HOXA10 and HOXA11 in Human Endometrial Benign Disorders: Unraveling Molecular Pathways and Their Impact on Reproduction
Abstract
:1. Introduction
2. Materials and Methods
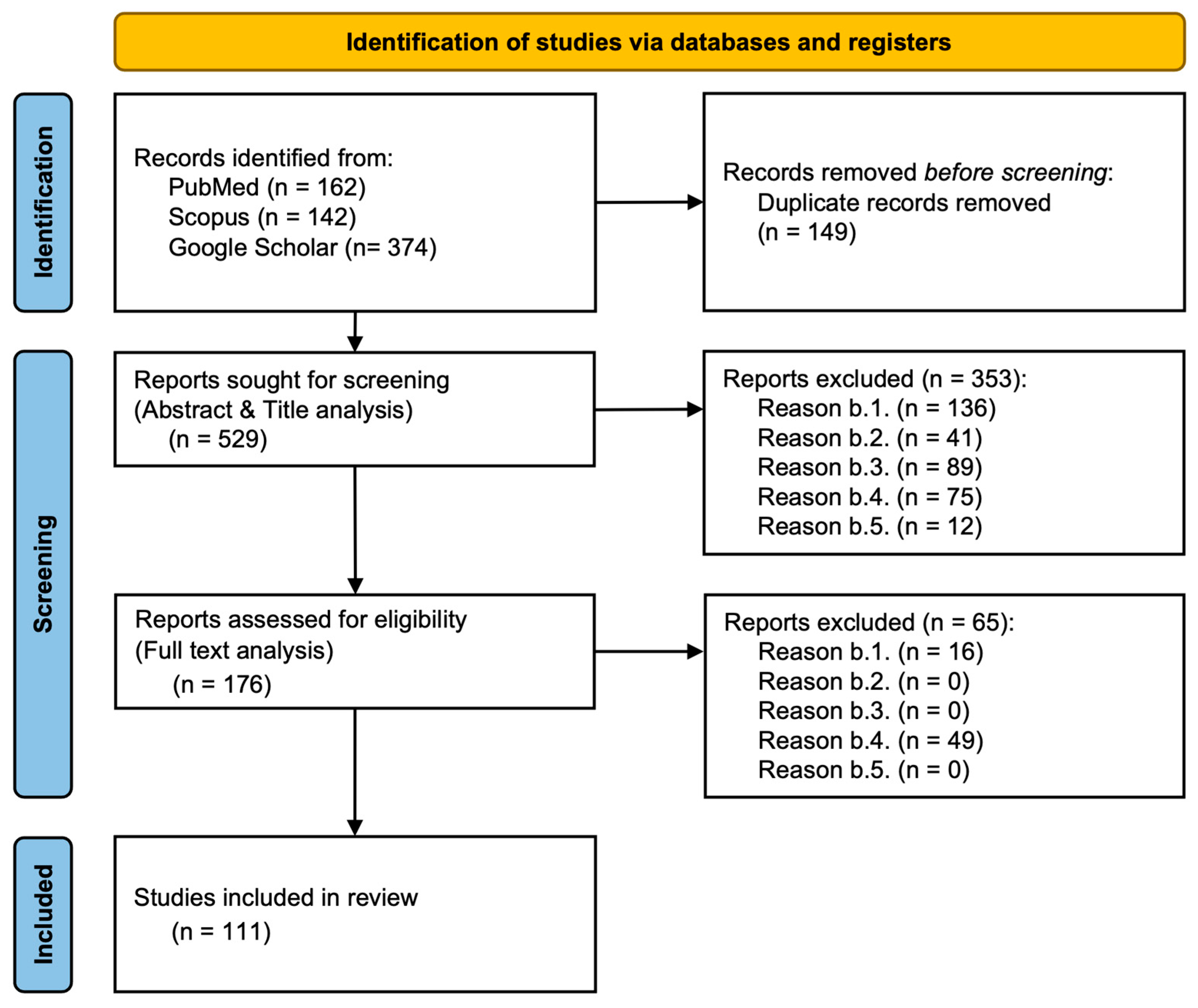
2.1. Literature Search Strategy and Database Selection
2.2. Selection Criteria Overview
- a.1.
- Studies exploring the role of HOXA10/HOXA11 genes in human benign endometrial disorders, fertility, or reproductive health.
- a.2.
- Human studies were prioritized, though relevant animal studies were considered.
- a.3.
- Publication date between 1 January 2004 and 31 December 2024, with an emphasis on molecular pathways, genetic regulation, and clinical conditions such as endometrial polyposis, adenomyosis, endometriosis, and infertility.
- a.4.
- Both experimental and observational studies were included, along with systematic reviews, meta-analyses, and randomized controlled trials to ensure a comprehensive analysis of the available literature.
- a.5.
- English-language publications to ensure accurate interpretation.
- b.1.
- Studies that focused on non-HOXA10/HOXA11 genes or non-reproductive health systems.
- b.2.
- Non-peer-reviewed articles, including opinion pieces and conference abstracts.
- b.3.
- Studies published before 1 January 2004.
- b.4.
- Animal studies were excluded from this review as our focus is solely on human reproductive health.
- b.5.
- Non-English publications due to potential translation and interpretation limitations.
2.3. Article Selection Process
3. Role of HOX Genes in Reproductive Health
3.1. Benign Disorders of the Reproductive System
3.2. Relationship Between Benign Endometrial Conditions and Fertility
4. Overview of HOX Genes and Endometrial Function
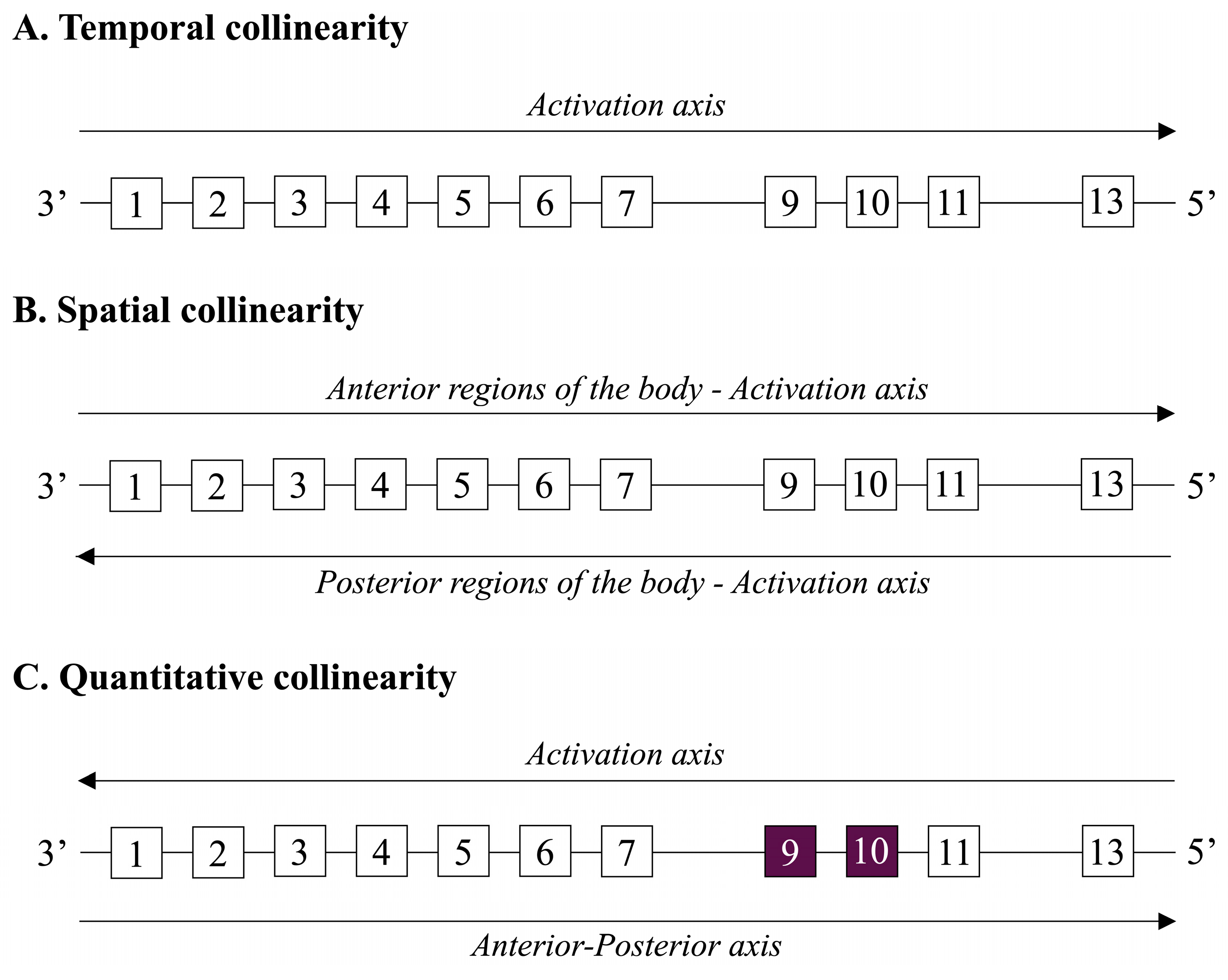
4.1. Overview of HOX Genes and Their Roles in Cellular Differentiation and Spatial Development
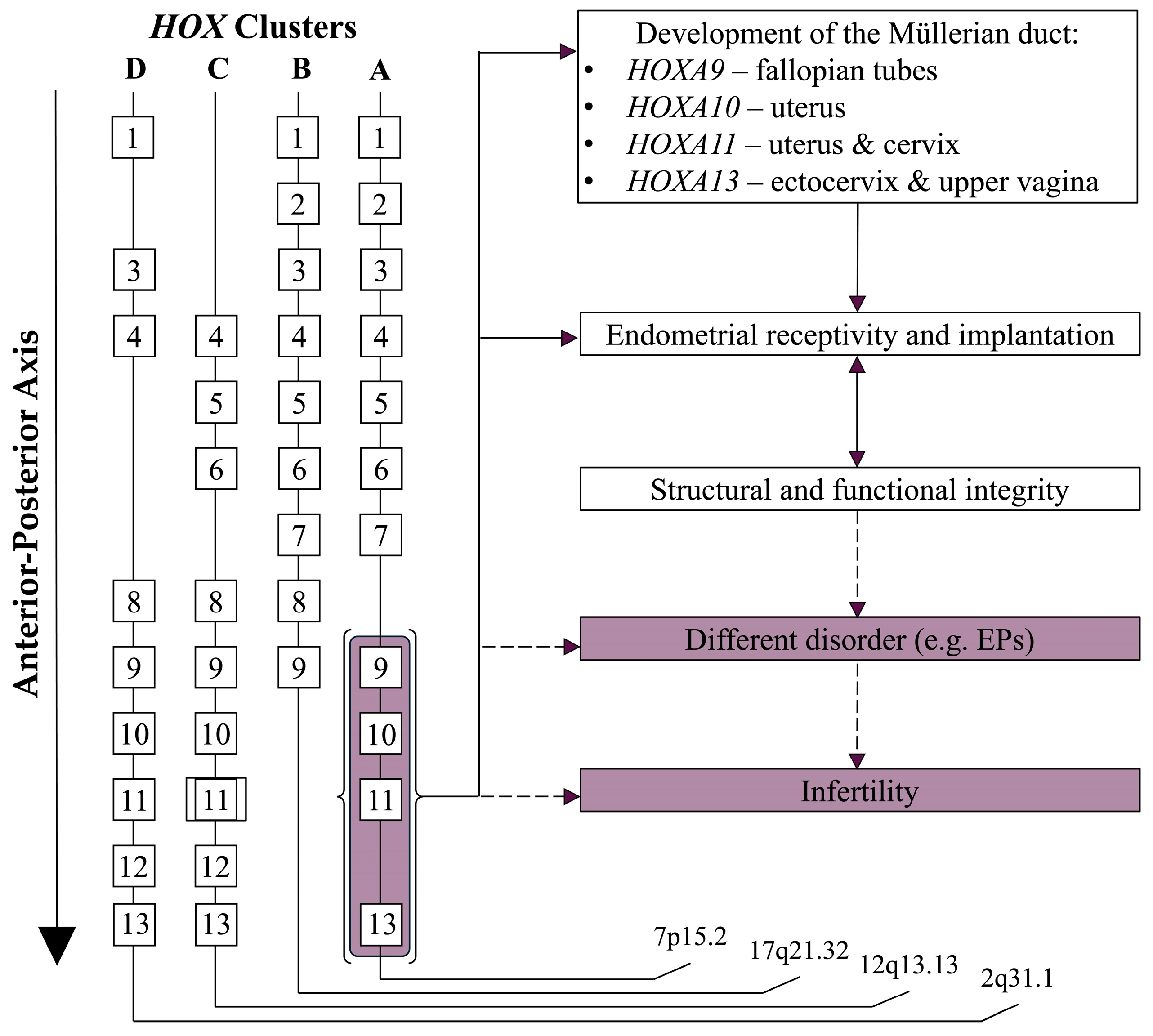
4.2. Specific Focus on HOXA10 and HOXA11 Genes
4.3. Regulation of Stromal Cell Differentiation by HOX Genes
4.4. Regulation of Epithelial Cell Differentiation by HOX Genes
5. HOX Gene Expression During the Menstrual Cycle and Implantation
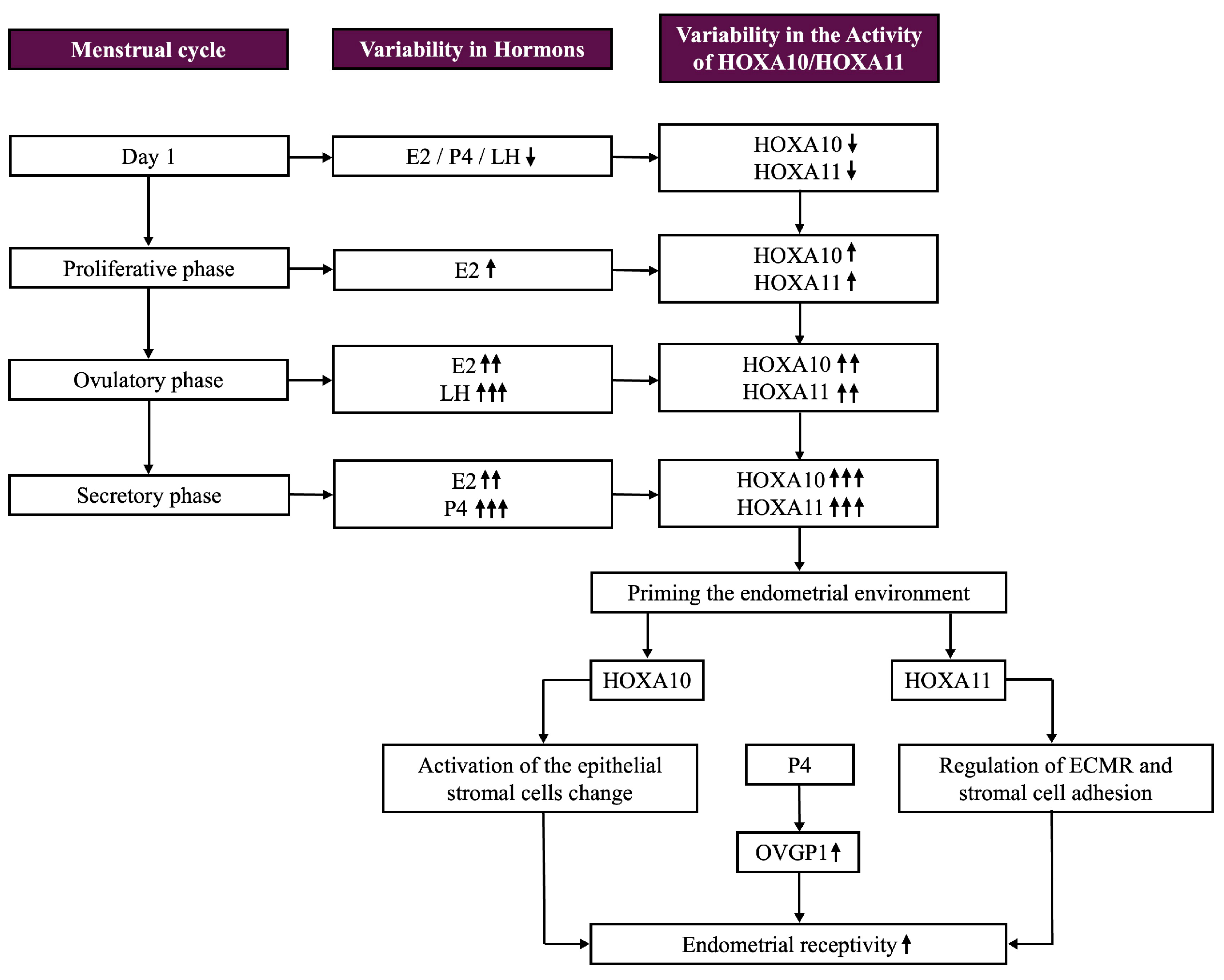
5.1. Regulation of HOXA Gene Expression
5.2. Role of HOXA10 and HOXA11 in Uterine Function and the Menstrual Cycle
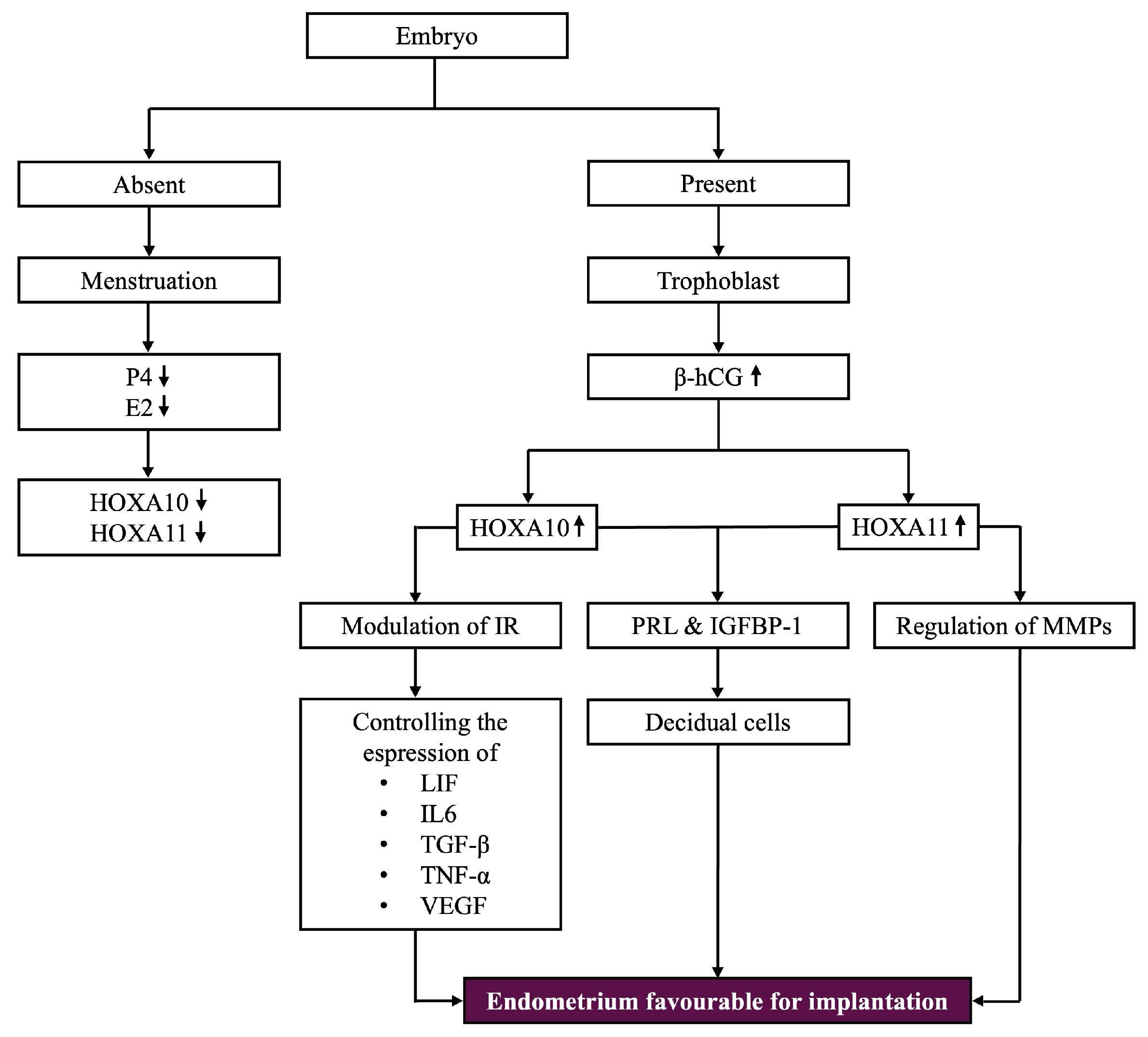
5.3. Expression Patterns in the Presence or Absence of Pregnancy
5.4. HOXA Genes in Lymphocyte Function and Immune Response
5.5. HOXA11’s Role in ECM Remodeling and Immune Regulation
5.6. Connection Between HOXA Genes and OVGP1
5.7. Vitamin D and HOXA Gene Expression
5.8. Glycodelin and HOX Genes in Implantation
6. Disrupted HOX Gene Expression in Endometrial Benign Disorders
6.1. HOXA10 Expression in Gynecological Conditions
6.2. Role of HOXA10 in Tissue Homeostasis and Differentiation
6.3. HOXA10 and Endometrial Hyperplasia
6.4. HOXA10 in Endometriosis
6.5. HOXA10/HOXA11 and Endometrial Polyps
7. Interplay Between NF-κB and HOX Genes
7.1. NF-κB Pathway Overview
7.2. NF-κB and HOX Genes: Crosstalk in Immunity and Development
7.3. HOXA10 and HOXA11 in Pregnancy
7.4. HOX Genes in Immune Responses
8. Clinical Implications and Future Directions
8.1. Diagnostic Potential of HOX Gene Expression Profiling
8.2. Therapeutic Approaches Targeting HOX Gene Pathways
8.3. Research Gaps and Future Directions
8.4. Need for Longitudinal Studies Examining the Impact of HOX-Targeted Therapies on Fertility Outcomes
9. Overview of Key Pathways and Genes Influencing HOX Function
10. Limitations of the Review
- Database Selection Bias: The study selection was based on articles retrieved from PubMed, Scopus, and Google Scholar. Relevant studies from other databases or gray literature may have been overlooked, limiting the comprehensiveness of this review.
- Exclusion Criteria Impact: Strict methodological and reporting criteria may have led to the omission of some relevant studies. Differences in study design, sample sizes, and analytical methods could have affected the generalizability of the findings.
- Title- and Abstract-Based Screening: The initial screening process was conducted based on article titles and abstracts, which may have led to the exclusion of studies that contained relevant information in the full text but lacked clarity in their abstracts. As a result, some valuable research may have been unintentionally disregarded.
- Language Restrictions: This review does not explicitly account for language limitations, meaning studies published in languages other than English may have been excluded, potentially reducing the diversity of perspectives.
- Publication Bias: Studies with significant or positive results are more likely to be published and included, while negative or inconclusive findings might be underrepresented, possibly skewing the overall conclusions.
- Dependence on Existing Literature: This review relies on previously published studies, meaning any inconsistencies, biases, or gaps in primary research are carried over into the synthesis. This limits the ability to provide entirely objective conclusions.
- Timeframe Constraints: This review may be restricted to studies published within a specific timeframe, potentially missing older but still relevant research that could provide additional context or alternative perspectives.
- Heterogeneity of Studies: The included studies may vary significantly in terms of research methods, sample sizes, and analytical approaches, making it challenging to draw uniform conclusions. This variability could introduce inconsistencies in the interpretation of findings.
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makker, A.; Goel, M.M.; Nigam, D.; Bhatia, V.; Mahdi, A.A.; Das, V.; Pandey, A. Endometrial expression of homeobox genes and cell adhesion molecules in infertile women with intramural fibroids during window of implantation. Reprod. Sci. 2016, 24, 435–444. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ni, Z.; Kong, S.; Lu, J.; Wang, H. Homeobox genes for embryo implantation: From mouse to human. Anim. Models Exp. Med. 2018, 1, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Taylor, H.S. The role of Hox Genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, challenges, and guiding treatment. J. Clin. Endocrinol. Metab. 2020, 106, e1071–e1083. [Google Scholar] [CrossRef]
- Polycystic Ovary Syndrome. Available online: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Polycystic-Ovary-Syndrome (accessed on 7 January 2025).
- The Lancet Regional Health—Europe. Polycystic Ovary Syndrome: What More Can Be Done Patients? Lancet Reg. Health Eur. 2022, 21, 100524. [Google Scholar] [CrossRef]
- Da Silva, M.C.M.; De Souza Ferreira, L.P.; Della Giustina, A. It is time to change the definition: Endometriosis is no longer a pelvic disease. Clinics 2024, 79, 100326. [Google Scholar] [CrossRef]
- Gunther, R.; Walker, C. Adenomyosis. StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539868/ (accessed on 7 January 2025).
- Wong, M.; Crnobrnja, B.; Liberale, V.; Dharmarajah, K.; Widschwendter, M.; Jurkovic, D. The natural history of endometrial polyps. Hum. Reprod. 2016, 32, 340–345. [Google Scholar] [CrossRef]
- Yanaihara, A.; Yorimitsu, T.; Motoyama, H.; Iwasaki, S.; Kawamura, T. Location of endometrial polyp and pregnancy rate in infertility patients. Fertil. Steril. 2007, 90, 180–182. [Google Scholar] [CrossRef]
- Al-Jefout, M.; Black, K.; Schulke, L.; Berbic, M.; Luscombe, G.; Tokushige, N.; Manconi, F.; Markham, R.; Fraser, I.S. Novel finding of high density of activated mast cells in endometrial polyps. Fertil. Steril. 2009, 92, 1104–1106. [Google Scholar] [CrossRef]
- Dreisler, E.; Sorensen, S.; Ibsen, P.H.; Lose, G. Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20–74 years. Ultrasound Obstet. Gynecol. 2008, 33, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Nijkang, N.P.; Anderson, L.; Markham, R.; Manconi, F. Endometrial polyps: Pathogenesis, sequelae and treatment. SAGE Open Medicine 2019, 7, 2050312119848247. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.-J.; Kim, M.S.; Lee, J.Y.; Nam, S.; Jeong, H.G.; Kim, T.; Park, H. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw. Open 2022, 5, e2243951. [Google Scholar] [CrossRef] [PubMed]
- Collée, J.; Mawet, M.; Tebache, L.; Nisolle, M.; Brichant, G. Polycystic ovarian syndrome and infertility: Overview and insights of the putative treatments. Gynecol. Endocrinol. 2021, 37, 869–874. [Google Scholar] [CrossRef]
- Bonavina, G.; Taylor, H.S. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front. Endocrinol. 2022, 13, 1020827. [Google Scholar] [CrossRef]
- Harada, T.; Khine, Y.M.; Kaponis, A.; Nikellis, T.; Decavalas, G.; Taniguchi, F. The impact of adenomyosis on women’s fertility. Obstet. Gynecol. Surv. 2016, 71, 557–568. [Google Scholar] [CrossRef]
- Chami, A.A.; Saridogan, E. Endometrial polyps and subfertility. J. Obstet. Gynecol. India 2016, 67, 9–14. [Google Scholar] [CrossRef]
- Clark, T.J.; Stevenson, H. Endometrial Polyps and Abnormal Uterine Bleeding (AUB-P): What is the relationship, how are they diagnosed and how are they treated? Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 40, 89–104. [Google Scholar] [CrossRef]
- Stamatellos, I.; Apostolides, A.; Stamatopoulos, P.; Bontis, J. Pregnancy rates after hysteroscopic polypectomy depending on the size or number of the polyps. Arch. Gynecol. Obstet. 2007, 277, 395–399. [Google Scholar] [CrossRef]
- Fang, W.; Li, K.; Ma, S.; Wei, F.; Hu, Y. Natural selection and convergent evolution of the HOX gene family in Carnivora. Front. Ecol. Evol. 2023, 11, 1107034. [Google Scholar] [CrossRef]
- Hubert, K.A.; Wellik, D.M. Hox genes in development and beyond. Development 2023, 150, dev192476. [Google Scholar] [CrossRef] [PubMed]
- Afzal, Z.; Krumlauf, R. Transcriptional regulation and implications for controlling Hox gene expression. J. Dev. Biol. 2022, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S. Hox Gene collinearity: From A-P patterning to radially symmetric animals. Curr. Genom. 2016, 17, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S. Physical laws shape up HOX gene collinearity. J. Dev. Biol. 2021, 9, 17. [Google Scholar] [CrossRef]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. Role of HOX genes in stem cell differentiation and cancer. Stem Cells Int. 2018, 2018, 3569493. [Google Scholar] [CrossRef]
- Zhao, L.; Lou, W.; Zhang, Y.; Han, Y.; Zhang, W. Comprehensive analysis of HOX family genes in endometrial cancer. Transl. Cancer Res. 2023, 12, 3728–3743. [Google Scholar] [CrossRef]
- Khan, I.; Amin, M.A.; Eklund, E.A.; Gartel, A.L. Regulation of HOX gene expression in AML. Blood Cancer J. 2024, 14, 42. [Google Scholar] [CrossRef]
- Ashary, N.; Laheri, S.; Modi, D. Homeobox genes in endometrium: From development to decidualization. Int. J. Dev. Biol. 2020, 64, 227–237. [Google Scholar] [CrossRef]
- Ekanayake, D.L.; Małopolska, M.M.; Schwarz, T.; Tuz, R.; Bartlewski, P.M. The roles and expression of HOXA/Hoxa10 gene: A prospective marker of mammalian female fertility? Reprod. Biol. 2022, 22, 100647. [Google Scholar] [CrossRef]
- Jiang, R.; Ding, L.; Zhou, J.; Huang, C.; Zhang, Q.; Jiang, Y.; Liu, J.; Yan, Q.; Zhen, X.; Sun, J.; et al. Enhanced HOXA10 sumoylation inhibits embryo implantation in women with recurrent implantation failure. Cell Death Discovery 2017, 3, 17057. [Google Scholar] [CrossRef]
- Kagan, M.; Pleniceanu, O.; Vivante, A. HOXA11 is another monogenic cause of congenital anomalies of the kidney and urinary tract—Reply. Pediatr. Nephrol. 2022, 38, 935. [Google Scholar] [CrossRef] [PubMed]
- De Keersmaecker, K.; Luna, P.J.R.; Della Gatta, G.; Palomero, T.; Castillo, M.; Sulis, M.L.; Tosello, V.; Gounari, F.; Kee, B.; Lenz, J.; et al. The HOX11/TLX1 transcription factor oncogene induces chromosomal aneuploidy in T-ALL. Blood 2009, 114, 142. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, C.; Wang, Y.; Ma, S.; Cao, W.; Guan, F. HOXC11 functions as a novel oncogene in human colon adenocarcinoma and kidney renal clear cell carcinoma. Life Sci. 2020, 243, 117230. [Google Scholar] [CrossRef]
- Daftary, G.S.; Taylor, H.S. Endocrine regulation of HOX genes. Endocr. Rev. 2006, 27, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Xu, B.; Mohankumar, K.M.; Goffin, V.; Perry, J.K.; Lobie, P.E.; Liu, D.-X. The prolactin receptor mediates HOXA1-stimulated oncogenicity in mammary carcinoma cells. Int. J. Oncol. 2012, 41, 2285–2295. [Google Scholar] [CrossRef]
- Saare, M.; Modhukur, V.; Suhorutshenko, M.; Rajashekar, B.; Rekker, K.; Sõritsa, D.; Karro, H.; Soplepmann, P.; Sõritsa, A.; Lindgren, C.M.; et al. The influence of menstrual cycle and endometriosis on endometrial methylome. Clin. Epigenet. 2016, 8, 2. [Google Scholar] [CrossRef]
- Daza, D.O.; Sundström, G.; Bergqvist, C.A.; Duan, C.; Larhammar, D. Evolution of the Insulin-Like Growth Factor binding Protein (IGFBP) family. Endocrinology 2011, 152, 2278–2289. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, S.; Yao, G.; Sun, Y. Identification of HOXA10 target genes in human endometrial stromal cells by RNA-seq analysis. Acta Biochim. Biophys. Sin. 2020, 53, 365–371. [Google Scholar] [CrossRef]
- Marquardt, R.M.; Kim, T.H.; Shin, J.-H.; Jeong, J.-W. Progesterone and estrogen signaling in the endometrium: What goes wrong in endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef]
- Bi, Y.; Huang, W.; Yuan, L.; Chen, S.; Liao, S.; Fu, X.; Liu, B.; Yang, Y. HOXA10 improves endometrial receptivity by upregulating E-cadherin. Biol. Reprod. 2022, 106, 992–999. [Google Scholar] [CrossRef]
- Shilpasree, A.; Kulkarni, V.; Shetty, P.; Bargale, A.; Goni, M.; Oli, A.; Sarathkumar, E.; Patil, V.; Desai, R. Induction of endometrial HOXA 10 gene expression by vitamin D and its possible influence on reproductive outcome of PCOS patients undergoing ovulation induction procedure. Indian J. Endocrinol. Metab. 2022, 26, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Modi, D. Role of HOXA10 in pathologies of the endometrium. Rev. Endocr. Metab. Disord. 2024, 26, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.-W.; Petraglia, F. Pathogenesis of adenomyosis: An update on molecular mechanisms. Reprod. BioMed. Online 2017, 35, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Sukumar, S. The Hox genes and their roles in oncogenesis. Nat. Rev. Cancer 2010, 10, 361–371. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, X.; Li, C.; Zhang, X.; Yang, D.; Liu, Y.; Li, L. DNA methylation of HOX genes and its clinical implications in cancer. Exp. Mol. Pathol. 2023, 134, 104871. [Google Scholar] [CrossRef]
- Fan, F.; Mo, H.; Zhang, H.; Dai, Z.; Wang, Z.; Qu, C.; Liu, F.; Zhang, L.; Luo, P.; Zhang, J.; et al. HOXA5: A crucial transcriptional factor in cancer and a potential therapeutic target. Biomed. Pharmacother. 2022, 155, 113800. [Google Scholar] [CrossRef]
- Ding, F.; Chen, P.; Bie, P.; Piao, W.; Cheng, Q. HOXA5 is recognized as a Prognostic-Related biomarker and promotes glioma progression through affecting cell cycle. Front. Oncol. 2021, 11, 633430. [Google Scholar] [CrossRef]
- Jin, Q.; Xu, L.; Wang, J.; Lin, J.; Lin, H. Pan-cancer analysis of Homeobox B9 as a predictor for prognosis and immunotherapy in human tumors. Aging 2023, 51, 5096–5124. [Google Scholar] [CrossRef]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. HOX genes and their role in the development of human cancers. J. Mol. Med. 2014, 92, 811–823. [Google Scholar] [CrossRef]
- Mishra, A.; Ganguli, N.; Majumdar, S.S.; Modi, D. Loss of HOXA10 causes endometrial hyperplasia progressing to endometrial cancer. J. Mol. Endocrinol. 2022, 69, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Makker, A.; Goel, M.M. Tumor progression, metastasis, and modulators of epithelial–mesenchymal transition in endometrioid endometrial carcinoma: An update. Endocr. Relat. Cancer 2015, 23, R85–R111. [Google Scholar] [CrossRef] [PubMed]
- Lazim, N.; Elias, M.H.; Sutaji, Z.; Karim, A.K.A.; Abu, M.A.; Ugusman, A.; Syafruddin, S.E.; Mokhtar, M.H.; Ahmad, M.F. Expression of HOXA10 Gene in Women with Endometriosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12869. [Google Scholar] [CrossRef]
- Zanatta, A.; Rocha, A.M.; Carvalho, F.M.; Pereira, R.M.A.; Taylor, H.S.; Motta, E.L.A.; Baracat, E.C.; Serafini, P.C. The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility: A review. J. Assist. Reprod. Genet. 2010, 27, 701–710. [Google Scholar] [CrossRef]
- Özcan, C.; Özdamar, Ö.; Gökbayrak, M.E.; Doğer, E.; Çakıroğlu, Y.; Çine, N. HOXA-10 gene expression in ectopic and eutopic endometrium tissues: Does it differ between fertile and infertile women with endometriosis? Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 233, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Camboni, A.; Marbaix, E. Ectopic Endometrium: The Pathologist’s perspective. Int. J. Mol. Sci. 2021, 22, 10974. [Google Scholar] [CrossRef]
- Kulebyakina, M.; Makarevich, P. Hox-Positive adult mesenchymal stromal cells: Beyond positional identity. Front. Cell Dev. Biol. 2020, 8, 624. [Google Scholar] [CrossRef]
- Elias, M.H.; Lazim, N.; Sutaji, Z.; Abu, M.A.; Karim, A.K.A.; Ugusman, A.; Syafruddin, S.E.; Mokhtar, M.H.; Ahmad, M.F. HOXA10 DNA Methylation Level in the Endometrium Women with Endometriosis: A Systematic Review. Biology 2023, 12, 474. [Google Scholar] [CrossRef]
- Ciscato, A.; Zare, S.Y.; Fadare, O. The significance of recurrence in endometrial polyps: A clinicopathologic analysis. Hum. Pathol. 2020, 100, 38–44. [Google Scholar] [CrossRef]
- Vaduva, C.C.; Constantinescu, C.; Serbanescu, M.; Dara, L.; Oancea, M.D.; Carp-Veliscu, A. The association between endometrial polyps, chronic endometritis, and IVF outcomes. PubMed 2023, 27, 8895–8904. [Google Scholar] [CrossRef]
- Munro, M.G. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil. Steril. 2019, 111, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, A.; Cialdella, M.; Cicinelli, R.; Santarsiero, C.M.; Greco, P.; Buzzaccarini, G.; Noventa, M.; Cicinelli, E. Association between Endometrial Polyps and Chronic Endometritis: Is It Time for a Paradigm Shift in the Pathophysiology of Endometrial Polyps in Pre-Menopausal Women? Results of a Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 2182. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Vitagliano, A.; Loizzi, V.; De Ziegler, D.; Fanelli, M.; Bettocchi, S.; Nardelli, C.; Trojano, G.; Cicinelli, R.; Minervini, C.F.; et al. Altered Gene Expression Encoding Cytochines, Grow Factors and Cell Cycle Regulators in the Endometrium of Women with Chronic Endometritis. Diagnostics 2021, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Bai, W. Higher Prevalence of Endometrial Polyps in Patients with Fallopian Tube Obstruction: A Case-control Study. J. Minim. Invasive Gynecol. 2018, 26, 935–940. [Google Scholar] [CrossRef]
- Holzer, I.; Ott, J.; Kurz, C.; Hofstetter, G.; Hager, M.; Kuessel, L.; Parry, J.P. Is Chronic Endometritis Associated with Tubal Infertility? A Prospective Cohort Study. J. Minim. Invasive Gynecol. 2021, 28, 1876–1881. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, Y.; Xu, S.; Zhang, C. Association between endometritis and endometrial polyp: A Mendelian randomization study. Int. J. Women Health 2023, 15, 1963–1970. [Google Scholar] [CrossRef]
- Kitaya, K.; Tada, Y.; Taguchi, S.; Funabiki, M.; Hayashi, T.; Nakamura, Y. Local mononuclear cell infiltrates in infertile patients with endometrial macropolyps versus micropolyps. Hum. Reprod. 2012, 27, 3474–3480. [Google Scholar] [CrossRef]
- Guo, L.; Gu, F.; Tan, J.; Luo, L.; Gao, J.; Zhou, C. Multiple endometrial polyps is associated with higher risk of chronic endometritis in reproductive-aged women. J. Obstet. Gynaecol. Res. 2020, 47, 389–396. [Google Scholar] [CrossRef]
- Gelardi, M.; Netti, G.S.; Giancaspro, R.; Spadaccino, F.; Pennella, A.; Fiore, V.; La Gatta, E.; Grilli, G.M.; Cassano, M.; Ranieri, E. Chronic rhinosinusitis with nasal polyposis (CRSwNP): The correlation between expression of Galectin-10 and Clinical-Cytological Grading (CCG). Am. J. Rhinol. Allergy 2021, 36, 229–237. [Google Scholar] [CrossRef]
- Ashktorab, H.; Brim, H.; Hassan, S.; Nouraie, M.; Gebreselassie, A.; Laiyemo, A.O.; Kibreab, A.; Aduli, F.; Latella, G.; Brant, S.R.; et al. Inflammatory polyps occur more frequently in inflammatory bowel disease than other colitis patients. BMC Gastroenterol. 2020, 20, 170. [Google Scholar] [CrossRef]
- Kitaya, K.; Tada, Y.; Hayashi, T.; Taguchi, S.; Funabiki, M.; Nakamura, Y. Comprehensive Endometrial Immunoglobulin Subclass Analysis in Infertile Women Suffering from Repeated Implantation Failure with or without Chronic Endometritis. Am. J. Reprod. Immunol. 2014, 72, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, S.; Huang, C.; Lian, R.; Chen, C.; Liu, S.; Li, L.; Diao, L.; Markert, U.R.; Zeng, Y. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil. Steril. 2019, 113, 187–196.e1. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G.; Basile, G.; Cucinella, G.; Greco, M.E.; Perino, A.; Chiantera, V.; Marinelli, S. Fresh vs. frozen embryo transfer in assisted reproductive techniques: A single center retrospective cohort study and ethical-legal implications. PubMed 2023, 27, 6809–6823. [Google Scholar] [CrossRef]
- Pai, P.; Sukumar, S. HOX genes and the NF-κB pathway: A convergence of developmental biology, inflammation and cancer biology. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1874, 188450. [Google Scholar] [CrossRef]
- Kanegae, Y.; Tavares, A.T.; Belmonte, J.C.I.; Verma, I.M. Role of Rel/NF-κB transcription factors during the outgrowth of the vertebrate limb. Nature 1998, 392, 611–614. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Z.; Wu, Q.; Wang, H.; Xia, H.; Sun, Y. Long non-coding RNA HOXA11-AS knockout inhibits proliferation and overcomes drug resistance in ovarian cancer. Bioengineered 2022, 13, 13893–13905. [Google Scholar] [CrossRef]
- Voutilainen, M.; Lindfors, P.H.; Lefebvre, S.; Ahtiainen, L.; Fliniaux, I.; Rysti, E.; Murtoniemi, M.; Schneider, P.; Schmidt-Ullrich, R.; Mikkola, M.L. Ectodysplasin regulates hormone-independent mammary ductal morphogenesis via NF-κB. Proc. Natl. Acad. Sci. USA 2012, 109, 5744–5749. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nature Reviews. Immunology 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Wang, W.; Nag, S.; Zhang, R. Targeting the NFκB Signaling Pathways for Breast Cancer Prevention and Therapy. Curr. Med. Chem. 2014, 22, 264–289. [Google Scholar] [CrossRef]
- Chen, Q.; Massagué, J. Molecular pathways: VCAM-1 as a potential therapeutic target in metastasis. Clin. Cancer Res. 2012, 18, 5520–5525. [Google Scholar] [CrossRef]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nature Reviews. Immunology 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Espín-Palazón, R.; Traver, D. The NF-κB family: Key players during embryonic development and HSC emergence. Exp. Hematol. 2016, 44, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Mulero, M.C.; Ferres-Marco, D.; Islam, A.; Margalef, P.; Pecoraro, M.; Toll, A.; Drechsel, N.; Charneco, C.; Davis, S.; Bellora, N.; et al. Chromatin-Bound IΚBA regulates a subset of polycomb target genes in differentiation and cancer. Cancer Cell 2013, 24, 151–166. [Google Scholar] [CrossRef]
- Sarno, J.; Schatz, F.; Huang, S.J.; Lockwood, C.; Taylor, H.S. Thrombin and interleukin-1 decrease HOX gene expression in human first trimester decidual cells: Implications for pregnancy loss. Mol. Hum. Reprod. 2009, 15, 451–457. [Google Scholar] [CrossRef]
- Manz, M.G.; Boettcher, S. Emergency granulopoiesis. Nat. Reviews. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bei, L.; Shah, C.A.; Hu, L.; Eklund, E.A. HOXA10 terminates emergency granulopoiesis by increasing expression of Triad1. J. Immunol. 2015, 194, 5375–5387. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Hayashi, K.; Ouchi, M.; Endou, H.; Anzai, N. HOXB9 acts as a negative regulator of activated human T cells in response to amino acid deficiency. Immunol. Cell Biol. 2016, 94, 612–617. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Yang, J.; Zhang, K.; Feng, X.; Sun, C.; Feng, Q.; Li, Z. Associations of Th1, Th2, Th17, and Treg cell levels and imbalance with recurrent spontaneous abortion: A meta-analysis. J. Obstet. Gynaecol. Res. 2025, 51, e16207. [Google Scholar] [CrossRef]
- Aguilar-Camacho, J.M.; Harry, N.D.; Zakas, C. Comparative Hox genes expression within the dimorphic annelid Streblospio benedicti reveals patterning variation during development. EvoDevo 2024, 15, 12. [Google Scholar] [CrossRef]
- Grier, D.; Thompson, A.; Kwasniewska, A.; McGonigle, G.; Halliday, H.; Lappin, T. Pathophysiol. HOX Genes Their Role Cancer. J. Pathol. 2005, 205, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W.; Wu, C.; Gao, H.; Yu, J.; Wang, X.; Li, B.; Jun, Z.; Zhang, W.; Zhou, P.; et al. HOXC10 promotes proliferation and invasion and induces immunosuppressive gene expression in glioma. FEBS J. 2018, 285, 2278–2291. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yan, B.; Lai, W.; Shi, Y.; Xiao, D.; Jia, J.; Liu, S.; Li, H.; Lu, J.; Li, Z.; et al. Repression of Hox genes by LMP1 in nasopharyngeal carcinoma and modulation of glycolytic pathway genes by HoxC8. Oncogene 2015, 34, 6079–6091. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-P.; Xie, M.; Pan, W.-X.; Zhang, Z.-Y.; Li, W.-F. HOXA10 Promotes the Development of Bladder Cancer through Regulating FOSL1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2945–2954. [Google Scholar] [CrossRef]
- Bruun, S.; Andersen, R.; Madsen, J.; Hansen, T.; Tabor, T.; Bechmann, T.; Kjær, I. Circulating methylated HOXA9 tumor DNA as a biomarker for mortality in recurrent breast cancer: An exploratory study. Oncol. Lett. 2024, 28, 581. [Google Scholar] [CrossRef]
- Celik, O.; Yurci, A.; Ersahin, A.; Gungor, N.D.; Celik, N.; Ozcil, M.D.; Dogan, S.; Dalkilic, S.; Dalkilic, L.; Ulug, U.; et al. Endometrial Injury Upregulates Expression of Receptivity Genes in Women with Implantation Failure. Int. J. Environ. Res. Public Health 2023, 20, 3942. [Google Scholar] [CrossRef]
- Makrigiannakis, A.; Makrygiannakis, F.; Vrekoussis, T. Approaches to improve endometrial receptivity in case of repeated implantation failures. Front. Cell Dev. Biol. 2021, 9, 613277. [Google Scholar] [CrossRef]
- Elgindy, E.A.; Abdelghany, A.A.; AbdAlsalam, H.S.; Mostafa, M.I. The novel incorporation of aromatase inhibitor in hormonal replacement therapy cycles: A randomized controlled trial. Reprod. BioMed. Online 2021, 44, 641–649. [Google Scholar] [CrossRef]
- Hashim, H.A. Aromatase Inhibitors for Endometriosis-Associated Infertility; Do we have sufficient evidence? DOAJ 2016, 10, 270–277. [Google Scholar] [CrossRef]
- Jin, K.; Sukumar, S. HOX genes: Major actors in resistance to selective endocrine response modifiers. Biochim. Biophys. Acta (BBA) Rev. Cancer 2016, 1865, 105–110. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, H.; Huang, W.; Bi, Y.; Qin, A.; Yang, Y. The function of metformin in endometrial receptivity (ER) of patients with polycyclic ovary syndrome (PCOS): A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2021, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Li, D.-J.; Wang, X.-Q. Towards a Better Understanding of Endometriosis-Related Infertility: A review on how Endometriosis affects endometrial receptivity. Biomolecules 2023, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-Z.; Yang, H.-L.; Shi, J.-W.; Shen, H.-H.; Wang, Y.; Chang, K.-K.; Zhang, T.; Ye, J.-F.; Sun, J.-S.; Qiu, X.-M.; et al. Protopanaxadiol improves endometriosis associated infertility and miscarriage in sex hormones receptors-dependent and independent manners. Int. J. Biol. Sci. 2021, 17, 1878–1894. [Google Scholar] [CrossRef] [PubMed]
- Auradkar, A.; Bulger, E.A.; Devkota, S.; McGinnis, W.; Bier, E. Dissecting the evolutionary role of the Hox gene proboscipedia in Drosophila mouthpart diversification by full locus replacement. Sci. Adv. 2021, 7, eabk1003. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, J.; Lv, Q.; Shi, C.; Qiu, M.; Xie, L.; Liu, W.; Yang, B.; Shan, W.; Cheng, Y.; et al. Endometrium-derived mesenchymal stem cells suppress progression of endometrial cancer via the DKK1-Wnt/β-catenin signaling pathway. Stem Cell Res. Ther. 2023, 14, 159. [Google Scholar] [CrossRef]
- Rungsiwiwut, R.; Virutamasen, P.; Pruksananonda, K. Mesenchymal stem cells for restoring endometrial function: An infertility perspective. Reprod. Med. Biol. 2020, 20, 13–19. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, L.; Luo, X.; Chen, X. Adult stem cells in endometrial regeneration: Molecular insights and clinical applications. Mol. Reprod. Dev. 2021, 88, 379–394. [Google Scholar] [CrossRef]
- Martinović, L.S.; Mladenić, T.; Lovrić, D.; Ostojić, S.; Pavlić, S.D. Decoding the epigenetics of infertility: Mechanisms, environmental influences, and therapeutic strategies. Epigenomes 2024, 8, 34. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, S.; Qi, J.; Wang, Y.; Ding, Y.; Zhu, Q.; He, Y.; Lu, Y.; Yao, Y.; Wang, S.; et al. Increased expression of HOXA11-AS attenuates endometrial decidualization in recurrent implantation failure patients. Mol. Ther. 2022, 30, 1706–1720. [Google Scholar] [CrossRef]

| Phase | HOXA10/HOXA11 Expression | Regulatory Factors | Impact on Endometrium |
|---|---|---|---|
| Proliferative Phase | Low | Estrogen | Induces growth and proliferation of endometrial lining |
| Ovulatory Phase | Moderate | Estrogen, LH | Primes the endometrial environment for progesterone responsiveness |
| Secretory Phase | High | Progesterone, Vitamin D, Relaxin | Supports decidualization, stromal differentiation, and implantation |
| Post-Implantation | Elevated | Progesterone, β-hCG | Facilitates decidual cell transformation and trophoblast invasion |
| Menstruation (if no implantation) | Decreased | Regression of Corpus Luteum | Leads to shedding of functional endometrial layer |
| Pathway/Regulatory Factor | Description | Impact |
|---|---|---|
| Estrogen and Progesterone Signaling | Works synergistically to modulate HOXA10/HOXA11 expression during menstrual phases, peaking in the secretory phase to prepare for implantation. | Key drivers of uterine receptivity, particularly during the secretory phase, ensuring proper endometrial preparation for implantation. |
| RA Pathway | Influences 3′ HOX gene expression, including HOXA9, critical for Müllerian duct development. | Regulates the development of the anterior–posterior axis and ensures proper organ formation along the reproductive tract. |
| VDR Pathway | Upregulates HOXA10 and HOXA11, enhancing uterine receptivity by modifying stromal cell adhesion and immune environment. | Enhances stromal cell adhesion and supports immune modulation to create a favorable implantation environment. |
| TGF-β and Cytokine Networks | TGF-β and pro-inflammatory cytokines like IL-6 and TNF-α regulate cellular differentiation, with disruption leading to infertility and endometriosis. | Critical in cellular differentiation and immune balance; disruption causes inflammation, tissue damage, and reduced reproductive capability. |
| MMPs | HOX genes, particularly HOXA10 and HOXA11, regulate MMPs critical for ECM remodeling during trophoblast invasion. | Essential for ECM remodeling, enabling embryo invasion into the endometrial lining during implantation. |
| Epigenetic Modifications | DNA methylation of HOXA10/HOXA11 promoters in pathological states (e.g., endometriosis, adenomyosis) results in decreased gene expression and altered endometrial receptivity. | Suppresses HOX gene expression, compromising decidualization and implantation success. |
| HOXA10 and HOXA11 Dysregulation | Lower expression in endometriosis reduces implantation success, while hypermethylation in adenomyosis affects decidualization and pregnancy outcomes. | Causes implantation failure and adverse pregnancy outcomes; central to many gynecological pathologies. |
| NF-κB-HOX Crosstalk | NF-κB pathways regulate inflammation and immunity, interacting with HOX genes to modulate embryonic patterning and immune responses. | Highlights the interconnectedness of inflammatory responses and developmental processes, with implications for reproductive disorders and systemic immune function. |
| Gene/Factor | Protein Function in Signaling | Molecular Pathway(s) Involved | Regulatory Factors | Associated Pathologies | Observations |
|---|---|---|---|---|---|
| HOXA10 | Facilitates stromal decidualization and regulates ECM remodeling | MMPs, LIF, VEGF signaling | Progesterone, estrogen, vitamin D, relaxin | Infertility, endometriosis, adenomyosis, EPs | Vitamin D increases HOXA10 expression, enhancing endometrial receptivity. Dysregulation leads to failed implantation |
| HOXA10 and HOXA11 (joint impact) | Orchestrates implantation and immune microenvironment | ECM remodeling, cytokine network (IL-6, LIF, TGF-β) | Progesterone, estrogen, vitamin D | Adenomyosis, endometriosis, infertility | Coordinate stromal and epithelial adhesion during implantation |
| NF-κB | Mediates inflammatory response and embryonic development | Canonical and non-canonical NF-κB pathways | Inflammatory cytokines (e.g., TNF-α, IL-1β), lipopolysaccharide (LPS) | Obesity, inflammatory diseases, atherosclerosis | Regulates dorsal/ventral patterning alongside HOX genes; interacts with HOXA10 during endometrial remodeling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pîrlog, L.-M.; Pătrășcanu, A.-A.; Ona, M.-D.; Cătană, A.; Rotar, I.C. HOXA10 and HOXA11 in Human Endometrial Benign Disorders: Unraveling Molecular Pathways and Their Impact on Reproduction. Biomolecules 2025, 15, 563. https://doi.org/10.3390/biom15040563
Pîrlog L-M, Pătrășcanu A-A, Ona M-D, Cătană A, Rotar IC. HOXA10 and HOXA11 in Human Endometrial Benign Disorders: Unraveling Molecular Pathways and Their Impact on Reproduction. Biomolecules. 2025; 15(4):563. https://doi.org/10.3390/biom15040563
Chicago/Turabian StylePîrlog, Lorin-Manuel, Andrada-Adelaida Pătrășcanu, Mara-Diana Ona, Andreea Cătană, and Ioana Cristina Rotar. 2025. "HOXA10 and HOXA11 in Human Endometrial Benign Disorders: Unraveling Molecular Pathways and Their Impact on Reproduction" Biomolecules 15, no. 4: 563. https://doi.org/10.3390/biom15040563
APA StylePîrlog, L.-M., Pătrășcanu, A.-A., Ona, M.-D., Cătană, A., & Rotar, I. C. (2025). HOXA10 and HOXA11 in Human Endometrial Benign Disorders: Unraveling Molecular Pathways and Their Impact on Reproduction. Biomolecules, 15(4), 563. https://doi.org/10.3390/biom15040563






