Hydroxyzine Effects on Post-Lanosterol Biosynthesis in Smith–Lemli–Opitz Syndrome (SLOS) Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Neuro2a Cell Cultures
2.3. Primary Neuronal and Astrocytes Cultures
2.4. Human Dermal Fibroblast Cultures
2.5. Post-Lanosterol LC-MS/MS Measurements
2.6. Statistical Analyses
3. Results
3.1. HYZ Effects on Neuro2a Cells
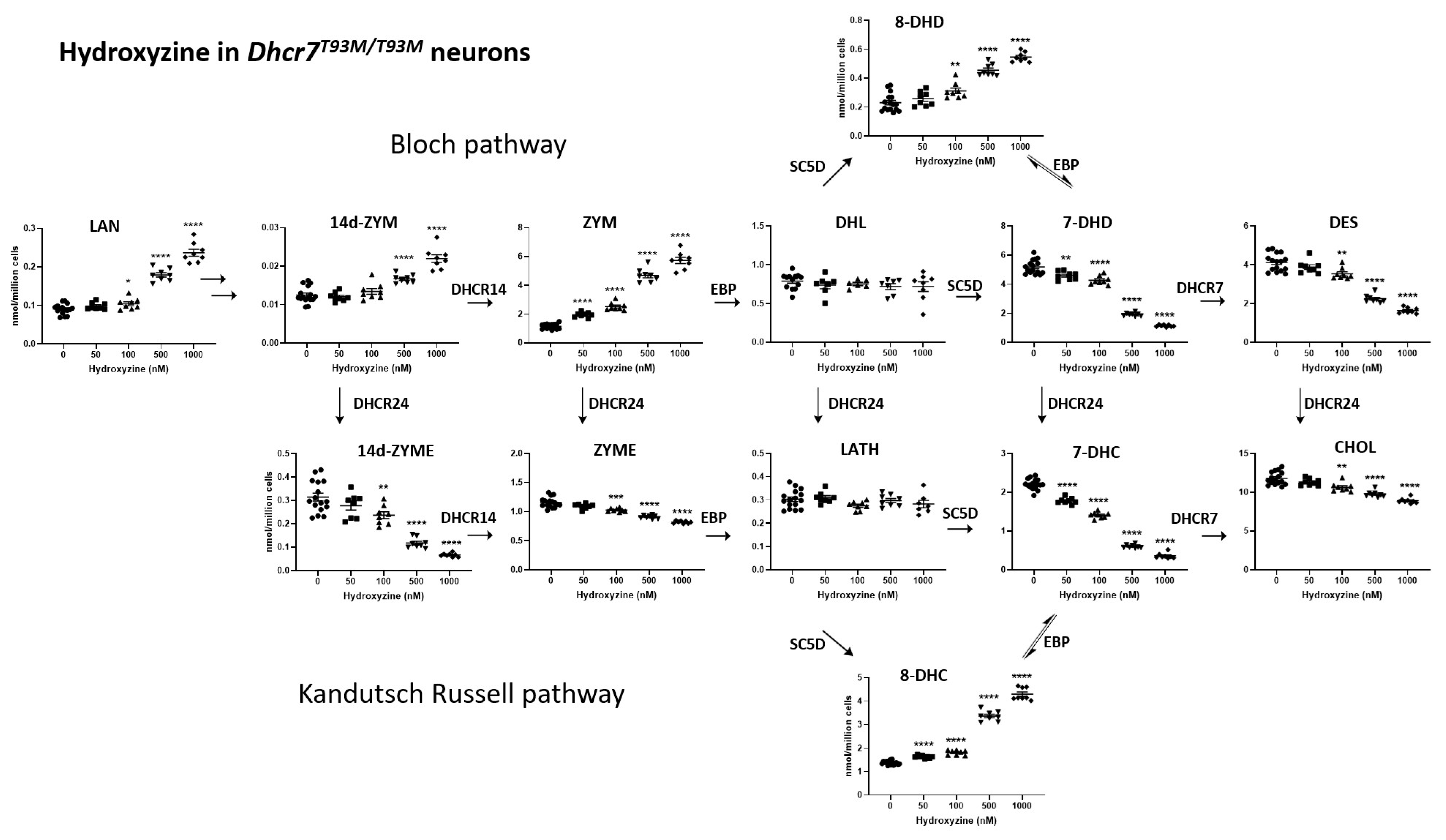
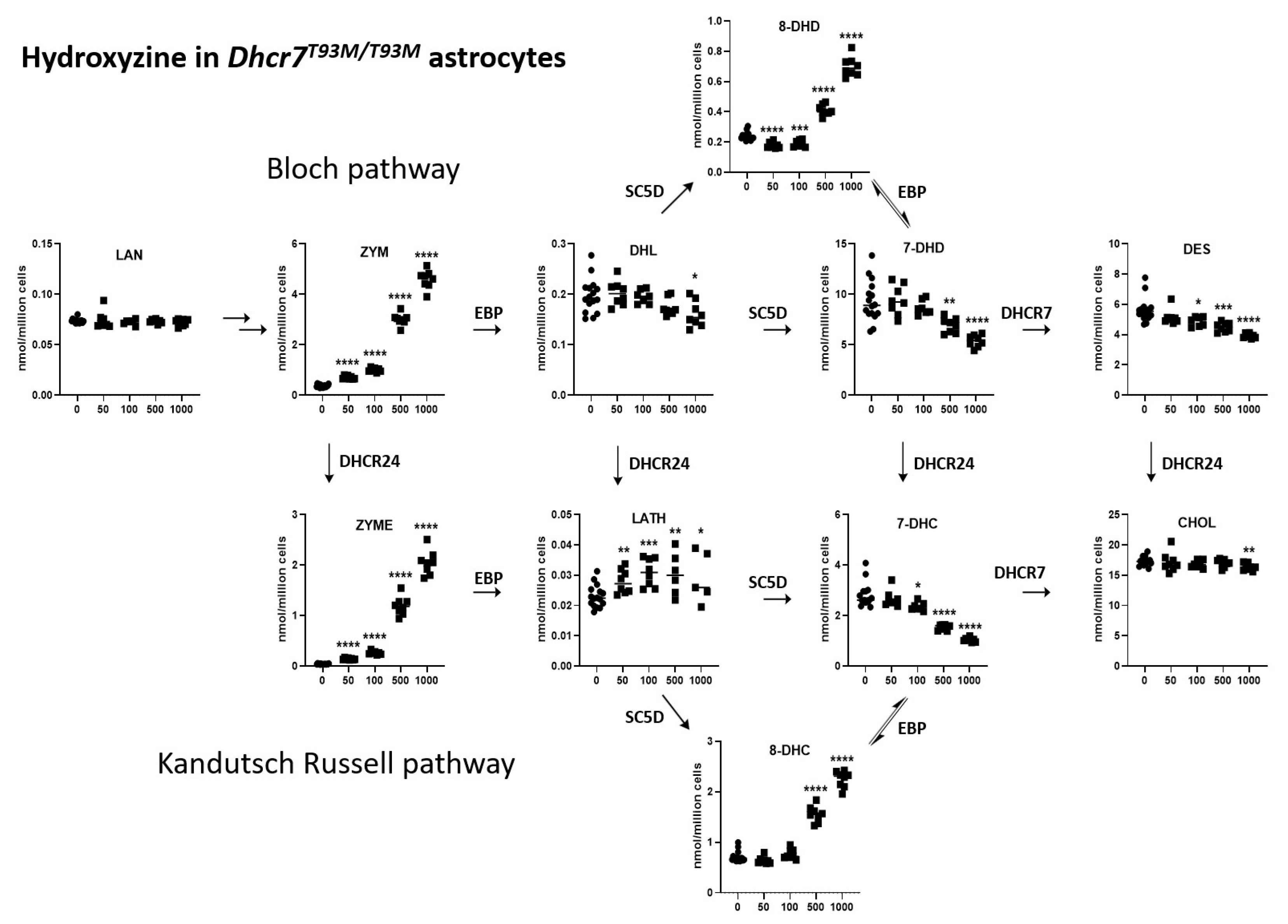
3.2. HYZ Effects on Dhcr7T93M/T93M Neurons and Glial Cells
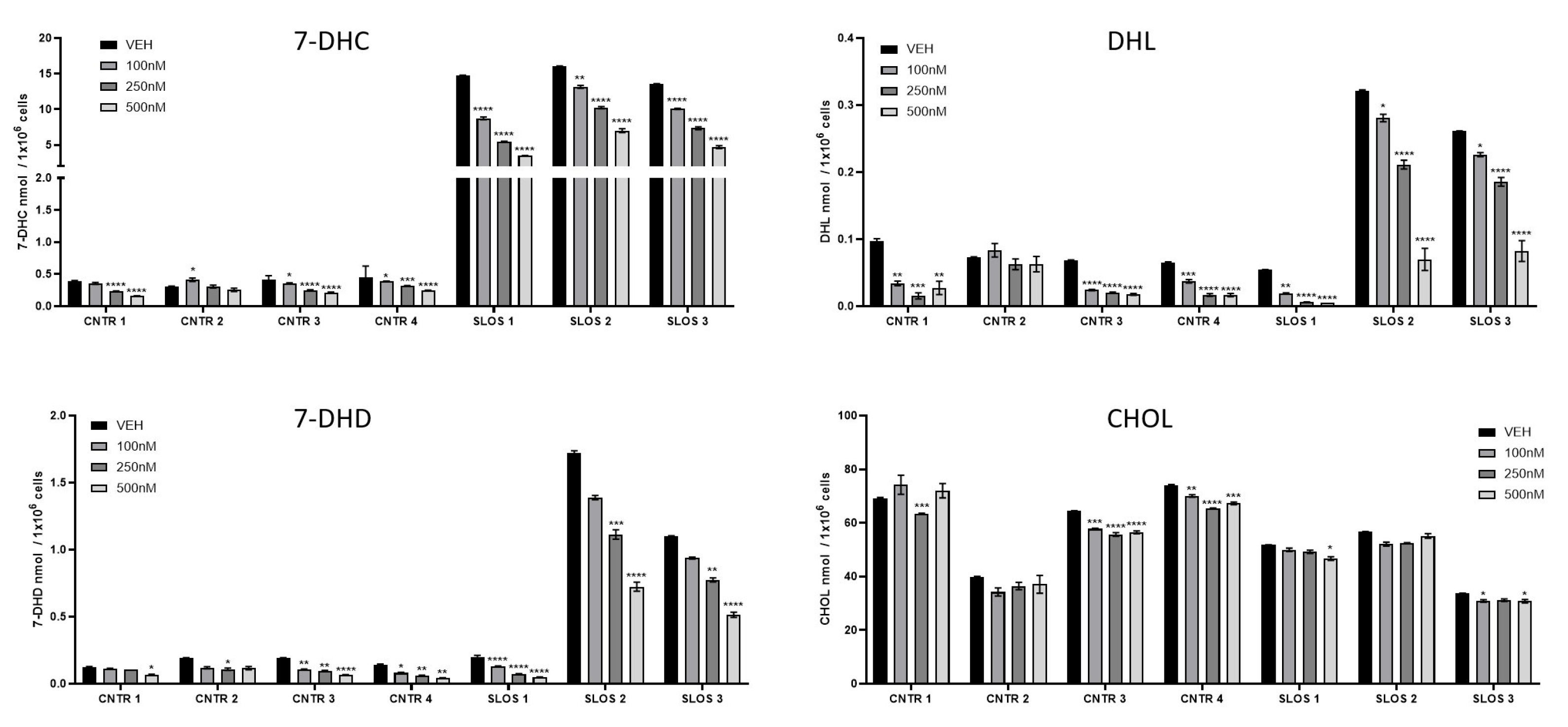
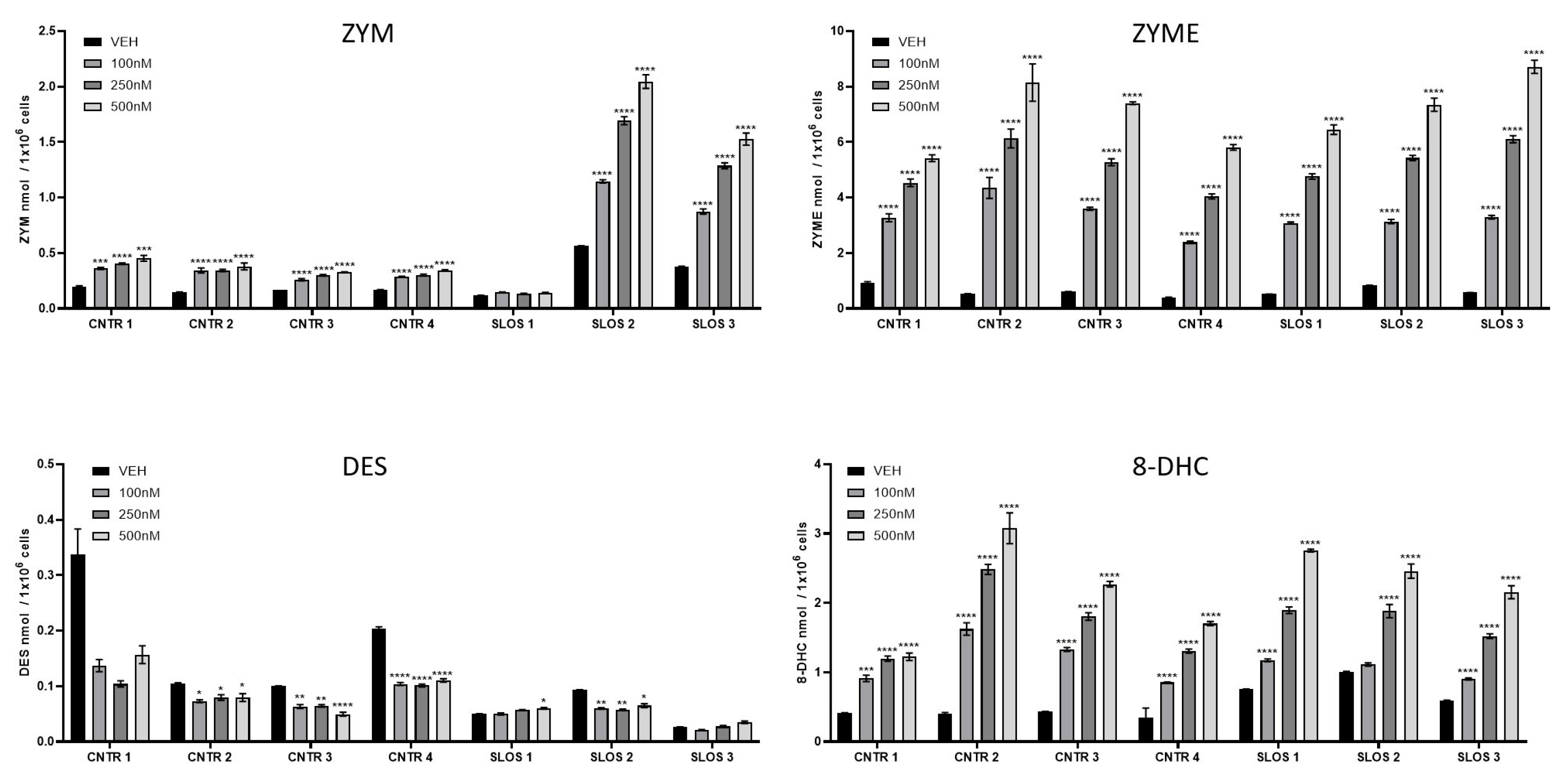
3.3. HYZ Effects on Human Dermal Fibroblasts (HDFs) from SLOS Patients and Controls
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLOS | Smith–Lemli–Opitz syndrome |
| HYZ | hydroxyzine |
| DHCR7 | Dehydrocholesterol reductase 7 |
| 7-DHC | 7-dehydrocholesterol |
| 8-DHC | 8-dehydrocholesterol |
| DES | desmosterol |
| LATH | lathosterol |
| LAN | lanosterol |
| ZYM | zymosterol |
| ZYME | zymostenol |
| DHL | dehydrolathosterol |
| 7-DHD | 7-dehydrodesmosterol |
| 8-DHD | 8-dehydrodesmosterol |
| SC5D | sterol-C5-desaturase |
| EBP | emopamil-binding protein |
| DHCR24 | Dehydrocholesterol reductase 24 |
References
- Porter, F.D. RSH/Smith-Lemli-Opitz syndrome: A multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol. Genet. Metab. 2000, 71, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Lemli, L.; Opitz, J.M. A Newly Recognized Syndrome of Multiple Congenital Anomalies. J. Pediatr. 1964, 64, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Sanghera, A.S.; Zeppieri, M. Smith-Lemli-Opitz Syndrome; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Yilmaz, M.; Bebek, O.; Turkyilmaz, A. Smith-Lemli-Opitz Syndrome with Biallelic c.1295A>G (p.Tyr432Cys) Variant in the DHCR7 Gene in a 73-Year-Old Woman: Report of the Oldest Patient. Mol. Syndromol. 2024, 15, 317–323. [Google Scholar] [CrossRef]
- Westbye, A.B.; Dizdarevic, L.L.; Dahl, S.R.; Asprusten, E.A.; Bliksrud, Y.T.; Sandblom, A.L.; Diczfalusy, U.; Thorsby, P.M.; Retterstøl, K. A sterol panel for rare lipid disorders: Sitosterolemia, cerebrotendinous xanthomatosis, and Smith-Lemli-Opitz syndrome. J. Lipid Res. 2024, 66, 100698. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, S.E.; Cross, J.L.; Wassif, C.A.; Porter, F.D. Pathogenesis, Epidemiology, Diagnosis and Clinical Aspects of Smith-Lemli-Opitz Syndrome. Expert. Opin. Orphan Drugs 2015, 3, 267–280. [Google Scholar] [CrossRef]
- Coupe, S.; Hertzog, A.; Foran, C.; Tolun, A.A.; Suthern, M.; Chung, C.W.T.; Ellaway, C. Keeping you on your toes: Smith-Lemli-Opitz Syndrome is an easily missed cause of developmental delays. Clin. Case Rep. 2023, 11, e6920. [Google Scholar] [CrossRef]
- Sanchez-Soler, M.J.; Serrano-Anton, A.T.; Lopez-Gonzalez, V.; Ballesta-Martinez, M.J.; Guillen-Navarro, E. Extremely variable expressivity in Smith-Lemli-Opitz syndrome: Review of 4 cases. Pediatr Engl. Ed. 2022, 96, 253–255. [Google Scholar] [CrossRef]
- Rozdzynska-Swiatkowska, A.; Ciara, E.; Halat-Wolska, P.; Krajewska-Walasek, M.; Jezela-Stanek, A. Anthropometric characteristics of 65 Polish Smith-Lemli-Opitz patients. J. Appl. Genet. 2021, 62, 469–475. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, T.; Ha, K.; Ki, C.S. Carrier frequency and incidence estimation of Smith-Lemli-Opitz syndrome in East Asian populations by Genome Aggregation Database (gnomAD) based analysis. Orphanet J. Rare Dis. 2021, 16, 166. [Google Scholar] [CrossRef]
- Lund, E.; Starck, L.; Venizelos, N. Detection of defective 3 beta-hydroxysterol delta 7-reductase activity in cultured human fibroblasts: A method for the diagnosis of Smith-Lemli-Opitz syndrome. J. Inherit. Metab. Dis. 1996, 19, 59–64. [Google Scholar] [CrossRef]
- Haas, D.; Armbrust, S.; Haas, J.P.; Zschocke, J.; Muhlmann, K.; Fusch, C.; Neumann, L.M. Smith-Lemli-Opitz syndrome with a classical phenotype, oesophageal achalasia and borderline plasma sterol concentrations. J. Inherit. Metab. Dis. 2005, 28, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, L.; Mitchell, G.; Giguere, R.; Lefebvre, F.; Melancon, S.B.; Lambert, M. Prenatal diagnosis of Smith-Lemli-Opitz syndrome is possible by measurement of 7-dehydrocholesterol in amniotic fluid. Prenat. Diagn. 1995, 15, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Tint, G.S.; Seller, M.; Hughes-Benzie, R.; Batta, A.K.; Shefer, S.; Genest, D.; Irons, M.; Elias, E.; Salen, G. Markedly increased tissue concentrations of 7-dehydrocholesterol combined with low levels of cholesterol are characteristic of the Smith-Lemli-Opitz syndrome. J. Lipid Res. 1995, 36, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Shefer, S.; Salen, G.; Batta, A.K.; Honda, A.; Tint, G.S.; Irons, M.; Elias, E.R.; Chen, T.C.; Holick, M.F. Markedly inhibited 7-dehydrocholesterol-delta 7-reductase activity in liver microsomes from Smith-Lemli-Opitz homozygotes. J. Clin. Investig. 1995, 96, 1779–1785. [Google Scholar] [CrossRef]
- Kelley, R.I. Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin. Chim. Acta 1995, 236, 45–58. [Google Scholar] [CrossRef]
- Honda, A.; Tint, G.S.; Salen, G.; Batta, A.K.; Chen, T.S.; Shefer, S. Defective conversion of 7-dehydrocholesterol to cholesterol in cultured skin fibroblasts from Smith-Lemli-Opitz syndrome homozygotes. J. Lipid Res. 1995, 36, 1595–1601. [Google Scholar] [CrossRef]
- Xu, L.; Korade, Z.; Rosado, D.A., Jr.; Liu, W.; Lamberson, C.R.; Porter, N.A. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011, 52, 1222–1233. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Sheflin, L.G.; Fliesler, S.J.; Porter, N.A. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: A model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011, 52, 1810–1820. [Google Scholar] [CrossRef]
- Xu, L.; Sheflin, L.G.; Porter, N.A.; Fliesler, S.J. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim. Biophys. Acta 2012, 1821, 877–883. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Lamberson, C.R.; Merkens, L.S.; Steiner, R.D.; Elias, E.R.; Haas, D.; Porter, N.A. Assays of plasma dehydrocholesteryl esters and oxysterols from Smith-Lemli-Opitz syndrome patients. J. Lipid Res. 2013, 54, 244–253. [Google Scholar] [PubMed]
- Shinkyo, R.; Xu, L.; Tallman, K.A.; Cheng, Q.; Porter, N.A.; Guengerich, F.P. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J. Biol. Chem. 2011, 286, 33021–33028. [Google Scholar] [PubMed]
- Xu, L.; Korade, Z.; Rosado, D.A., Jr.; Mirnics, K.; Porter, N.A. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J. Lipid Res. 2013, 54, 1135–1143. [Google Scholar] [PubMed]
- Francis, K.R.; Ton, A.N.; Xin, Y.; O’Halloran, P.E.; Wassif, C.A.; Malik, N.; Williams, I.M.; Cluzeau, C.V.; Trivedi, N.S.; Pavan, W.J.; et al. Modeling Smith-Lemli-Opitz syndrome with induced pluripotent stem cells reveals a causal role for Wnt/beta-catenin defects in neuronal cholesterol synthesis phenotypes. Nat. Med. 2016, 22, 388–396. [Google Scholar]
- Freel, B.A.; Kelvington, B.A.; Sengupta, S.; Mukherjee, M.; Francis, K.R. Sterol dysregulation in Smith-Lemli-Opitz syndrome causes astrocyte immune reactivity through microglia crosstalk. Dis. Model. Mech. 2022, 15, dmm049843. [Google Scholar]
- Korade, Z.; Xu, L.; Shelton, R.; Porter, N.A. Biological activities of 7-dehydrocholesterol-derived oxysterols: Implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2010, 51, 3259–3269. [Google Scholar]
- Pfeffer, B.A.; Xu, L.; Fliesler, S.J. Transcriptomic Changes Associated with Loss of Cell Viability Induced by Oxysterol Treatment of a Retinal Photoreceptor-Derived Cell Line: An In Vitro Model of Smith-Lemli-Opitz Syndrome. Int. J. Mol. Sci. 2021, 22, 2339. [Google Scholar] [CrossRef]
- Xu, L.; Mirnics, K.; Bowman, A.B.; Liu, W.; Da, J.; Porter, N.A.; Korade, Z. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol. Dis. 2012, 45, 923–929. [Google Scholar]
- Gaoua, W.; Chevy, F.; Roux, C.; Wolf, C. Oxidized derivatives of 7-dehydrocholesterol induce growth retardation in cultured rat embryos: A model for antenatal growth retardation in the Smith-Lemli-Opitz syndrome. J. Lipid Res. 1999, 40, 456–463. [Google Scholar]
- Correa-Cerro, L.S.; Wassif, C.A.; Kratz, L.; Miller, G.F.; Munasinghe, J.P.; Grinberg, A.; Fliesler, S.J.; Porter, F.D. Development and characterization of a hypomorphic Smith-Lemli-Opitz syndrome mouse model and efficacy of simvastatin therapy. Hum. Mol. Genet. 2006, 15, 839–851. [Google Scholar]
- Li, A.; Hines, K.M.; Ross, D.H.; MacDonald, J.W.; Xu, L. Temporal changes in the brain lipidome during neurodevelopment of Smith-Lemli-Opitz syndrome mice. Analyst 2022, 147, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Thurm, A.; Tierney, E.; Farmer, C.; Albert, P.; Joseph, L.; Swedo, S.; Bianconi, S.; Bukelis, I.; Wheeler, C.; Sarphare, G.; et al. Development, behavior, and biomarker characterization of Smith-Lemli-Opitz syndrome: An update. J. Neurodev. Disord. 2016, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Hines, K.M.; Herron, J.M.; Li, A.; Baggett, D.W.; Xu, L. 7-Dehydrocholesterol-derived oxysterols cause neurogenic defects in Smith-Lemli-Opitz syndrome. eLife 2022, 11, e67141. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Tomita, H.; Xu, L. Temporal gene expression changes and affected pathways in neurodevelopment of a mouse model of Smith-Lemli-Opitz syndrome. bioRxiv 2023. [Google Scholar] [CrossRef]
- Li, A.; Xu, L. MALDI-IM-MS Imaging of Brain Sterols and Lipids in a Mouse Model of Smith-Lemli-Opitz Syndrome. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gabor, K.; Mesev, E.V.; Madenspacher, J.; Meacham, J.; Rai, P.; Moon, S.; Wassif, C.A.; Shaikh, S.R.; Tucker, C.; Karmaus, P.; et al. Sterol biosynthesis regulates TLR signaling and the innate immune response in a Smith-Lemli-Opitz syndrome model. J. Clin. Investig. 2024, 134, e167633. [Google Scholar] [CrossRef]
- Dietschy, J.M. Central nervous system: Cholesterol turnover, brain development and neurodegeneration. Biol. Chem. 2009, 390, 287–293. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001, 12, 105–112. [Google Scholar] [CrossRef]
- Bjorkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef]
- Mitsche, M.A.; McDonald, J.G.; Hobbs, H.H.; Cohen, J.C. Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways. eLife 2015, 4, e07999. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Anderson, A.; Allen, L.B.; Korade, Z.; Mirnics, K. Cholesterol Biosynthesis and Uptake in Developing Neurons. ACS Chem. Neurosci. 2019, 10, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Tierney, E.; Conley, S.K.; Goodwin, H.; Porter, F.D. Analysis of short-term behavioral effects of dietary cholesterol supplementation in Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. A 2010, 152A, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wassif, C.A.; Kratz, L.; Sparks, S.E.; Wheeler, C.; Bianconi, S.; Gropman, A.; Calis, K.A.; Kelley, R.I.; Tierney, E.; Porter, F.D. A placebo-controlled trial of simvastatin therapy in Smith-Lemli-Opitz syndrome. Genet. Med. 2017, 19, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Elias, E.R.; Orth, L.E.; Li, A.; Xu, L.; Jones, S.M.; Rizzo, W.B. Cholic acid increases plasma cholesterol in Smith-Lemli-Opitz syndrome: A pilot study. Mol. Genet. Metab. Rep. 2024, 38, 101030. [Google Scholar] [CrossRef]
- Xu, G.; Salen, G.; Shefer, S.; Ness, G.C.; Chen, T.S.; Zhao, Z.; Salen, L.; Tint, G. Treatment of the cholesterol biosynthetic defect in Smith-Lemli-Opitz syndrome reproduced in rats by BM 15.766. Gastroenterology 1995, 109, 1301–1307. [Google Scholar] [CrossRef]
- Starck, L.; Lovgren-Sandblom, A.; Bjorkhem, I. Cholesterol treatment forever? The first Scandinavian trial of cholesterol supplementation in the cholesterol-synthesis defect Smith-Lemli-Opitz syndrome. J. Intern. Med. 2002, 252, 314–321. [Google Scholar] [CrossRef]
- Korade, Z.; Kim, H.Y.; Tallman, K.A.; Liu, W.; Koczok, K.; Balogh, I.; Xu, L.; Mirnics, K.; Porter, N.A. The Effect of Small Molecules on Sterol Homeostasis: Measuring 7-Dehydrocholesterol in Dhcr7-Deficient Neuro2a Cells and Human Fibroblasts. J. Med. Chem. 2016, 59, 1102–1115. [Google Scholar] [CrossRef]
- Burgazli, C.R.; Rana, K.B.; Brown, J.N.; Tillman, F., 3rd. Efficacy and safety of hydroxyzine for sleep in adults: Systematic review. Hum. Psychopharmacol. 2023, 38, e2864. [Google Scholar] [CrossRef]
- Ferreri, M.; Hantouche, E.G. Recent clinical trials of hydroxyzine in generalized anxiety disorder. Acta Psychiatr. Scand. Suppl. 1998, 393, 102–108. [Google Scholar] [CrossRef]
- Guaiana, G.; Barbui, C.; Cipriani, A. Hydroxyzine for generalised anxiety disorder. Cochrane Database Syst. Rev. 2010, 12, CD006815. [Google Scholar] [CrossRef]
- Kim, T.; Kim, K.; Kim, S.; Kim, J. Safety of hydroxyzine in the sedation of pediatric dental patients. J. Dent. Anesth. Pain. Med. 2022, 22, 395–404. [Google Scholar] [CrossRef] [PubMed]
- ClinCalc. Hydroxyzine Drug Usage Statistics, United States, 2013–2022; Clin Calc: Chicago, IL, USA, 2022. [Google Scholar]
- Gengo, F.M.; Dabronzo, J.; Yurchak, A.; Love, S.; Miller, J.K. The relative antihistaminic and psychomotor effects of hydroxyzine and cetirizine. Clin. Pharmacol. Ther. 1987, 42, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Jotaki, S.; Murotani, K.; Hiraki, T. Preventive Effect of Hydroxyzine on Postoperative Nausea and Vomiting: A Single-Center, Retrospective, Observational Cohort Study. J. Clin. Med. 2024, 13, 7807. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S. Hydroxyzine may be safe and effective in generalised anxiety disorder. Evid. Based Ment. Health 2003, 6, 91. [Google Scholar] [CrossRef]
- Llorca, P.M.; Spadone, C.; Sol, O.; Danniau, A.; Bougerol, T.; Corruble, E.; Faruch, M.; Macher, J.-P.; Sermet, E.; Servant, D. Efficacy and safety of hydroxyzine in the treatment of generalized anxiety disorder: A 3-month double-blind study. J. Clin. Psychiatry 2002, 63, 1020–1027. [Google Scholar] [CrossRef]
- Paton, D.M.; Webster, D.R. Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines). Clin. Pharmacokinet. 1985, 10, 477–497. [Google Scholar] [CrossRef]
- Simons, F.E.; Simons, K.J.; Frith, E.M. The pharmacokinetics and antihistaminic of the H1 receptor antagonist hydroxyzine. J. Allergy Clin. Immunol. 1984, 73 Pt 1, 69–75. [Google Scholar] [CrossRef]
- Foye, W.O.; Lemke, T.L.; Williams, D.A. Foye’s Principles of Medicinal Chemistry, 7th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Korade, Z.; Kenworthy, A.K.; Mirnics, K. Molecular consequences of altered neuronal cholesterol biosynthesis. J. Neurosci. Res. 2009, 87, 866–875. [Google Scholar] [CrossRef]
- Korade, Z.; Xu, L.; Harrison, F.E.; Ahsen, R.; Hart, S.E.; Folkes, O.M.; Mirnics, K.; Porter, N.A. Antioxidant supplementation ameliorates molecular deficits in Smith-Lemli-Opitz syndrome. Biol. Psychiatry 2014, 75, 215–222. [Google Scholar] [CrossRef]
- Correa-Cerro, L.S.; Porter, F.D. 3beta-hydroxysterol Delta7-reductase and the Smith-Lemli-Opitz syndrome. Mol. Genet. Metab. 2005, 84, 112–126. [Google Scholar] [CrossRef]
- Korade, Z.; Mi, Z.; Portugal, C.; Schor, N.F. Expression and p75 neurotrophin receptor dependence of cholesterol synthetic enzymes in adult mouse brain. Neurobiol. Aging 2007, 28, 1522–1531. [Google Scholar] [PubMed]
- Goudriaan, A.; Camargo, N.; Carney, K.E.; Oliet, S.H.; Smit, A.B.; Verheijen, M.H. Novel cell separation method for molecular analysis of neuron-astrocyte co-cultures. Front. Cell Neurosci. 2014, 8, 12. [Google Scholar] [PubMed]
- Brovkovych, V.; Aldrich, A.; Li, N.; Atilla-Gokcumen, G.E.; Frasor, J. Removal of Serum Lipids and Lipid-Derived Metabolites to Investigate Breast Cancer Cell Biology. Proteomics 2019, 19, e1800370. [Google Scholar] [PubMed]
- Tallman, K.A.; Allen, L.B.; Klingelsmith, K.B.; Anderson, A.; Genaro-Mattos, T.C.; Mirnics, K.; Porter, N.A.; Korade, Z. Prescription Medications Alter Neuronal and Glial Cholesterol Synthesis. ACS Chem. Neurosci. 2021, 12, 735–745. [Google Scholar]
- Bukelis, I.; Porter, F.D.; Zimmerman, A.W.; Tierney, E. Smith-Lemli-Opitz syndrome and autism spectrum disorder. Am. J. Psychiatry 2007, 164, 1655–1661. [Google Scholar]
- Haas, D.; Garbade, S.F.; Vohwinkel, C.; Muschol, N.; Trefz, F.K.; Penzien, J.M.; Zschocke, J.; Hoffmann, G.F.; Burgard, P. Effects of cholesterol and simvastatin treatment in patients with Smith-Lemli-Opitz syndrome (SLOS). J. Inherit. Metab. Dis. 2007, 30, 375–387. [Google Scholar]
- Korade, Z.; Genaro-Mattos, T.C.; Tallman, K.A.; Liu, W.; Garbett, K.A.; Koczok, K.; Balogh, I.; Mirnics, K.; Porter, N.A. Vulnerability of DHCR7(+/−) mutation carriers to aripiprazole and trazodone exposure. J. Lipid Res. 2017, 58, 2139–2146. [Google Scholar]
- Balog, M.; Anderson, A.C.; Heffer, M.; Korade, Z.; Mirnics, K. Effects of Psychotropic Medication on Somatic Sterol Biosynthesis of Adult Mice. Biomolecules 2022, 12, 1535. [Google Scholar] [CrossRef]
- Kim, H.Y.; Korade, Z.; Tallman, K.A.; Liu, W.; Weaver, C.D.; Mirnics, K.; Porter, N.A. Inhibitors of 7-Dehydrocholesterol Reductase: Screening of a Collection of Pharmacologically Active Compounds in Neuro2a Cells. Chem. Res. Toxicol. 2016, 29, 892–900. [Google Scholar]
- Korade, Z.; Heffer, M.; Mirnics, K. Medication effects on developmental sterol biosynthesis. Mol. Psychiatry 2022, 27, 490–501. [Google Scholar]
- Peeples, E.S.; Mirnics, K.; Korade, Z. Chemical Inhibition of Sterol Biosynthesis. Biomolecules 2024, 14, 410. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Maternal aripiprazole exposure interacts with 7-dehydrocholesterol reductase mutations and alters embryonic neurodevelopment. Mol. Psychiatry 2019, 24, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Korade, Z.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Genaro-Mattos, T.C.; Porter, N.A.; Mirnics, K. Trazodone effects on developing brain. Transl. Psychiatry 2021, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Korade, Z.; Liu, W.; Warren, E.B.; Armstrong, K.; Porter, N.A.; Konradi, C. Effect of psychotropic drug treatment on sterol metabolism. Schizophr. Res. 2017, 187, 74–81. [Google Scholar] [CrossRef]
- Boland, M.R.; Tatonetti, N.P. Investigation of 7-dehydrocholesterol reductase pathway to elucidate off-target prenatal effects of pharmaceuticals: A systematic review. Pharmacogenom. J. 2016, 16, 411–429. [Google Scholar] [CrossRef]
- Cross, J.L.; Iben, J.; Simpson, C.L.; Thurm, A.; Swedo, S.; Tierney, E.; Bailey-Wilson, J.; Biesecker, L.; Porter, F.; Wassif, C. Determination of the allelic frequency in Smith-Lemli-Opitz syndrome by analysis of massively parallel sequencing data sets. Clin. Genet. 2015, 87, 570–575. [Google Scholar] [CrossRef]
- Lalovic, A.; Merkens, L.; Russell, L.; Arsenault-Lapierre, G.; Nowaczyk, M.J.; Porter, F.D.; Steiner, R.D.; Turecki, G. Cholesterol metabolism and suicidality in Smith-Lemli-Opitz syndrome carriers. Am. J. Psychiatry 2004, 161, 2123–2126. [Google Scholar] [CrossRef]
- Ikegawa, S.; Ohashi, H.; Ogata, T.; Honda, A.; Tsukahara, M.; Kubo, T.; Kimizuka, M.; Shimode, M.; Hasegawa, T.; Nishimura, G.; et al. Novel and recurrent EBP mutations in X-linked dominant chondrodysplasia punctata. Am. J. Med. Genet. 2000, 94, 300–305. [Google Scholar] [CrossRef]
- Kumble, S.; Savarirayan, R. Chondrodysplasia Punctata 2, X.-Linked. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korade, Z.; Anderson, A.C.; Balog, M.; Tallman, K.A.; Porter, N.A.; Mirnics, K. Hydroxyzine Effects on Post-Lanosterol Biosynthesis in Smith–Lemli–Opitz Syndrome (SLOS) Models. Biomolecules 2025, 15, 562. https://doi.org/10.3390/biom15040562
Korade Z, Anderson AC, Balog M, Tallman KA, Porter NA, Mirnics K. Hydroxyzine Effects on Post-Lanosterol Biosynthesis in Smith–Lemli–Opitz Syndrome (SLOS) Models. Biomolecules. 2025; 15(4):562. https://doi.org/10.3390/biom15040562
Chicago/Turabian StyleKorade, Zeljka, Allison C. Anderson, Marta Balog, Keri A. Tallman, Ned A. Porter, and Karoly Mirnics. 2025. "Hydroxyzine Effects on Post-Lanosterol Biosynthesis in Smith–Lemli–Opitz Syndrome (SLOS) Models" Biomolecules 15, no. 4: 562. https://doi.org/10.3390/biom15040562
APA StyleKorade, Z., Anderson, A. C., Balog, M., Tallman, K. A., Porter, N. A., & Mirnics, K. (2025). Hydroxyzine Effects on Post-Lanosterol Biosynthesis in Smith–Lemli–Opitz Syndrome (SLOS) Models. Biomolecules, 15(4), 562. https://doi.org/10.3390/biom15040562







