool Samples. mSystems 2019, 4, e00289-19. [Google Scholar] [CrossRef]
- Silva, C.A.C.; Fidelle, M.; Birebent, R.; Dalban, C.; Zoppi, S.; Reni, A.; Rauber, C.; Lahmar, I.; Mallard De La Varende, A.-L.; Rioux-Leclercq, N.; et al. Serum Soluble MAdCAM-1: A New Biomarker for Cancer Immunotherapy. J. Clin. Oncol. 2023, 41, 4548. [Google Scholar] [CrossRef]
- Goubet, A.-G.; Rouanne, M.; Derosa, L.; Kroemer, G.; Zitvogel, L. From Mucosal Infection to Successful Cancer Immunotherapy. Nat. Rev. Urol. 2023, 20, 682–700. [Google Scholar] [CrossRef]
- Thomas, S.; Thélémaque, C.; Forsberg, S.; Rucevic, M.; Lebba, V.; Durand, S.; Derosa, L.; Fidelle, M.; Zitvogel, L. 1273 Fecal microbiota transplantation impact in response to immunotherapy assessed by multi-omics. J. Immunother. Cancer 2024, 12, A1427. [Google Scholar] [CrossRef]
- Joachim, L.; Göttert, S.; Sax, A.; Steiger, K.; Neuhaus, K.; Heinrich, P.; Fan, K.; Orberg, E.T.; Kleigrewe, K.; Ruland, J.; et al. The Microbial Metabolite Desaminotyrosine Enhances T-Cell Priming and Cancer Immunotherapy with Immune Checkpoint Inhibitors. eBioMedicine 2023, 97, 104834. [Google Scholar] [CrossRef]
- Saldanha, E.F.; Lau, S.C.M.; Laister, R.C.; Wang, B.X.; Penny, S.; Pinto, D.; Sacher, A.G.; Saibil, S. Circulating Metabolic Profiling as a Biomarker for Immune Checkpoint Blockade Efficacy. J. Clin. Oncol. 2024, 42, 2564. [Google Scholar] [CrossRef]
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The Microbial Metabolite Desaminotyrosine Protects from Influenza through Type I Interferon. Science 2017, 357, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Thiele Orberg, E.; Göttert, S.; Hiergeist, A.; Meedt, E.; Kleigrewe, K.; Herr, W.; Bassermann, F.; Gessner, A.; Holler, E.; Heidegger, S.; et al. Microbial-Derived Metabolites Induce Epithelial Recovery via the Sting Pathway in Mice and Men and Protect from Graft-Versus-Host Disease. Blood 2021, 138, 87. [Google Scholar] [CrossRef]
- Thiele Orberg, E.; Meedt, E.; Hiergeist, A.; Xue, J.; Heinrich, P.; Ru, J.; Ghimire, S.; Miltiadous, O.; Lindner, S.; Tiefgraber, M.; et al. Bacteria and Bacteriophage Consortia Are Associated with Protective Intestinal Metabolites in Patients Receiving Stem Cell Transplantation. Nat. Cancer 2024, 5, 187–208. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative Association of Antibiotics on Clinical Activity of Immune Checkpoint Inhibitors in Patients with Advanced Renal Cell and Non-Small-Cell Lung Cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Elkrief, A.; Derosa, L.; Kroemer, G.; Zitvogel, L.; Routy, B. The Negative Impact of Antibiotics on Outcomes in Cancer Patients Treated with Immunotherapy: A New Independent Prognostic Factor? Ann. Oncol. 2019, 30, 1572–1579. [Google Scholar] [CrossRef]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment with Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients with Cancer. JAMA Oncol. 2019, 5, 1774. [Google Scholar] [CrossRef]
- Cantarelli, L.; Gutiérrez Nicolás, F.; García Gil, S.; Morales Barrios, J.A.; Oramas Rodriguez, J.; Nazco Casariego, G.J. Effect of Concomitant Use of Proton Pump Inhibitors on Immunotherapy Clinical Response in Advanced Cancer Patients: Real-Life Setting. J. Immunother. 2023, 47, 21–31. [Google Scholar] [CrossRef]
- Rousseau, A.; Foulon, S.; Simon-Tillaux, N.; Michiels, S.; Lolivier, A.; Bonastre, J.; Planchard, D.; Barlesi, F.; Remon Masip, J.; Lavaud, P.; et al. 1319P Impact of Concomitant Co-Medications on Survival with First-Line Pembrolizumab in 43,000 French Patients with Advanced NSCLC. Ann. Oncol. 2024, 35, S839–S840. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Tan, Q.; Jiang, G.; Jia, J. Antibiotic Use Is a Negative Predictor of the Efficacy and Toxicity of Epidermal Growth Factor Receptor-targeted Therapy in Advanced Non-small Cell Lung Cancer. Oncol. Lett. 2019, 18, 2677–2683. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, N.; Zhou, C.; Nahm, S.; Rack, S.; Tan, G.C.L.; Lorigan, P.; Blackhall, F.; Cook, N. Antibiotic Use Reduces Efficacy of Tyrosine Kinase Inhibitors in Patients with Advanced Melanoma and Non-Small-Cell Lung Cancer. ESMO Open 2022, 7, 100430. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Koh, J.-Y.; Shin, S.-J.; Shin, J.-H.; Hong, M.; Chung, H.C.; Rha, S.Y.; Kim, H.S.; Lee, C.-K.; Lee, J.H.; et al. Prior Antibiotic Administration Disrupts Anti-PD-1 Responses in Advanced Gastric Cancer by Altering the Gut Microbiome and Systemic Immune Response. CR Med. 2023, 4, 101251. [Google Scholar] [CrossRef]
- Løfling, L.L.; Støer, N.C.; Sloan, E.K.; Chang, A.; Gandini, S.; Ursin, G.; Botteri, E. β-Blockers and Breast Cancer Survival by Molecular Subtypes: A Population-Based Cohort Study and Meta-Analysis. Br. J. Cancer 2022, 127, 1086–1096. [Google Scholar] [CrossRef]
- Caparica, R.; Bruzzone, M.; Agostinetto, E.; De Angelis, C.; Fêde, Â.; Ceppi, M.; De Azambuja, E. Beta-Blockers in Early-Stage Breast Cancer: A Systematic Review and Meta-Analysis. ESMO Open 2021, 6, 100066. [Google Scholar] [CrossRef]
- Alves Costa Silva, C.; Derosa, L.; Dalban, C.; Colomba, E.; Negrier, S.; Chevreau, C.M.; Gravis, G.; Oudard, S.M.; Laguerre, B.; Barthelemy, P.; et al. 697P Impact of β-Blockers (BB) on Outcomes of Metastatic Renal Cell Carcinoma (mRCC) Patients Treated with Nivolumab (N). Ann. Oncol. 2021, 32, S710. [Google Scholar] [CrossRef]
- Parker, W.P.; Lohse, C.M.; Zaid, H.B.; Cheville, J.C.; Boorjian, S.A.; Leibovich, B.C.; Thompson, R.H. Evaluation of Beta-Blockers and Survival among Hypertensive Patients with Renal Cell Carcinoma. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 36.e1–36.e6. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, G.; Kim, Y.; Cho, B.; Kim, S.-Y.; Do, E.-J.; Bae, D.-J.; Kweon, M.-N.; Song, J.S.; Park, H. Fecal Microbiota Transplantation Combined with Anti-PD-1 Inhibitor for Unresectable or Metastatic Solid Cancers Refractory to Anti-PD-1 Inhibitor. J. Clin. Oncol. 2023, 41, 105. [Google Scholar] [CrossRef]
- Carlson, P.E. Regulatory Considerations for Fecal Microbiota Transplantation Products. Cell Host Microbe 2020, 27, 173–175. [Google Scholar] [CrossRef]
- Rodriguez, J.; Cordaillat-Simmons, M.; Badalato, N.; Berger, B.; Breton, H.; De Lahondès, R.; Deschasaux-Tanguy, M.; Desvignes, C.; D’Humières, C.; Kampshoff, S.; et al. Microbiome Testing in Europe: Navigating Analytical, Ethical and Regulatory Challenges. Microbiome 2024, 12, 258. [Google Scholar] [CrossRef]
- Duttagupta, S.; Messaoudene, M.; Jamal, R.; Mihalcioiu, C.; Belanger, K.; Lenehan, J.; Belkaid, W.; Parvathy, S.N.; Maillou, J.; Hu, Y.; et al. Abstract CT258: Phase II Trial of Fecal Microbiota Transplantation in Combination with Ipilimumab and Nivolumab in Patients with Advanced Cutaneous Melanoma (FMT-LUMINate Trial). Cancer Res. 2024, 84, CT258. [Google Scholar] [CrossRef]
- Elkrief, A.; Duttagupta, S.; Jamal, R.; Marcoux, N.; Desilets, A.; Messaoudene, M.; Mihalcioiu, C.; Durand, S.; Tehfe, M.; Blais, N.; et al. 1068P Phase II Trial of Fecal Microbiota Transplantation (FMT) plus Immune Checkpoint Inhibition (ICI) in Advanced Non-Small Cell Lung Cancer and Cutaneous Melanoma (FMT-LUMINate). Ann. Oncol. 2024, 35, S707–S708. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.; Kim, S.; Cho, B.; Kim, S.-Y.; Do, E.-J.; Bae, D.-J.; Kim, S.; Kweon, M.-N.; Song, J.S.; et al. Fecal Microbiota Transplantation Improves Anti-PD-1 Inhibitor Efficacy in Unresectable or Metastatic Solid Cancers Refractory to Anti-PD-1 Inhibitor. Cell Host Microbe 2024, 32, 1380–1393.e9. [Google Scholar] [CrossRef] [PubMed]
- Porcari, S.; Ciccarese, C.; Pinto, F.; Quaranta, G.; De Giorgi, S.; Rondinella, D.; Settanni, C.R.; Cortesi, E.; Roberto, M.; Primi, F.; et al. Fecal Microbiota Transplantation to Improve Efficacy of Immune Checkpoint Inhibitors in Renal Cell Carcinoma (TACITO Trial). J. Clin. Oncol. 2022, 40, TPS407. [Google Scholar] [CrossRef]
- Spreafico, A.; Heirali, A.A.; Araujo, D.V.; Tan, T.J.; Oliva, M.; Schneeberger, P.H.H.; Chen, B.; Wong, M.K.; Stayner, L.-A.; Hansen, A.R.; et al. First-in-Class Microbial Ecosystem Therapeutic 4 (MET4) in Combination with Immune Checkpoint Inhibitors in Patients with Advanced Solid Tumors (MET4-IO Trial). Ann. Oncol. 2023, 34, 520–530. [Google Scholar] [CrossRef]
- Tomita, Y.; Ikeda, T.; Sakata, S.; Saruwatari, K.; Sato, R.; Iyama, S.; Jodai, T.; Akaike, K.; Ishizuka, S.; Saeki, S.; et al. Association of Probiotic Clostridium Butyricum Therapy with Survival and Response to Immune Checkpoint Blockade in Patients with Lung Cancer. Cancer Immunol. Res. 2020, 8, 1236–1242. [Google Scholar] [CrossRef]
- Tomita, Y.; Goto, Y.; Sakata, S.; Imamura, K.; Minemura, A.; Oka, K.; Hayashi, A.; Jodai, T.; Akaike, K.; Anai, M.; et al. Clostridium Butyricum Therapy Restores the Decreased Efficacy of Immune Checkpoint Blockade in Lung Cancer Patients Receiving Proton Pump Inhibitors. Oncoimmunology 2022, 11, 2081010. [Google Scholar] [CrossRef]
- Tomita, Y.; Sakata, S.; Imamura, K.; Iyama, S.; Jodai, T.; Saruwatari, K.; Hamada, S.; Akaike, K.; Anai, M.; Fukusima, K.; et al. Association of Clostridium Butyricum Therapy Using the Live Bacterial Product CBM588 with the Survival of Patients with Lung Cancer Receiving Chemoimmunotherapy Combinations. Cancers 2023, 16, 47. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary Fiber and Probiotics Influence the Gut Microbiome and Melanoma Immunotherapy Response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Reimer, R.A. Establishing the Role of Diet in the Microbiota–Disease Axis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Farias, R.; Holly, A.E.; Levy, E.J.; Klein, L.; Rains, J.; Sirmans, E.; Burton, E.M.; Wang, J.; Amaria, R.N.; et al. The DIET Study: A Randomized Controlled Trial of a High Fiber Diet Intervention (HFDI) in Patients (Pts) with Melanoma Receiving Immune Checkpoint Blockade (ICB). J. Clin. Oncol. 2024, 42, 9558. [Google Scholar] [CrossRef]
- Jiang, Y. Abstract LB348: A Controlled High Fiber Dietary Intervention Alters Metabolome and Gut Microbiome in Melanoma Survivors. Cancer Res. 2023, 83, LB348. [Google Scholar] [CrossRef]
- Link, V.M.; Subramanian, P.; Cheung, F.; Han, K.L.; Stacy, A.; Chi, L.; Sellers, B.A.; Koroleva, G.; Courville, A.B.; Mistry, S.; et al. Differential Peripheral Immune Signatures Elicited by Vegan versus Ketogenic Diets in Humans. Nat. Med. 2024, 30, 560–572. [Google Scholar] [CrossRef]
- Ferrere, G.; Tidjani Alou, M.; Liu, P.; Goubet, A.-G.; Fidelle, M.; Kepp, O.; Durand, S.; Iebba, V.; Fluckiger, A.; Daillère, R.; et al. Ketogenic Diet and Ketone Bodies Enhance the Anticancer Effects of PD-1 Blockade. JCI Insight 2021, 6, e145207. [Google Scholar] [CrossRef]
- Richard, J.; Beauvillain, C.; Benoit, M.; Barth, M.; Aubert, C.; Rolley, C.; Bellal, S.; Bourreau, J.; Ferragu, M.; Lebdai, S.; et al. Ketogenic Diet Enhances the Anti-Cancer Effects of PD-L1 Blockade in Renal Cell Carcinoma. Front. Endocrinol. 2024, 15, 1344891. [Google Scholar] [CrossRef]
- Collins, M.D.; Gibson, G.R. Probiotics, Prebiotics, and Synbiotics: Approaches for Modulating the Microbial Ecology of the Gut. Am. J. Clin. Nutr. 1999, 69, 1052S–1057S. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Messaoudene, M.; Pidgeon, R.; Richard, C.; Ponce, M.; Diop, K.; Benlaifaoui, M.; Nolin-Lapalme, A.; Cauchois, F.; Malo, J.; Belkaid, W.; et al. A Natural Polyphenol Exerts Antitumor Activity and Circumvents Anti–PD-1 Resistance through Effects on the Gut Microbiota. Cancer Discov. 2022, 12, 1070–1087. [Google Scholar] [CrossRef]
- McCulloch, J.A.; Davar, D.; Rodrigues, R.R.; Badger, J.H.; Fang, J.R.; Cole, A.M.; Balaji, A.K.; Vetizou, M.; Prescott, S.M.; Fernandes, M.R.; et al. Intestinal Microbiota Signatures of Clinical Response and Immune-Related Adverse Events in Melanoma Patients Treated with Anti-PD-1. Nat. Med. 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Silva, C.A.C.; Fidelle, M.; Almonte, A.A.; Derosa, L.; Zitvogel, L. Gut Microbiota–Related Biomarkers in Immuno-Oncology. Annu. Rev. Pharmacol. Toxicol. 2024, 65, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Hassani, Z.; Alves Costa Silva, C.; Betsou, F.; Carraturo, F.; Fasano, A.; Israelsen, M.; Iyappan, A.; Krag, A.; Metwaly, A.; et al. State of the Art and the Future of Microbiome-Based Biomarkers: A Multidisciplinary Delphi Consensus. Lancet Microbe 2024, 6, 100948. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves Costa Silva, C.; Almonte, A.A.; Zitvogel, L. Oncobiomics: Leveraging Microbiome Translational Research in Immuno-Oncology for Clinical-Practice Changes. Biomolecules 2025, 15, 504. https://doi.org/10.3390/biom15040504
Alves Costa Silva C, Almonte AA, Zitvogel L. Oncobiomics: Leveraging Microbiome Translational Research in Immuno-Oncology for Clinical-Practice Changes. Biomolecules. 2025; 15(4):504. https://doi.org/10.3390/biom15040504
Chicago/Turabian StyleAlves Costa Silva, Carolina, Andrew A. Almonte, and Laurence Zitvogel. 2025. "Oncobiomics: Leveraging Microbiome Translational Research in Immuno-Oncology for Clinical-Practice Changes" Biomolecules 15, no. 4: 504. https://doi.org/10.3390/biom15040504
APA StyleAlves Costa Silva, C., Almonte, A. A., & Zitvogel, L. (2025). Oncobiomics: Leveraging Microbiome Translational Research in Immuno-Oncology for Clinical-Practice Changes. Biomolecules, 15(4), 504. https://doi.org/10.3390/biom15040504






