Fungal Warriors: Effects of Beauveria bassiana and Purpureocillium lilacinum on CCYV-Carrying Whiteflies
Abstract
:1. Introduction
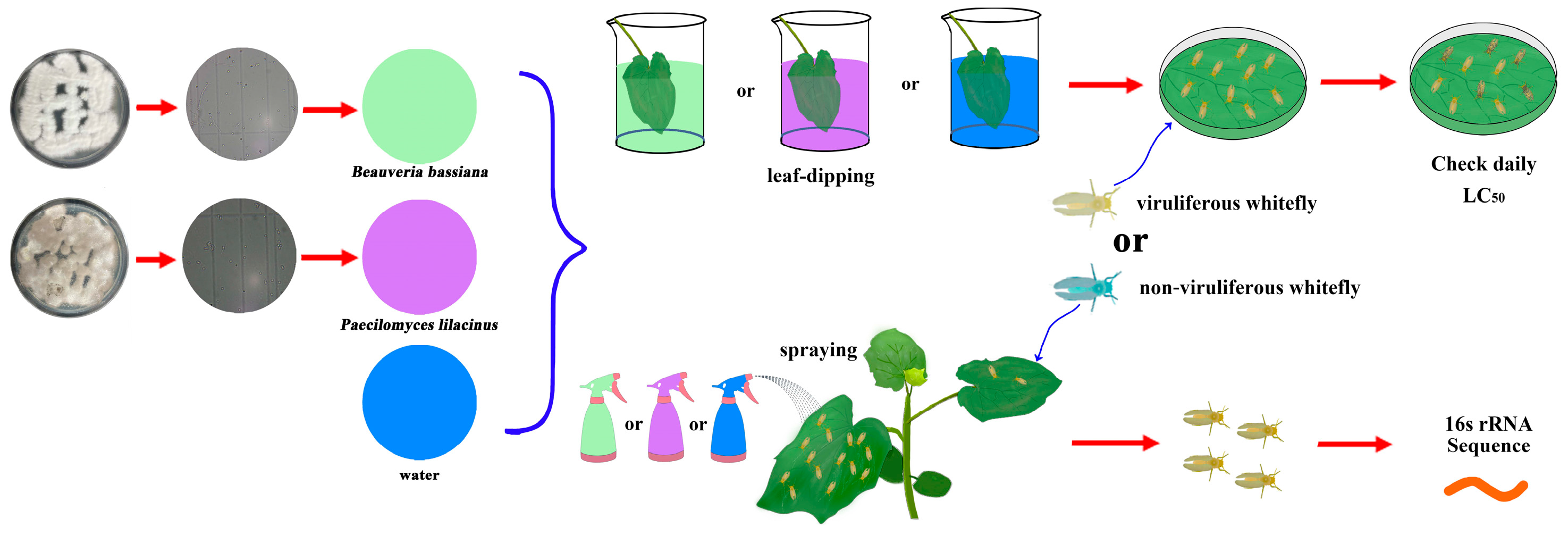
2. Materials and Methods
2.1. Plants, Virus, Fungi, and Insects
2.2. Entomopathogenic Fungi Bioassay of Non-Viruliferous and Viruliferous Whiteflies
2.3. Effect of B. bassiana and P. lilacinum on the Symbiotic Bacteria to Non-Viruliferous and Viruliferous Whiteflies
2.4. Quantification of Bacteria Communities
2.5. Bioinformatics and Statistical Analyses
3. Results
3.1. B. bassiana and P. lilacinum Pathogenicity Against Whiteflies
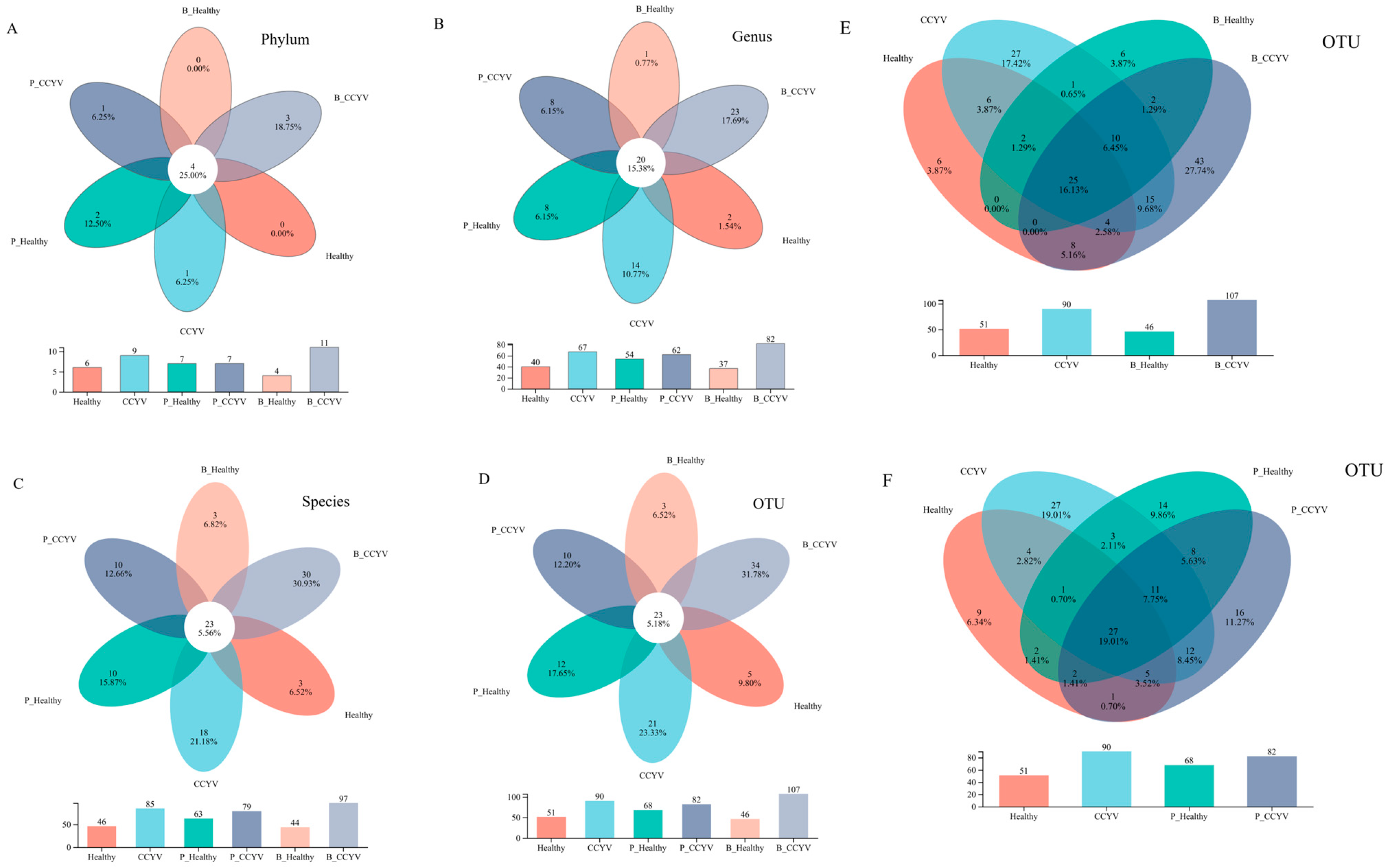
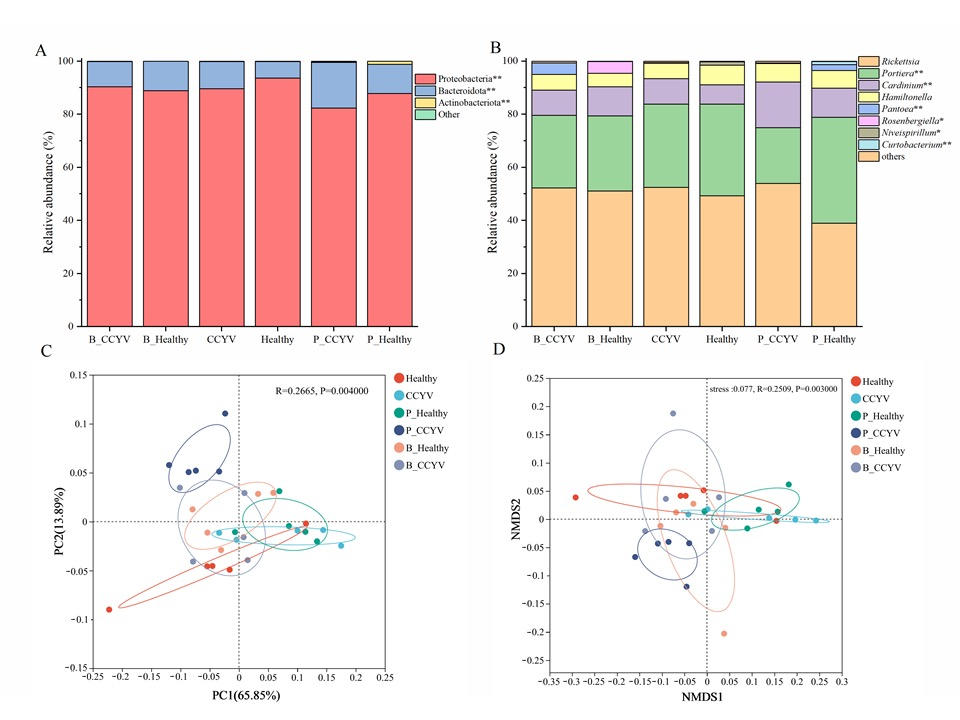
3.2. Overview of Microbiotas in Whiteflies Fed on B. bassiana and P. lilacinum
3.3. Effect of B. bassiana and P. lilacinum on Microbiome Diversity in Whiteflies
3.4. Effect of B. bassiana and P. lilacinum on Gene Experssion in Whiteflies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, J.; Guo, Z.; Yang, Z.; Han, H.; Wang, S.; Xu, H.; Yang, X.; Yang, F.; Wu, Q.; Xie, W.; et al. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693–1705.e17. [Google Scholar] [CrossRef]
- Jain, R.G.; Fletcher, S.J.; Manzie, N.; Robinson, K.E.; Li, P.; Lu, E.; Brosnan, C.A.; Xu, Z.P.; Mitter, N. Foliar application of clay-delivered RNA interference for whitefly control. Nat. Plants 2022, 8, 535–548. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Z.; Gong, C.; Xia, J.; Hu, Y.; Zhong, J.; Yang, X.; Xie, W.; Wang, S.; Wu, Q.; et al. Two horizontally acquired bacterial genes steer the exceptionally efficient and flexible nitrogenous waste cycling in whiteflies. Sci. Adv. 2024, 10, eadi3105. [Google Scholar] [CrossRef]
- Wang, X.-W.; Blanc, S. Insect Transmission of Plant Single-Stranded DNA Viruses. Annu. Rev. Entomol. 2021, 66, 389–405. [Google Scholar] [CrossRef]
- Zhang, Z.; He, H.; Yan, M.; Zhao, C.; Lei, C.; Li, J.; Yan, F. Widely targeted analysis of metabolomic changes of Cucumis sativus induced by cucurbit chlorotic yellows virus. BMC Plant Biol. 2022, 22, 158. [Google Scholar] [CrossRef]
- He, H.; Li, J.; Zhang, Z.; Yan, M.; Zhang, B.; Zhu, C.; Yan, W.; Shi, B.; Wang, Y.; Zhao, C.; et al. A plant virus enhances odorant-binding protein 5 (OBP5) in the vector whitefly for more actively olfactory orientation to the host plant. Pest Manag. Sci. 2022, 79, 1410–1419. [Google Scholar] [CrossRef]
- Mao, L.; Zhang, L.; Wang, S.; Zhang, Y.; Zhu, L.; Jiang, H.; Liu, X. Application of insecticides by soil drenching before seedling transplanting combined with anti-insect nets to control tobacco whitefly in tomato greenhouses. Sci. Rep. 2022, 12, 15939. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Palmieri, M.; Herrera, M.P.; Dubón, A.L. Integrated Pest Management of Whitefly Crop: Free Periods Can Reduce Begomovirus Transmission in Tomato. In Agricultural, Forestry and Bioindustry Biotechnology and Biodiscovery; Chong, P.A., Newman, D.J., Steinmacher, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 433–454. [Google Scholar]
- Conboy, N.J.A.; McDaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Gatehouse, A.M.R.; Tosh, C.R. Volatile Organic Compounds as Insect Repellents and Plant Elicitors: An Integrated Pest Management (IPM) Strategy for Glasshouse Whitefly (Trialeurodes vaporariorum). J. Chem Ecol. 2020, 46, 1090–1104. [Google Scholar] [CrossRef]
- Morin, S.; Atkinson, P.W.; Walling, L.L. Whitefly–Plant Interactions: An Integrated Molecular Perspective. Annu. Rev. Entomol. 2024, 69, 503–525. [Google Scholar] [CrossRef]
- Fu, B.; Liang, J.; Hu, J.; Du, T.; Tan, Q.; He, C.; Wei, X.; Gong, P.; Yang, J.; Liu, S.; et al. GPCR-MAPK signaling pathways underpin fitness trade-offs in whitefly. Proc. Natl. Acad Sci. USA 2024, 121, e2402407121. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, J.; Hu, J.; Yang, F.; Liang, J.; Xue, H.; Wei, X.; Fu, B.; Huang, M.; Du, H.; et al. Glutathione S-transferase directly metabolizes imidacloprid in the whitefly, Bemisia tabaci. Pestic Biochem. Physiol. 2024, 201, 105863. [Google Scholar] [CrossRef]
- Hu, J.; Fu, B.; Liang, J.; Zhang, R.; Wei, X.; Yang, J.; Tan, Q.; Xue, H.; Gong, P.; Liu, S.; et al. CYP4CS5-mediated thiamethoxam and clothianidin resistance is accompanied by fitness cost in the whitefly Bemisia tabaci. Pest Manag. Sci. 2024, 80, 910–921. [Google Scholar] [CrossRef]
- Yang, X.; Deng, S.; Wei, X.; Yang, J.; Zhao, Q.; Yin, C.; Du, T.; Guo, Z.; Xia, J.; Yang, Z.; et al. MAPK-directed activation of the whitefly transcription factor CREB leads to P450-mediated imidacloprid resistance. Proc. Natl. Acad Sci. USA 2020, 117, 10246–10253. [Google Scholar] [CrossRef]
- Yan, M.; He, H.; Zhang, Z.; Zhang, B.; Zhu, C.; Yan, W.; Zhao, C.; Li, J.; Yan, F. Molecular basis of mutual benefits between Cucurbit chlorotic yellows virus (CCYV) transmission and imidacloprid resistance in Bemisia tabaci. J. Pest Sci. 2023, 96, 489–497. [Google Scholar] [CrossRef]
- Yan, W.; Wang, S.; Liu, J.; Zhai, D.; Lu, H.; Li, J.; Bai, R.; Lei, C.; Song, L.; Zhao, C.; et al. Managing Super Pests: Interplay between Pathogens and Symbionts Informs Biocontrol of Whiteflies. Microorganisms 2024, 12, 887. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic Value of Biological Control in Integrated Pest Management of Managed Plant Systems. Annu. Rev. Entomol. 2015, 60, 621–645. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, C.; Zang, L.-S.; Gu, M.; Zhang, R.; Eleftherianos, I.; Mohamed, A.A. Entomopathogenic fungal-derived metabolites alter innate immunity and gut microbiota in the migratory locust. J. Pest Sci. 2024, 97, 853–872. [Google Scholar] [CrossRef]
- Konopická, J.; Skoková Habuštová, O.; Jánová, N.; Žurovcová, M.; Doležal, P.; Zemek, R. Isolation and identification of entomopathogenic fungi strains for Colorado potato beetle (Leptinotarsa decemlineata) control. J. Appl. Microbiol. 2024, 135, lxae213. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S. Insect Pathogenic Fungi: Genomics, Molecular Interactions, and Genetic Improvements. Annu. Rev. Entomol. 2017, 62, 73–90. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, Q.; He, L.; Wang, Z.; Li, G.; Wang, Z.; Liu, H. Biological and physiological effects in Bemisia tabaci feeding on tomatoes endophytically colonized by Beauveria bassiana. Pest Manag. Sci. 2024, 80, 4085–4097. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; Wang, X.; Shi, Y.; Gu, Q.; Kuo, Y.W.; Falk, B.W.; Yan, F. Direct evidence for the semipersistent transmission of Cucurbit chlorotic yellows virus by a whitefly vector. Sci. Rep. 2016, 6, 36604. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, Y.; Zhang, Y.; Wu, Q.; Wang, S. Primer screening and amplification protocol optimization of rapid detection technique for Cucurbit chlorotic yellows virus. Acta Entomol. Sin. 2020, 63, 149–158. [Google Scholar]
- Shi, P.-Q.; Wang, L.; Chen, X.-Y.; Wang, K.; Wu, Q.-J.; Turlings, T.C.J.; Zhang, P.-J.; Qiu, B.-L. Rickettsia transmission from whitefly to plants benefits herbivore insects but is detrimental to fungal and viral pathogens. mBio 2024, 15, e0244823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Luo, J.; Jiang, W.; Wu, L.; Zhang, L.; Ji, J.; Wang, L.; Ma, Y.; Cui, J. Response of the bacterial community of Propylea japonica (Thunberg) to Cry2Ab protein. Environ. Pollut. 2019, 254 Pt B, 113063. [Google Scholar] [CrossRef]
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host–vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Luan, J.B.; Yao, D.M.; Zhang, T.; Walling, L.L.; Yang, M.; Wang, Y.J.; Liu, S.S. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef]
- Dong, Y.; Li, T.; Hou, Y.; Wilson, K.; Wang, X.; Su, C.; Li, Y.; Ren, G.; Xu, P. Densovirus infection facilitates plant–virus transmission by an aphid. New Phytol. 2024, 243, 1539–1553. [Google Scholar] [CrossRef]
- Wang, H.; Xu, D.; Pu, L.; Zhou, G. Southern rice black-streaked dwarf virus alters insect vectors’ host orientation preferences to enhance spread and increase rice ragged stunt virus co-infection. Phytopathology 2014, 104, 196–201. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Lu, C.; Tian, H.; Lin, S.; Wang, L.; Linghu, T.; Zheng, X.; Wei, H.; Fan, X.; et al. Chemosensory protein regulates the behavioural response of Frankliniella intonsa and Frankliniella occidentalis to tomato zonate spot virus-Infected pepper (Capsicum annuum). PLoS Pathog. 2023, 19, e1011380. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Wu, N.; Wang, H.; Zhang, Z.; Liu, Y.; Wang, X. Southern rice black-streaked dwarf virus induces incomplete autophagy for persistence in gut epithelial cells of its vector insect. PLoS Pathog. 2023, 19, e1011134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, S.-S.; Wang, X.-W.; Yang, J.-G.; Pan, L.-L. Manipulation of Whitefly Behavior by Plant Viruses. Microorganisms 2022, 10, 2410. [Google Scholar] [CrossRef]
- Wen, Z.; Feng, J.; Zhu, B.; Xu, W.; Xu, F.; Tan, H.; Chu, D.; Guo, L. Pyrifluquinazon baseline susceptibility and inhibition of Tomato chlorosis virus transmission by Bemisia tabaci. Pest Manag. Sci. 2023, 79, 3520–3528. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Preisser, E.L.; Jiao, X.; Zhang, Y. Tomato yellow leaf curl virus infection alters Bemisia tabaci MED (Hemiptera: Aleyrodidae) vulnerability to Flupyradifurone. J. Econ. Entomol. 2020, 113, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Li, Z.; Lin, H.; Cheng, Y.; Wang, H.; Jiang, Z.; Ji, Z.; Huang, Z.; Chen, H.; Wei, T. A phytoplasma effector destabilizes chloroplastic glutamine synthetase inducing chlorotic leaves that attract leafhopper vectors. Proc. Natl. Acad. Sci. USA 2024, 121, e2402911121. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Y.; Li, Y.; Sun, P.; Jiang, J.; Zhou, H.; Liu, J.; Wang, S. Expression of mosquito miRNAs in entomopathogenic fungus induces pathogen-mediated host RNA interference and increases fungal efficacy. Cell Rep. 2022, 41, 111527. [Google Scholar] [CrossRef]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Extracellular enzyme activity of entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae and their pathogenicity potential as a bio-control agent against whitefly pests, Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). BMC Res. Notes 2022, 15, 117. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microbial. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef]
- Sain, S.K.; Monga, D.; Hiremani, N.S.; Nagrale, D.T.; Kranthi, S.; Kumar, R.; Kranthi, K.R.; Tuteja, O.P.; Waghmare, V.N. Evaluation of bioefficacy potential of entomopathogenic fungi against the whitefly (Bemisia tabaci Genn.) on cotton under polyhouse and field conditions. J. Invertebr. Pathol. 2021, 183, 107618. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Zhu-Salzman, K.; Ge, F.; Sun, Y. PEBP balances apoptosis and autophagy in whitefly upon arbovirus infection. Nat. Commun. 2022, 13, 846. [Google Scholar] [CrossRef]
- Barman, M.; Samanta, S.; Upadhyaya, G.; Thakur, H.; Chakraborty, S.; Samanta, A.; Tarafdar, J. Unraveling the basis of neonicotinoid resistance in whitefly species complex: Role of endosymbiotic bacteria and insecticide resistance genes. Front. Microbiol. 2022, 13, 901793. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Guo, H.; Di, J.; Xie, L.; Zhu-Salzman, K.; Ge, F.; Zhao, Z.; Sun, Y. A complete DNA repair system assembled by two endosymbionts restores heat tolerance of the insect host. Proc. Natl. Acad. Sci. USA 2024, 121, e2415651121. [Google Scholar] [CrossRef]
- Liu, Z.; Lyu, B.; Lu, H.; Tang, J.; Zhang, Q.; Jiao, B. The toxicological mechanism of emamectin benzoate in Spodoptera frugiperda via regulating gut microbiota. Entomol. Gen. 2025, 45, 253–263. [Google Scholar] [CrossRef]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.T.; Li, Y.; Li, T.P.; Liang, Y.; Hu, L.; Zhang, D.; Zhou, C.Y.; Yang, C.; Zhang, X.; Zha, S.S.; et al. Stable Introduction of Plant-Virus-Inhibiting Wolbachia into Planthoppers for Rice Protection. Curr. Biol. 2020, 30, 4837–4845.e5. [Google Scholar] [CrossRef]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.; Zhang, N.; Miao, Y.; Shen, Q.; Zhang, R. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 2021, 9, 35. [Google Scholar] [CrossRef]
- Barman, M.; Samanta, S.; Thakur, H.; Chakraborty, S.; Samanta, A.; Ghosh, A.; Tarafdar, J. Effect of Neonicotinoids on Bacterial Symbionts and Insecticide-Resistant Gene in Whitefly, Bemisia tabaci. Insects 2021, 12, 742. [Google Scholar] [CrossRef]
- Kliot, A.; Johnson, R.S.; MacCoss, M.J.; Kontsedalov, S.; Lebedev, G.; Czosnek, H.; Heck, M.; Ghanim, M. A proteomic approach reveals possible molecular mechanisms and roles for endosymbiotic bacteria in begomovirus transmission by whiteflies. GigaScience 2020, 9, giaa124. [Google Scholar] [CrossRef]
- Lei, T.; Zhao, J.; Wang, H.L.; Liu, Y.Q.; Liu, S.S. Impact of a novel Rickettsia symbiont on the life history and virus transmission capacity of its host whitefly (Bemisia tabaci). Insect Sci. 2021, 28, 377–391. [Google Scholar] [CrossRef]
- Kliot, A.; Kontsedalov, S.; Lebedev, G.; Czosnek, H.; Ghanim, M. Combined infection with Tomato yellow leaf curl virus and Rickettsia influences fecundity, attraction to infected plants and expression of immunity-related genes in the whitefly Bemisia tabaci. J. Gen. Virol. 2019, 100, 721–731. [Google Scholar] [CrossRef]
- Kontsedalov, S.; Zchori-Fein, E.; Chiel, E.; Gottlieb, Y.; Inbar, M.; Ghanim, M. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Karaca, M.M.; Döker, İ.; Karut, K. Monitoring insecticide resistance and endosymbiont composition in greenhouse populations of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) from Mersin, Turkey. Phytoparasitica 2020, 48, 659–672. [Google Scholar] [CrossRef]
- Su, Q.; Oliver, K.M.; Xie, W.; Wu, Q.; Wang, S.; Zhang, Y. The whitefly-associated facultative symbiont Hamiltonella defensa suppresses induced plant defences in tomato. Funct. Ecol. 2015, 29, 1007–1018. [Google Scholar] [CrossRef]
- Li, Q.; Sun, J.; Qin, Y.; Fan, J.; Zhang, Y.; Tan, X.; Hou, M.; Chen, J. Reduced insecticide susceptibility of the wheat aphid Sitobion miscanthi after infection by the secondary bacterial symbiont Hamiltonella defensa. Pest Manag. Sci. 2021, 77, 1936–1944. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Jin, D.; Gong, P.; Li, Q.; Zhang, Y.; Li, X.; Deng, Y.; Cernava, T.; Zhu, X. Implications of environmentally shaped microbial communities for insecticide resistance in Sitobion miscanthi. Environ. Res. 2022, 215, 114409. [Google Scholar] [CrossRef]
- Karut, K.; Castle, S.J.; Karut, Ş.T.; Karaca, M.M. Secondary endosymbiont diversity of Bemisia tabaci and its parasitoids. Infect. Genet. Evol. 2020, 78, 104104. [Google Scholar] [CrossRef]
- Sloan, D.B.; Moran, N.A. Endosymbiotic bacteria as a source of carotenoids in whiteflies. Biol. Lett. 2012, 8, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, K.; Wang, J.; Chu, D. Cardinium infection alters cotton defense and detoxification metabolism of its whitefly host. Insect Sci. 2023, 30, 473–485. [Google Scholar] [CrossRef]
- Ying, L.; Baiming, L.; Hongran, L.; Tianbo, D.; Yunli, T.; Dong, C. Effect of Cardinium Infection on the Probing Behavior of Bemisia tabaci (Hemiptera: Aleyrodidae) MED. J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef]
- Hu, W.; Gao, H.; Cui, C.; Wang, L.; Wang, Y.; Li, Y.; Li, F.; Zheng, Y.; Xia, T.; Wang, S. Harnessing engineered symbionts to combat concurrent malaria and arboviruses transmission. Nat. Commun. 2025, 16, 2104. [Google Scholar] [CrossRef]
- Keppanan, R.; Karuppannasamy, A.; Nagaraja, B.C.; Thiruvengadam, V.; Kesavan, S.; Dhawane, Y.A.; Ramasamy, A. Effectiveness of chitosan nanohydrogel mediated encapsulation of EcR dsRNA against the whitefly, Bemisia tabaci Asia-I (Gennedius) (Hemiptera: Aleyordidae). Pestic. Biochem. Physiol. 2024, 198, 105712. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, D.; Lu, H.; Liu, S.; Liu, J.; Zhang, W.; Wu, J.; Li, J.; Bai, R.; Yan, F.; Zhao, C. Fungal Warriors: Effects of Beauveria bassiana and Purpureocillium lilacinum on CCYV-Carrying Whiteflies. Biomolecules 2025, 15, 593. https://doi.org/10.3390/biom15040593
Zhai D, Lu H, Liu S, Liu J, Zhang W, Wu J, Li J, Bai R, Yan F, Zhao C. Fungal Warriors: Effects of Beauveria bassiana and Purpureocillium lilacinum on CCYV-Carrying Whiteflies. Biomolecules. 2025; 15(4):593. https://doi.org/10.3390/biom15040593
Chicago/Turabian StyleZhai, Dan, Hang Lu, Suyao Liu, Jialei Liu, Wanyu Zhang, Jingjing Wu, Jingjing Li, Rune Bai, Fengming Yan, and Chenchen Zhao. 2025. "Fungal Warriors: Effects of Beauveria bassiana and Purpureocillium lilacinum on CCYV-Carrying Whiteflies" Biomolecules 15, no. 4: 593. https://doi.org/10.3390/biom15040593
APA StyleZhai, D., Lu, H., Liu, S., Liu, J., Zhang, W., Wu, J., Li, J., Bai, R., Yan, F., & Zhao, C. (2025). Fungal Warriors: Effects of Beauveria bassiana and Purpureocillium lilacinum on CCYV-Carrying Whiteflies. Biomolecules, 15(4), 593. https://doi.org/10.3390/biom15040593





