Improving Fat Graft Survival Using Soluble Molecule Preconditioning
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Articles describing only graft survival enhancement techniques that did not involve growth factors/molecules;
- (2)
- Reviews and meta-analyses;
- (3)
- Studies combining molecules and cell-based therapy.
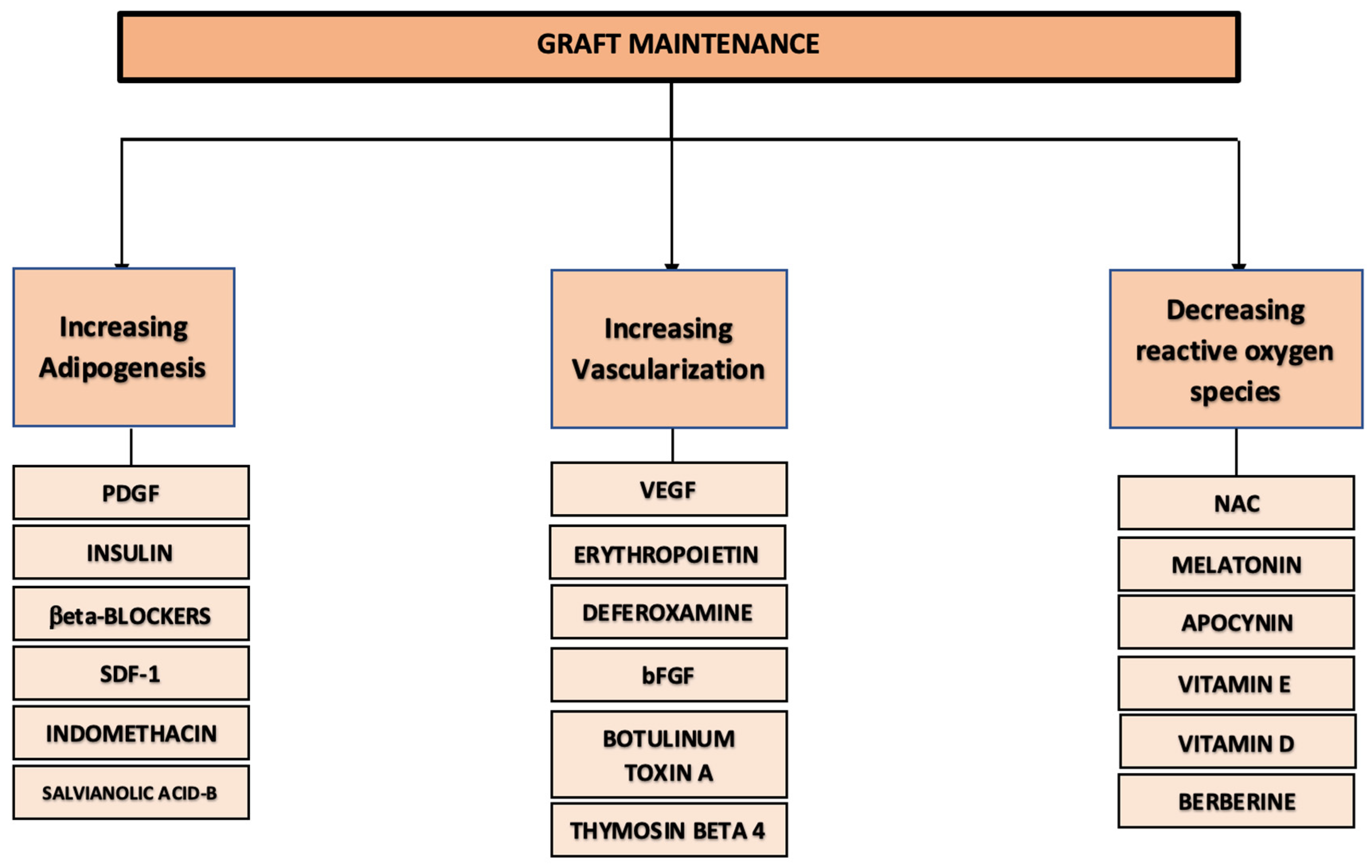
3. Discussion
3.1. Stimulating Adipogenesis
3.1.1. Platelet-Derived Growth Factor (PDGF)
3.1.2. Insulin
3.1.3. Beta-Blockers
3.1.4. Other Molecules of Interest Impacting Adipogenesis and Adipocytes
3.2. Improving Vascularization
3.2.1. Vascular Endothelial Growth Factor (VEGF)
3.2.2. Erythropoietin (EPO)
3.2.3. Deferoxamine (DFX)
3.2.4. Basic Fibroblast Growth Factor (bFGF)
3.2.5. Botulinum Toxin A (BTX)
3.2.6. Thymosin Beta 4 (TB4)
3.3. Reducing Oxidative Stress
3.3.1. N-Acetylcysteine (NAC)
3.3.2. Other Antioxidants of Interest
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Model | Reference | Injection Site | Graft Treatment | Graft Analysis | Findings |
|---|---|---|---|---|---|
| PDGF | |||||
| Human patients | Fontdevila et al. (2014) [17] | Cheeks (various planes) | A total of 2 groups of HIV patients suffering from facial lipodystrophy. Autologous lipoaspirate mixed with PRP rich in PDGF (n = 29 patients) or left untreated (n = 20 patients). Volumes were specific for each patient. |
|
|
| Rodent models | Craft et al. (2007) [22] | Scalp (subcutaneous) | A total of 3 groups (n = 8 mice/group). A total of 1 mL of human fat was left untreated or mixed with free PDGF, PDGF bound to gelatin microspheres, or blank gelatin microspheres. |
|
|
| INSULIN | |||||
| Human patients | Cervelli et al. (2012) [35] | Different zones of soft tissue defect depending on the patient | A total of n = 39 patients. Autologous lipoaspirate mixed with variable volumes of PRP. Volumes of lipoaspirate varied between patients. A total of 10 patients were locally injected with insulin 7 and 15 days after fat grafting. |
|
|
| Rodent models | Olaru et al. (2020) [36] | Dorsum (subcutaneous) | A total of 4 groups (n = 6 rats/group). A total of 1 mL of fat alone or mixed with EPO, insulin, or a mix of insulin + EPO. |
|
|
| Okyay et al. (2019) [30] | Dorsum. A total of 4 injections, 1 site for each treatment (subcutaneous) | A total of 4 groups (n = 22 rats). A total of 1 mL of autologous fat injected after being incubated for 5 min in a solution of insulin, metoprolol, deferoxamine (DFX), or PBS. |
|
| |
| Hong et al. (2010) [28] | Dorsum. A total of 4 injections, 1 site for each treatment (subcutaneous) | A total of 4 groups (n = 24 rabbits). A total of 2 mg of autologous fat injected after being soaked for 5 min in PBS, DMEM, DMEM + insulin, or DMEM + insulin + bFGF. |
|
| |
| Lu et al. (2009) [31] | Dorsum. A total of 4 injections, 1 site for each treatment (subcutaneous) | A total of 4 groups (n = 18 mice). A total of 0.3 mL of human lipoaspirate was mixed with adenovirally VEGF transduced ASCs, control ASCs, insulin, or DMEM. |
|
| |
| Ayhan et al. (2001) [32] | Dorsum. A total of 2 injections, 1 site for each treatment (supramuscular) | A total of 2 groups (n = 10/group).
|
|
| |
| Yuksel et al. (1999) [29] | Dorsum (under the panniculus carnosus) | A total of 7 groups (n = 6 mice/group). A total of 500 mg of autologous fat left untreated or mixed with PLGA containing different growth factors: insulin or IGF-1 or bFGF or insulin + IGF-1 or insulin + IGF-1 + bFGF or empty. |
|
| |
| Moscona et al. (1994) [33] | Cheeks (subcutaneous) | Four groups (n = 3 rats/group):
|
|
| |
| Nguyen et al. (1990) [34] | Ear (subcutaneous) and rectus muscle (intramuscular) | A total of 2 groups (n = 28 rabbits). A total of 3 to 4 mL of autologous fat mixed with insulin and injected into the same animal (ear and rectus muscle). The other side was used as control and injected with untreated fat. |
| No histopathologic differences in the adipocytes transplanted with or without insulin in all examined grafts. | |
| BETA-BLOCKERS (Metoprolol/Metapyrolol) | |||||
| Rodent models | Okyay et al. (2023) [39] | Dorsum. A total of 4 injections, 1 site for each treatment (subcutaneous) | A total of 4 groups (n = 10 rats). A total of 0.5 g of autologous fat injected after being incubated for 5 min in a solution as follows:
|
|
|
| Okyay et al. (2019) [30] | Dorsum. A total of 4 injections, 1 site for each treatment (subcutaneous) | A total of 4 groups (n = 22 rats). A total of 1 mL of autologous fat injected after being incubated for 5 min in a solution of insulin, metoprolol, deferoxamine, or PBS. |
|
| |
| Ayhan et al. (2001) [32] | Dorsum. A total of 2 injections, 1 site for each treatment (supramuscular) | A total of 2 groups (n = 10 rats/group).
|
|
| |
| SDF-1 | |||||
| Rodent models | Hamed et al. (2012) [43] | Scalp (subcutaneous) | A total of 3 groups (n = 10 mice/group) A total of 1 mL of autologous fat mixed with PBS, SDF-1, or monoclonal antibody against SDF-1 and injected into diabetic mice. |
|
|
| INDOMETHACIN | |||||
| Rodent models | Zhan et al. (2017) [45] | Dorsum (subcutaneous) | A total of 4 groups (n = 5 mice/group):
|
|
|
| SALVIANOLIC ACID B (SAL-B) | |||||
| Rodent models | Sun et al. (2023) [48] | Dorsum (subcutaneous) | A total of 3 groups (n = 15 mice/group):
|
|
|
| Sun et al. (2021) [47] | Dorsum (subcutaneous). The same injection on both sides | A total of 3 groups (n = 6 mice/group):
|
|
Sal-B groups had lower fibrosis and inflammatory cell infiltration. Adipocyte viability was increased in treatment groups. | |
Appendix B
| Model | Reference | Injection Site | Graft Treatment | Graft Analysis | Findings |
|---|---|---|---|---|---|
| VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) | |||||
| Rodent models | Zhang et al. (2021) [161] | Dorsum. A total of 4 injections, 1 site for each treatment (subcutaneous) | A total of 4 treatments (n = 12 mice/treatment). A total of 0.8 mL of human lipoaspirate mixed with different ratios of Liquid Phase Concentrated Growth Factors (LPCGFs) or different ratios of PRP. |
|
|
| Ding et al. (2015) [59] | Dorsum. A total of 4 injections, 1 site for each treatment (subcutaneous) | A total of 4 groups (n = 24 mice). A total of 0.2 mL of human lipoaspirate mixed with VEGF calcium alginate (CA) microspheres, empty CA microspheres, free VEGF, or DMEM. |
|
| |
| Zhang et al. (2014) [57] | Dorsum. Four injections; one site for each treatment (subcutaneous) | A total of 4 groups (n = 28 mice). A total of 0.2 mL of human lipoaspirate mixed with VEGF chitosan nanospheres, empty nanospheres, free VEGF, or DMEM. |
|
| |
| Topcu et al. (2012) [58] | Dorsum (under the panniculus carnosus) | A total of 4 groups (n = 6 mice/group) A total of 0.5 g of autologous fat left untreated or mixed with VEGF-enriched calcium alginate (CA) microspheres or empty CA microspheres. Another group received VEGF microsphere injection at recipient site 21 days prior to grafting. |
|
| |
| Chung et al. (2012) [55] | Flank (subcutaneous) | A total of 3 groups (n = 6 mice/group). A total of 1 mL of human lipoaspirate untreated or mixed with VEGF-loaded PLGA microspheres or empty microspheres. |
|
| |
| Hamed et al. (2010) [54] | Scalp (subcutaneous) | First experiment A total of 3 groups (n = 10 mice/group). A total of 1 mL of human lipoaspirate mixed with PBS, EPO, or VEGF. Grafts then received injections of the same additive every 3 days for 18 days. Second experiment A total of 2 groups (n = 10 mice/group). Untreated fat grafts received PBS or VEGF injections every 3 days for 18 days. |
|
| |
| Lei et al. (2008) [162] | Dorsum (subcutaneous) | A total of 3 groups (n = 16 rats/group). After injection of 0.2 g of autologous fat, recipient sites were injected with normal saline, plasmid DNA encoding rhVEGF, or control plasmid DNA. |
|
| |
| ERYTHROPOIETIN (EPO) | |||||
| Rodent models | Olaru et al. (2020) [36] | Dorsum (subcutaneous) | A total of 4 groups (n = 6 rats/group). A total of 1 mL of fat alone or mixed with EPO, insulin or insulin + EPO. |
|
|
| Hamed et al. (2010) [54] | Scalp (subcutaneous) | First experiment A total of 3 groups (n = 10 mice/group) A total of 1 mL of human lipoaspirate mixed with PBS, low-dose EPO (1000 IU/kg), or high-dose EPO (5000 IU/kg). Grafts then received injections of the same additive every 3 days for 18 days. Second experiment A total of 2 groups (n = 10 mice/group). Untreated fat grafts receiving PBS or VEGF injections every 3 days for 18 days. |
|
| |
| DEFEROXAMINE (DFX) | |||||
| Rodent models | Lin et al. (2023) [85] | Dorsum. Four injections; one site for each treatment (subcutaneous) | A total of 4 treatments (n = 25 mice). A total of 0.3 mL of autologous fat injected after being mixed with different concentrations of DFX:
|
|
|
| Okyay et al. (2019) [30] | Dorsum. Four injections; one site for each treatment (subcutaneous) | A total of 4 groups (n = 22 rats). A total of 0.5 g of autologous fat injected after being incubated for 5 min in a solution of insulin, metoprolol, DFX, or PBS. |
|
| |
| Kim et al. (2019) [76] | Scalp (supramuscular) | A total of 3 groups (n = 6 rats/group). Injection site was left untreated (negative controls) or preconditioned with serial injections of DFX or saline every 2 days (5 treatments in total). |
|
| |
| Flacco et al. (2018) [77] | Scalp (irradiated skin) (subcutaneous) | A total of 2 groups (n = 6 mice/group). After 6 doses of radiation to the scalp, mice either received DFX injections on the scalp every 2 days (7 doses) or PBS injections in the irradiated zone. A total of 0.2 mL of human lipoaspirate fat grafting was performed afterwards. |
|
| |
| Temiz et al. (2016) [73] | Scalp (subcutaneous) | A total of 3 groups (n = 8 rats/group):
|
|
| |
| FIBROBLAST GROWTH FACTOR (bFGF or FGF-2) | |||||
| Human patients | Tamura et al. (2015) [97] | Vocal fold. One vocal cord for each treatment (intramuscular) | A total of 2 groups. Autologous fat alone (n = 36 patients) or mixed with a collagen sponge containing bFGF PLGA microspheres (N = 8 patients) (mean volume of injection: 0.2 mL). |
|
|
| Canine model | Tamura et al. (2007) [96] | Vocal fold. One vocal cord for each treatment (intramuscular) | A total of 2 groups (n = 12 dogs). A total of 0.5 mL of autologous fat alone or mixed with bFGF gelatin microspheres. |
|
|
| Rodent models | Hong et al. (2010) [28] | Dorsum. Four injections; one site for each treatment (subcutaneous) | A total of 4 groups (n = 24 rabbits). A total of 2 mg of autologous fat injected after being soaked for 5 min in PBS, DMEM, DMEM + insulin, or DMEM + insulin + bFGF. |
|
|
| Nakamura et al. (2010) [95] | Dorsum. Two injections; one site for each treatment (subcutaneous) | A total of 2 groups (n = 48 rats). A total of 0.8 g of autologous fat mixed with empty fragmin/protamin (FP) microspheres or bFGF-loaded FP microspheres. |
|
| |
| Yuksel et al. (1999) [29] | Dorsum (under the panniculus carnosus) | A total of 7 groups (n = 6 mice/group). A total of 0.5 g of autologous fat left untreated or mixed with PLGA containing different growth factors: insulin or IGF-1 or bFGF or insulin + IGF-1 or insulin + IGF-1 + bFGF or empty. |
|
| |
| Eppley et al. (1992) [98] | Dorsum Four injections; one site for each treatment (subcutaneous) | A total of 4 groups (n = 40 rats). A total of 500 mg of autologous fat left untreated or mixed with blank dextran microspheres, dextran microspheres soaked in cytochrome C (control solution), or dextran microspheres soaked in bFGF solution for 1 min. |
|
| |
| Eppley et al. (1992) [100] | Cheeks. Two injections; one side for each treatment (subcutaneous) | A total of 2 groups (n = 20 rats). A total of 250 mg of autologous fat mixed with bFGF solution or dextran microspheres soaked in bFGF solution for 15 min. |
|
| |
| Eppley et al. (1991) [99] | Face. Two injections; one side for each treatment (subcutaneous) | A total of 2 groups (n = 15 rats). An average of 0.3 g of autologous fat alone or mixed with dextran microspheres soaked in bFGF solution for 1 min. |
|
| |
| BOTULINUM TOXIN A (BTX) | |||||
| Human patients | Liu et al. (2024) [107] | Breasts | A total of 2 groups (n = 16 women patients). One breast received autologous lipoaspirate alone, and the other breast received autologous lipoaspirate mixed with 100 U of BTX. |
|
|
| Rodent models | Yoon et al. (2021) [110] | Center of the ear | A total of 4 groups (n = 10 rabbits/group). A total of 1.5 mL of human lipoaspirate mixed with saline, BTX, prostaglandin E2, or polydeoxyribonucleotides (PDRNs). |
|
|
| Wu et al. (2020) [111] | Quadriceps (supramuscular) | A total of 0.3 mL of human lipoaspirate injected onto the surface of the right quadriceps. A total of 3 groups (n = 24 mice/group):
|
|
| |
| Shi et al. (2019) [113] | Limbs: quadriceps femoris and gastrocnemius (subcutaneous and intramuscular) | A total of 2 treatments (n = 12 rats). Rats received 0.2 mL of fat mixed with BTX in one limb and fat mixed with PBS on the other one. For each side, intramuscular and subcutaneous injections were performed. |
|
| |
| Tang et al. (2017) [108] | Dorsum. Two injections; one site for each treatment (supramuscular) | A total of 2 groups (n = 6 rats). Autologous fat (mean: 1 mL) alone or mixed with BTX. In vitro, ASCs were isolated from fat of other rats and incubated with various BTX concentrations for a day. |
|
| |
| Baek et al. (2012) [109] | Dorsum Two injections; one site for each treatment (supramuscular) | A total of 2 groups (n = 8 mice). A total of 0.5 mL of fat from rats mixed with BTX or PBS before injection onto mice backs. |
|
| |
| THYMOSIN BETA 4 (TB4) | |||||
| Rodent models | Qu et al. (2020) [122] | Ears (subcutaneous) | A total of 3 groups (n = 6 rabbits/group).
|
|
|
Appendix C
| Model | Reference | Injection Site | Graft Treatment | Graft Analysis | Findings |
|---|---|---|---|---|---|
| N-ACETYLCYSTEIN (NAC) | |||||
| Human patients | Pietruski et al. (2021) [126] | Breasts (each breast receiving one of the two treatments) | A total of 2 groups (n = 15 women). A total of 145 mL of autologous lipoaspirate harvested using normal tumescent solution in one thigh and NAC-enriched tumescent solution on the other. |
|
|
| Rodent model | Gillis et al. (2015) [123] | Scalp (subcutaneous) | A total of 2 groups (n = 15 mice/group). A total of 0.2 mL of autologous fat harvested using tumescent solution with or without NAC. |
|
|
| MELATONIN | |||||
| Rodent model | Cinar et al. (2023) [139] | Dorsum (subcutaneous) | A total of 4 groups (n = 8 rats/group) After transplantation of autologous fat (volume varying depending on the amount of fat collected), rats underwent a specific diet every day for 3 months:
|
|
|
| Dang et al. (2023) [133] | Scalp (subcutaneous) | A total of 4 groups (n = 18 mice/group) After transplantation of 0.3 g of autologous fat, mice were treated every day for 2 weeks with different doses of oral melatonin.
|
|
| |
| VITAMIN E | |||||
| Rodent model | Cinar et al. (2023) [139] | Dorsum (subcutaneous) | A total of 4 groups (n = 8 rats/group). After transplantation of fat, rats underwent a specific diet every day for 3 months:
|
|
|
| Abbas et al. (2022) [137] | Scalp (subcutaneous) | A total of 4 groups (n = 10 mice/group): mice underwent scalp irradiation and recovered for 4 weeks.
|
|
| |
| VITAMIN D (CALCITRIOL) | |||||
| Rodent model | Loder et al. (2023) [140] | Dorsum (subcutaneous) | A total of 0.3 mL of human lipoaspirate with one of four different treatments:
|
|
|
| APOCYNIN | |||||
| Rodent model | Keskin et al. (2021) [146] | Scalp (subcutaneous) | A total of 3 groups (n = 7 rats/group): a total of 0.4 to 0.8 g of autologous fat. After grafting, there were 3 groups of treatment.
|
|
|
| BERBERINE (BBR) | |||||
| Rodent model | Pang et al. (2023) [149] | Dorsum and neck (subcutaneous) | A total of 2 groups (n = 10 mice/group): 0.2 mL of human lipoaspirate. Treated grafts were soaked in BBR for 10 min. After transplantation, grafts had daily injections of saline or BBR (4 mM). |
|
|
References
- Mazzola, R.F.; Mazzola, I.C. The fascinating history of fat grafting. J. Craniofacial Surg. 2013, 24, 1069–1071. [Google Scholar]
- Chappell, A.G.; Yuksel, S.; Sasson, D.C.; Wescott, A.B.; Connor, L.M.; Ellis, M.F. Post-Mastectomy Pain Syndrome: An Up-to-Date Review of Treatment Outcomes. JPRAS Open 2021, 30, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Cederna, P.S.; Rubin, J.P.; Coleman, S.R.; Levi, B. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast. Reconstr. Surg. 2015, 136, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Saffari, T.M.; Saffari, S.; Vyas, K.S.; Mardini, S.; Shin, A.Y. Role of adipose tissue grafting and adipose-derived stem cells in peripheral nerve surgery. Neural Regen. Res. 2022, 17, 2179–2184. [Google Scholar] [CrossRef]
- Laloze, J.; Varin, A.; Bertheuil, N.; Grolleau, J.L.; Vaysse, C.; Chaput, B. Cell-assisted lipotransfer: Current concepts. Ann. Chir. Plast. Esthet. 2017, 62, 609–616. [Google Scholar] [CrossRef]
- Carpaneda, C.A.; Ribeiro, M.T. Study of the histologic alterations and viability of the adipose graft in humans. Aesthetic Plast. Surg. 1993, 17, 43–47. [Google Scholar] [CrossRef]
- Pu, L.L. Mechanisms of Fat Graft Survival. Ann. Plast. Surg. 2016, 77 (Suppl. S1), S84–S86. [Google Scholar] [CrossRef]
- Zhao, J.; Yi, C.; Li, L.; Zheng, Y.; Wu, K.; Liang, L.; Xia, W.; Guo, S. Observations on the survival and neovascularization of fat grafts interchanged between C57BL/6-gfp and C57BL/6 mice. Plast. Reconstr. Surg. 2012, 130, 398e–406e. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Y.; Mu, D. Fat Processing Techniques: A Narrative Review. Aesthetic Plast. Surg. 2021, 45, 730–739. [Google Scholar] [CrossRef]
- Trotzier, C.; Sequeira, I.; Auxenfans, C.; Mojallal, A.A. Fat Graft Retention: Adipose Tissue, Adipose-Derived Stem Cells, and Aging. Plast. Reconstr. Surg. 2023, 151, 420e–431e. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, J.; Lu, F.; Dong, Z. Survival Mechanisms and Retention Strategies in Large-Volume Fat Grafting: A Comprehensive Review and Future Perspectives. Aesthetic Plast. Surg. 2024, 48, 4178–4193. [Google Scholar] [CrossRef]
- Eto, H.; Kato, H.; Suga, H.; Aoi, N.; Doi, K.; Kuno, S.; Yoshimura, K. The fate of adipocytes after nonvascularized fat grafting: Evidence of early death and replacement of adipocytes. Plast. Reconstr. Surg. 2012, 129, 1081–1092. [Google Scholar] [CrossRef]
- Molitor, M.; Trávníčková, M.; Měšťák, O.; Christodoulou, P.; Sedlář, A.; Bačáková, L.; Lucchina, S. The Influence of High and Low Negative Pressure Liposuction and Various Harvesting Techniques on the Viability and Function of Harvested Cells-a Systematic Review of Animal and Human Studies. Aesthetic Plast. Surg. 2021, 45, 2379–2394. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mo, M.; Wang, J.; Sadia, S.; Shi, B.; Fu, X.; Yu, L.; Tredget, E.E.; Wu, Y. Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cell. Mol. Life Sci. 2018, 75, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, M.; Löffler, G. Influence of growth factors on growth and differentiation of 3T3-L1 preadipocytes in serum-free conditions. Eur. J. Cell Biol. 1995, 68, 323–329. [Google Scholar] [PubMed]
- Staiger, H.; Löffler, G. The role of PDGF-dependent suppression of apoptosis in differentiating 3T3-L1 preadipocytes. Eur. J. Cell Biol. 1998, 77, 220–227. [Google Scholar] [CrossRef]
- Fontdevila, J.; Guisantes, E.; Martínez, E.; Prades, E.; Berenguer, J. Double-blind clinical trial to compare autologous fat grafts versus autologous fat grafts with PDGF: No effect of PDGF. Plast. Reconstr. Surg. 2014, 134, 219e–230e. [Google Scholar] [CrossRef]
- Guzman, N.; Vijayan, V. HIV-associated Lipodystrophy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lee, S.J. Cytokine delivery and tissue engineering. Yonsei Med. J. 2000, 41, 704–719. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Wang, Y.; Wang, Q.; Yao, M.; Zhao, L.; Shi, J.; Guan, F.; Ma, S. PDGF-BB/SA/Dex injectable hydrogels accelerate BMSC-mediated functional full thickness skin wound repair by promoting angiogenesis. J. Mater. Chem. B 2021, 9, 6176–6189. [Google Scholar] [CrossRef]
- Kang, S.; Yoon, J.S.; Lee, J.Y.; Kim, H.J.; Park, K.; Kim, S.E. Long-term local PDGF delivery using porous microspheres modified with heparin for tendon healing of rotator cuff tendinitis in a rabbit model. Carbohydr. Polym. 2019, 209, 372–381. [Google Scholar] [CrossRef]
- Craft, R.O.; Rophael, J.; Morrison, W.A.; Vashi, A.V.; Mitchell, G.M.; Penington, A.J. Effect of local, long-term delivery of platelet-derived growth factor (PDGF) on injected fat graft survival in severe combined immunodeficient (SCID) mice. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Artemenko, Y.; Gagnon, A.; Aubin, D.; Sorisky, A. Anti-adipogenic effect of PDGF is reversed by PKC inhibition. J. Cell. Physiol. 2005, 204, 646–653. [Google Scholar] [CrossRef]
- Blüher, S.; Kratzsch, J.; Kiess, W. Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 577–587. [Google Scholar] [CrossRef]
- Thong, F.S.; Dugani, C.B.; Klip, A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology 2005, 20, 271–284. [Google Scholar] [CrossRef]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef]
- Yuksel, E.; Weinfeld, A.B.; Cleek, R.; Waugh, J.M.; Jensen, J.; Boutros, S.; Shenaq, S.M.; Spira, M. De novo adipose tissue generation through long-term, local delivery of insulin and insulin-like growth factor-1 by PLGA/PEG microspheres in an in vivo rat model: A novel concept and capability. Plast. Reconstr. Surg. 2000, 105, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Lee, J.H.; Hong, S.M.; Park, C.H. Enhancing the viability of fat grafts using new transfer medium containing insulin and beta-fibroblast growth factor in autologous fat transplantation. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, E.; Weinfeld, A.B.; Cleek, R.; Wamsley, S.; Jensen, J.; Boutros, S.; Waugh, J.M.; Shenaq, S.M.; Spira, M. Increased free fat-graft survival with the long-term, local delivery of insulin, insulin-like growth factor-I, and basic fibroblast growth factor by PLGA/PEG microspheres. Plast. Reconstr. Surg. 2000, 105, 1712–1720. [Google Scholar] [CrossRef]
- Okyay, M.F.; Kömürcü, H.; Bağhaki, S.; Demiröz, A.; Aydın, Ö.; Arslan, H. Effects of Insulin, Metoprolol and Deferoxamine on Fat Graft Survival. Aesthetic Plast. Surg. 2019, 43, 845–852. [Google Scholar] [CrossRef]
- Lu, F.; Li, J.; Gao, J.; Ogawa, R.; Ou, C.; Yang, B.; Fu, B. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast. Reconstr. Surg. 2009, 124, 1437–1446. [Google Scholar] [CrossRef]
- Ayhan, M.; Senen, D.; Adanali, G.; Görgü, M.; Erdoğan, B.; Albayrak, B. Use of beta blockers for increasing survival of free fat grafts. Aesthetic Plast. Surg. 2001, 25, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Moscona, R.; Shoshani, O.; Lichtig, H.; Karnieli, E. Viability of adipose tissue injected and treated by different methods: An experimental study in the rat. Ann. Plast. Surg. 1994, 33, 500–506. [Google Scholar] [CrossRef]
- Nguyen, A.; Pasyk, K.A.; Bouvier, T.N.; Hassett, C.A.; Argenta, L.C. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast. Reconstr. Surg. 1990, 85, 378–386; discussion 387–379. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Scioli, M.G.; Gentile, P.; Doldo, E.; Bonanno, E.; Spagnoli, L.G.; Orlandi, A. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl. Med. 2012, 1, 206–220. [Google Scholar] [CrossRef]
- Olaru, I.; Sava, A.; Tamaş, C.; Costea, C.F.; Dumitrescu, G.F.; Paşca, A.S.; Olaru, F.; Stamate, T. The significance of erythropoietin and insulin administration on survival of fat tissue after autologous fat transplantation in Wistar rats. An experimental study. Rom. J. Morphol. Embryol. 2020, 61, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fong, C.; Chen, Y.; Cai, G.; Yang, M. Beta-adrenergic signals regulate adipogenesis of mouse mesenchymal stem cells via cAMP/PKA pathway. Mol. Cell. Endocrinol. 2010, 323, 201–207. [Google Scholar] [CrossRef]
- Louis, S.N.; Jackman, G.P.; Nero, T.L.; Iakovidis, D.; Louis, W.J. Role of beta-adrenergic receptor subtypes in lipolysis. Cardiovasc. Drugs Ther. 2000, 14, 565–577. [Google Scholar] [CrossRef]
- Okyay, M.F.; Oztermeli, A. Evaluation of the Effect of Metoprolol Dosage on Fat Graft Survival. Aesthetic Plast. Surg. 2023, 47, 1598–1608. [Google Scholar] [CrossRef]
- Penn, M.S.; Pastore, J.; Miller, T.; Aras, R. SDF-1 in myocardial repair. Gene Ther. 2012, 19, 583–587. [Google Scholar] [CrossRef]
- Ji, J.F.; He, B.P.; Dheen, S.T.; Tay, S.S. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 2004, 22, 415–427. [Google Scholar] [CrossRef]
- Guo, R.; Chai, L.; Chen, L.; Chen, W.; Ge, L.; Li, X.; Li, H.; Li, S.; Cao, C. Stromal cell-derived factor 1 (SDF-1) accelerated skin wound healing by promoting the migration and proliferation of epidermal stem cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Egozi, D.; Dawood, H.; Keren, A.; Kruchevsky, D.; Ben-Nun, O.; Gilhar, A.; Brenner, B.; Ullmann, Y. The chemokine stromal cell-derived factor-1α promotes endothelial progenitor cell-mediated neovascularization of human transplanted fat tissue in diabetic immunocompromised mice. Plast. Reconstr. Surg. 2013, 132, 239e–250e. [Google Scholar] [CrossRef] [PubMed]
- Styner, M.; Sen, B.; Xie, Z.; Case, N.; Rubin, J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J. Cell. Biochem. 2010, 111, 1042–1050. [Google Scholar] [CrossRef]
- Zhan, W.; Tan, S.S.; Han, X.; Palmer, J.A.; Mitchell, G.M.; Morrison, W.A. Indomethacin Enhances Fat Graft Retention by Up-Regulating Adipogenic Genes and Reducing Inflammation. Plast. Reconstr. Surg. 2017, 139, 1093e–1104e. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhao, W.; Zhao, D.; Wang, C.; Yu, N.; An, T.; Mo, F.; Liu, J.; Miao, J.; Lv, B.; et al. Salvianolic Acid B Improves Mitochondrial Function in 3T3-L1 Adipocytes Through a Pathway Involving PPARγ Coactivator-1α (PGC-1α). Front. Pharmacol. 2018, 9, 671. [Google Scholar] [CrossRef]
- Sun, J.M.; Ho, C.K.; Gao, Y.; Chong, C.H.; Zheng, D.N.; Zhang, Y.F.; Yu, L. Salvianolic acid-B improves fat graft survival by promoting proliferation and adipogenesis. Stem Cell Res. Ther. 2021, 12, 507. [Google Scholar] [CrossRef]
- Sun, J.M.; Ho, C.K.; Gao, Y.; Chong, C.H.; Liu, Y.D.; Liu, Y.X.; Zheng, D.N.; Zhang, Y.F.; Yu, L. Salvianolic Acid B Reduces the Inflammation of Fat Grafts by Inhibiting the NF-Kb Signalling Pathway in Macrophages. Aesthetic Surg. J. 2023, 43, Np372–Np390. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Matsumoto, F.; Bujo, H.; Shibasaki, M.; Takahashi, K.; Yoshimoto, S.; Ichinose, M.; Saito, Y. Revascularization determines volume retention and gene expression by fat grafts in mice. Exp. Biol. Med. 2005, 230, 742–748. [Google Scholar] [CrossRef]
- Gurunluoglu, R.; Ozer, K.; Skugor, B.; Lubiatowski, P.; Carnevale, K.; Siemionow, M. Effect of transfection time on the survival of epigastric skin flaps pretreated with adenovirus encoding the VEGF gene. Ann. Plast. Surg. 2002, 49, 161–169. [Google Scholar] [CrossRef]
- He, Y.; Yu, X.; Chen, Z.; Li, L. Stromal vascular fraction cells plus sustained release VEGF/Ang-1-PLGA microspheres improve fat graft survival in mice. J. Cell. Physiol. 2019, 234, 6136–6146. [Google Scholar] [CrossRef]
- Murohara, T.; Horowitz, J.R.; Silver, M.; Tsurumi, Y.; Chen, D.; Sullivan, A.; Isner, J.M. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation 1998, 97, 99–107. [Google Scholar] [CrossRef]
- Simón-Yarza, T.; Formiga, F.R.; Tamayo, E.; Pelacho, B.; Prosper, F.; Blanco-Prieto, M.J. Vascular endothelial growth factor-delivery systems for cardiac repair: An overview. Theranostics 2012, 2, 541–552. [Google Scholar] [CrossRef]
- Hamed, S.; Egozi, D.; Kruchevsky, D.; Teot, L.; Gilhar, A.; Ullmann, Y. Erythropoietin improves the survival of fat tissue after its transplantation in nude mice. PLoS ONE 2010, 5, e13986. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.W.; Marra, K.G.; Li, H.; Leung, A.S.; Ward, D.H.; Tan, H.; Kelmendi-Doko, A.; Rubin, J.P. VEGF microsphere technology to enhance vascularization in fat grafting. Ann. Plast. Surg. 2012, 69, 213–219. [Google Scholar] [CrossRef]
- Li, L.; Pan, S.; Ni, B.; Lin, Y. Improvement in autologous human fat transplant survival with SVF plus VEGF-PLA nano-sustained release microspheres. Cell Biol. Int. 2014, 38, 962–970. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Ding, S.L.; Tang, S.J.; Yang, H.; Shi, H.F.; Shen, X.Z.; Tan, W.Q. Effect of chitosan nanospheres loaded with VEGF on adipose tissue transplantation: A preliminary report. Tissue Eng. Part A 2014, 20, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Topcu, A.; Aydin, O.E.; Ünlü, M.; Barutcu, A.; Atabey, A. Increasing the viability of fat grafts by vascular endothelial growth factor. Arch. Facial Plast. Surg. 2012, 14, 270–276. [Google Scholar] [CrossRef]
- Ding, S.L.; Zhang, M.Y.; Tang, S.J.; Yang, H.; Tan, W.Q. Effect of Calcium Alginate Microsphere Loaded with Vascular Endothelial Growth Factor on Adipose Tissue Transplantation. Ann. Plast. Surg. 2015, 75, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Kang, J.Q.; Maiese, K. Erythropoietin: Cytoprotection in vascular and neuronal cells. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2003, 3, 141–154. [Google Scholar] [CrossRef]
- Warren, J.S.; Zhao, Y.; Yung, R.; Desai, A. Recombinant human erythropoietin suppresses endothelial cell apoptosis and reduces the ratio of Bax to Bcl-2 proteins in the aortas of apolipoprotein E-deficient mice. J. Cardiovasc. Pharmacol. 2011, 57, 424–433. [Google Scholar] [CrossRef]
- Ribatti, D.; Presta, M.; Vacca, A.; Ria, R.; Giuliani, R.; Dell’Era, P.; Nico, B.; Roncali, L.; Dammacco, F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood 1999, 93, 2627–2636. [Google Scholar]
- Nakano, M.; Satoh, K.; Fukumoto, Y.; Ito, Y.; Kagaya, Y.; Ishii, N.; Sugamura, K.; Shimokawa, H. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ. Res. 2007, 100, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, K.; Iso, Y.; Sato, T.; Wakabayashi, K.; Kobayashi, Y.; Takeyama, Y.; Suzuki, H. Effects of erythropoietin on angiogenesis after myocardial infarction in porcine. Heart Vessel. 2012, 27, 79–88. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, F.; He, Q.; Tsang, W.P.; Lu, L.; Li, Q.; Wu, Z.; Qiu, G.; Zhou, G.; Wan, C. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis. PLoS ONE 2014, 9, e102010. [Google Scholar] [CrossRef]
- Undén, J.; Sjölund, C.; Länsberg, J.K.; Wieloch, T.; Ruscher, K.; Romner, B. Post-ischemic continuous infusion of erythropoeitin enhances recovery of lost memory function after global cerebral ischemia in the rat. BMC Neurosci. 2013, 14, 27. [Google Scholar] [CrossRef]
- Kükrek, H.; Aitzetmüller, M.; Vodiškar, M.; Moog, P.; Machens, H.G.; Duscher, D. Erythropoetin can partially restore cigarette smoke induced effects on Adipose derived Stem Cells. Clin. Hemorheol. Microcirc. 2021, 77, 27–36. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Y.; Zhang, Y.; Xiong, Z.; Li, G.; Cui, L. Local injection of deferoxamine improves neovascularization in ischemic diabetic random flap by increasing HIF-1α and VEGF expression. PLoS ONE 2014, 9, e100818. [Google Scholar] [CrossRef]
- Wang, K.; Jing, Y.; Xu, C.; Zhao, J.; Gong, Q.; Chen, S. HIF-1α and VEGF Are Involved in Deferoxamine-Ameliorated Traumatic Brain Injury. J. Surg. Res. 2020, 246, 419–426. [Google Scholar] [CrossRef]
- Weinstein, G.S.; Maves, M.D.; McCormack, M.L. Deferoxamine decreases necrosis in dorsally based pig skin flaps. Otolaryngol. Head Neck Surg. 1989, 101, 559–561. [Google Scholar] [CrossRef]
- Morselli, P.G.; Sorbi, G.; Feliziani, C.; Muscari, C. Deferoxamine Protects Stromal/Stem Cells of “Lull pgm System”-Processed Lipoaspirates Against Damages Induced by Mitochondrial Respiration Inhibition. Aesthetic Plast. Surg. 2020, 44, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Temiz, G.; Sirinoglu, H.; Yesiloglu, N.; Filinte, D.; Kaçmaz, C. Effects of Deferoxamine on Fat Graft Survival. Facial Plast. Surg. 2016, 32, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Tevlin, R.; Longaker, M.T.; Wan, D.C. Deferoxamine to Minimize Fibrosis During Radiation Therapy. Adv. Wound Care 2022, 11, 548–559. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ozono, I.; Tajima, S.; Imao, M.; Horinouchi, Y.; Izawa-Ishizawa, Y.; Kihira, Y.; Miyamoto, L.; Ishizawa, K.; Tsuchiya, K.; et al. Iron chelation by deferoxamine prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. PLoS ONE 2014, 9, e89355. [Google Scholar] [CrossRef]
- Kim, J.; Park, M.; Jeong, W.; Lee, H.W.; Lee, G.; Suk Lee, K.; Park, S.W.; Choi, J. Recipient-Site Preconditioning with Deferoxamine Increases Fat Graft Survival by Inducing VEGF and Neovascularization in a Rat Model. Plast. Reconstr. Surg. 2019, 144, 619e–629e. [Google Scholar] [CrossRef]
- Flacco, J.; Chung, N.; Blackshear, C.P.; Irizarry, D.; Momeni, A.; Lee, G.K.; Nguyen, D.; Gurtner, G.C.; Longaker, M.T.; Wan, D.C. Deferoxamine Preconditioning of Irradiated Tissue Improves Perfusion and Fat Graft Retention. Plast. Reconstr. Surg. 2018, 141, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gutierrez, E.; Kovacevic, Z.; Saletta, F.; Obeidy, P.; Suryo Rahmanto, Y.; Richardson, D.R. Iron chelators for the treatment of cancer. Curr. Med. Chem. 2012, 19, 2689–2702. [Google Scholar] [CrossRef]
- Komoto, K.; Nomoto, T.; El Muttaqien, S.; Takemoto, H.; Matsui, M.; Miura, Y.; Nishiyama, N. Iron chelation cancer therapy using hydrophilic block copolymers conjugated with deferoxamine. Cancer Sci. 2021, 112, 410–421. [Google Scholar] [CrossRef]
- Ozer, U. The role of Iron on breast cancer stem-like cells. Cell. Mol. Biol. 2016, 62, 25–30. [Google Scholar]
- Duscher, D.; Neofytou, E.; Wong, V.W.; Maan, Z.N.; Rennert, R.C.; Inayathullah, M.; Januszyk, M.; Rodrigues, M.; Malkovskiy, A.V.; Whitmore, A.J.; et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc. Natl. Acad. Sci. USA 2015, 112, 94–99. [Google Scholar] [CrossRef]
- Lintel, H.; Abbas, D.B.; Lavin, C.V.; Griffin, M.; Guo, J.L.; Guardino, N.; Churukian, A.; Gurtner, G.C.; Momeni, A.; Longaker, M.T.; et al. Transdermal deferoxamine administration improves excisional wound healing in chronically irradiated murine skin. J. Transl. Med. 2022, 20, 274. [Google Scholar] [CrossRef]
- Vignesh, S.; Sivashanmugam, A.; Mohandas Annapoorna Janarthanan, R.; Iyer Subramania Nair Shantikumar, V.; Jayakumar, R. Injectable deferoxamine nanoparticles loaded chitosan-hyaluronic acid coacervate hydrogel for therapeutic angiogenesis. Colloids Surf. B Biointerfaces 2018, 161, 129–138. [Google Scholar] [CrossRef]
- Takpradit, C.; Viprakasit, V.; Narkbunnam, N.; Vathana, N.; Phuakpet, K.; Pongtanakul, B.; Sanpakit, K.; Buaboonnam, J. Using of deferasirox and deferoxamine in refractory iron overload thalassemia. Pediatr. Int. 2021, 63, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, X.; Li, H.; Mu, D. Deferoxamine Mesylate Improves the Survival Rate of Transplanted Fat by Promoting Angiogenesis. Aesthetic Surg. J. 2023, 43, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Khouri, R.K.; Brown, D.M.; Leal-Khouri, S.M.; Tark, K.C.; Shaw, W.W. The effect of basic fibroblast growth factor on the neovascularisation process: Skin flap survival and staged flap transfers. Br. J. Plast. Surg. 1991, 44, 585–588. [Google Scholar] [CrossRef]
- Yan, L.; Wu, W.; Wang, Z.; Li, C.; Lu, X.; Duan, H.; Zhou, J.; Wang, X.; Wan, P.; Song, Y.; et al. Comparative study of the effects of recombinant human epidermal growth factor and basic fibroblast growth factor on corneal epithelial wound healing and neovascularization in vivo and in vitro. Ophthalmic Res. 2013, 49, 150–160. [Google Scholar] [CrossRef]
- Tabata, Y.; Miyao, M.; Inamoto, T.; Ishii, T.; Hirano, Y.; Yamaoki, Y.; Ikada, Y. De novo formation of adipose tissue by controlled release of basic fibroblast growth factor. Tissue Eng. 2000, 6, 279–289. [Google Scholar] [CrossRef]
- Navre, M.; Ringold, G.M. A growth factor-repressible gene associated with protein kinase C-mediated inhibition of adipocyte differentiation. J. Cell Biol. 1988, 107, 279–286. [Google Scholar] [CrossRef]
- Inoue, S.; Imamura, M.; Tabata, Y. Adipogenic differentiation of adipo-stromal cells incubated with basic fibroblast growth factor in solution and coated form. J. Biomater. Sci. Polym. Ed. 2009, 20, 483–494. [Google Scholar] [CrossRef]
- Quang, T.; Marquez, M.; Blanco, G.; Zhao, Y. Dosage and cell line dependent inhibitory effect of bFGF supplement in human pluripotent stem cell culture on inactivated human mesenchymal stem cells. PLoS ONE 2014, 9, e86031. [Google Scholar] [CrossRef]
- Aoki, S.; Toda, S.; Sakemi, T.; Sugihara, H. Coculture of endothelial cells and mature adipocytes actively promotes immature preadipocyte development in vitro. Cell Struct. Funct. 2003, 28, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, N.; Toriyama, K.; Nicodemou-Lena, E.; Inou, K.; Torii, S.; Kitagawa, Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc. Natl. Acad. Sci. USA 1998, 95, 1062–1066. [Google Scholar] [CrossRef]
- Fukumura, D.; Ushiyama, A.; Duda, D.G.; Xu, L.; Tam, J.; Krishna, V.; Chatterjee, K.; Garkavtsev, I.; Jain, R.K. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ. Res. 2003, 93, e88–e97. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ishihara, M.; Takikawa, M.; Murakami, K.; Kishimoto, S.; Nakamura, S.; Yanagibayashi, S.; Mori, Y.; Fujita, M.; Kubo, S.; et al. Increased survival of free fat grafts and vascularization in rats with local delivery of fragmin/protamine microparticles containing FGF-2 (F/P MP-F). J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 234–241. [Google Scholar] [CrossRef]
- Tamura, E.; Fukuda, H.; Tabata, Y. Adipose tissue formation in response to basic fibroblast growth factor. Acta Otolaryngol. 2007, 127, 1327–1331. [Google Scholar] [CrossRef]
- Tamura, E.; Tabata, Y.; Yamada, C.; Okada, S.; Iida, M. Autologous fat augmentation of the vocal fold with basic fibroblast growth factor: Computed tomographic assessment of fat tissue survival after augmentation. Acta Otolaryngol. 2015, 135, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Sidner, R.A.; Platis, J.M.; Sadove, A.M. Bioactivation of free-fat transfers: A potential new approach to improving graft survival. Plast. Reconstr. Surg. 1992, 90, 1022–1030. [Google Scholar] [CrossRef]
- Eppley, B.L.; Sadove, A.M. A physicochemical approach to improving free fat graft survival: Preliminary observations. Aesthetic Plast. Surg. 1991, 15, 215–218. [Google Scholar] [CrossRef]
- Eppley, B.L.; Snyders, R.V., Jr.; Winkelmann, T.; Delfino, J.J. Autologous facial fat transplantation: Improved graft maintenance by microbead bioactivation. J. Oral Maxillofac. Surg. 1992, 50, 477–482; discussion 482–483. [Google Scholar] [CrossRef]
- Park, H.; Williams, R.; Goldman, N.; Choe, H.; Kobler, J.; Lopez-Guerra, G.; Heaton, J.T.; Langer, R.; Zeitels, S.M. Comparison of effects of 2 harvesting methods on fat autograft. Laryngoscope 2008, 118, 1493–1499. [Google Scholar] [CrossRef]
- Lo, C.; Cao, L.; Lin, Y.; Wang, H. The Effect of Botulinum Toxin Type A in the Autologous Fat Grafting: A Review. J. Cosmet. Dermatol. 2024, 23, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yuan, Y.; Dong, Z.; Gao, J.; Lu, F. The Fate of Fat Grafts in Different Recipient Areas: Subcutaneous Plane, Fat Pad, and Muscle. Dermatol. Surg. 2016, 42, 535–542. [Google Scholar] [CrossRef]
- Ratner, D. Skin grafting: From here to there. Dermatol. Clin. 1998, 16, 75–90. [Google Scholar] [CrossRef]
- Li, G.; Fu, N.; Yang, X.; Li, M.; Ba, K.; Wei, X.; Fu, Y.; Yao, Y.; Cai, X.; Lin, Y. Mechanical compressive force inhibits adipogenesis of adipose stem cells. Cell Prolif. 2013, 46, 586–594. [Google Scholar] [CrossRef]
- Cho, S.W.; Song, K.W.; Rhie, J.W.; Park, M.H.; Choi, C.Y.; Kim, B.S. Engineered adipose tissue formation enhanced by basic fibroblast growth factor and a mechanically stable environment. Cell Transpl. 2007, 16, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qiang, S.; Wang, N.; Wei, S.; Qiu, L.; Xiong, S.; Ma, X.; Zhang, Z.; Yi, C. Improving the Retention Rate of Fat Grafting by Botulinum Toxin A: A Randomized, Self-controlled, Clinical Trial. Aesthetic Plast. Surg. 2024, 48, 5342–5349. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, C.; Wang, X.; Li, W.; Zhang, Y.; Wang, M.; Jing, W.; Wang, H.; Guo, W.; Tian, W. Botulinum toxin A improves adipose tissue engraftment by promoting cell proliferation, adipogenesis and angiogenesis. Int. J. Mol. Med. 2017, 40, 713–720. [Google Scholar] [CrossRef]
- Baek, R.M.; Park, S.O.; Jeong, E.C.; Oh, H.S.; Kim, S.W.; Minn, K.W.; Lee, S.Y. The effect of botulinum toxin A on fat graft survival. Aesthetic Plast. Surg. 2012, 36, 680–686. [Google Scholar] [CrossRef]
- Hoon, S.Y.; Gao, J.; Xu, L.; Yu, Z.; Jiang, T.; Kang, B.K.; Zhang, R.; Cao, D. Effect of additive-assisted fat transplantation on fat graft survival rate: A preliminary experimental study based on a rabbit animal model. Ann. Chir. Plast. Esthet. 2021, 66, 440–446. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Wang, Z.; Feng, J.; Wang, J.; Xiao, X.; Lu, F.; Dong, Z. Botulinum Toxin A Improves Supramuscular Fat Graft Retention by Enhancing Angiogenesis and Adipogenesis. Dermatol. Surg. 2020, 46, 646–652. [Google Scholar] [CrossRef]
- Shi, N.; Guo, S.; Su, Y.; Zhang, Z.; Qiu, L.; Yu, Z.; Yang, Q.; Wang, N.; Yi, C. Improvement in the Retention Rate of Transplanted Fat in Muscle by Denervation. Aesthetic Surg. J. 2018, 38, 1026–1034. [Google Scholar] [CrossRef]
- Shi, N.; Su, Y.; Guo, S.; Zhang, Z.; Qiu, L.; Yi, C. Improving the Retention Rate of Fat Grafts in Recipient Areas via Botulinum Toxin A Treatment. Aesthetic Surg. J. 2019, 39, 1436–1444. [Google Scholar] [CrossRef]
- Baran, C.N.; Celebioğlu, S.; Sensöz, O.; Ulusoy, G.; Civelek, B.; Ortak, T. The behavior of fat grafts in recipient areas with enhanced vascularity. Plast. Reconstr. Surg. 2002, 109, 1646–1650. [Google Scholar] [CrossRef]
- Aygit, A.C.; Sarikaya, A.; Doganay, L.; Top, H.; Cakir, B.; Firat, M.F. The fate of intramuscularly injected fat autografts: An experimental study in rabbits. Aesthetic Plast. Surg. 2004, 28, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.B.; Greco, T. Injectable treatments for the aging face. Facial Plast. Surg. 2006, 22, 140–146. [Google Scholar] [CrossRef]
- Zheng, Z.; Hao, Y.; Yin, J.; Lei, X.; Cheng, B.; Huang, W. Autogenous Fat Transplantation and Botulinum Toxin Injection Into the Masseter Muscle to Create an Ideal Oval Face. Aesthetic Surg. J. 2021, 41, Np579–Np588. [Google Scholar] [CrossRef]
- Lv, S.; Cai, H.; Xu, Y.; Dai, J.; Rong, X.; Zheng, L. Thymosin-β 4 induces angiogenesis in critical limb ischemia mice via regulating Notch/NF-κB pathway. Int. J. Mol. Med. 2020, 46, 1347–1358. [Google Scholar] [CrossRef]
- Jeon, B.J.; Yang, Y.; Kyung Shim, S.; Yang, H.M.; Cho, D.; Ik Bang, S. Thymosin beta-4 promotes mesenchymal stem cell proliferation via an interleukin-8-dependent mechanism. Exp. Cell Res. 2013, 319, 2526–2534. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, R.; Zhou, F.; Streubel, P.N.; Chen, S.; Duan, B. Electrospun thymosin Beta-4 loaded PLGA/PLA nanofiber/ microfiber hybrid yarns for tendon tissue engineering application. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110268. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, I.R.; Park, C.Y.; Joo, H.J.; Noh, J.M.; Choi, S.C.; Hong, S.J.; Lim, D.S. Thymosin β4-Enhancing Therapeutic Efficacy of Human Adipose-Derived Stem Cells in Mouse Ischemic Hindlimb Model. Int. J. Mol. Sci. 2020, 21, 2166. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, Q.; Fu, S.; Guo, X.; Luan, J.; Mu, D. The Effect of Thymosin beta4 on the Survival of Autologous Fat Grafting: A Preliminary Study. Aesthetic Surg. J. 2020, 40, Np519–Np529. [Google Scholar] [CrossRef]
- Gillis, J.; Gebremeskel, S.; Phipps, K.D.; MacNeil, L.A.; Sinal, C.J.; Johnston, B.; Hong, P.; Bezuhly, M. Effect of N-Acetylcysteine on Adipose-Derived Stem Cell and Autologous Fat Graft Survival in a Mouse Model. Plast. Reconstr. Surg. 2015, 136, 179e–188e. [Google Scholar] [CrossRef]
- Millea, P.J. N-acetylcysteine: Multiple clinical applications. Am. Fam. Physician 2009, 80, 265–269. [Google Scholar] [PubMed]
- Kelly, G.S. Clinical applications of N-acetylcysteine. Altern. Med. Rev. 1998, 3, 114–127. [Google Scholar]
- Pietruski, P.; Paskal, W.; Paluch, Ł.; Paskal, A.M.; Nitek, Ż.; Włodarski, P.; Walecki, J.; Noszczyk, B. The Impact of N-Acetylcysteine on Autologous Fat Graft: First-in-Human Pilot Study. Aesthetic Plast. Surg. 2021, 45, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef]
- Luchetti, F.; Canonico, B.; Betti, M.; Arcangeletti, M.; Pilolli, F.; Piroddi, M.; Canesi, L.; Papa, S.; Galli, F. Melatonin signaling and cell protection function. FASEB J. 2010, 24, 3603–3624. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef]
- Luchetti, F.; Canonico, B.; Bartolini, D.; Arcangeletti, M.; Ciffolilli, S.; Murdolo, G.; Piroddi, M.; Papa, S.; Reiter, R.J.; Galli, F. Melatonin regulates mesenchymal stem cell differentiation: A review. J. Pineal. Res. 2014, 56, 382–397. [Google Scholar] [CrossRef]
- Tan, S.S.; Han, X.; Sivakumaran, P.; Lim, S.Y.; Morrison, W.A. Melatonin Protects Human Adipose-Derived Stem Cells from Oxidative Stress and Cell Death. Arch. Plast. Surg. 2016, 43, 237–241. [Google Scholar] [CrossRef]
- Zhao, J.; Young, Y.K.; Fradette, J.; Eliopoulos, N. Melatonin pretreatment of human adipose tissue-derived mesenchymal stromal cells enhances their prosurvival and protective effects on human kidney cells. Am. J. Physiol. Renal. Physiol. 2015, 308, F1474–F1483. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Yu, Z.; Wang, T.; Jiao, Y.; Wang, K.; Dou, W.; Yi, C.; Song, B. Effects of Melatonin on Fat Graft Retention Through Browning of Adipose Tissue and Alternative Macrophage Polarization. Aesthetic Plast. Surg. 2023, 47, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, M.; Sánchez-Vera, I.; Sevillano, J.; Herrero, L.; Serra, D.; Ramos, M.P.; Viana, M. Vitamin E reduces adipose tissue fibrosis, inflammation, and oxidative stress and improves metabolic profile in obesity. Obesity 2015, 23, 1598–1606. [Google Scholar] [CrossRef]
- Chiao, T.B.; Lee, A.J. Role of pentoxifylline and vitamin E in attenuation of radiation-induced fibrosis. Ann. Pharmacother. 2005, 39, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Kaidar-Person, O.; Marks, L.B.; Jones, E.L. Pentoxifylline and vitamin E for treatment or prevention of radiation-induced fibrosis in patients with breast cancer. Breast J. 2018, 24, 816–819. [Google Scholar] [CrossRef]
- Abbas, D.B.; Lavin, C.V.; Fahy, E.J.; Griffin, M.; Guardino, N.J.; Nazerali, R.S.; Nguyen, D.H.; Momeni, A.; Longaker, M.T.; Wan, D.C. Fat Grafts Augmented with Vitamin E Improve Volume Retention and Radiation-Induced Fibrosis. Aesthetic Surg. J. 2022, 42, 946–955. [Google Scholar] [CrossRef]
- Zingg, J.M.; Meydani, M.; Azzi, A. α-Tocopheryl phosphate--an activated form of vitamin E important for angiogenesis and vasculogenesis? Biofactors 2012, 38, 24–33. [Google Scholar] [CrossRef]
- Cinar, F.; Yalcin, C.E.; Ayas, G.; Celik, U.; Okyay, M.F.; Demiröz, A.; Bağhaki, S.; Cetinkale, O. Increased Total Antioxidant Capacity Value Improves Survival of Fat Grafts in a Rat Model. Plast. Reconstr. Surg. 2023, 153, 1307–1316. [Google Scholar] [CrossRef]
- Loder, S.; Wang, S.; Amurgis, C.; DeSanto, M.; Stavros, A.G.; Patadji, S.; Olevian, D.; Lee, P.; Guerrero, D.; Gusenoff, J.A.; et al. Active Vitamin D3 (Calcitriol) Increases Adipose Graft Retention in a Xenograft Model. Aesthetic Surg. J. 2023, 43, Np449–Np465. [Google Scholar] [CrossRef]
- Savla, S.R.; Laddha, A.P.; Kulkarni, Y.A. Pharmacology of apocynin: A natural acetophenone. Drug Metab. Rev. 2021, 53, 542–562. [Google Scholar] [CrossRef]
- Chocry, M.; Leloup, L. The NADPH Oxidase Family and Its Inhibitors. Antioxid. Redox Signal. 2020, 33, 332–353. [Google Scholar] [CrossRef]
- Du, Z.D.; Yu, S.; Qi, Y.; Qu, T.F.; He, L.; Wei, W.; Liu, K.; Gong, S.S. NADPH oxidase inhibitor apocynin decreases mitochondrial dysfunction and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging model in rats. Neurochem. Int. 2019, 124, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Sun, F.; Huang, R.; Sun, W.; Zhang, D.; Wang, Q. Inhibition of NADPH oxidase by apocynin prevents learning and memory deficits in a mouse Parkinson’s disease model. Redox Biol. 2019, 22, 101134. [Google Scholar] [CrossRef]
- Yu, J.; Weïwer, M.; Linhardt, R.J.; Dordick, J.S. The role of the methoxyphenol apocynin, a vascular NADPH oxidase inhibitor, as a chemopreventative agent in the potential treatment of cardiovascular diseases. Curr. Vasc. Pharmacol. 2008, 6, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Keskin, E.R.; Çakan, D. The Effect of Apocynin on Fat Graft Survival. Aesthetic Plast. Surg. 2021, 45, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Purwaningsih, I.; Maksum, I.P.; Sumiarsa, D.; Sriwidodo, S. A Review of Fibraurea tinctoria and Its Component, Berberine, as an Antidiabetic and Antioxidant. Molecules 2023, 28, 1294. [Google Scholar] [CrossRef]
- Ghorbani, A.; Baradaran Rahimi, V.; Sadeghnia, H.R.; Hosseini, A. Effect of berberine on the viability of adipose tissue-derived mesenchymal stem cells in nutrients deficient condition. Nat. Prod. Res. 2018, 32, 592–595. [Google Scholar] [CrossRef]
- Pang, H.; Zhou, Y.; Wang, J.; Wu, H.; Liu, X.; Gao, F.; Xiao, Z. Berberine Influences the Survival of Fat Grafting by Inhibiting Autophagy and Apoptosis of Human Adipose Derived Mesenchymal Stem Cells. Drug Des. Devel. Ther. 2021, 15, 4795–4809. [Google Scholar] [CrossRef]
- Xining, Z.; Sai, L. The Evolving Function of Vasculature and Pro-angiogenic Therapy in Fat Grafting. Cell Transpl. 2024, 33, 9636897241264976. [Google Scholar] [CrossRef]
- Debuc, B.; Gendron, N.; Cras, A.; Rancic, J.; Philippe, A.; Cetrulo, C.L., Jr.; Lellouch, A.G.; Smadja, D.M. Improving Autologous Fat Grafting in Regenerative Surgery through Stem Cell-Assisted Lipotransfer. Stem Cell Rev. Rep. 2023, 19, 1726–1754. [Google Scholar] [CrossRef]
- Tan, S.S.; Zhan, W.; Poon, C.J.; Han, X.; Marre, D.; Boodhun, S.; Palmer, J.A.; Mitchell, G.M.; Morrison, W.A. Melatonin promotes survival of nonvascularized fat grafts and enhances the viability and migration of human adipose-derived stem cells via down-regulation of acute inflammatory cytokines. J. Tissue Eng. Regen. Med. 2018, 12, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Li, M.; Duan, W.; Dong, Y.; Wang, Y. Improvement of the survival of human autologous fat transplantation by adipose-derived stem-cells-assisted lipotransfer combined with bFGF. Sci. World J. 2015, 2015, 968057. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.L.; Li, J.X.; Liu, H.W.; Li, S.H.; Wu, Y.Y.; Liao, X.; Rao, C.Q. Improved fat transplantation survival by using the conditioned medium of vascular endothelial growth factor transfected human adipose-derived stem cells. Kaohsiung J. Med. Sci. 2017, 33, 379–384. [Google Scholar] [CrossRef]
- Chang, L.; Wang, J.; Zheng, D.; Zhang, B.; Fan, Q.; Zhu, C.; Yu, L. Improvement of the survival of autologous free-fat transplants in rats using vascular endothelial growth factor 165-transfected bone mesenchymal stem cells. Ann. Plast. Surg. 2014, 72, 355–362. [Google Scholar] [CrossRef]
- Liu, M.; Wang, G.; Jin, W.; Wu, H.; Liu, N.; Zhen, Y.; An, Y. Poloxamer 188 washing of lipoaspirate improves fat graft survival: A comparative study in nude mice. J. Plast. Reconstr. Aesthetic Surg. 2024, 95, 357–367. [Google Scholar] [CrossRef]
- Medina, M.A., 3rd; Nguyen, J.T.; Kirkham, J.C.; Lee, J.H.; McCormack, M.C.; Randolph, M.A.; Austen, W.G., Jr. Polymer therapy: A novel treatment to improve fat graft viability. Plast. Reconstr. Surg. 2011, 127, 2270–2282. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Hong, K.Y.; Ju, U.I.; Choi, B.G.; Jin, U.S.; Chun, Y.S.; Chang, H. Compact Fat Grafting: A Novel Method to Improve Graft Retention Through Modulation of Adipocyte Size. Aesthetic Surg. J. 2021, 41, Np653–Np661. [Google Scholar] [CrossRef]
- Yu, P.; Yang, Z.; Lu, H.; Jin, X.; Yang, X.; Qi, Z. Quercetin May Improve Fat Graft Survival by Promoting Fat Browning Peripherally. Aesthetic Plast. Surg. 2022, 46, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cao, H.B.; Li, W.J.; Zhao, L. The CXCL12 (SDF-1)/CXCR4 chemokine axis: Oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin. J. Nat. Med. 2018, 16, 801–810. [Google Scholar] [CrossRef]
- Zhang, T.; Dai, J.; Xu, Y.; Yu, L.; Wang, X. Liquid Phase Concentrated Growth Factor Improves Autologous Fat Graft Survival In Vivo in Nude Mice. Aesthetic Plast. Surg. 2021, 45, 2417–2422. [Google Scholar] [CrossRef]
- Lei, M.; Liu, S.Q.; Peng, H.; Liu, Y.L. Effect of rhVEGF gene transfection on survival of grafts after autologous free granular fat transplantation in rats. Chin. J. Traumatol. 2008, 11, 49–53. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amraoui, N.; Xu, I.; Robles Cortés, J.; Beaudoin Cloutier, C.; Fradette, J. Improving Fat Graft Survival Using Soluble Molecule Preconditioning. Biomolecules 2025, 15, 526. https://doi.org/10.3390/biom15040526
Amraoui N, Xu I, Robles Cortés J, Beaudoin Cloutier C, Fradette J. Improving Fat Graft Survival Using Soluble Molecule Preconditioning. Biomolecules. 2025; 15(4):526. https://doi.org/10.3390/biom15040526
Chicago/Turabian StyleAmraoui, Nabil, Isabelle Xu, Jorge Robles Cortés, Chanel Beaudoin Cloutier, and Julie Fradette. 2025. "Improving Fat Graft Survival Using Soluble Molecule Preconditioning" Biomolecules 15, no. 4: 526. https://doi.org/10.3390/biom15040526
APA StyleAmraoui, N., Xu, I., Robles Cortés, J., Beaudoin Cloutier, C., & Fradette, J. (2025). Improving Fat Graft Survival Using Soluble Molecule Preconditioning. Biomolecules, 15(4), 526. https://doi.org/10.3390/biom15040526






