Biocontrol Mechanism of Bacillus thuringiensis GBAC46 Against Diseases and Pests Caused by Fusarium verticillioides and Spodoptera frugiperda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Phytophagous Insects, and Plant Materials
2.2. Antifungal Activity Assay
2.3. Determination of Reactive Oxygen Species
2.4. Insecticidal Activity Assay
2.5. Greenhouse Experiments
2.6. Field Experiment and Determination of Fumonisins B1
2.7. Extraction of Total RNA and Expression Analysis by RT-qPCR
2.8. Statistical Analysis
3. Results
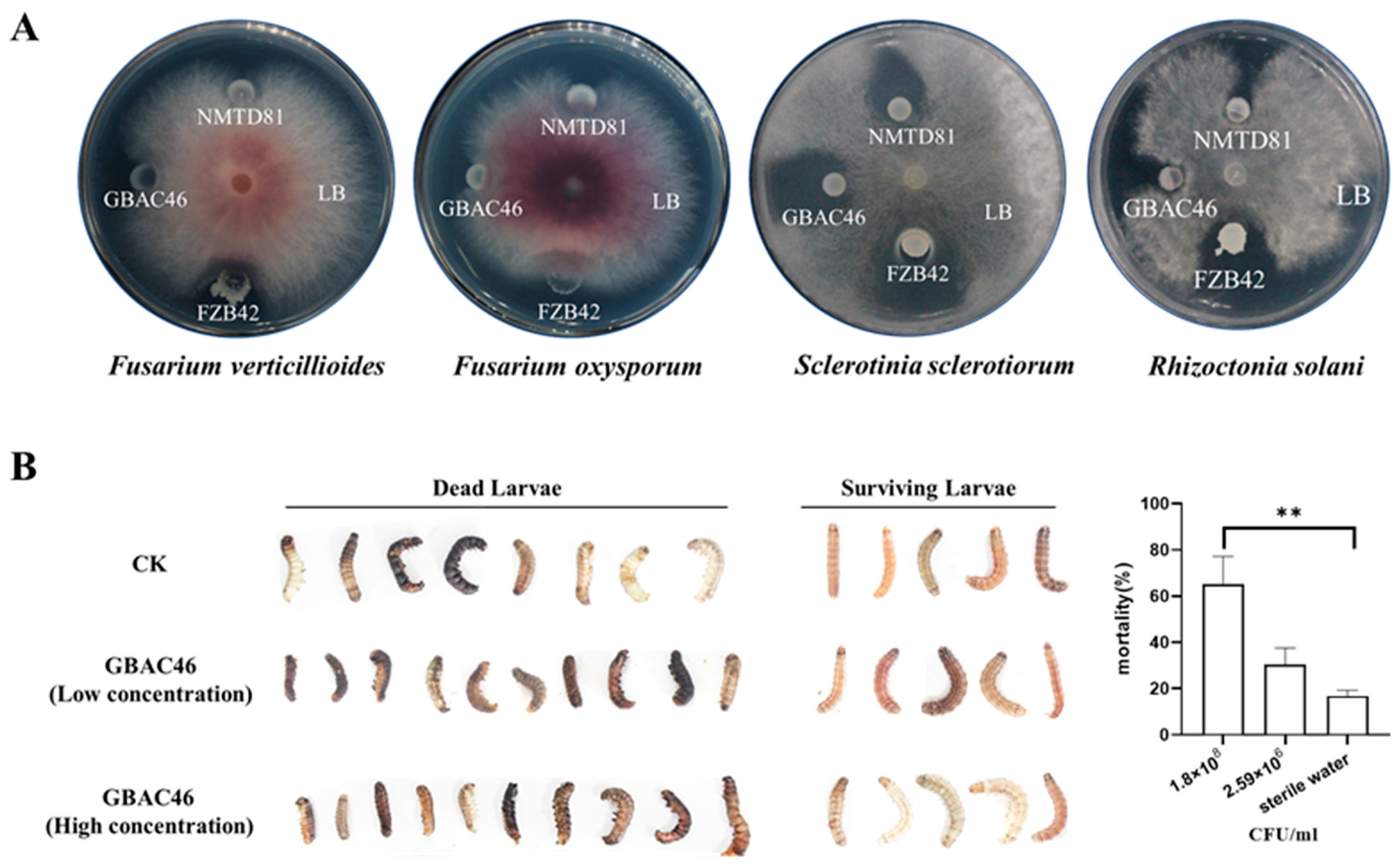
3.1. Bt Strain GBAC46 Exhibits Significant Antifungal Activity
3.2. Insecticidal Activity of Bt Strain GBAC46 Against S. frugiperda
3.3. Reactive Oxygen Species Assays
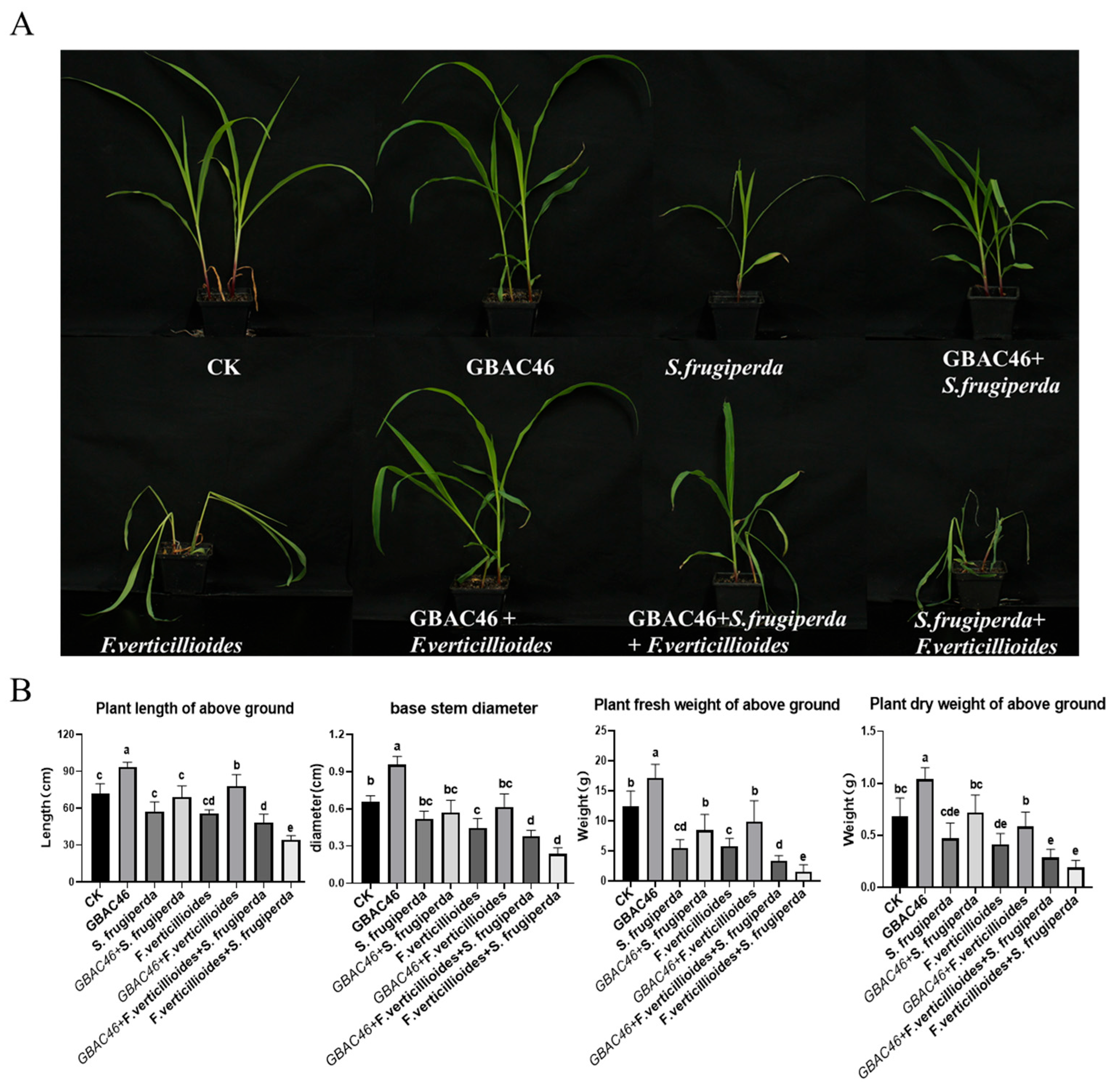
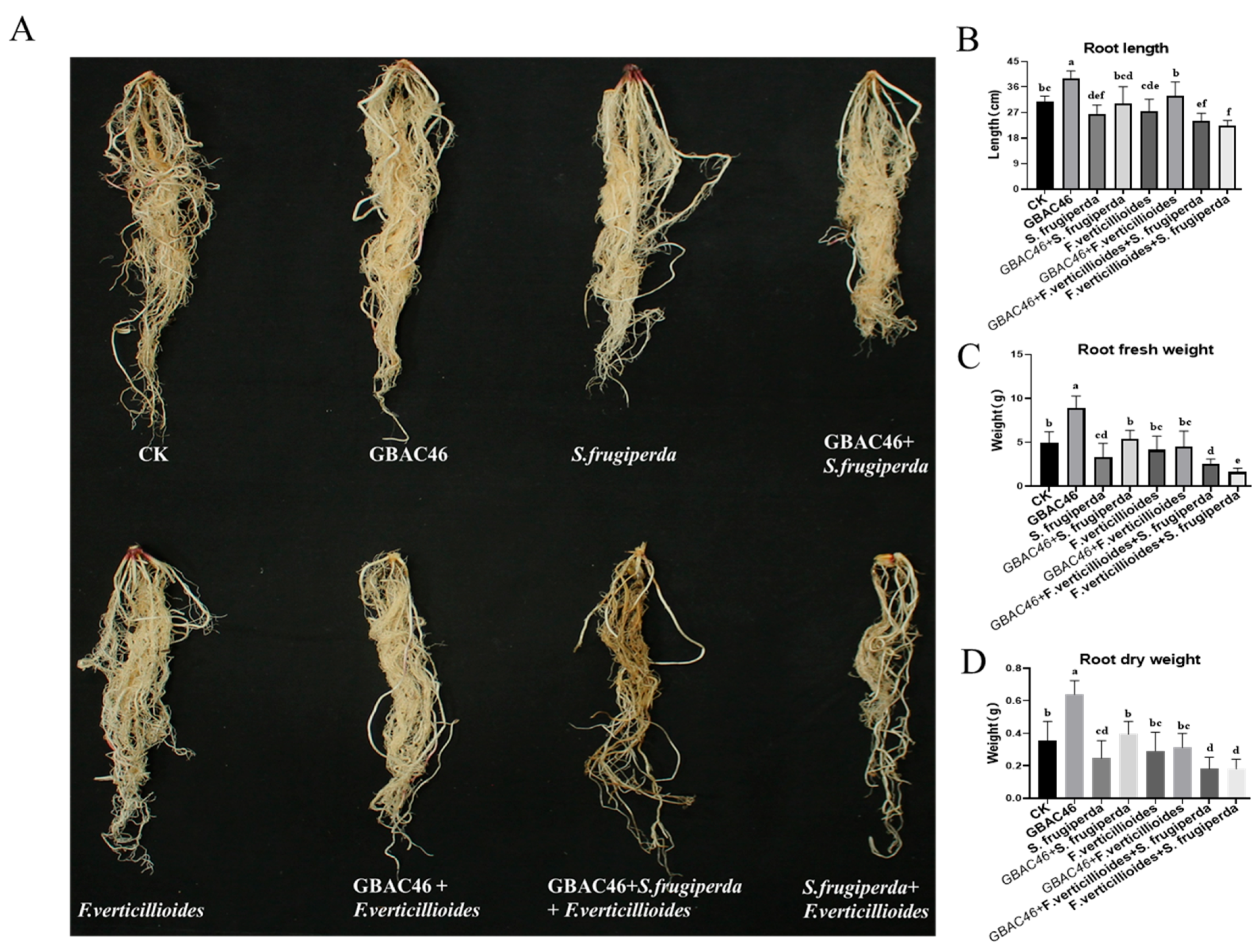
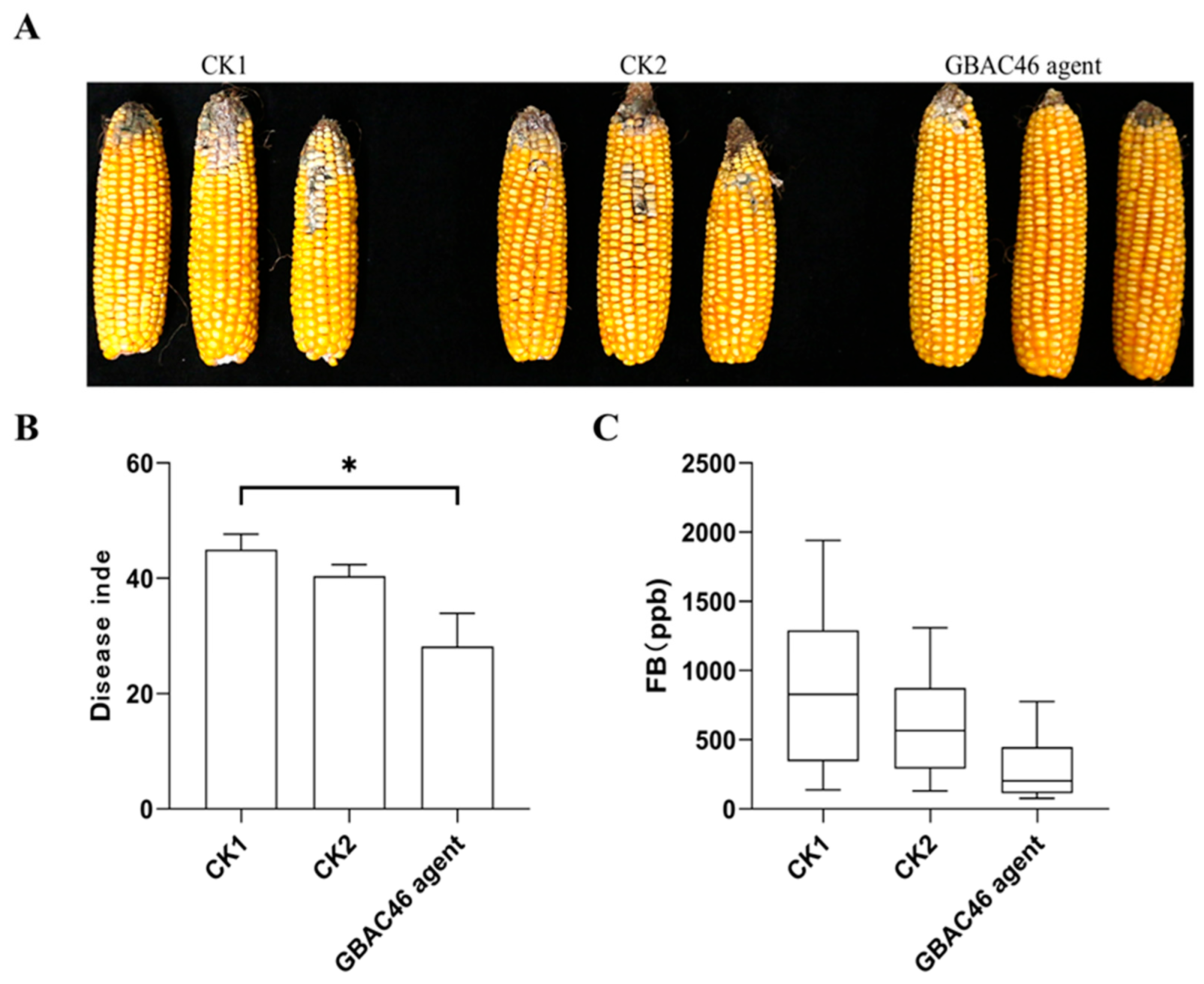
3.4. Biocontrol Activity of GBAC46 Against Co-Occurring Diseases and Pests Caused by F. verticillioides and S. frugiperda
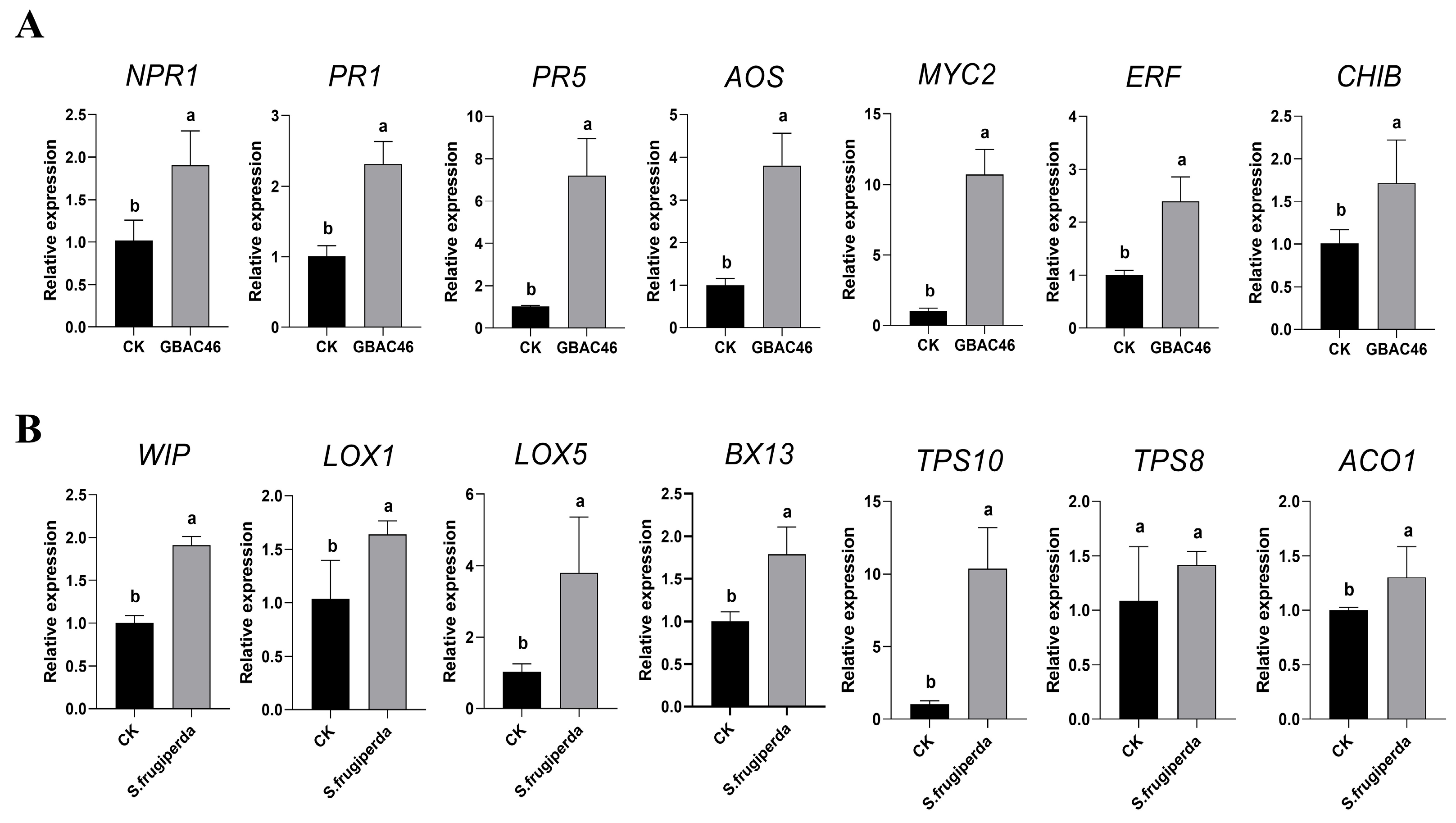
3.5. Induced Systemic Resistance
3.6. Effect of Biocontrol Potential of the GBAC46 Strain on Maize Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, R.N.; Costa Alves, G.S.; Van Sluys, M.A. Plant immunity: Unravelling the complexity of plant responses to biotic stresses. Ann. Bot. 2017, 119, 681–687. [Google Scholar] [PubMed]
- Ali, Q.; Ali, M.; Jing, H.; Hussain, A.; Manghwar, H.; Ali, M.; Raza, W.; Mundra, S. Power of plant microbiome: A sustainable approach for agricultural resilience. Plant Stress 2024, 14, 100681. [Google Scholar]
- Franco, F.P.; Moura, D.S.; Vivanco, J.M.; Silva-Filho, M.C. Plant-insect-pathogen interactions: A naturally complex ménage à trois. Curr. Opin. Microbiol. 2017, 37, 54–60. [Google Scholar] [PubMed]
- Willsey, T.; Chatterton, S.; Cárcamo, H. Interactions of root-feeding insects with fungal and oomycete plant pathogens. Front. Plant Sci. 2017, 8, 1764. [Google Scholar]
- Nicoletti, R.; Becchimanzi, A. Ecological and molecular interactions between insects and fungi. Microorganisms 2022, 10, 96. [Google Scholar] [CrossRef]
- Rayamajhi, M.B.; Van, T.K.; Pratt, P.D.; Center, T.D. Interactive association between Puccinia psidii and Oxyops vitiosa, two introduced natural enemies of Melaleuca quinquenervia in Florida. Biol. Control. 2006, 37, 56–67. [Google Scholar]
- Ariss, J.J.; Rhodes, L.H.; Sulc, R.M.; Hammond, R.B. Potato leafhopper injury and fusarium crown rot effects on three alfalfa populations. Crop Sci. 2007, 47, 1661–1671. [Google Scholar]
- Friedli, J.; Bacher, S. Mutualistic interaction between a weevil and a rust fungus, two parasites of the weed Cirsium arvense. Oecologia 2001, 129, 571–576. [Google Scholar]
- Mondy, N.; Corio-Costet, M.F. Feeding insects with a phytopathogenic fungus influences their diapause and population dynamics. Ecol. Entomol. 2004, 29, 711–717. [Google Scholar]
- Shapiro, L.; De Moraes, C.M.; Stephenson, A.G.; Mescher, M.C. Pathogen effects on vegetative and floral odors mediate vector attraction and host exposure in a complex pathosystem. Ecol. Lett. 2012, 15, 1430–1438. [Google Scholar]
- Tack, A.J.M.; Gripenberg, S.; Roslin, T. Cross-kingdom interactions matter: Fungal-mediated interactions structure an insect community on oak. Ecol. Lett. 2012, 15, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, B.; Karlovsky, P.; Vidal, S. Interaction between western corn rootworm (Coleoptera: Chrysomelidae) larvae and root-infecting Fusarium verticillioides. Environ. Entomol. 2010, 39, 1532–1538. [Google Scholar] [PubMed]
- Eyles, A.; Chorbadjian, R.; Wallis, C.; Hansen, R.; Cipollini, D.; Herms, D.; Bonello, P. Cross induction of systemic induced resistance between an insect and a fungal pathogen in Austrian pine over a fertility gradient. Oecologia 2007, 153, 365–374. [Google Scholar]
- Yang, J.W.; Yi, H.S.; Kim, H.; Lee, B.; Lee, S.; Ghim, S.Y.; Ryu, C.M. Whitefly infestation of pepper plants elicits defense responses against bacterial pathogens in leaves and roots and changes the below-ground microflora. J. Ecol. 2011, 99, 46–56. [Google Scholar] [CrossRef]
- Islam, W.; Noman, A.; Naveed, H.; Alamri, S.A.; Hashem, M.; Huang, Z.Q.; Chen, H.Y.H. Plant insect vector-virus interactions under environmental change. Sci. Total Environ. 2020, 701, 135044. [Google Scholar] [CrossRef]
- Li, Y.; Duan, T.; Li, Y. Research progress in the interactions of fungal pathogens and insect pests during host plant colonization. J. Plant Dis. Prot. 2021, 128, 633–647. [Google Scholar]
- Liang, Z.; Ali, Q.; Wang, Y.; Mu, G.; Kan, X.; Ren, Y.; Manghwar, H.; Gu, Q.; Wu, H.; Gao, X. Toxicity of Bacillus thuringiensis strains derived from the novel crystal protein Cry31Aa with high nematicidal activity against rice parasitic nematode Aphelenchoides besseyi. Int. J. Mol. Sci. 2022, 23, 8189. [Google Scholar] [CrossRef]
- Peng, Q.; Yu, Q.; Song, F. Expression of cry genes in Bacillus thuringiensis biotechnology. Appl. Microbiol. Biotechnol. 2019, 103, 1617–1626. [Google Scholar] [CrossRef]
- Wan, L.T.; Lin, J.; Du, H.W.; Zhang, Y.L.; Bravo, A.; Soberon, M.; Sun, M.; Peng, D.H. Bacillus thuringiensis targets the host intestinal epithelial junctions for successful infection of Caenorhabditis elegans. Environ. Microbiol. 2019, 21, 1086–1098. [Google Scholar]
- Xiao, Y.; Wu, K. Recent progress on the interaction between insects and Bacillus thuringiensis crops. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 4, 20180316. [Google Scholar]
- Akram, W.; Mahboob, A.; Javed, A.A. Bacillus thuringiensis strain 199 can induce systemic resistance in tomato against Fusarium wilt. Eur. J. Microbiol. Immunol. 2013, 3, 275–280. [Google Scholar]
- Reyes-Ramírez, A.; Escudero-Abarca, B.I.; Aguilar-Uscanga, G.; Hayward-Jones, P.M.; Barboza-Corona, J.E. Antifungal activity of Bacillus thuringiensis chitinase and its potential for the biocontrol of phytopathogenic fungi in soybean seeds. J. Food Sci. 2004, 69, M131–M134. [Google Scholar]
- Sadfi, N.; Cherif, M.; Fliss, I.; Boudabbous, A.; Antoun, H. Evaluation of bacterial isolates from salty soils and Bacillus thuringiensis strains for the biocontrol of Fusarium dry rot of potato tubers. J. Plant Pathol. 2001, 83, 101–117. [Google Scholar]
- Shrestha, A.; Sultana, R.; Chae, J.C.; Kim, K.; Lee, K.J. Bacillus thuringiensis C25 which is rich in cell wall degrading enzymes efficiently controls lettuce drop caused by Sclerotinia minor. Eur. J. Plant Pathol. 2015, 142, 577–589. [Google Scholar]
- Tang, Y.; Zou, J.; Zhang, L.; Li, Z.; Ma, C.; Ma, N. Anti-fungi activities of Bacillus thuringiensis H3 chitinase and immobilized chitinase particles and their effects on rice seedling defensive enzymes. J. Nanosci. Nanotechnol. 2012, 12, 8081–8086. [Google Scholar]
- Zheng, M.; Shi, J.; Shi, J.; Wang, Q.; Li, Y. Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the an-thracnose pathogen in postharvest mangos. Biol. Control 2013, 65, 200–206. [Google Scholar]
- Wu, H.J.; Gu, Q.; Xie, Y.L.; Lou, Z.Y.; Xue, P.Q.; Fang, L.; Yu, C.J.; Jia, D.D.; Huang, G.C.; Zhu, B.C.; et al. Cold-adapted Bacilli isolated from the Qinghai-Tibetan Plateau are able to promote plant growth in extreme environments. Environ. Microbiol. 2019, 21, 3505–3526. [Google Scholar]
- Mirocha, C.J.; Kolaczkowski, E.; Xie, W.; Yu, H.; Jelen, H. Analysis of deoxynivalenol and its derivatives (batch and single kernel) using gas chromatography/mass spectrometry. J. Agric. Food Chem. 1998, 46, 1414–1418. [Google Scholar]
- Liang, Z.; Qiao, J.Q.; Li, P.P.; Zhang, L.L.; Qiao, Z.X.; Lin, L.; Yu, C.J.; Yang, Y.; Zubair, M.; Gu, Q.; et al. A novel Rap-Phr system in Bacillus velezensis NAU-B3 regulates surfactin production and sporulation via interaction with ComA. Appl. Microbiol. Biotechnol. 2020, 104, 10059–10074. [Google Scholar]
- Hilker, M.; Meiners, T. How do plants “notice” attack by herbivorous arthropods? Biol. Rev. 2010, 85, 267–280. [Google Scholar]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [PubMed]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [PubMed]
- Mithofer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar]
- Bamisile, B.S.; Afolabi, O.G.; Siddiqui, J.A.; Xu, Y. Endophytic insect pathogenic fungi-host plant-herbivore mutualism: Elucidating the mechanisms involved in the tripartite interactions. World J. Microbiol. Biotechnol. 2023, 39, 326. [Google Scholar]
- Gossner, M.M.; Beenken, L.; Arend, K.; Begerow, D.; Peršoh, D. Insect herbivory facilitates the establishment of an invasive plant pathogen. ISME Commun. 2021, 22, 6. [Google Scholar]
- English-Loeb, G.; Norton, A.P.; Gadoury, D.M.; Seem, R.C.; Wilcox, W.F. Control of powdery mildew in wild and cultivated grapes by a tydeid mite. Biol. Control. 1999, 14, 97–103. [Google Scholar]
- Hao, Z.P.; Sheng, L.; Feng, Z.B.; Fei, W.X.; Hou, S.M. Aphids may facilitate the spread of Sclerotinia Stem Rot in Oilseed Rape by Carrying and Depositing Ascospores. J Fungi 2024, 10, 202. [Google Scholar]
- Medeiros, A.H.; Franco, F.P.; Matos, J.L.; de Castro, P.A.; Santos-Silva, L.K.; Henrique-Silva, F.; Goldman, G.H.; Moura, D.S.; Silva-Filho, M.C. Sugarwin: A sugarcane insect-induced gene with antipathogenic activity. Mol. Plant Microbe Interact. 2012, 25, 613–624. [Google Scholar]
- Guo, J.; Guo, J.; He, K.; Bai, S.; Zhang, T.; Zhao, J.; Wang, Z. Physiological responses induced by Ostrinia furnacalis (Lepidoptera: Crambidae) feeding in Maize and their effects on O. furnacalis performance. J. Econ. Entomol. 2017, 110, 739–747. [Google Scholar]
- Ji, M.; Bui, H.; Ramirez, R.A.; Clark, R.M. Concerted cis and trans effects underpin heightened defense gene expression in multi-herbivore-resistant maize lines. Plant J. 2022, 111, 508–528. [Google Scholar]
- Tack, A.J.M.; Dicke, M. Plant pathogens structure arthropod communities across multiple spatial and temporal scales. Funct. Ecol. 2013, 27, 633–645. [Google Scholar]
- Rostas, M.; Simon, M.; Hilker, M. Ecological cross-effects of induced plant responses towards herbivores and phytopathogenic fungi. Basic. Appl. Ecol. 2003, 4, 43–62. [Google Scholar]
- Ueda, H.; Kugimiya, S.; Tabata, J.; Kitamoto, H.; Mitsuhara, I. Accumulation of salicylic acid in tomato plants under biological stress affects oviposition preference of Bemisia tabaci. J. Plant Interact. 2019, 14, 73–78. [Google Scholar]
- Hatcher, P.E.; Paul, N.D.; Ayres, P.G.; Whittaker, J.B. The effect of a foliar disease (rust) on the development of Gastrophysa-viridula (Coleoptera, chrysomelidae). Ecol. Entomol. 1994, 19, 349–360. [Google Scholar]
- Guo, S.X.; Liu, M.; Peng, D.H.; Ji, S.S.; Wang, P.X.; Yu, Z.N.; Sun, M. New strategy for isolating novel nematicidal crystal protein genes from Bacillus thuringiensis strain YBT-1518. Appl. Environ. Microb. 2008, 74, 6997–7001. [Google Scholar]
- Ali, Q.; Khan, A.R.; Tao, S.; Rajer, F.U.; Ayaz, M.; Abro, M.A.; Gu, Q.; Wu, H.; Kuptsov, V.; Kolomiets, E.; et al. Broad-spectrum antagonistic potential of Bacillus spp. volatiles against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Physiol. Plant. 2023, 175, e14087. [Google Scholar]
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711. [Google Scholar]
- Ostry, V.; Ovesna, J.; Skarkova, J.; Pouchova, V.; Ruprich, J. A review on comparative data concerning Fusarium mycotoxins in Bt maize and non-Bt isogenic maize. Mycotoxin Res. 2010, 26, 141–145. [Google Scholar]
- Barroso, V.M.; Rocha, L.O.; Reis, T.A.; Reis, G.M.; Duarte, A.P.; Michelotto, M.D.; Correa, B. Fusarium verticillioides and fumonisin contamination in Bt and non-Bt maize cultivated in Brazil. Mycotoxin Res. 2017, 33, 121–127. [Google Scholar]
- Cawoy, H.; Debois, D.; Franzil, L.; De Pauw, E.; Thonart, P.; Ongena, M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2015, 8, 281–295. [Google Scholar]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Sheikh, T.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the induction of systemic resistance and regulation of antioxidant pathways in tomato using fengycin produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 16, 613. [Google Scholar]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [PubMed]
- Gao, H.; Li, P.; Xu, X.; Zeng, Q.; Guan, W. Research on volatile organic compounds from Bacillus subtilis CF-3: Biocontrol effects on fruit fungal pathogens and dynamic changes during fermentation. Front. Microbiol. 2018, 14, 456. [Google Scholar]
- Zhang, L.; Sun, C. Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 2018, 84, e00445-18. [Google Scholar]
- Wang, Y.; Zhang, C.; Liang, J.; Wang, L.; Gao, W.; Jiang, J.; Chang, R. Surfactin and fengycin B extracted from Bacillus pumilus W-7 provide protection against potato late blight via distinct and synergistic mechanisms. Appl. Microbiol. Biotechnol. 2020, 104, 7467–7481. [Google Scholar]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar]
- Ayaz, M.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Ali, F.; Rashid, K.A.; Cao, S.; He, Y.-Q.; Bukero, A.A.; Huang, W.-K.; et al. Exploring plant growth promoting traits and biocontrol potential of new isolated Bacillus subtilis BS-2301 strain in suppressing Sclerotinia sclerotiorum through various mechanisms. Front. Plant Sci. 2024, 15, 1444328. [Google Scholar]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Hanif, A.; Tahir, H.A.S.; Gao, X. Marker assisted detection and LC-MS analysis of antimicrobial compounds in different Bacillus strains and their antifungal effect on Sclerotinia sclerotiorum. Boil. Control 2019, 133, 91–102. [Google Scholar]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Ali, Q.; Wu, H.; Gu, Q.; Liu, X.; Sun, H.; Gao, X. Biocontrol Mechanism of Bacillus thuringiensis GBAC46 Against Diseases and Pests Caused by Fusarium verticillioides and Spodoptera frugiperda. Biomolecules 2025, 15, 519. https://doi.org/10.3390/biom15040519
Liang Z, Ali Q, Wu H, Gu Q, Liu X, Sun H, Gao X. Biocontrol Mechanism of Bacillus thuringiensis GBAC46 Against Diseases and Pests Caused by Fusarium verticillioides and Spodoptera frugiperda. Biomolecules. 2025; 15(4):519. https://doi.org/10.3390/biom15040519
Chicago/Turabian StyleLiang, Zhao, Qurban Ali, Huijun Wu, Qin Gu, Xin Liu, Houjun Sun, and Xuewen Gao. 2025. "Biocontrol Mechanism of Bacillus thuringiensis GBAC46 Against Diseases and Pests Caused by Fusarium verticillioides and Spodoptera frugiperda" Biomolecules 15, no. 4: 519. https://doi.org/10.3390/biom15040519
APA StyleLiang, Z., Ali, Q., Wu, H., Gu, Q., Liu, X., Sun, H., & Gao, X. (2025). Biocontrol Mechanism of Bacillus thuringiensis GBAC46 Against Diseases and Pests Caused by Fusarium verticillioides and Spodoptera frugiperda. Biomolecules, 15(4), 519. https://doi.org/10.3390/biom15040519





