The Emerging Roles of Vacuolar-Type ATPase-Dependent Lysosomal Acidification in Cardiovascular Disease
Abstract
1. Introduction
2. Lysosome Function
2.1. Lysosomes and Their Role in Cellular Degradation
2.2. The Importance of Maintaining Acidic pH Within Lysosomes for Proper Enzymatic Activity
3. V-ATPase and Its Structure
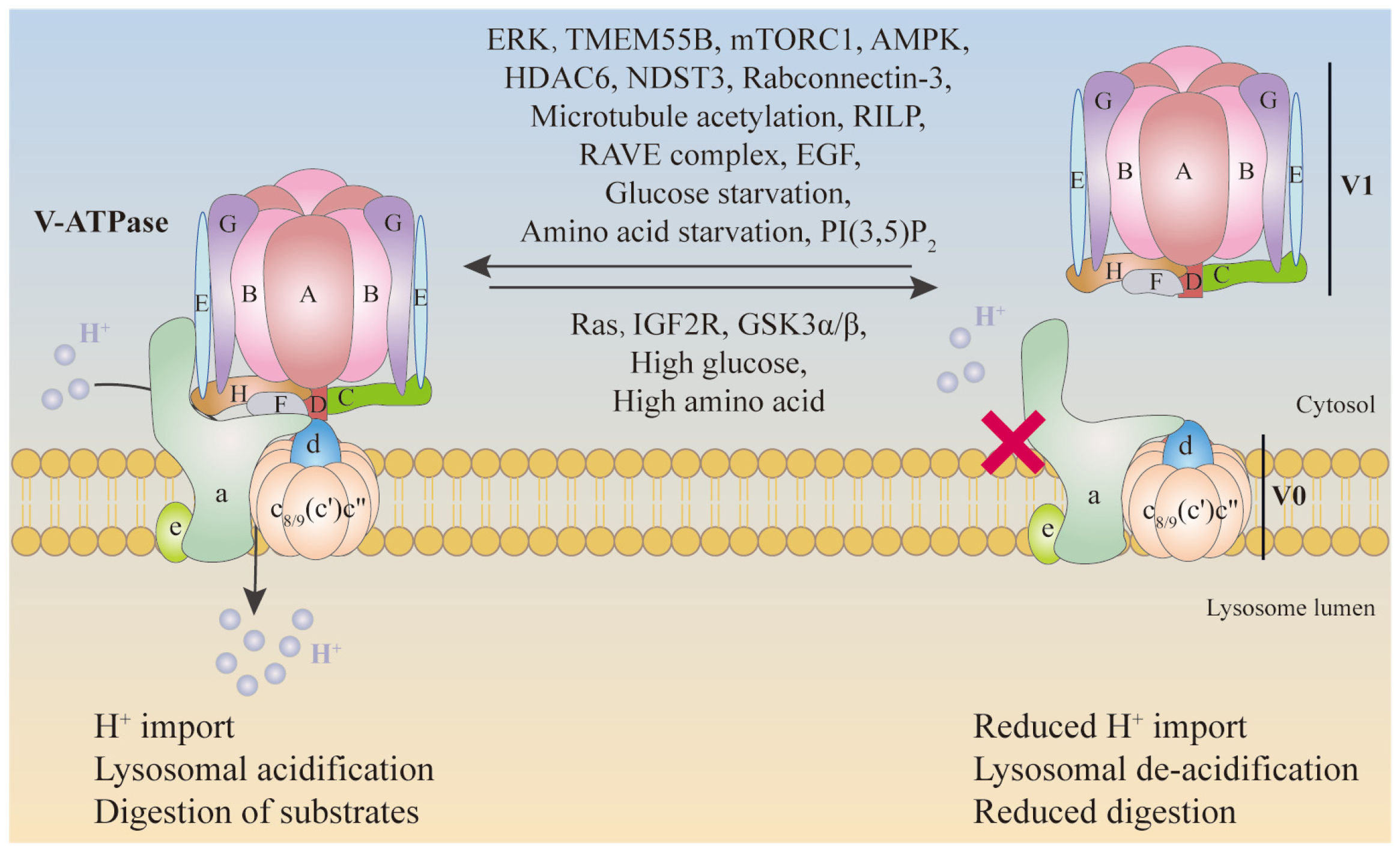
4. Regulation of V-ATPase
4.1. Regulated Assembly
4.2. Regulated Trafficking
4.3. Other Forms of Regulation
5. Role of V-ATPase in the Cardiovascular System
5.1. Vesicle Loading and Coupled Transport
5.2. pH Acidification and Sensing
5.3. Nutrient Signaling
5.4. Others
6. Dysfunction of V-ATPase-Dependent Lysosomal Acidification in Cardiovascular Diseases
6.1. V-ATPase-Dependent Lysosomal Acidification and Myocardial Disease
6.2. V-ATPase-Dependent Lysosomal Acidification and Diabetic Cardiomyopathy
6.3. V-ATPase-Dependent Lysosomal Acidification and Hypertension
6.4. V-ATPase-Dependent Lysosomal Acidification and Atherosclerosis
6.5. V-ATPase-Dependent Lysosomal Acidification and Vascular Inflammation
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nayak, S.; Mindell, J.A.; Grabe, M. A model of lysosomal pH regulation. J. Gen. Physiol. 2013, 141, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Colacurcio, D.J.; Nixon, R.A. Disorders of lysosomal acidification-The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res. Rev. 2016, 32, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Song, S.B. Impaired Autophagic Flux in Glucose-Deprived Cells: An Outcome of Lysosomal Acidification Failure Exacerbated by Mitophagy Dysfunction. Mol. Cells 2023, 46, 655–663. [Google Scholar] [CrossRef]
- Nishi, T.; Forgac, M. The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002, 3, 94–103. [Google Scholar] [CrossRef]
- Song, Q.; Meng, B.; Xu, H.; Mao, Z. The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases. Transl. Neurodegener. 2020, 9, 17. [Google Scholar] [CrossRef]
- Bagh, M.B.; Peng, S.; Chandra, G.; Zhang, Z.; Singh, S.P.; Pattabiraman, N.; Liu, A.; Mukherjee, A.B. Misrouting of v-ATPase subunit V0a1 dysregulates lysosomal acidification in a neurodegenerative lysosomal storage disease model. Nat. Commun. 2017, 8, 14612. [Google Scholar] [CrossRef]
- Santos-Pereira, C.; Rodrigues, L.R.; Côrte-Real, M. Emerging insights on the role of V-ATPase in human diseases: Therapeutic challenges and opportunities. Med. Res. Rev. 2021, 41, 1927–1964. [Google Scholar] [CrossRef]
- Yim, W.W.-Y.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Heather, L.C.; Luiken, J.J.F.P. CD36 as a gatekeeper of myocardial lipid metabolism and therapeutic target for metabolic disease. Physiol. Rev. 2024, 104, 727–764. [Google Scholar] [CrossRef]
- Chen, Q.; Kou, H.; Demy, D.L.; Liu, W.; Li, J.; Wen, Z.; Herbomel, P.; Huang, Z.; Zhang, W.; Xu, J. The different roles of V-ATPase a subunits in phagocytosis/endocytosis and autophagy. Autophagy 2024, 20, 2297–2313. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper metabolism in cell death and autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef]
- Rossi, A.; Malvagia, S.; la Marca, G.; Parenti, G.; Brunetti-Pierri, N. Biomarkers for gene therapy clinical trials of lysosomal storage disorders. Mol. Ther. 2024, 32, 2930–2938. [Google Scholar] [CrossRef] [PubMed]
- Chitsamankhun, C.; Siritongtaworn, N.; Fournier, B.P.J.; Sriwattanapong, K.; Theerapanon, T.; Samaranayake, L.; Porntaveetus, T. Cathepsin C in health and disease: From structural insights to therapeutic prospects. J. Transl. Med. 2024, 22, 777. [Google Scholar] [CrossRef]
- Cai, Z.; Xu, S.; Liu, C. Cathepsin B in cardiovascular disease: Underlying mechanisms and therapeutic strategies. J. Cell Mol. Med. 2024, 28, e70064. [Google Scholar] [CrossRef]
- Ferrari, V.; Tedesco, B.; Cozzi, M.; Chierichetti, M.; Casarotto, E.; Pramaggiore, P.; Cornaggia, L.; Mohamed, A.; Patelli, G.; Piccolella, M.; et al. Lysosome quality control in health and neurodegenerative diseases. Cell Mol. Biol. Lett. 2024, 29, 116. [Google Scholar] [CrossRef]
- Lee, J.; Ou, J.H.J. HCV-induced autophagy and innate immunity. Front. Immunol. 2024, 15, 1305157. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zheng, H.; Zhang, Z.; Fan, P.; Yang, X. Mechanism and therapeutic targets of the involvement of a novel lysosomal proton channel TMEM175 in Parkinson’s disease. Ageing Res. Rev. 2024, 100, 102373. [Google Scholar] [CrossRef]
- Trojani, M.-C.; Santucci-Darmanin, S.; Breuil, V.; Carle, G.F.; Pierrefite-Carle, V. Lysosomal exocytosis: From cell protection to protumoral functions. Cancer Lett. 2024, 597, 217024. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Liu, S.; Xu, H. The Acid Gate in the Lysosome. Autophagy 2023, 19, 1368–1370. [Google Scholar] [CrossRef]
- Hu, M.; Feng, X.; Liu, Q.; Liu, S.; Huang, F.; Xu, H. The ion channels of endomembranes. Physiol. Rev. 2024, 104, 1335–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, W.; Han, Y.; Lee, W.-R.; Liou, J.; Jiang, Y. Lysosomal LAMP proteins regulate lysosomal pH by direct inhibition of the TMEM175 channel. Mol. Cell 2023, 83, 2524–2539. [Google Scholar] [CrossRef] [PubMed]
- Coupland, C.E.; Karimi, R.; Bueler, S.A.; Liang, Y.; Courbon, G.M.; Di Trani, J.M.; Wong, C.J.; Saghian, R.; Youn, J.-Y.; Wang, L.-Y.; et al. High-resolution electron cryomicroscopy of V-ATPase in native synaptic vesicles. Science 2024, 385, 168–174. [Google Scholar] [CrossRef]
- Vasanthakumar, T.; Rubinstein, J.L. Structure and Roles of V-type ATPases. Trends Biochem. Sci. 2020, 45, 295–307. [Google Scholar] [CrossRef]
- Kane, P.M. Regulation of V-ATPases by reversible disassembly. FEBS Lett. 2000, 469, 137–141. [Google Scholar] [PubMed]
- Wang, N.; Ren, L.; Danser, A.H.J. Vacuolar H+-ATPase in Diabetes, Hypertension, and Atherosclerosis. Microcirculation 2024, 31, e12855. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Liu, J.; Tang, D. The V-ATPases in cancer and cell death. Cancer Gene Ther. 2022, 29, 1529–1541. [Google Scholar] [CrossRef]
- Mijaljica, D.; Prescott, M.; Devenish, R.J. V-ATPase engagement in autophagic processes. Autophagy 2011, 7, 666–668. [Google Scholar] [CrossRef]
- Merkulova, M.; Hurtado-Lorenzo, A.; Hosokawa, H.; Zhuang, Z.; Brown, D.; Ausiello, D.A.; Marshansky, V. Aldolase directly interacts with ARNO and modulates cell morphology and acidic vesicle distribution. Am. J. Physiol. Cell Physiol. 2011, 300, C1442–C1455. [Google Scholar] [CrossRef]
- Lu, M.; Ammar, D.; Ives, H.; Albrecht, F.; Gluck, S.L. Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump. J. Biol. Chem. 2007, 282, 24495–24503. [Google Scholar] [CrossRef]
- Bond, S.; Forgac, M. The Ras/cAMP/protein kinase A pathway regulates glucose-dependent assembly of the vacuolar (H+)-ATPase in yeast. J. Biol. Chem. 2008, 283, 36513–36521. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Liu, M.; Liu, Y.; Hwang, R.-D.; Zhang, T.; Wang, J. NDST3 deacetylates α-tubulin and suppresses V-ATPase assembly and lysosomal acidification. EMBO J. 2021, 40, e107204. [Google Scholar] [CrossRef] [PubMed]
- Kane, P.M. Disassembly and reassembly of the yeast vacuolar H+-ATPase in vivo. J. Biol. Chem. 1995, 270, 17025–17032. [Google Scholar] [CrossRef] [PubMed]
- Parra, K.J.; Hayek, S.R. A lysosomal proton pump turns on when glucose runs out. J. Biol. Chem. 2018, 293, 9124–9125. [Google Scholar] [CrossRef]
- McGuire, C.M.; Forgac, M. Glucose starvation increases V-ATPase assembly and activity in mammalian cells through AMP kinase and phosphatidylinositide 3-kinase/Akt signaling. J. Biol. Chem. 2018, 293, 9113–9123. [Google Scholar] [CrossRef]
- Stransky, L.A.; Forgac, M. Amino Acid Availability Modulates Vacuolar H+-ATPase Assembly. J. Biol. Chem. 2015, 290, 27360–27369. [Google Scholar] [CrossRef]
- Smardon, A.M.; Tarsio, M.; Kane, P.M. The RAVE complex is essential for stable assembly of the yeast V-ATPase. J. Biol. Chem. 2002, 277, 13831–13839. [Google Scholar]
- Xu, Y.; Parmar, A.; Roux, E.; Balbis, A.; Dumas, V.; Chevalier, S.; Posner, B.I. Epidermal growth factor-induced vacuolar (H+)-atpase assembly: A role in signaling via mTORC1 activation. J. Biol. Chem. 2012, 287, 26409–26422. [Google Scholar] [CrossRef]
- De Luca, M.; Cogli, L.; Progida, C.; Nisi, V.; Pascolutti, R.; Sigismund, S.; Di Fiore, P.P.; Bucci, C. RILP regulates vacuolar ATPase through interaction with the V1G1 subunit. J. Cell Sci. 2014, 127, 2697–2708. [Google Scholar] [CrossRef]
- Coen, K.; Flannagan, R.S.; Baron, S.; Carraro-Lacroix, L.R.; Wang, D.; Vermeire, W.; Michiels, C.; Munck, S.; Baert, V.; Sugita, S.; et al. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 2012, 198, 23–35. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Shirane, M.; Nakayama, K.I. TMEM55B contributes to lysosomal homeostasis and amino acid-induced mTORC1 activation. Genes. Cells 2018, 23, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, L.; Lan, B.; Wang, Y.; Du, L.; Chen, X.; Li, Q.; Liu, K.; Hu, M.; Xue, Y.; et al. IGF2R-initiated proton rechanneling dictates an anti-inflammatory property in macrophages. Sci. Adv. 2020, 6, eabb7389. [Google Scholar] [CrossRef]
- Long, Z.; Ge, C.; Zhao, Y.; Liu, Y.; Zeng, Q.; Tang, Q.; Dong, Z.; He, G. Enhanced autophagic clearance of amyloid-β via HDAC6-mediated V-ATPase assembly and lysosomal acidification protects against Alzheimer’s disease in vitro and in vivo. Neural Regen. Res. 2024, 20, 2633–2644. [Google Scholar] [CrossRef] [PubMed]
- Hallows, K.R.; Alzamora, R.; Li, H.; Gong, F.; Smolak, C.; Neumann, D.; Pastor-Soler, N.M. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am. J. Physiol. Cell Physiol. 2009, 296, C672–C681. [Google Scholar] [CrossRef]
- Alzamora, R.; Al-Bataineh, M.M.; Liu, W.; Gong, F.; Li, H.; Thali, R.F.; Joho-Auchli, Y.; Brunisholz, R.A.; Satlin, L.M.; Neumann, D.; et al. AMP-activated protein kinase regulates the vacuolar H+-ATPase via direct phosphorylation of the A subunit (ATP6V1A) in the kidney. Am. J. Physiol. Renal Physiol. 2013, 305, F943–F956. [Google Scholar] [CrossRef]
- Pastor-Soler, N.M.; Hallows, K.R.; Smolak, C.; Gong, F.; Brown, D.; Breton, S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am. J. Physiol. Cell Physiol. 2008, 294, C488–C494. [Google Scholar] [PubMed]
- Alzamora, R.; Thali, R.F.; Gong, F.; Smolak, C.; Li, H.; Baty, C.J.; Bertrand, C.A.; Auchli, Y.; Brunisholz, R.A.; Neumann, D.; et al. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J. Biol. Chem. 2010, 285, 24676–24685. [Google Scholar] [CrossRef]
- Păunescu, T.G.; Ljubojevic, M.; Russo, L.M.; Winter, C.; McLaughlin, M.M.; Wagner, C.A.; Breton, S.; Brown, D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am. J. Physiol. Renal Physiol. 2010, 298, F643–F654. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Onogi, Y.; Krueger, M.; Oeckl, J.; Karlina, R.; Singh, I.; Hauck, S.M.; Feederle, R.; Li, Y.; Ussar, S. PAT2 regulates vATPase assembly and lysosomal acidification in brown adipocytes. Mol. Metab. 2022, 61, 101508. [Google Scholar] [CrossRef] [PubMed]
- Peña-Llopis, S.; Vega-Rubin-de-Celis, S.; Schwartz, J.C.; Wolff, N.C.; Tran, T.A.T.; Zou, L.; Xie, X.-J.; Corey, D.R.; Brugarolas, J. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 2011, 30, 3242–3258. [Google Scholar] [CrossRef]
- Farsi, Z.; Gowrisankaran, S.; Krunic, M.; Rammner, B.; Woehler, A.; Lafer, E.M.; Mim, C.; Jahn, R.; Milosevic, I. Clathrin coat controls synaptic vesicle acidification by blocking vacuolar ATPase activity. eLife 2018, 7, e32569. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Wu, L.; Huang, K.; Wang, Z.; Zheng, Y.; Zheng, C.; Zhang, Z.; Chen, J.; Wei, J.; et al. Ubiquitin ligase subunit FBXO9 inhibits V-ATPase assembly and impedes lung cancer metastasis. Exp. Hematol. Oncol. 2024, 13, 32. [Google Scholar] [CrossRef]
- Zhang, W.; Sha, Z.; Tang, Y.; Jin, C.; Gao, W.; Chen, C.; Yu, L.; Lv, N.; Liu, S.; Xu, F.; et al. Defective Lamtor5 Leads to Autoimmunity by Deregulating v-ATPase and Lysosomal Acidification. Adv. Sci. 2024, 11, e2400446. [Google Scholar] [CrossRef]
- Cao, Y.; Li, R.; Shen, M.; Li, C.; Zou, Y.; Jiang, Q.; Liu, S.; Lu, C.; Li, H.; Liu, H.; et al. DDRGK1, a crucial player of ufmylation system, is indispensable for autophagic degradation by regulating lysosomal function. Cell Death Dis. 2021, 12, 416. [Google Scholar] [CrossRef]
- Schiffer, I.; Gerisch, B.; Kawamura, K.; Laboy, R.; Hewitt, J.; Denzel, M.S.; Mori, M.A.; Vanapalli, S.; Shen, Y.; Symmons, O.; et al. miR-1 coordinately regulates lysosomal v-ATPase and biogenesis to impact proteotoxicity and muscle function during aging. Elife 2021, 10, e66768. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Han, Y.-H.; Kim, J.-Y.; Lee, M.-O. RORα Enhances Lysosomal Acidification and Autophagic Flux in the Hepatocytes. Hepatol. Commun. 2021, 5, 2121–2138. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Zeng, Q.; Hu, Y.; He, R.; Ma, W.; Qian, C.; Hua, T.; Song, F.; Cai, Y.; et al. Prosapogenin A induces GSDME-dependent pyroptosis of anaplastic thyroid cancer through vacuolar ATPase activation-mediated lysosomal over-acidification. Cell Death Dis. 2024, 15, 586. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Forgac, M. Inhibition of vacuolar H+-ATPase by disulfide bond formation between cysteine 254 and cysteine 532 in subunit A. J. Biol. Chem. 1994, 269, 13224–13230. [Google Scholar] [CrossRef] [PubMed]
- Shao, E.; Nishi, T.; Kawasaki-Nishi, S.; Forgac, M. Mutational analysis of the non-homologous region of subunit A of the yeast V-ATPase. J. Biol. Chem. 2003, 278, 12985–12991. [Google Scholar] [CrossRef]
- Kawasaki-Nishi, S.; Nishi, T.; Forgac, M. Yeast V-ATPase complexes containing different isoforms of the 100-kDa a-subunit differ in coupling efficiency and in vivo dissociation. J. Biol. Chem. 2001, 276, 17941–17948. [Google Scholar] [CrossRef]
- Namkoong, S.; Lee, K.I.; Lee, J.I.; Park, R.; Lee, E.-J.; Jang, I.-S.; Park, J. The integral membrane protein ITM2A, a transcriptional target of PKA-CREB, regulates autophagic flux via interaction with the vacuolar ATPase. Autophagy 2015, 11, 756–768. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Caddye, E.; Najafov, A.; Houde, V.P.; Johnson, C.; Dissanayake, K.; Toth, R.; Campbell, D.G.; Prescott, A.R.; MacKintosh, C. The E3 ubiquitin ligase ZNRF2 is a substrate of mTORC1 and regulates its activation by amino acids. eLife 2016, 5, e12278. [Google Scholar] [CrossRef]
- Günther, W.; Lüchow, A.; Cluzeaud, F.; Vandewalle, A.; Jentsch, T.J. ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc. Natl. Acad. Sci. USA 1998, 95, 8075–8080. [Google Scholar]
- Johnson, D.E.; Ostrowski, P.; Jaumouillé, V.; Grinstein, S. The position of lysosomes within the cell determines their luminal pH. J. Cell Biol. 2016, 212, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Jaskolka, M.C.; Tarsio, M.; Smardon, A.M.; Khan, M.M.; Kane, P.M. Defining steps in RAVE-catalyzed V-ATPase assembly using purified RAVE and V-ATPase subcomplexes. J. Biol. Chem. 2021, 296, 100703. [Google Scholar] [CrossRef]
- Jaskolka, M.C.; Winkley, S.R.; Kane, P.M. RAVE and Rabconnectin-3 Complexes as Signal Dependent Regulators of Organelle Acidification. Front. Cell Dev. Biol. 2021, 9, 698190. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Forgac, M. Microtubules are involved in glucose-dependent dissociation of the yeast vacuolar [H+]-ATPase in vivo. J. Biol. Chem. 2001, 276, 24855–24861. [Google Scholar]
- Manolson, M.F.; Proteau, D.; Preston, R.A.; Stenbit, A.; Roberts, B.T.; Hoyt, M.A.; Preuss, D.; Mulholland, J.; Botstein, D.; Jones, E.W. The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H+-ATPase. J. Biol. Chem. 1992, 267, 14294–14303. [Google Scholar]
- Manolson, M.F.; Wu, B.; Proteau, D.; Taillon, B.E.; Roberts, B.T.; Hoyt, M.A.; Jones, E.W. STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H+-ATPase subunit Vph1p. J. Biol. Chem. 1994, 269, 14064–14074. [Google Scholar]
- Kawasaki-Nishi, S.; Bowers, K.; Nishi, T.; Forgac, M.; Stevens, T.H. The amino-terminal domain of the vacuolar proton-translocating ATPase a subunit controls targeting and in vivo dissociation, and the carboxyl-terminal domain affects coupling of proton transport and ATP hydrolysis. J. Biol. Chem. 2001, 276, 47411–47420. [Google Scholar]
- Miranda, K.C.; Karet, F.E.; Brown, D. An extended nomenclature for mammalian V-ATPase subunit genes and splice variants. PLoS ONE 2010, 5, e9531. [Google Scholar] [CrossRef]
- Bodzęta, A.; Kahms, M.; Klingauf, J. The Presynaptic v-ATPase Reversibly Disassembles and Thereby Modulates Exocytosis but Is Not Part of the Fusion Machinery. Cell Rep. 2017, 20, 1348–1359. [Google Scholar] [CrossRef]
- Hurtado-Lorenzo, A.; Skinner, M.; El Annan, J.; Futai, M.; Sun-Wada, G.-H.; Bourgoin, S.; Casanova, J.; Wildeman, A.; Bechoua, S.; Ausiello, D.A.; et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 2006, 8, 124–136. [Google Scholar] [CrossRef]
- Morel, N.; Dedieu, J.-C.; Philippe, J.-M. Specific sorting of the a1 isoform of the V-H+ATPase a subunit to nerve terminals where it associates with both synaptic vesicles and the presynaptic plasma membrane. J. Cell Sci. 2003, 116, 4751–4762. [Google Scholar] [CrossRef] [PubMed]
- Toyomura, T.; Murata, Y.; Yamamoto, A.; Oka, T.; Sun-Wada, G.-H.; Wada, Y.; Futai, M. From lysosomes to the plasma membrane: Localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J. Biol. Chem. 2003, 278, 22023–22030. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Sekiya, M.; Tohyama, K.; Ishiyama-Matsuura, E.; Sun-Wada, G.-H.; Wada, Y.; Futai, M.; Nakanishi-Matsui, M. Essential Role of the a3 Isoform of V-ATPase in Secretory Lysosome Trafficking via Rab7 Recruitment. Sci. Rep. 2018, 8, 6701. [Google Scholar] [CrossRef]
- Saw, N.M.N.; Kang, S.-Y.A.; Parsaud, L.; Han, G.A.; Jiang, T.; Grzegorczyk, K.; Surkont, M.; Sun-Wada, G.-H.; Wada, Y.; Li, L.; et al. Vacuolar H+-ATPase subunits Voa1 and Voa2 cooperatively regulate secretory vesicle acidification, transmitter uptake, and storage. Mol. Biol. Cell 2011, 22, 3394–3409. [Google Scholar] [CrossRef]
- Sun-Wada, G.-H.; Tabata, H.; Kawamura, N.; Aoyama, M.; Wada, Y. Direct recruitment of H+-ATPase from lysosomes for phagosomal acidification. J. Cell Sci. 2009, 122, 2504–2513. [Google Scholar] [CrossRef]
- Oka, T.; Murata, Y.; Namba, M.; Yoshimizu, T.; Toyomura, T.; Yamamoto, A.; Sun-Wada, G.H.; Hamasaki, N.; Wada, Y.; Futai, M. a4, a unique kidney-specific isoform of mouse vacuolar H+-ATPase subunit a. J. Biol. Chem. 2001, 276, 40050–40054. [Google Scholar] [CrossRef]
- Nishi, T.; Forgac, M. Molecular cloning and expression of three isoforms of the 100-kDa a subunit of the mouse vacuolar proton-translocating ATPase. J. Biol. Chem. 2000, 275, 6824–6830. [Google Scholar] [CrossRef]
- Pietrement, C.; Sun-Wada, G.H.; Silva, N.D.; McKee, M.; Marshansky, V.; Brown, D.; Futai, M.; Breton, S. Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol. Reprod. 2006, 74, 185–194. [Google Scholar] [CrossRef]
- Esmail, S.; Yao, Y.; Kartner, N.; Li, J.; Reithmeier, R.A.F.; Manolson, M.F. N-Linked Glycosylation Is Required for Vacuolar H+-ATPase (V-ATPase) a4 Subunit Stability, Assembly, and Cell Surface Expression. J. Cell Biochem. 2016, 117, 2757–2768. [Google Scholar] [CrossRef]
- Hinton, A.; Bond, S.; Forgac, M. V-ATPase functions in normal and disease processes. Pflugers Arch. 2009, 457, 589–598. [Google Scholar] [PubMed]
- Forgac, M. Vacuolar ATPases: Rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 2007, 8, 917–929. [Google Scholar] [PubMed]
- Smith, A.N.; Skaug, J.; Choate, K.A.; Nayir, A.; Bakkaloglu, A.; Ozen, S.; Hulton, S.A.; Sanjad, S.A.; Al-Sabban, E.A.; Lifton, R.P.; et al. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat. Genet. 2000, 26, 71–75. [Google Scholar]
- Shine, L.; Kilty, C.; Gross, J.; Kennedy, B. Vacuolar ATPases and their role in vision. Adv. Exp. Med. Biol. 2014, 801, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A.; Finberg, K.E.; Breton, S.; Marshansky, V.; Brown, D.; Geibel, J.P. Renal vacuolar H+-ATPase. Physiol. Rev. 2004, 84, 1263–1314. [Google Scholar]
- Breton, S.; Brown, D. Regulation of luminal acidification by the V-ATPase. Physiology 2013, 28, 318–329. [Google Scholar] [CrossRef]
- Levin, L.R.; Buck, J. Physiological roles of acid-base sensors. Annu. Rev. Physiol. 2015, 77, 347–362. [Google Scholar] [CrossRef]
- Rahman, N.; Ramos-Espiritu, L.; Milner, T.A.; Buck, J.; Levin, L.R. Soluble adenylyl cyclase is essential for proper lysosomal acidification. J. Gen. Physiol. 2016, 148, 325–339. [Google Scholar] [CrossRef]
- Vasanthakumar, T.; Bueler, S.A.; Wu, D.; Beilsten-Edmands, V.; Robinson, C.V.; Rubinstein, J.L. Structural comparison of the vacuolar and Golgi V-ATPases from Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2019, 116, 7272–7277. [Google Scholar] [CrossRef]
- Florey, O.; Gammoh, N.; Kim, S.E.; Jiang, X.; Overholtzer, M. V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation. Autophagy 2015, 11, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Forgac, M. A novel mechanism for regulation of vacuolar acidification. J. Biol. Chem. 1992, 267, 19769–19772. [Google Scholar] [PubMed]
- Milosevic, I. Revisiting the Role of Clathrin-Mediated Endoytosis in Synaptic Vesicle Recycling. Front. Cell Neurosci. 2018, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, G.; Cecchetti, S.; Bocchinfuso, G.; Radio, F.C.; Leoni, C.; Onesimo, R.; Calligari, P.; Pietrantoni, A.; Ciolfi, A.; Ferilli, M.; et al. Dominantly acting variants in ATP6V1C1 and ATP6V1B2 cause a multisystem phenotypic spectrum by altering lysosomal and/or autophagosome function. HGG Adv. 2024, 5, 100349. [Google Scholar] [CrossRef]
- Ren, X.; Wang, J.; Wei, H.; Li, X.; Tian, Y.; Wang, Z.; Yin, Y.; Guo, Z.; Qin, Z.; Wu, M.; et al. Impaired TFEB-mediated autophagy-lysosome fusion promotes tubular cell cycle G2/M arrest and renal fibrosis by suppressing ATP6V0C expression and interacting with SNAREs. Int. J. Biol. Sci. 2024, 20, 1905–1926. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Kamp, M.A.; Siapich, S.A.; Hescheler, J.; Lüke, M.; Schneider, T. Ca(v)2.3 Ca2+ channel interacts with the G1-subunit of V-ATPase. Cell Physiol. Biochem. 2011, 27, 421–432. [Google Scholar] [CrossRef]
- Abuammar, H.; Bhattacharjee, A.; Simon-Vecsei, Z.; Blastyák, A.; Csordás, G.; Páli, T.; Juhász, G. Ion Channels and Pumps in Autophagy: A Reciprocal Relationship. Cells 2021, 10, 3537. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, Y.; Zhang, Z.; Zhang, R.; Song, X.; Malek, S.N.; Tang, D.; Klionsky, D.J. Big1 is a newly identified autophagy regulator that is critical for a fully functional V-ATPase. Mol. Biol. Cell 2024, 35, br20. [Google Scholar] [CrossRef]
- Johnson, D.L.; Kumar, R.; Kakhniashvili, D.; Pfeffer, L.M.; Laribee, R.N. Ccr4-Not ubiquitin ligase signaling regulates ribosomal protein homeostasis and inhibits 40S ribosomal autophagy. J. Biol. Chem. 2024, 107582. [Google Scholar] [CrossRef]
- Jefferies, K.C.; Cipriano, D.J.; Forgac, M. Function, structure and regulation of the vacuolar (H+)-ATPases. Arch. Biochem. Biophys. 2008, 476, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, Y.; Jiang, C.; Qu, R.; Wren, J.D.; Georgescu, C.; Wang, X.; Reuter, D.N.; Liu, B.; Giles, C.B.; et al. CD151 Maintains Endolysosomal Protein Quality to Inhibit Vascular Inflammation. Circ. Res. 2024, 134, 1330–1347. [Google Scholar] [CrossRef] [PubMed]
- Sobota, J.A.; Bäck, N.; Eipper, B.A.; Mains, R.E. Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal- and secretory-pathway proteins. J. Cell Sci. 2009, 122, 3542–3553. [Google Scholar] [CrossRef]
- Mauvezin, C.; Nagy, P.; Juhász, G.; Neufeld, T.P. Autophagosome-lysosome fusion is independent of V-ATPase-mediated acidification. Nat. Commun. 2015, 6, 7007. [Google Scholar] [CrossRef]
- Kissing, S.; Hermsen, C.; Repnik, U.; Nesset, C.K.; von Bargen, K.; Griffiths, G.; Ichihara, A.; Lee, B.S.; Schwake, M.; De Brabander, J.; et al. Vacuolar ATPase in phagosome-lysosome fusion. J. Biol. Chem. 2015, 290, 14166–14180. [Google Scholar] [CrossRef]
- Scott, C.C.; Gruenberg, J. Ion flux and the function of endosomes and lysosomes: pH is just the start: The flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays 2011, 33, 103–110. [Google Scholar] [CrossRef]
- Ratto, E.; Chowdhury, S.R.; Siefert, N.S.; Schneider, M.; Wittmann, M.; Helm, D.; Palm, W. Direct control of lysosomal catabolic activity by mTORC1 through regulation of V-ATPase assembly. Nat. Commun. 2022, 13, 4848. [Google Scholar] [CrossRef]
- Li, T.Y.; Wang, Q.; Gao, A.W.; Li, X.; Sun, Y.; Mottis, A.; Shong, M.; Auwerx, J. Lysosomes mediate the mitochondrial UPR via mTORC1-dependent ATF4 phosphorylation. Cell Discov. 2023, 9, 92. [Google Scholar] [CrossRef]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef]
- Chung, C.Y.-S.; Shin, H.R.; Berdan, C.A.; Ford, B.; Ward, C.C.; Olzmann, J.A.; Zoncu, R.; Nomura, D.K. Covalent targeting of the vacuolar H+-ATPase activates autophagy via mTORC1 inhibition. Nat. Chem. Biol. 2019, 15, 776–785. [Google Scholar] [CrossRef]
- Groszer, M. Rare human ATP6V1A variants provide unique insights into V-ATPase functions. Brain 2022, 145, 2626–2628. [Google Scholar] [CrossRef] [PubMed]
- Marshansky, V. The V-ATPase a2-subunit as a putative endosomal pH-sensor. Biochem. Soc. Trans. 2007, 35, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Kim, D.-S.; Kim, H.-T.; Lee, J.-W.; Chung, C.-H.; Ahn, T.; Lim, J.M.; Kim, I.-K.; Chae, H.-J.; Kim, H.-R. Enhanced lysosomal activity is involved in Bax inhibitor-1-induced regulation of the endoplasmic reticulum (ER) stress response and cell death against ER stress: Involvement of vacuolar H+-ATPase (V-ATPase). J. Biol. Chem. 2011, 286, 24743–24753. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, R.; Haginaka, J.; Tanimoto, T.; Kuroda, Y. Maintenance of luminal pH and protease activity in lysosomes/late endosomes by vacuolar ATPase in chlorpromazine-treated RAW264 cells accumulating phospholipids. Cell Biol. Toxicol. 2014, 30, 67–77. [Google Scholar] [CrossRef]
- Zhang, C.-S.; Jiang, B.; Li, M.; Zhu, M.; Peng, Y.; Zhang, Y.-L.; Wu, Y.-Q.; Li, T.Y.; Liang, Y.; Lu, Z.; et al. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 2014, 20, 526–540. [Google Scholar] [CrossRef]
- Bar-Peled, L.; Schweitzer, L.D.; Zoncu, R.; Sabatini, D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012, 150, 1196–1208. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Wyant, G.A.; Kim, C.; Laqtom, N.N.; Abbasi, M.; Chan, S.H.; Freinkman, E.; Sabatini, D.M. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 2017, 358, 807–813. [Google Scholar] [CrossRef]
- Kimura, T.; Nada, S.; Takegahara, N.; Okuno, T.; Nojima, S.; Kang, S.; Ito, D.; Morimoto, K.; Hosokawa, T.; Hayama, Y.; et al. Erratum: Polarization of M2 macrophages requires Lamtor1 that integrates cytokine and amino-acid signals. Nat. Commun. 2017, 8, 14711. [Google Scholar] [CrossRef]
- Collins, M.P.; Stransky, L.A.; Forgac, M. AKT Ser/Thr kinase increases V-ATPase-dependent lysosomal acidification in response to amino acid starvation in mammalian cells. J. Biol. Chem. 2020, 295, 9433–9444. [Google Scholar] [CrossRef]
- Lei, Y.; Klionsky, D.J. The coordination of V-ATPase and ATG16L1 is part of a common mechanism of non-canonical autophagy. Autophagy 2022, 18, 2267–2269. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Hong, L.; Yang, Z.; Cai, X.; Chen, X.; Fu, Y.; Lin, Y.; Wen, W.; Li, S.; et al. Golgi-associated LC3 lipidation requires V-ATPase in noncanonical autophagy. Cell Death Dis. 2016, 7, e2330. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Sakurai, M.; Wang, Y.; Saito, C.; Yoshii, G.; Wileman, T.; Mizushima, N.; Kuwahara, T.; Iwatsubo, T. The V-ATPase-ATG16L1 axis recruits LRRK2 to facilitate the lysosomal stress response. J. Cell Biol. 2024, 223, e202302067. [Google Scholar] [CrossRef]
- Figueras-Novoa, C.; Timimi, L.; Marcassa, E.; Ulferts, R.; Beale, R. Conjugation of ATG8s to single membranes at a glance. J. Cell Sci. 2024, 137, jcs261031. [Google Scholar] [CrossRef]
- Vaccari, T.; Duchi, S.; Cortese, K.; Tacchetti, C.; Bilder, D. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development 2010, 137, 1825–1832. [Google Scholar] [CrossRef]
- Miles, A.L.; Burr, S.P.; Grice, G.L.; Nathan, J.A. The vacuolar-ATPase complex and assembly factors, TMEM199 and CCDC115, control HIF1α prolyl hydroxylation by regulating cellular iron levels. Elife 2017, 6, e22693. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Guo, X.; Liu, J.; Chen, L.; Chen, H.; Zhao, Y.; Li, H.; Rong, S.; Yao, P. Refining the Rab7-V1G1 axis to mitigate iron deposition: Protective effects of quercetin in alcoholic liver disease. J. Nutr. Biochem. 2024, 135, 109767. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Park, H.W.; Sciarretta, S.; Mo, J.-S.; Jewell, J.L.; Russell, R.C.; Wu, X.; Sadoshima, J.; Guan, K.-L. Rag GTPases are cardioprotective by regulating lysosomal function. Nat. Commun. 2014, 5, 4241. [Google Scholar] [CrossRef]
- Jewell, J.L.; Kim, Y.C.; Russell, R.C.; Yu, F.-X.; Park, H.W.; Plouffe, S.W.; Tagliabracci, V.S.; Guan, K.-L. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 2015, 347, 194–198. [Google Scholar] [CrossRef]
- Milkereit, R.; Persaud, A.; Vanoaica, L.; Guetg, A.; Verrey, F.; Rotin, D. LAPTM4b recruits the LAT1-4F2hc Leu transporter to lysosomes and promotes mTORC1 activation. Nat. Commun. 2015, 6, 7250. [Google Scholar] [CrossRef]
- Kinouchi, K.; Ichihara, A.; Sano, M.; Sun-Wada, G.-H.; Wada, Y.; Kurauchi-Mito, A.; Bokuda, K.; Narita, T.; Oshima, Y.; Sakoda, M.; et al. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ. Res. 2010, 107, 30–34. [Google Scholar] [CrossRef]
- Morissette, G.; Ammoury, A.; Rusu, D.; Marguery, M.C.; Lodge, R.; Poubelle, P.E.; Marceau, F. Intracellular sequestration of amiodarone: Role of vacuolar ATPase and macroautophagic transition of the resulting vacuolar cytopathology. Br. J. Pharmacol. 2009, 157, 1531–1540. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Wen, Y.; Li, Z.; Chen, F.; Zou, Y.; Dong, X.; Liu, X.; Wang, J. Epirubicin induces cardiotoxicity through disrupting ATP6V0A2-dependent lysosomal acidification and triggering ferroptosis in cardiomyocytes. Cell Death Discov. 2024, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Steinbusch, L.K.M.; Nabben, M.; Kapsokalyvas, D.; van Zandvoort, M.; Schönleitner, P.; Antoons, G.; Simons, P.J.; Coumans, W.A.; Geomini, A.; et al. Palmitate-Induced Vacuolar-Type H+-ATPase Inhibition Feeds Forward Into Insulin Resistance and Contractile Dysfunction. Diabetes 2017, 66, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Schianchi, F.; Neumann, D.; Wong, L.-Y.; Sun, A.; van Nieuwenhoven, F.A.; Zeegers, M.P.; Strzelecka, A.; Col, U.; Glatz, J.F.C.; et al. Specific amino acid supplementation rescues the heart from lipid overload-induced insulin resistance and contractile dysfunction by targeting the endosomal mTOR-v-ATPase axis. Mol. Metab. 2021, 53, 101293. [Google Scholar] [CrossRef]
- Wang, S.; Han, Y.; Liu, R.; Hou, M.; Neumann, D.; Zhang, J.; Wang, F.; Li, Y.; Zhao, X.; Schianchi, F.; et al. Glycolysis-Mediated Activation of v-ATPase by Nicotinamide Mononucleotide Ameliorates Lipid-Induced Cardiomyopathy by Repressing the CD36-TLR4 Axis. Circ. Res. 2024, 134, 505–525. [Google Scholar] [CrossRef]
- Ichihara, A.; Kaneshiro, Y.; Takemitsu, T.; Sakoda, M.; Nakagawa, T.; Nishiyama, A.; Kawachi, H.; Shimizu, F.; Inagami, T. Contribution of nonproteolytically activated prorenin in glomeruli to hypertensive renal damage. J. Am. Soc. Nephrol. 2006, 17, 2495–2503. [Google Scholar] [PubMed]
- Ichihara, A.; Kaneshiro, Y.; Takemitsu, T.; Sakoda, M.; Suzuki, F.; Nakagawa, T.; Nishiyama, A.; Inagami, T.; Hayashi, M. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension 2006, 47, 894–900. [Google Scholar]
- Ichihara, A. (Pro)renin receptor and vacuolar H+-ATPase. Keio J. Med. 2012, 61, 73–78. [Google Scholar]
- Xia, Y.; Liu, N.; Xie, X.; Bi, G.; Ba, H.; Li, L.; Zhang, J.; Deng, X.; Yao, Y.; Tang, Z.; et al. The macrophage-specific V-ATPase subunit ATP6V0D2 restricts inflammasome activation and bacterial infection by facilitating autophagosome-lysosome fusion. Autophagy 2019, 15, 960–975. [Google Scholar] [CrossRef]
- Gan, Z.; Guo, Y.; Zhao, M.; Ye, Y.; Liao, Y.; Liu, B.; Yin, J.; Zhou, X.; Yan, Y.; Yin, Y.; et al. Excitatory amino acid transporter supports inflammatory macrophage responses. Sci. Bull. 2024, 69, 2405–2419. [Google Scholar] [CrossRef]
- Banerjee, S.; Kane, P.M. Regulation of V-ATPase Activity and Organelle pH by Phosphatidylinositol Phosphate Lipids. Front. Cell Dev. Biol. 2020, 8, 510. [Google Scholar] [CrossRef]
- Mitra, C.; Winkley, S.; Kane, P.M. Human V-ATPase a-subunit isoforms bind specifically to distinct phosphoinositide phospholipids. J. Biol. Chem. 2023, 299, 105473. [Google Scholar] [CrossRef]
- Larsen, L.E.; van den Boogert, M.A.W.; Rios-Ocampo, W.A.; Jansen, J.C.; Conlon, D.; Chong, P.L.E.; Levels, J.H.M.; Eilers, R.E.; Sachdev, V.V.; Zelcer, N.; et al. Defective Lipid Droplet-Lysosome Interaction Causes Fatty Liver Disease as Evidenced by Human Mutations in TMEM199 and CCDC115. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-Q.; Sun, X.-J.; Zhu, Y.; Liu, N.-F. PDK4 promotes vascular calcification by interfering with autophagic activity and metabolic reprogramming. Cell Death Dis. 2020, 11, 991. [Google Scholar] [CrossRef]
- Rath, S.; Liebl, J.; Fürst, R.; Vollmar, A.M.; Zahler, S. Regulation of endothelial signaling and migration by v-ATPase. Angiogenesis 2014, 17, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ma, J.; Hao, Z.; Li, W. HPS6 Regulates the Biogenesis of Weibel-Palade Body in Endothelial Cells Through Trafficking v-ATPase to Its Limiting Membrane. Front. Cell Dev. Biol. 2021, 9, 743124. [Google Scholar] [CrossRef]
- Sava, I.; Davis, L.J.; Gray, S.R.; Bright, N.A.; Luzio, J.P. Reversible assembly and disassembly of V-ATPase during the lysosome regeneration cycle. Mol. Biol. Cell 2024, 35, ar63. [Google Scholar] [CrossRef]

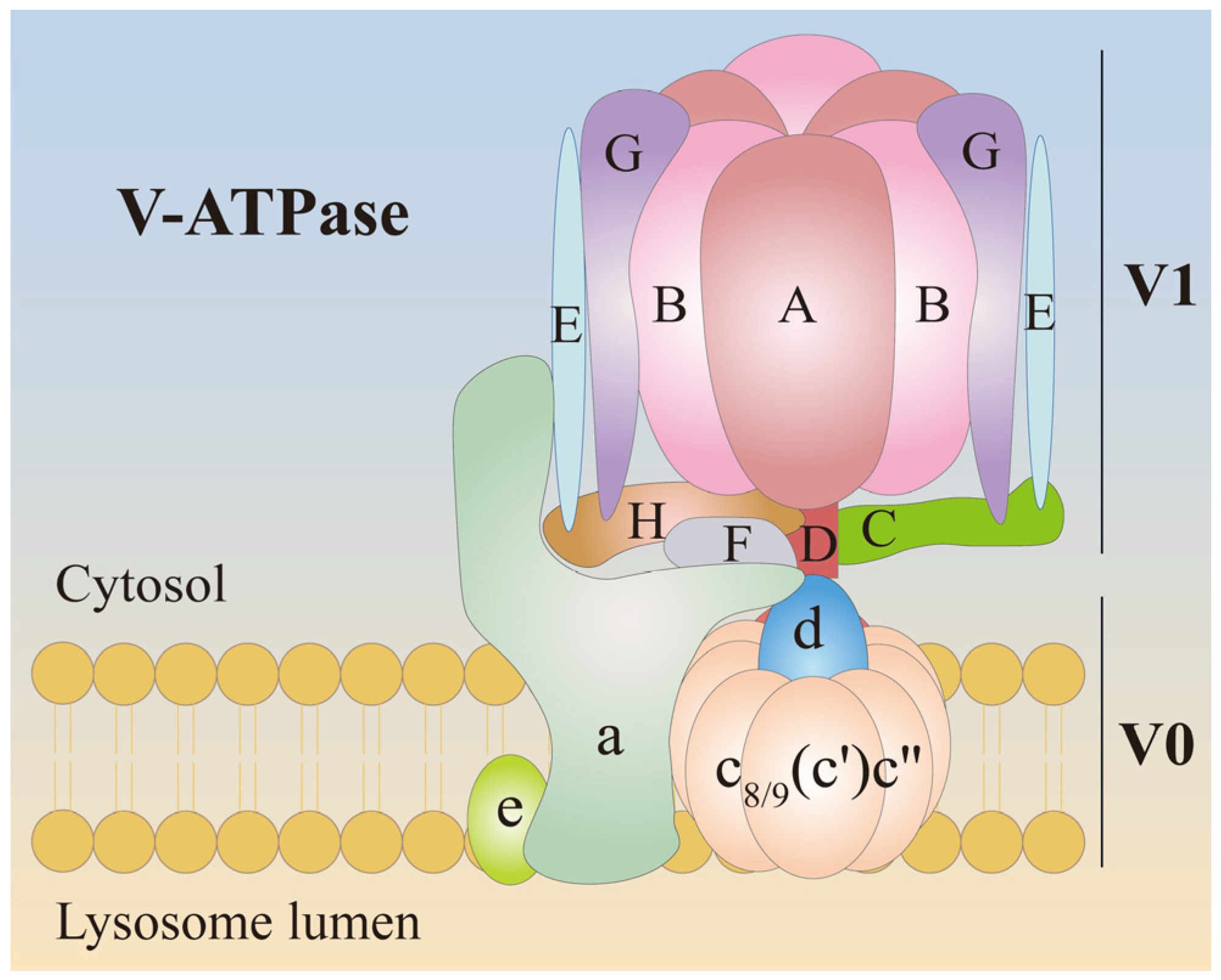

| Type | Regulator | V-ATPase Activity | Location/Cell | Regulation Mechanism | Ref. |
|---|---|---|---|---|---|
| Regulated Assembly | Aldolase | - | Endosome/lysosome, HeLa cells | Aldolase engages with the transmembrane a subunit within the V0 sector and the soluble E and B subunits of the V1 sector | [29] |
| Aldolase | ↑ | Yeast | Disruption of the binding between aldolase and the B subunit of V-ATPase results in disassembly and malfunction of V-ATPase | [30] | |
| The Ras/cAMP/PKA pathway | ↑ | Yeast | Active Ras2 blocks V-ATPase dissociation, PKA regulates V-ATPase assembly, and Active Ras2 affects the aldolase/V-ATPase interaction | [31] | |
| NDST3 | ↓ | Human retina pigmented epithelial (RPE1) cells | Loss of NDST3 enhances the assembly of the V-ATPase holoenzyme on the lysosomal membrane via microtubule acetylation | [32] | |
| Glucose | ↑ | Yeast | Depriving the yeast cells of glucose, even for as little as 5 min, caused dissociation of approximately 70% of the assembled enzyme complexes into separate V1 and V0 subcomplexes | [33,34] | |
| Glucose | ↓ | Mammalian cells | Glucose starvation can enhance the assembly of V-ATPase via the AMPK and PI3K/Akt signaling pathways | [35] | |
| Amino acid | ↑ | HEK293T cells | Starvation for amino acids can enhance the assembly of the V-ATPase complex | [36] | |
| RAVE complex | ↑ | Yeast | The RAVE complex associates reversibly with V1 subcomplexes | [37] | |
| EGF | ↑ | Primary rat hepatocytes | EGF induces V-ATPase assembly | [38] | |
| RILP | ↑ | Hela cells | RILP recruits the ATP6V1G1 subunit to the membranes of late endosomes and lysosomes, and ensures the ATP6V1G1 stability | [39] | |
| PSEN1 | ↓ | Neurons | PSEN1 plays a crucial role in facilitating the N-glycosylation process of the V0a1 subunit within the endoplasmic reticulum | [40] | |
| TMEM55B | ↑ | Neuro2A cells | TMEM55B interacts with V-ATPase to result in the assembly of the V-ATPase complex in the lysosomal membrane lipid rafts | [41] | |
| IGF2R | ↓ | C57BL/6 mice, bone marrow–nucleated cells, and THP1 cells | IGF2R induces Dnmt3a-mediated DNA methylation by activating GSK3α/β and subsequently blocks expression and assembly of V-ATPase | [42] | |
| HDAC6 | ↑ | C57BL/6 mice, HT22 | HDAC6 mediates V-ATPase assembly | [43] | |
| Regulated Trafficking | AMPK | ↓ | The apical membrane in renal and epididymal cells; the renal cells | AMPK was found to block PKA-induced apical accumulation of the V-ATPase; AMPK directly phosphorylated subunit A | [44,45] |
| cAMP | ↑ | The apical membrane in renal | The elevation of cAMP prompts the insertion of V-ATPase into the apical membrane, a process in which PKA-dependent phosphorylation of the subunit a plays a pivotal role | [46,47,48] | |
| PAT2 | ↑ | Brown adipocytes | PAT2 facilitates the assembly of the lysosomal V-ATPase complex by bringing cytosolic V1 subunits to the lysosomal membrane | [49] | |
| Regulation of Subunit Expression Levels | mTORC1 | ↑ | Endosome/lysosome, mouse embryo fibroblasts | V-ATPases are regulated transcriptionally by mTORC1 through Tfeb | [50] |
| Clathrin coat | ↓ | Mouse brain | Clathrin coat controls synaptic vesicle acidification by blocking vacuolar ATPase activity | [51] | |
| FBXO9 | ↓ | A549 and H1299 cells | FBXO9 promotes the ubiquitination of subunit ATP6V1A to hinder the V-ATPase assembly | [52] | |
| Lamtor5 | ↑ | Macrophages and peripheral blood mononuclear cells (PBMCs) from gender and age-matched systemic lupus erythematosus (SLE) patients | Lamtor5 is physically associated with ATP6V1A to promoting the V0/V1 assembly | [53] | |
| DDRGK1 | ↑ | Mouse embryonic cells | DDRGK1 inhibits ubiquitin–proteasome-mediated degradation of V-ATPase subunits (including ATP6V0d1 and ATP6V1A) and maintains the stable expression of them | [54] | |
| miR-1 | ↓ | Muscle tissue | miR-1 directly down-regulates the subunit vha-13/ATP6V1A via its 3′UTR | [55] | |
| RORα | ↑ | C57BL/6 mice | RORα induces the transcription of ATP6V1G1 | [56] | |
| Prosapogenin A | ↑ | 8505C and KHM-5M cells | Prosapogenin A significantly upregulates ATP6V1A, ATP6V1B2, and ATP6V0C | [57] | |
| Post-Translational Modifications | The disulfide bond | ↓ | Bovine | The disulfide bond formed between cysteine 254 and cysteine 532 in the A subunit of bovine V-ATPase | [58] |
| Adjustments in Coupling Efficiency | Specific mutations in distinct non-homologous areas of subunit A | ↓ | Yeast | Specific mutations in distinct non-homologous areas of subunit A can enhance this coupling efficiency | [59] |
| Subunit a isoform | ↑ | Yeast | - | [60] | |
| Protein–Protein Interaction | ITM2A | ↓ | HEK293T cells | ITM2A interacts with V-ATPase to negatively regulate the activity of V-ATPase | [61] |
| ZNRF2 | ↑ | Mouse embryonic fibroblasts | ZNRF2 interacts with V-ATPase and positively regulates its function | [62] | |
| Changes in Counterion Conductance | The ClC-5 chloride channel | ↓ | Active kidney cells | - | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.; Liu, C.-X.; Liu, H.-X.; Wen, S.-Y. The Emerging Roles of Vacuolar-Type ATPase-Dependent Lysosomal Acidification in Cardiovascular Disease. Biomolecules 2025, 15, 525. https://doi.org/10.3390/biom15040525
Chen Y-Y, Liu C-X, Liu H-X, Wen S-Y. The Emerging Roles of Vacuolar-Type ATPase-Dependent Lysosomal Acidification in Cardiovascular Disease. Biomolecules. 2025; 15(4):525. https://doi.org/10.3390/biom15040525
Chicago/Turabian StyleChen, Yan-Yan, Cai-Xia Liu, Hai-Xin Liu, and Shi-Yuan Wen. 2025. "The Emerging Roles of Vacuolar-Type ATPase-Dependent Lysosomal Acidification in Cardiovascular Disease" Biomolecules 15, no. 4: 525. https://doi.org/10.3390/biom15040525
APA StyleChen, Y.-Y., Liu, C.-X., Liu, H.-X., & Wen, S.-Y. (2025). The Emerging Roles of Vacuolar-Type ATPase-Dependent Lysosomal Acidification in Cardiovascular Disease. Biomolecules, 15(4), 525. https://doi.org/10.3390/biom15040525





