Exploring the Roles of Liver X Receptors in Lipid Metabolism and Immunity in Atherosclerosis
Abstract
1. Introduction
2. Immune Cells in Atherosclerotic Plaques
2.1. Monocytes and Macrophages
2.2. Neutrophils
2.3. Dendritic Cells
2.4. Lymphocytes
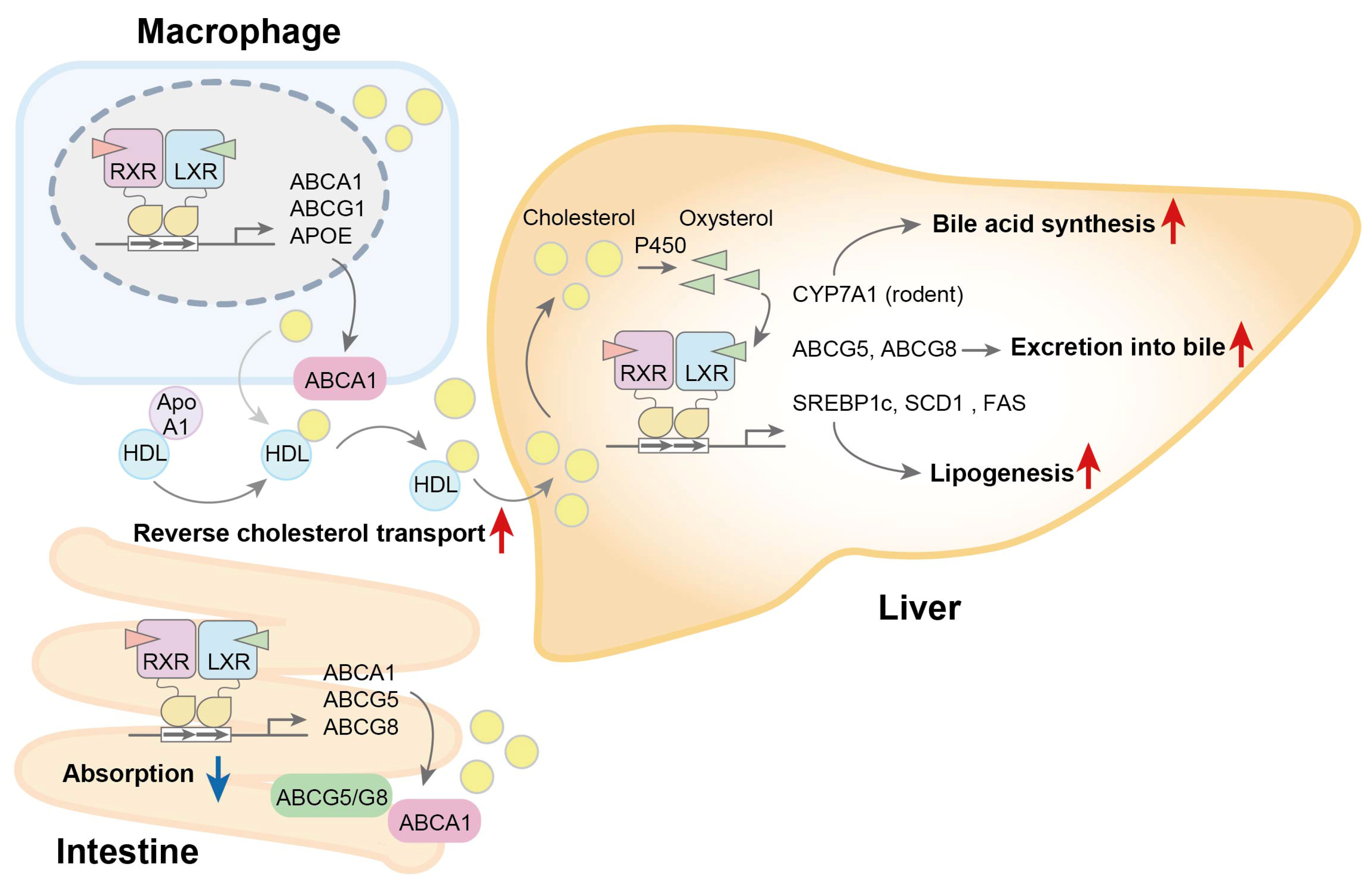
3. LXRs Regulate Lipid Metabolism and Immune Function
3.1. Regulation of Lipid Metabolism
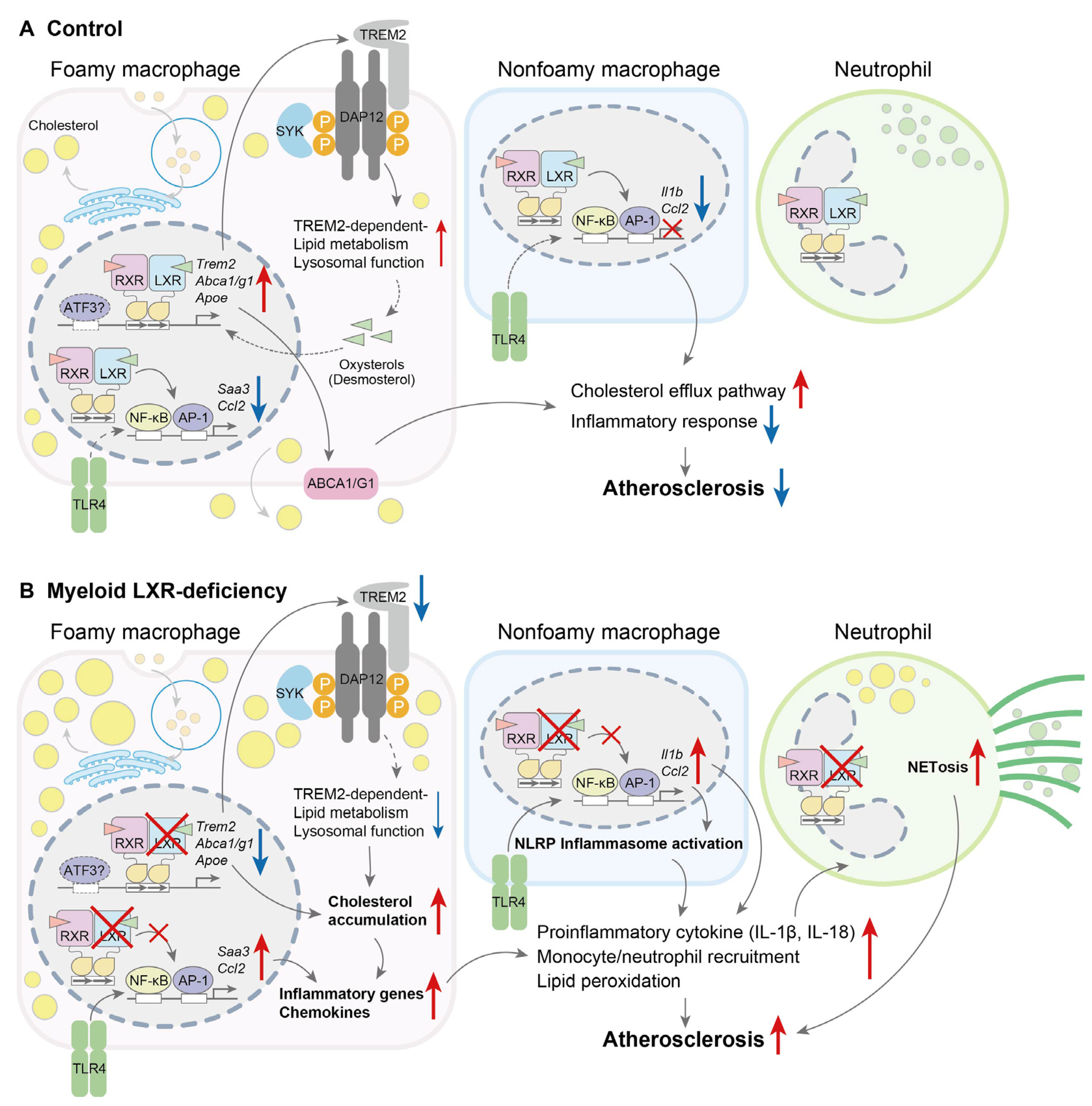
3.2. Regulation of Immune Response
3.3. Phagocytosis and Apoptosis
4. Effect of Cholesterol Accumulation in Atherosclerotic Plaques
5. Pharmacological and Endogenous LXR Activation in Animal Models of Atherosclerosis
5.1. Pharmacological LXR Activation
| Mice | Model | LXR | Diet | Treatment | Findings | Ref |
|---|---|---|---|---|---|---|
| LDLR(-/-) and Apo E(-/-) | Progression | Pharmacological activation | WTD (12 weeks) | GW3965 (1 or 10 mpk) | Lesion size↓ ABCA1, ABCG1↑ (aorta) | [139] |
| LDLR(-/-) | Progression | Pharmacological activation | WTD (8 weeks) | T0901317 (3 or 10 mpk) | Lesion size↓ ABCA1, ABCG1↑ (plaque) | [140] |
| Apo E(-/-) | Progression | Pharmacological activation | WTD (4 weeks) | GW3965 (10 mpk) | Lesion size↓ Macrophage apoptosis↓, efferocytosis↑ Endoplasmic reticulum stress↓ | [142] |
| LDLR(-/-) | Regression | Pharmacological activation | WTD (14 weeks) →ND (3 weeks) | T0901317 (25 mpk) and/or K/BxN serum with ND | Plaque regression and stability↑ Arthritic clinical score↓ Inflammation↓ | [143] |
| LDLR(-/-) | Progression | Pharmacological activation | WTD (12 weeks) | BMT (myeloid ABCA1/G1(-/-)) T0901317 or GW3965 (10 mpk) | Lesion size↓ Immune cells↓ (aorta) Plasma chemokine↓ | [144] |
| LDLR(-/-) | Progression | Pharmacological activation | WTD (8 weeks) | GW3965-treated endothelial progenitor cells | Lesion size↓ Expression of vascular cell adhesion molecule 1↓ | [145] |
| Apo E(-/-) | Regression | Pharmacological activation by nanoparticle-mediated delivery | WTD (14 weeks) →ND (6 weeks) | T0901317 (1.5 mpk) in synthetic HDL nanoparticles (30 mpk) | Lesion size↓ Hepatic lipogenic gene, plasma TG→ | [146] |
| Apo E(-/-) | Progression | Pharmacological activation by nanoparticle-mediated delivery | WTD (16 weeks) | T0901317 (5 mpk) in nanofiber hydrogel (0.2%) | Lesion size↓ Hepatic lipogenic gene→ | [147] |
| Apo E(-/-) | Progression | Pharmacological activation | WTD (16 weeks) | T0901317 (1 mpk) and/or metformin (100 mpk) | Lesion size: T0901317↓, T0901317 + metformin↓ Plaque stability↑ Hepatic lipogenesis→ | [148] |
| Apo E(-/-) or LDLR(-/-) | Progression | Pharmacological activation | WTD (16 or 20 weeks) | T0901317 (1 mpk) and/or U0126 (3 mpk) | Lesion size↓(T0901317) ↓(T0901317 + U0126) Hepatic lipogenesis→ | [149,150] |
| Apo E(-/-) | Progression | Pharmacological activation | WTD (13 weeks) | T0901317 (1.5 mpk) and/or rHDL (30 mpk) | Lesion size↓ (T0901317 + reconstituted HDL) Cholesterol efflux↑ | [151] |
| Apo E(-/-) | Progression | Pharmacological activation | WTD (11 weeks) | N,N-dimethyl-3β-hydroxy-cholenamide (8 mpk) | Lesion size↓ Macrophage infiltration↓ Hepatic lipogenic gene→ | [153] |
| Apo E(-/-) | Progression | Pharmacological activation | WTD (12 weeks) | Nagilactone B (10 and 30 mpk) | Lesion size↓ ABCA1↑ (plaque macrophage) Hepatic lipogenic gene→ | [154] |
| LDLR(-/-) | Progression | Pharmacological activation | WTD (8 weeks) | WAY-252623 (LXR-623) (15 mpk) | Lesion size↓ Hepatic lipogenic gene→ | [155] |
| Apo E(-/-); Lipid droplet-associated hydrolase-transgenic | Progression | Endogenous activation | WTD (20 weeks) | Lesion size↓ LXR target genes↑ | [156] | |
| LDLR(-/-) | Progression | Endogenous activation | WTD (13 weeks) | SH42 (0.5 mpk) | Desmosterol levels↑ Lesion size→ | [157] |
| LDLR(-/-) | Progression | Liver-specific overexpression | WTD (12 weeks) | Adeno-associated virus gene transfer of LXRα | Lesion size↓ LXR target genes↑ | [158] |
| LDLR(-/-); Intestinal-specific VP16-LXRα transgenic | Progression | Intestine-specific overexpression | WTD (16 weeks) | Lesion size↓ Cholesterol absorption↓ Reverse cholesterol transport↑ | [159] |
5.2. Endogenous LXR Ligands: Oxysterols
6. LXR Deletion in Mouse Models of Atherosclerosis
| Mice | Model | LXR | Diet | Treatment | Findings | Ref. |
|---|---|---|---|---|---|---|
| Apo E(-/-); LXRα(-/-) | Progression | Genetic deletion | WTD (14 or 34 weeks) | GW3965 (20 mpk) | Cholesterol accumulation in tissues Lesion size↑ GW3965 administration reduces lesion size | [172] |
| LDLR(-/-); LXRα(-/-) LDLR(-/-); LXRβ(-/-) | Progression | Genetic deletion | WTD (20 weeks) | T0901317 (10→3 mpk) | Lesion size↑ [LXRα(-/-)] Lesion size→ [LXRβ(-/-)] T0901317 administration reduces lesion size [LXRα(-/-) and LXRβ(-/-)] | [173] |
| Apo E(-/-); LXRα(-/-) Apo E(-/-); LXRβ(-/-) | Progression | Genetic deletion | WTD (15 weeks) | Lesion size↑ [LXRα(-/-)] Lesion size→ [LXRβ(-/-)] | [174] | |
| LDLR(-/-); Liver-specific LXRα(-/-) | Progression | Genetic deletion (liver) | WTD (20 weeks) | Cholesterol excretion↓ Lesion size↑ | [175] | |
| Apo E(-/-) and LDLR(-/-) | Progression | BMT [donor: LXRα(-/-); LXRβ(-/-)] | WTD (8 or 16 weeks) | T0901317 (10 mpk) | Lesion size↑ No effect on T0901317 treatment | [177,178] |
| LDLR(-/-) | Progression | BMT [donor: myeloid-specific LXRα(-/-); LXRβ(-/-)] | WTD (10 weeks) | Lesion size↑ Foamy macrophage activation↑ NETosis↑ | [179] | |
| Apo E(-/-) | Regression | BMT [donor: Apo E(-/-); LXRα(-/-) or Apo E(-/-); LXRβ(-/-)] | WTD (16 weeks) | Aortic arch transplantation to wild-type mice impairs plaque regression CCL7 expression↓ | [180] |
7. Post-Translational Modification of LXR in Atherosclerosis
| Mice | Model | LXR | Diet | Findings | Ref. |
|---|---|---|---|---|---|
| LDLR(-/-); Myeloid specific LXRα S196A knock-in | Progression | Genetic modification (disruption of LXRα phosphorylation) | WTD (12 weeks) | Lesion size↑ Macrophage proliferation↑ Necrotic core↓ Efferocytosis↓ | [190] |
| LDLR(-/-) | Progression | BMT [LXRα(-/-) S196A] (disruption of LXRα phosphorylation) | WTD (16 weeks) | Macrophage proliferation↓ Monocyte recruitment↓ Apoptosis↓ | [191] |
| LDLR(-/-); TTC39B(-/-) | Progression | Endogenous LXRα protein stability↑ | WTD (16 weeks) | Lesion size↓ Plaque severity↓ HDL-C↑, LDL-C↓ | [185] |
8. Effects of Other Nuclear Receptors on Atherosclerosis
8.1. FXR
8.2. PPARs
9. Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| Apo E | Apolipoprotein E |
| BMT | Bone marrow transplantation |
| CCL | C-C chemokine ligand |
| CCR | C-C chemokine receptor |
| CITE-seq | Cellular indexing of transcriptomes and epitopes by sequencing |
| CH25H | Cholesterol 25-hydroxylase |
| CYP7A1 | Cholesterol 7α-hydroxylase |
| cDC | Conventional dendritic cell |
| DC | Dendritic cell |
| DHCR24 | 24-dehydrocholesterol reductase |
| FXR | Farnesoid X receptor |
| FAS | Fatty acid synthase |
| HDL | High-density lipoprotein |
| iNOS | Inducible NO synthase |
| IFN | Interferon |
| IL | Interleukin |
| LBP | Lipopolysaccharide-binding protein |
| LXR | Liver X receptor |
| LDL | Low-density lipoprotein |
| LDLR | Low-density lipoprotein receptor |
| M-CSF | Macrophage colony-stimulating factor |
| MHC | Major histocompatibility complex |
| NK | Natural killer |
| NET | Neutrophil extracellular trap |
| NADPH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| NLRP3 | NLR family pyrin domain containing 3 |
| pDC | Plasmacytoid dendritic cell |
| PAD | Peptidylarginine deiminase |
| PPAR | Peroxisome proliferator-activated receptor |
| rHDL | Reconstituted high-density lipoprotein |
| RAR | Retinoic acid receptor |
| SCD1 | Stearoyl-CoA desaturase-1 |
| scRNA-seq | Single-cell RNA sequencing |
| SREBP1c | Sterol regulatory element-binding protein-1c |
| SUMO | Small ubiquitin-like modifier |
| TLR | Toll-like receptor |
| TTC39B | Tetratricopeptide repeat domain protein 39B |
| TGM2 | Transglutaminase 2 |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
References
- Tamargo, I.A.; Baek, K.I.; Kim, Y.; Park, C.; Jo, H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat. Rev. Cardiol. 2023, 20, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, B.; Otterdal, K.; Dahl, T.B.; Skjelland, M.; Gullestad, L.; Øie, E.; Aukrust, P. Atherosclerotic plaque stability—What determines the fate of a plaque? Prog. Cardiovasc. Dis. 2008, 51, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Apfel, R.; Benbrook, D.; Lernhardt, E.; Ortiz, M.A.; Salbert, G.; Pfahl, M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell Biol. 1994, 14, 7025–7035. [Google Scholar] [CrossRef] [PubMed]
- Teboul, M.; Enmark, E.; Li, Q.; Wikström, A.C.; Pelto-Huikko, M.; Gustafsson, J.A. OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 2096–2100. [Google Scholar] [CrossRef]
- Willy, P.J.; Umesono, K.; Ong, E.S.; Evans, R.M.; Heyman, R.A.; Mangelsdorf, D.J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995, 9, 1033–1045. [Google Scholar] [CrossRef]
- Bookout, A.L.; Jeong, Y.; Downes, M.; Yu, R.T.; Evans, R.M.; Mangelsdorf, D.J. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 2006, 126, 789–799. [Google Scholar] [CrossRef]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef]
- Janowski, B.A.; Grogan, M.J.; Jones, S.A.; Wisely, G.B.; Kliewer, S.A.; Corey, E.J.; Mangelsdorf, D.J. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA 1999, 96, 266–271. [Google Scholar] [CrossRef]
- Fu, X.; Menke, J.G.; Chen, Y.; Zhou, G.; MacNaul, K.L.; Wright, S.D.; Sparrow, C.P.; Lund, E.G. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J. Biol. Chem. 2001, 276, 38378–38387. [Google Scholar] [CrossRef]
- Endo-Umeda, K.; Yasuda, K.; Sugita, K.; Honda, A.; Ohta, M.; Ishikawa, M.; Hashimoto, Y.; Sakaki, T.; Makishima, M. 7-Dehydrocholesterol metabolites produced by sterol 27-hydroxylase (CYP27A1) modulate liver X receptor activity. J. Steroid Biochem. Mol. Biol. 2014, 140, 7–16. [Google Scholar] [CrossRef]
- Chen, W.; Chen, G.; Head, D.L.; Mangelsdorf, D.J.; Russell, D.W. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007, 5, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; McDonald, J.G.; Patel, A.; Zhang, Y.; Umetani, M.; Xu, F.; Westover, E.J.; Covey, D.F.; Mangelsdorf, D.J.; Cohen, J.C.; et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 2006, 281, 27816–27826. [Google Scholar] [CrossRef] [PubMed]
- Spann, N.J.; Garmire, L.X.; McDonald, J.G.; Myers, D.S.; Milne, S.B.; Shibata, N.; Reichart, D.; Fox, J.N.; Shaked, I.; Heudobler, D.; et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 2012, 151, 138–152. [Google Scholar] [CrossRef]
- Willy, P.J.; Mangelsdorf, D.J. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR. Genes Dev. 1997, 11, 289–298. [Google Scholar] [CrossRef]
- Calkin, A.C.; Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012, 13, 213–224. [Google Scholar] [CrossRef]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Pelisek, J.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.E.; Zernecke, A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018, 122, 1661–1674. [Google Scholar] [CrossRef]
- Kim, K.; Shim, D.; Lee, J.S.; Zaitsev, K.; Williams, J.W.; Kim, K.W.; Jang, M.Y.; Seok Jang, H.; Yun, T.J.; Lee, S.H.; et al. Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ. Res. 2018, 123, 1127–1142. [Google Scholar] [CrossRef]
- Winkels, H.; Ehinger, E.; Vassallo, M.; Buscher, K.; Dinh, H.Q.; Kobiyama, K.; Hamers, A.A.J.; Cochain, C.; Vafadarnejad, E.; Saliba, A.E.; et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ. Res. 2018, 122, 1675–1688. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef]
- Depuydt, M.A.C.; Prange, K.H.M.; Slenders, L.; Örd, T.; Elbersen, D.; Boltjes, A.; de Jager, S.C.A.; Asselbergs, F.W.; de Borst, G.J.; Aavik, E.; et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ. Res. 2020, 127, 1437–1455. [Google Scholar] [CrossRef]
- Wirka, R.C.; Wagh, D.; Paik, D.T.; Pjanic, M.; Nguyen, T.; Miller, C.L.; Kundu, R.; Nagao, M.; Coller, J.; Koyano, T.K.; et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 2019, 25, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Bashore, A.C.; Yan, H.; Xue, C.; Zhu, L.Y.; Kim, E.; Mawson, T.; Coronel, J.; Chung, A.; Sachs, N.; Ho, S.; et al. High-Dimensional Single-Cell Multimodal Landscape of Human Carotid Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Libby, P.; Aikawa, E.; Alcaide, P.; Luscinskas, F.W.; Weissleder, R.; Pittet, M.J. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Investig. 2007, 117, 195–205. [Google Scholar] [CrossRef]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.; Mack, M.; van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef]
- Robbins, C.S.; Hilgendorf, I.; Weber, G.F.; Theurl, I.; Iwamoto, Y.; Figueiredo, J.L.; Gorbatov, R.; Sukhova, G.K.; Gerhardt, L.M.; Smyth, D.; et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013, 19, 1166–1172. [Google Scholar] [CrossRef]
- Weinberger, T.; Esfandyari, D.; Messerer, D.; Percin, G.; Schleifer, C.; Thaler, R.; Liu, L.; Stremmel, C.; Schneider, V.; Vagnozzi, R.J.; et al. Ontogeny of arterial macrophages defines their functions in homeostasis and inflammation. Nat. Commun. 2020, 11, 4549. [Google Scholar] [CrossRef]
- Lin, J.D.; Nishi, H.; Poles, J.; Niu, X.; McCauley, C.; Rahman, K.; Brown, E.J.; Yeung, S.T.; Vozhilla, N.; Weinstock, A.; et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 2019, 4, e124574. [Google Scholar] [CrossRef]
- Ensan, S.; Li, A.; Besla, R.; Degousee, N.; Cosme, J.; Roufaiel, M.; Shikatani, E.A.; El-Maklizi, M.; Williams, J.W.; Robins, L.; et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat. Immunol. 2016, 17, 159–168. [Google Scholar] [CrossRef]
- Williams, J.W.; Zaitsev, K.; Kim, K.W.; Ivanov, S.; Saunders, B.T.; Schrank, P.R.; Kim, K.; Elvington, A.; Kim, S.H.; Tucker, C.G.; et al. Limited proliferation capacity of aortic intima resident macrophages requires monocyte recruitment for atherosclerotic plaque progression. Nat. Immunol. 2020, 21, 1194–1204. [Google Scholar] [CrossRef]
- Patterson, M.T.; Firulyova, M.M.; Xu, Y.; Hillman, H.; Bishop, C.; Zhu, A.; Hickok, G.H.; Schrank, P.R.; Ronayne, C.E.; Caillot, Z.; et al. Trem2 promotes foamy macrophage lipid uptake and survival in atherosclerosis. Nat. Cardiovasc. Res. 2023, 2, 1015–1031. [Google Scholar] [CrossRef]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef] [PubMed]
- Deczkowska, A.; Weiner, A.; Amit, I. The Physiology, Pathology, and Potential Therapeutic Applications of the TREM2 Signaling Pathway. Cell 2020, 181, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e1217. [Google Scholar] [CrossRef]
- Nugent, A.A.; Lin, K.; van Lengerich, B.; Lianoglou, S.; Przybyla, L.; Davis, S.S.; Llapashtica, C.; Wang, J.; Kim, D.J.; Xia, D.; et al. TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge. Neuron 2020, 105, 837–854.e839. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef]
- Jaitin, D.A.; Adlung, L.; Thaiss, C.A.; Weiner, A.; Li, B.; Descamps, H.; Lundgren, P.; Bleriot, C.; Liu, Z.; Deczkowska, A.; et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178, 686–698.e614. [Google Scholar] [CrossRef]
- Seidman, J.S.; Troutman, T.D.; Sakai, M.; Gola, A.; Spann, N.J.; Bennett, H.; Bruni, C.M.; Ouyang, Z.; Li, R.Z.; Sun, X.; et al. Niche-Specific Reprogramming of Epigenetic Landscapes Drives Myeloid Cell Diversity in Nonalcoholic Steatohepatitis. Immunity 2020, 52, 1057–1074.e1057. [Google Scholar] [CrossRef]
- Piollet, M.; Porsch, F.; Rizzo, G.; Kapser, F.; Schulz, D.J.J.; Kiss, M.G.; Schlepckow, K.; Morenas-Rodriguez, E.; Sen, M.O.; Gropper, J.; et al. TREM2 protects from atherosclerosis by limiting necrotic core formation. Nat. Cardiovasc. Res. 2024, 3, 269–282. [Google Scholar] [CrossRef]
- Patterson, M.T.; Xu, Y.; Hillman, H.; Osinski, V.; Schrank, P.R.; Kennedy, A.E.; Barrow, F.; Zhu, A.; Tollison, S.; Shekhar, S.; et al. Trem2 Agonist Reprograms Foamy Macrophages to Promote Atherosclerotic Plaque Stability-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1646–1657. [Google Scholar] [CrossRef]
- Zernecke, A.; Erhard, F.; Weinberger, T.; Schulz, C.; Ley, K.; Saliba, A.E.; Cochain, C. Integrated single-cell analysis-based classification of vascular mononuclear phagocytes in mouse and human atherosclerosis. Cardiovasc. Res. 2023, 119, 1676–1689. [Google Scholar] [CrossRef] [PubMed]
- Bashore, A.C.; Xue, C.; Kim, E.; Yan, H.; Zhu, L.Y.; Pan, H.; Kissner, M.; Ross, L.S.; Zhang, H.; Li, M.; et al. Monocyte Single-Cell Multimodal Profiling in Cardiovascular Disease Risk States. Circ. Res. 2024, 135, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.; Saigusa, R.; Gulati, R.; Armstrong Suthahar, S.S.; Suryawanshi, V.; Alimadadi, A.; Durant, C.P.; Ghosheh, Y.; Roy, P.; Ehinger, E.; et al. Combined protein and transcript single-cell RNA sequencing in human peripheral blood mononuclear cells. BMC Biol. 2022, 20, 193. [Google Scholar] [CrossRef]
- Drechsler, M.; Megens, R.T.; van Zandvoort, M.; Weber, C.; Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010, 122, 1837–1845. [Google Scholar] [CrossRef]
- Rotzius, P.; Thams, S.; Soehnlein, O.; Kenne, E.; Tseng, C.N.; Björkström, N.K.; Malmberg, K.J.; Lindbom, L.; Eriksson, E.E. Distinct infiltration of neutrophils in lesion shoulders in ApoE-/- mice. Am. J. Pathol. 2010, 177, 493–500. [Google Scholar] [CrossRef]
- Döring, Y.; Drechsler, M.; Wantha, S.; Kemmerich, K.; Lievens, D.; Vijayan, S.; Gallo, R.L.; Weber, C.; Soehnlein, O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ. Res. 2012, 110, 1052–1056. [Google Scholar] [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef]
- Franck, G.; Mawson, T.L.; Folco, E.J.; Molinaro, R.; Ruvkun, V.; Engelbertsen, D.; Liu, X.; Tesmenitsky, Y.; Shvartz, E.; Sukhova, G.K.; et al. Roles of PAD4 and NETosis in Experimental Atherosclerosis and Arterial Injury: Implications for Superficial Erosion. Circ. Res. 2018, 123, 33–42. [Google Scholar] [CrossRef]
- Tsourouktsoglou, T.D.; Warnatsch, A.; Ioannou, M.; Hoving, D.; Wang, Q.; Papayannopoulos, V. Histones, DNA, and Citrullination Promote Neutrophil Extracellular Trap Inflammation by Regulating the Localization and Activation of TLR4. Cell Rep. 2020, 31, 107602. [Google Scholar] [CrossRef]
- Naruko, T.; Ueda, M.; Haze, K.; van der Wal, A.C.; van der Loos, C.M.; Itoh, A.; Komatsu, R.; Ikura, Y.; Ogami, M.; Shimada, Y.; et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation 2002, 106, 2894–2900. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Kumon, Y.; Kobayashi, T.; Enzan, H.; Nishioka, Y.; Yuri, K.; Wakiguchi, H.; Sugiura, T. Neutrophil infiltration and oxidant-production in human atherosclerotic carotid plaques. Histol. Histopathol. 2011, 26, 11. [Google Scholar] [CrossRef]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Macritchie, N.; Grassia, G.; Sabir, S.R.; Maddaluno, M.; Welsh, P.; Sattar, N.; Ialenti, A.; Kurowska-Stolarska, M.; McInnes, I.B.; Brewer, J.M.; et al. Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2569–2579. [Google Scholar] [CrossRef]
- Döring, Y.; Manthey, H.D.; Drechsler, M.; Lievens, D.; Megens, R.T.; Soehnlein, O.; Busch, M.; Manca, M.; Koenen, R.R.; Pelisek, J.; et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 2012, 125, 1673–1683. [Google Scholar] [CrossRef]
- Sage, A.P.; Murphy, D.; Maffia, P.; Masters, L.M.; Sabir, S.R.; Baker, L.L.; Cambrook, H.; Finigan, A.J.; Ait-Oufella, H.; Grassia, G.; et al. MHC Class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation 2014, 130, 1363–1373. [Google Scholar] [CrossRef]
- Niessner, A.; Sato, K.; Chaikof, E.L.; Colmegna, I.; Goronzy, J.J.; Weyand, C.M. Pathogen-Sensing Plasmacytoid Dendritic Cells Stimulate Cytotoxic T-Cell Function in the Atherosclerotic Plaque Through Interferon-α. Circulation 2006, 114, 2482–2489. [Google Scholar] [CrossRef]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Lu, S.; Zhang, C.; Ma, Z.; Su, R.; Li, Y.; Sun, T.; Li, Y.; Hong, M.; et al. Pairing of single-cell RNA analysis and T cell antigen receptor profiling indicates breakdown of T cell tolerance checkpoints in atherosclerosis. Nat. Cardiovasc. Res. 2023, 2, 290–306. [Google Scholar] [CrossRef]
- Horstmann, H.; Michel, N.A.; Sheng, X.; Hansen, S.; Lindau, A.; Pfeil, K.; Fernández, M.C.; Marchini, T.; Winkels, H.; Mitre, L.S.; et al. Cross-species single-cell RNA sequencing reveals divergent phenotypes and activation states of adaptive immunity in human carotid and experimental murine atherosclerosis. Cardiovasc. Res. 2024, 120, 1713–1726. [Google Scholar] [CrossRef]
- Nakai, Y.; Iwabuchi, K.; Fujii, S.; Ishimori, N.; Dashtsoodol, N.; Watano, K.; Mishima, T.; Iwabuchi, C.; Tanaka, S.; Bezbradica, J.S.; et al. Natural killer T cells accelerate atherogenesis in mice. Blood 2004, 104, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Tupin, E.; Nicoletti, A.; Elhage, R.; Rudling, M.; Ljunggren, H.G.; Hansson, G.K.; Berne, G.P. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J. Exp. Med. 2004, 199, 417–422. [Google Scholar] [CrossRef]
- Pattarabanjird, T.; Li, C.; McNamara, C. B Cells in Atherosclerosis: Mechanisms and Potential Clinical Applications. JACC Basic Transl. Sci. 2021, 6, 546–563. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Herbin, O.; Bouaziz, J.D.; Binder, C.J.; Uyttenhove, C.; Laurans, L.; Taleb, S.; Van Vré, E.; Esposito, B.; Vilar, J.; et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010, 207, 1579–1587. [Google Scholar] [CrossRef]
- Repa, J.J.; Berge, K.E.; Pomajzl, C.; Richardson, J.A.; Hobbs, H.; Mangelsdorf, D.J. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002, 277, 18793–18800. [Google Scholar] [CrossRef]
- Peet, D.J.; Turley, S.D.; Ma, W.; Janowski, B.A.; Lobaccaro, J.M.; Hammer, R.E.; Mangelsdorf, D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 1998, 93, 693–704. [Google Scholar] [CrossRef]
- Alberti, S.; Schuster, G.; Parini, P.; Feltkamp, D.; Diczfalusy, U.; Rudling, M.; Angelin, B.; Björkhem, I.; Pettersson, S.; Gustafsson, J.A. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J. Clin. Investig. 2001, 107, 565–573. [Google Scholar] [CrossRef]
- Endo-Umeda, K.; Nakashima, H.; Umeda, N.; Seki, S.; Makishima, M. Dysregulation of Kupffer Cells/Macrophages and Natural Killer T Cells in Steatohepatitis in LXRα Knockout Male Mice. Endocrinology 2018, 159, 1419–1432. [Google Scholar] [CrossRef]
- Zelcer, N.; Hong, C.; Boyadjian, R.; Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 2009, 325, 100–104. [Google Scholar] [CrossRef]
- Calkin, A.C.; Lee, S.D.; Kim, J.; Van Stijn, C.M.; Wu, X.H.; Lusis, A.J.; Hong, C.; Tangirala, R.I.; Tontonoz, P. Transgenic expression of dominant-active IDOL in liver causes diet-induced hypercholesterolemia and atherosclerosis in mice. Circ. Res. 2014, 115, 442–449. [Google Scholar] [CrossRef]
- Ding, J.; Nguyen, A.T.; Lohman, K.; Hensley, M.T.; Parker, D.; Hou, L.; Taylor, J.; Voora, D.; Sawyer, J.K.; Boudyguina, E.; et al. LXR signaling pathways link cholesterol metabolism with risk for prediabetes and diabetes. J. Clin. Investig. 2024, 134, e173278. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Liang, G.; Ou, J.; Bashmakov, Y.; Lobaccaro, J.M.; Shimomura, I.; Shan, B.; Brown, M.S.; Goldstein, J.L.; Mangelsdorf, D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000, 14, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Shimano, H.; Amemiya-Kudo, M.; Yahagi, N.; Hasty, A.H.; Matsuzaka, T.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol. Cell Biol. 2001, 21, 2991–3000. [Google Scholar] [CrossRef]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Collins, J.L.; Osborne, T.F.; Tontonoz, P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef]
- Schultz, J.R.; Tu, H.; Luk, A.; Repa, J.J.; Medina, J.C.; Li, L.; Schwendner, S.; Wang, S.; Thoolen, M.; Mangelsdorf, D.J.; et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000, 14, 2831–2838. [Google Scholar] [CrossRef]
- Repa, J.J.; Turley, S.D.; Lobaccaro, J.A.; Medina, J.; Li, L.; Lustig, K.; Shan, B.; Heyman, R.A.; Dietschy, J.M.; Mangelsdorf, D.J. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 2000, 289, 1524–1529. [Google Scholar] [CrossRef]
- Costet, P.; Luo, Y.; Wang, N.; Tall, A.R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 2000, 275, 28240–28245. [Google Scholar] [CrossRef]
- Laffitte, B.A.; Repa, J.J.; Joseph, S.B.; Wilpitz, D.C.; Kast, H.R.; Mangelsdorf, D.J.; Tontonoz, P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 507–512. [Google Scholar] [CrossRef]
- Naik, S.U.; Wang, X.; Da Silva, J.S.; Jaye, M.; Macphee, C.H.; Reilly, M.P.; Billheimer, J.T.; Rothblat, G.H.; Rader, D.J. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation 2006, 113, 90–97. [Google Scholar] [CrossRef]
- Joseph, S.B.; Castrillo, A.; Laffitte, B.A.; Mangelsdorf, D.J.; Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003, 9, 213–219. [Google Scholar] [CrossRef]
- Ghisletti, S.; Huang, W.; Ogawa, S.; Pascual, G.; Lin, M.E.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol. Cell 2007, 25, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, S.M.; Kim, O.S.; Lee, C.S.; Woo, J.H.; Park, S.J.; Joe, E.H.; Jou, I. Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Mol. Cell 2009, 35, 806–817. [Google Scholar] [CrossRef]
- Venteclef, N.; Jakobsson, T.; Ehrlund, A.; Damdimopoulos, A.; Mikkonen, L.; Ellis, E.; Nilsson, L.M.; Parini, P.; Jänne, O.A.; Gustafsson, J.A.; et al. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev. 2010, 24, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hong, C.; Rong, X.; Zhu, X.; Tarling, E.J.; Hedde, P.N.; Gratton, E.; Parks, J.; Tontonoz, P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife 2015, 4, e08009. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.M.; Torrecilla-Parra, M.; Pardo-Marqués, V.; de-Frutos, M.F.; Pérez-García, A.; Tabraue, C.; de la Rosa, J.V.; Martín-Rodriguez, P.; Díaz-Sarmiento, M.; Nuñez, U.; et al. Crosstalk Between LXR and Caveolin-1 Signaling Supports Cholesterol Efflux and Anti-Inflammatory Pathways in Macrophages. Front. Endocrinol. 2021, 12, 635923. [Google Scholar] [CrossRef]
- Thomas, D.G.; Doran, A.C.; Fotakis, P.; Westerterp, M.; Antonson, P.; Jiang, H.; Jiang, X.C.; Gustafsson, J.; Tabas, I.; Tall, A.R. LXR Suppresses Inflammatory Gene Expression and Neutrophil Migration through cis-Repression and Cholesterol Efflux. Cell Rep. 2018, 25, 3774–3785.e3774. [Google Scholar] [CrossRef]
- Pascual-García, M.; Carbó, J.M.; León, T.; Matalonga, J.; Out, R.; Van Berkel, T.; Sarrias, M.R.; Lozano, F.; Celada, A.; Valledor, A.F. Liver X receptors inhibit macrophage proliferation through downregulation of cyclins D1 and B1 and cyclin-dependent kinases 2 and 4. J. Immunol. 2011, 186, 4656–4667. [Google Scholar] [CrossRef]
- Ramón-Vázquez, A.; de la Rosa, J.V.; Tabraue, C.; Lopez, F.; Díaz-Chico, B.N.; Bosca, L.; Tontonoz, P.; Alemany, S.; Castrillo, A. Common and Differential Transcriptional Actions of Nuclear Receptors Liver X Receptors α and β in Macrophages. Mol. Cell Biol. 2019, 39, e00376-18. [Google Scholar] [CrossRef]
- Lavin, Y.; Winter, D.; Blecher-Gonen, R.; David, E.; Keren-Shaul, H.; Merad, M.; Jung, S.; Amit, I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014, 159, 1312–1326. [Google Scholar] [CrossRef]
- A-Gonzalez, N.; Guillen, J.A.; Gallardo, G.; Diaz, M.; de la Rosa, J.V.; Hernandez, I.H.; Casanova-Acebes, M.; Lopez, F.; Tabraue, C.; Beceiro, S.; et al. The nuclear receptor LXRα controls the functional specialization of splenic macrophages. Nat. Immunol. 2013, 14, 831–839. [Google Scholar] [CrossRef]
- Scott, C.L.; T’Jonck, W.; Martens, L.; Todorov, H.; Sichien, D.; Soen, B.; Bonnardel, J.; De Prijck, S.; Vandamme, N.; Cannoodt, R.; et al. The Transcription Factor ZEB2 Is Required to Maintain the Tissue-Specific Identities of Macrophages. Immunity 2018, 49, 312–325.e315. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Troutman, T.D.; Seidman, J.S.; Ouyang, Z.; Spann, N.J.; Abe, Y.; Ego, K.M.; Bruni, C.M.; Deng, Z.; Schlachetzki, J.C.M.; et al. Liver-Derived Signals Sequentially Reprogram Myeloid Enhancers to Initiate and Maintain Kupffer Cell Identity. Immunity 2019, 51, 655–670.e658. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Tsai, R.; Cummins, C.L. Loss of the Liver X Receptors Disrupts the Balance of Hematopoietic Populations, With Detrimental Effects on Endothelial Progenitor Cells. J. Am. Heart Assoc. 2018, 7, e007787. [Google Scholar] [CrossRef]
- Murphy, A.J.; Akhtari, M.; Tolani, S.; Pagler, T.; Bijl, N.; Kuo, C.L.; Wang, M.; Sanson, M.; Abramowicz, S.; Welch, C.; et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Investig. 2011, 121, 4138–4149. [Google Scholar] [CrossRef]
- Endo-Umeda, K.; Nakashima, H.; Komine-Aizawa, S.; Umeda, N.; Seki, S.; Makishima, M. Liver X receptors regulate hepatic F4/80 (+) CD11b(+) Kupffer cells/macrophages and innate immune responses in mice. Sci. Rep. 2018, 8, 9281. [Google Scholar] [CrossRef]
- Joseph, S.B.; Bradley, M.N.; Castrillo, A.; Bruhn, K.W.; Mak, P.A.; Pei, L.; Hogenesch, J.; O’Connell, R.M.; Cheng, G.; Saez, E.; et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 2004, 119, 299–309. [Google Scholar] [CrossRef]
- Fontaine, C.; Rigamonti, E.; Nohara, A.; Gervois, P.; Teissier, E.; Fruchart, J.C.; Staels, B.; Chinetti-Gbaguidi, G. Liver X receptor activation potentiates the lipopolysaccharide response in human macrophages. Circ. Res. 2007, 101, 40–49. [Google Scholar] [CrossRef]
- Ménégaut, L.; Thomas, C.; Jalil, A.; Julla, J.B.; Magnani, C.; Ceroi, A.; Basmaciyan, L.; Dumont, A.; Le Goff, W.; Mathew, M.J.; et al. Interplay between Liver X Receptor and Hypoxia Inducible Factor 1α Potentiates Interleukin-1β Production in Human Macrophages. Cell Rep. 2020, 31, 107665. [Google Scholar] [CrossRef]
- Ménégaut, L.; Jalil, A.; Pilot, T.; van Dongen, K.; Crespy, V.; Steinmetz, E.; Pais de Barros, J.P.; Geissler, A.; Le Goff, W.; Venteclef, N.; et al. Regulation of glycolytic genes in human macrophages by oxysterols: A potential role for liver X receptors. Br. J. Pharmacol. 2021, 178, 3124–3139. [Google Scholar] [CrossRef]
- González de la Aleja, A.; Herrero, C.; Torres-Torresano, M.; de la Rosa, J.V.; Alonso, B.; Capa-Sardón, E.; Muller, I.B.; Jansen, G.; Puig-Kröger, A.; Vega, M.A.; et al. Activation of LXR Nuclear Receptors Impairs the Anti-Inflammatory Gene and Functional Profile of M-CSF-Dependent Human Monocyte-Derived Macrophages. Front. Immunol. 2022, 13, 835478. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Sonntag, G.V.H.; Braun, L.C.; Lagache, S.M.M.; Liebmann, M.; Klotz, L.; Godfrey, R.; Kahles, F.; Waltenberger, J.; Findeisen, H.M. LXR Activation Induces a Proinflammatory Trained Innate Immunity-Phenotype in Human Monocytes. Front. Immunol. 2020, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Saas, P.; Chagué, C.; Maraux, M.; Cherrier, T. Toward the characterization of human pro-resolving macrophages? Front. Immunol. 2020, 11, 593300. [Google Scholar] [CrossRef] [PubMed]
- Geyeregger, R.; Zeyda, M.; Bauer, W.; Kriehuber, E.; Säemann, M.D.; Zlabinger, G.J.; Maurer, D.; Stulnig, T.M. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood 2007, 109, 4288–4295. [Google Scholar] [CrossRef]
- Töröcsik, D.; Baráth, M.; Benko, S.; Széles, L.; Dezso, B.; Póliska, S.; Hegyi, Z.; Homolya, L.; Szatmári, I.; Lányi, A.; et al. Activation of liver X receptor sensitizes human dendritic cells to inflammatory stimuli. J. Immunol. 2010, 184, 5456–5465. [Google Scholar] [CrossRef]
- Beceiro, S.; Pap, A.; Czimmerer, Z.; Sallam, T.; Guillén, J.A.; Gallardo, G.; Hong, C.; A-Gonzalez, N.; Tabraue, C.; Diaz, M.; et al. Liver X Receptor Nuclear Receptors Are Transcriptional Regulators of Dendritic Cell Chemotaxis. Mol. Cell Biol. 2018, 38, e00534-17. [Google Scholar] [CrossRef]
- Nunomura, S.; Okayama, Y.; Matsumoto, K.; Hashimoto, N.; Endo-Umeda, K.; Terui, T.; Makishima, M.; Ra, C. Activation of LXRs using the synthetic agonist GW3965 represses the production of pro-inflammatory cytokines by murine mast cells. Allergol. Int. 2015, 64 (Suppl. S1), 11–17. [Google Scholar] [CrossRef]
- Hong, C.; Kidani, Y.; A-Gonzalez, N.; Phung, T.; Ito, A.; Rong, X.; Ericson, K.; Mikkola, H.; Beaven, S.W.; Miller, L.S.; et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J. Clin. Investig. 2012, 122, 337–347. [Google Scholar] [CrossRef]
- Bosteels, V.; Maréchal, S.; De Nolf, C.; Rennen, S.; Maelfait, J.; Tavernier, S.J.; Vetters, J.; Van De Velde, E.; Fayazpour, F.; Deswarte, K.; et al. LXR signaling controls homeostatic dendritic cell maturation. Sci. Immunol. 2023, 8, eadd3955. [Google Scholar] [CrossRef]
- Ceroi, A.; Delettre, F.A.; Marotel, C.; Gauthier, T.; Asgarova, A.; Biichlé, S.; Duperrier, A.; Mourey, G.; Perruche, S.; Lagrost, L.; et al. The anti-inflammatory effects of platelet-derived microparticles in human plasmacytoid dendritic cells involve liver X receptor activation. Haematologica 2016, 101, e72–e76. [Google Scholar] [CrossRef]
- Ceroi, A.; Masson, D.; Roggy, A.; Roumier, C.; Chagué, C.; Gauthier, T.; Philippe, L.; Lamarthée, B.; Angelot-Delettre, F.; Bonnefoy, F.; et al. LXR agonist treatment of blastic plasmacytoid dendritic cell neoplasm restores cholesterol efflux and triggers apoptosis. Blood 2016, 128, 2694–2707. [Google Scholar] [CrossRef]
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D.; et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.T.; Fenn, A.M.; Harder, N.K.; Mindur, J.E.; McAlpine, C.S.; Patel, J.; Valet, C.; Rattik, S.; Iwamoto, Y.; He, S.; et al. Liver X receptors are required for thymic resilience and T cell output. J. Exp. Med. 2020, 217, e20200318. [Google Scholar] [CrossRef] [PubMed]
- Endo-Umeda, K.; Nakashima, H.; Uno, S.; Toyoshima, S.; Umeda, N.; Komine-Aizawa, S.; Seki, S.; Makishima, M. Liver X receptors regulate natural killer T cell population and antitumor activity in the liver of mice. Sci. Rep. 2021, 11, 22595. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wagoner, G.; Douglas, J.C.; Drew, P.D. Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J. Leukoc. Biol. 2009, 86, 401–409. [Google Scholar] [CrossRef]
- Cui, G.; Qin, X.; Wu, L.; Zhang, Y.; Sheng, X.; Yu, Q.; Sheng, H.; Xi, B.; Zhang, J.Z.; Zang, Y.Q. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J. Clin. Investig. 2011, 121, 658–670. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Lee, J.E.; Choi, G.; Chung, H.; Kim, D.; Park, M.J.; Gye, Y.S.; Shin, K.S.; Kang, C.Y.; et al. Liver X receptor controls follicular helper T cell differentiation via repression of TCF-1. Proc. Natl. Acad. Sci. USA 2023, 120, e2213793120. [Google Scholar] [CrossRef]
- Michaels, A.J.; Campbell, C.; Bou-Puerto, R.; Rudensky, A.Y. Nuclear receptor LXRβ controls fitness and functionality of activated T cells. J. Exp. Med. 2021, 218, e20201311. [Google Scholar] [CrossRef]
- Heine, G.; Dahten, A.; Hilt, K.; Ernst, D.; Milovanovic, M.; Hartmann, B.; Worm, M. Liver X receptors control IgE expression in B cells. J. Immunol. 2009, 182, 5276–5282. [Google Scholar] [CrossRef]
- Ito, A.; Hong, C.; Oka, K.; Salazar, J.V.; Diehl, C.; Witztum, J.L.; Diaz, M.; Castrillo, A.; Bensinger, S.J.; Chan, L.; et al. Cholesterol Accumulation in CD11c(+) Immune Cells Is a Causal and Targetable Factor in Autoimmune Disease. Immunity 2016, 45, 1311–1326. [Google Scholar] [CrossRef]
- Castrillo, A.; Joseph, S.B.; Vaidya, S.A.; Haberland, M.; Fogelman, A.M.; Cheng, G.; Tontonoz, P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell 2003, 12, 805–816. [Google Scholar] [CrossRef]
- A-Gonzalez, N.; Bensinger, S.J.; Hong, C.; Beceiro, S.; Bradley, M.N.; Zelcer, N.; Deniz, J.; Ramirez, C.; Díaz, M.; Gallardo, G.; et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 2009, 31, 245–258. [Google Scholar] [CrossRef] [PubMed]
- A-Gonzalez, N.; Quintana, J.A.; García-Silva, S.; Mazariegos, M.; González de la Aleja, A.; Nicolás-Ávila, J.A.; Walter, W.; Adrover, J.M.; Crainiciuc, G.; Kuchroo, V.K.; et al. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J. Exp. Med. 2017, 214, 1281–1296. [Google Scholar] [CrossRef] [PubMed]
- Ait-Oufella, H.; Pouresmail, V.; Simon, T.; Blanc-Brude, O.; Kinugawa, K.; Merval, R.; Offenstadt, G.; Lesèche, G.; Cohen, P.L.; Tedgui, A.; et al. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1429–1431. [Google Scholar] [CrossRef]
- Doran, A.C.; Ozcan, L.; Cai, B.; Zheng, Z.; Fredman, G.; Rymond, C.C.; Dorweiler, B.; Sluimer, J.C.; Hsieh, J.; Kuriakose, G.; et al. CAMKIIγ suppresses an efferocytosis pathway in macrophages and promotes atherosclerotic plaque necrosis. J. Clin. Investig. 2017, 127, 4075–4089. [Google Scholar] [CrossRef]
- Rébé, C.; Raveneau, M.; Chevriaux, A.; Lakomy, D.; Sberna, A.L.; Costa, A.; Bessède, G.; Athias, A.; Steinmetz, E.; Lobaccaro, J.M.; et al. Induction of transglutaminase 2 by a liver X receptor/retinoic acid receptor alpha pathway increases the clearance of apoptotic cells by human macrophages. Circ. Res. 2009, 105, 393–401. [Google Scholar] [CrossRef]
- Sarang, Z.; Joós, G.; Garabuczi, É.; Rühl, R.; Gregory, C.D.; Szondy, Z. Macrophages engulfing apoptotic cells produce nonclassical retinoids to enhance their phagocytic capacity. J. Immunol. 2014, 192, 5730–5738. [Google Scholar] [CrossRef]
- Arai, S.; Shelton, J.M.; Chen, M.; Bradley, M.N.; Castrillo, A.; Bookout, A.L.; Mak, P.A.; Edwards, P.A.; Mangelsdorf, D.J.; Tontonoz, P.; et al. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005, 1, 201–213. [Google Scholar] [CrossRef]
- Hamada, M.; Nakamura, M.; Tran, M.T.; Moriguchi, T.; Hong, C.; Ohsumi, T.; Dinh, T.T.; Kusakabe, M.; Hattori, M.; Katsumata, T.; et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat. Commun. 2014, 5, 3147. [Google Scholar] [CrossRef]
- Sallam, T.; Ito, A.; Rong, X.; Kim, J.; van Stijn, C.; Chamberlain, B.T.; Jung, M.E.; Chao, L.C.; Jones, M.; Gilliland, T.; et al. The macrophage LBP gene is an LXR target that promotes macrophage survival and atherosclerosis. J. Lipid Res. 2014, 55, 1120–1130. [Google Scholar] [CrossRef]
- Christ, A.; Günther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175.e114. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Welch, C.; Pagler, T.A.; Ranalletta, M.; Lamkanfi, M.; Han, S.; Ishibashi, M.; Li, R.; Wang, N.; Tall, A.R. Increased inflammatory gene expression in ABC transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 2008, 118, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Murphy, A.J.; Wang, M.; Pagler, T.A.; Vengrenyuk, Y.; Kappus, M.S.; Gorman, D.J.; Nagareddy, P.R.; Zhu, X.; Abramowicz, S.; et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ. Res. 2013, 112, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Sallam, T.; Jones, M.; Thomas, B.J.; Wu, X.; Gilliland, T.; Qian, K.; Eskin, A.; Casero, D.; Zhang, Z.; Sandhu, J.; et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat. Med. 2018, 24, 304–312. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Westerterp, M.; Fotakis, P.; Ouimet, M.; Bochem, A.E.; Zhang, H.; Molusky, M.M.; Wang, W.; Abramowicz, S.; la Bastide-van Gemert, S.; Wang, N.; et al. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis. Circulation 2018, 138, 898–912. [Google Scholar] [CrossRef]
- Yalcinkaya, M.; Fotakis, P.; Liu, W.; Endo-Umeda, K.; Dou, H.; Abramowicz, S.; Xiao, T.; Libby, P.; Wang, N.; Tall, A.R.; et al. Cholesterol accumulation in macrophages drives NETosis in atherosclerotic plaques via IL-1β secretion. Cardiovasc. Res. 2023, 119, 969–981. [Google Scholar] [CrossRef]
- Joseph, S.B.; McKilligin, E.; Pei, L.; Watson, M.A.; Collins, A.R.; Laffitte, B.A.; Chen, M.; Noh, G.; Goodman, J.; Hagger, G.N.; et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 7604–7609. [Google Scholar] [CrossRef]
- Terasaka, N.; Hiroshima, A.; Koieyama, T.; Ubukata, N.; Morikawa, Y.; Nakai, D.; Inaba, T. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 2003, 536, 6–11. [Google Scholar] [CrossRef]
- Spyridon, M.; Moraes, L.A.; Jones, C.I.; Sage, T.; Sasikumar, P.; Bucci, G.; Gibbins, J.M. LXR as a novel antithrombotic target. Blood 2011, 117, 5751–5761. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Xiao, Q.; Song, W.; Zhang, H.; Sun, B.; Geng, N.; Tao, Z.; Shao, Q.; Pu, J. Protective Functions of Liver X Receptor α in Established Vulnerable Plaques: Involvement of Regulating Endoplasmic Reticulum-Mediated Macrophage Apoptosis and Efferocytosis. J. Am. Heart Assoc. 2021, 10, e018455. [Google Scholar] [CrossRef] [PubMed]
- Dragoljevic, D.; Lee, M.K.S.; Pernes, G.; Morgan, P.K.; Louis, C.; Shihata, W.; Huynh, K.; Kochetkova, A.A.; Bell, P.W.; Mellett, N.A.; et al. Administration of an LXR agonist promotes atherosclerotic lesion remodelling in murine inflammatory arthritis. Clin. Transl. Immunol. 2023, 12, e1446. [Google Scholar] [CrossRef] [PubMed]
- Kappus, M.S.; Murphy, A.J.; Abramowicz, S.; Ntonga, V.; Welch, C.L.; Tall, A.R.; Westerterp, M. Activation of liver X receptor decreases atherosclerosis in Ldlr⁻/⁻ mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 279–284. [Google Scholar] [CrossRef]
- Rasheed, A.; Shawky, S.A.; Tsai, R.; Jung, R.G.; Simard, T.; Saikali, M.F.; Hibbert, B.; Rayner, K.J.; Cummins, C.L. The secretome of liver X receptor agonist-treated early outgrowth cells decreases atherosclerosis in Ldlr-/- mice. Stem Cells Transl. Med. 2021, 10, 479–491. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, W.; Yu, B.; Kuai, R.; Hu, W.; Morin, E.E.; Garcia-Barrio, M.T.; Zhang, J.; Moon, J.J.; Schwendeman, A.; et al. Synthetic High-Density Lipoprotein-Mediated Targeted Delivery of Liver X Receptors Agonist Promotes Atherosclerosis Regression. eBioMedicine 2018, 28, 225–233. [Google Scholar] [CrossRef]
- Ma, C.; Feng, K.; Yang, X.; Yang, Z.; Wang, Z.; Shang, Y.; Fan, G.; Liu, L.; Yang, S.; Li, X.; et al. Targeting macrophage liver X receptors by hydrogel-encapsulated T0901317 reduces atherosclerosis without effect on hepatic lipogenesis. Br. J. Pharmacol. 2021, 178, 1620–1638. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Yang, X.; Liu, Y.; Liu, L.; Feng, K.; Zhang, X.; Yang, S.; Sun, L.; Yu, M.; et al. Functional interplay between liver X receptor and AMP-activated protein kinase α inhibits atherosclerosis in apolipoprotein E-deficient mice—A new anti-atherogenic strategy. Br. J. Pharmacol. 2018, 175, 1486–1503. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, Y.; Yang, X.; Sun, L.; Liu, M.; Wang, Q.; Ma, X.; Zhang, W.; Li, X.; Hu, W.; et al. Inhibition of ERK1/2 and activation of LXR synergistically reduce atherosclerotic lesions in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 948–959. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; Yang, X.; Duan, Y.; Zeng, P.; Yang, S.; Ma, C.; Li, X.; Han, J.; Chen, Y. Combination of MEK1/2 inhibitor and LXR ligand synergistically inhibit atherosclerosis in LDLR deficient mice. Biochem. Biophys. Res. Commun. 2020, 522, 512–517. [Google Scholar] [CrossRef]
- Morin, E.E.; Guo, Y.; He, H.; Yuan, W.; Souery, W.N.; Fawaz, M.V.; Chen, Y.E.; Schwendeman, A. Synergetic Effect of rHDL and LXR Agonist on Reduction of Atherosclerosis in Mice. Front. Pharmacol. 2020, 11, 513031. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, P.; Kothari, V.; Thomas, D.G.; Westerterp, M.; Molusky, M.M.; Altin, E.; Abramowicz, S.; Wang, N.; He, Y.; Heinecke, J.W.; et al. Anti-Inflammatory Effects of HDL (High-Density Lipoprotein) in Macrophages Predominate Over Proinflammatory Effects in Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e253–e272. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, A.; Buchebner, M.; Pfeifer, T.; Becker, T.M.; Uray, G.; Miyazaki, M.; Miyazaki-Anzai, S.; Ebner, B.; Chandak, P.G.; Kadam, R.S.; et al. Synthetic LXR agonist attenuates plaque formation in apoE-/- mice without inducing liver steatosis and hypertriglyceridemia. J. Lipid Res. 2009, 50, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Yao, S.; Yan, H.; Hu, L.; Yu, C.; Gao, F.; Xi, C.; Li, H.; Ye, Y.; Wang, Y. A novel small molecule liver X receptor transcriptional regulator, nagilactone B, suppresses atherosclerosis in apoE-deficient mice. Cardiovasc. Res. 2016, 112, 502–514. [Google Scholar] [CrossRef]
- Quinet, E.M.; Basso, M.D.; Halpern, A.R.; Yates, D.W.; Steffan, R.J.; Clerin, V.; Resmini, C.; Keith, J.C.; Berrodin, T.J.; Feingold, I.; et al. LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J. Lipid Res. 2009, 50, 2358–2370. [Google Scholar] [CrossRef]
- Goo, Y.H.; Plakkal Ayyappan, J.; Cheeran, F.D.; Bangru, S.; Saha, P.K.; Baar, P.; Schulz, S.; Lydic, T.A.; Spengler, B.; Wagner, A.H.; et al. Lipid droplet-associated hydrolase mobilizes stores of liver X receptor sterol ligands and protects against atherosclerosis. Nat. Commun. 2024, 15, 6540. [Google Scholar] [CrossRef]
- Ge, X.; Slütter, B.; Lambooij, J.M.; Zhou, E.; Ying, Z.; Agirman, C.; Heijink, M.; Rimbert, A.; Guigas, B.; Kuiper, J.; et al. DHCR24 inhibitor SH42 increases desmosterol without preventing atherosclerosis development in mice. iScience 2024, 27, 109830. [Google Scholar] [CrossRef]
- Lehrke, M.; Lebherz, C.; Millington, S.C.; Guan, H.P.; Millar, J.; Rader, D.J.; Wilson, J.M.; Lazar, M.A. Diet-dependent cardiovascular lipid metabolism controlled by hepatic LXRalpha. Cell Metab. 2005, 1, 297–308. [Google Scholar] [CrossRef][Green Version]
- Lo Sasso, G.; Murzilli, S.; Salvatore, L.; D’Errico, I.; Petruzzelli, M.; Conca, P.; Jiang, Z.Y.; Calabresi, L.; Parini, P.; Moschetta, A. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010, 12, 187–193. [Google Scholar] [CrossRef]
- Aoyama, A.; Endo-Umeda, K.; Kishida, K.; Ohgane, K.; Noguchi-Yachide, T.; Aoyama, H.; Ishikawa, M.; Miyachi, H.; Makishima, M.; Hashimoto, Y. Design, synthesis, and biological evaluation of novel transrepression-selective liver X receptor (LXR) ligands with 5,11-dihydro-5-methyl-11-methylene-6H-dibenz[b,e]azepin-6-one skeleton. J. Med. Chem. 2012, 55, 7360–7377. [Google Scholar] [CrossRef]
- Nomura, S.; Endo-Umeda, K.; Aoyama, A.; Makishima, M.; Hashimoto, Y.; Ishikawa, M. Styrylphenylphthalimides as Novel Transrepression-Selective Liver X Receptor (LXR) Modulators. ACS Med. Chem. Lett. 2015, 6, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Endo-Umeda, K.; Makishima, M.; Hashimoto, Y.; Ishikawa, M. Development of Tetrachlorophthalimides as Liver X Receptor β (LXRβ)-Selective Agonists. ChemMedChem 2016, 11, 2347–2360. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, B.E.M.; Goher, S.S.; Hegazy, L.S.; Arief, M.M.H.; Burris, T.P. Recent Advances in the Medicinal Chemistry of Liver X Receptors. J. Med. Chem. 2018, 61, 10935–10956. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Udata, C.; Ott, E.; Hickey, L.; Burczynski, M.E.; Burghart, P.; Vesterqvist, O.; Meng, X. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J. Clin. Pharmacol. 2009, 49, 643–649. [Google Scholar] [CrossRef]
- Kirchgessner, T.G.; Sleph, P.; Ostrowski, J.; Lupisella, J.; Ryan, C.S.; Liu, X.; Fernando, G.; Grimm, D.; Shipkova, P.; Zhang, R.; et al. Beneficial and Adverse Effects of an LXR Agonist on Human Lipid and Lipoprotein Metabolism and Circulating Neutrophils. Cell Metab. 2016, 24, 223–233. [Google Scholar] [CrossRef]
- Zhang, X.; McDonald, J.G.; Aryal, B.; Canfrán-Duque, A.; Goldberg, E.L.; Araldi, E.; Ding, W.; Fan, Y.; Thompson, B.M.; Singh, A.K.; et al. Desmosterol suppresses macrophage inflammasome activation and protects against vascular inflammation and atherosclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2107682118. [Google Scholar] [CrossRef]
- Li, Z.; Martin, M.; Zhang, J.; Huang, H.Y.; Bai, L.; Zhang, J.; Kang, J.; He, M.; Li, J.; Maurya, M.R.; et al. Krüppel-Like Factor 4 Regulation of Cholesterol-25-Hydroxylase and Liver X Receptor Mitigates Atherosclerosis Susceptibility. Circulation 2017, 136, 1315–1330. [Google Scholar] [CrossRef]
- Canfrán-Duque, A.; Rotllan, N.; Zhang, X.; Andrés-Blasco, I.; Thompson, B.M.; Sun, J.; Price, N.L.; Fernández-Fuertes, M.; Fowler, J.W.; Gómez-Coronado, D.; et al. Macrophage-Derived 25-Hydroxycholesterol Promotes Vascular Inflammation, Atherogenesis, and Lesion Remodeling. Circulation 2023, 147, 388–408. [Google Scholar] [CrossRef]
- Umetani, M.; Ghosh, P.; Ishikawa, T.; Umetani, J.; Ahmed, M.; Mineo, C.; Shaul, P.W. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014, 20, 172–182. [Google Scholar] [CrossRef]
- Yu, L.; Xu, L.; Chu, H.; Peng, J.; Sacharidou, A.; Hsieh, H.H.; Weinstock, A.; Khan, S.; Ma, L.; Durán, J.G.B.; et al. Macrophage-to-endothelial cell crosstalk by the cholesterol metabolite 27HC promotes atherosclerosis in male mice. Nat. Commun. 2023, 14, 4101. [Google Scholar] [CrossRef]
- Schuster, G.U.; Parini, P.; Wang, L.; Alberti, S.; Steffensen, K.R.; Hansson, G.K.; Angelin, B.; Gustafsson, J.A. Accumulation of foam cells in liver X receptor-deficient mice. Circulation 2002, 106, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.N.; Hong, C.; Chen, M.; Joseph, S.B.; Wilpitz, D.C.; Wang, X.; Lusis, A.J.; Collins, A.; Hseuh, W.A.; Collins, J.L.; et al. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J. Clin. Investig. 2007, 117, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, E.D.; Daige, C.L.; Petrowski, M.; Dedman, H.; Pattison, J.; Juliano, J.; Li, A.C.; Schulman, I.G. Non-redundant roles for LXRalpha and LXRbeta in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J. Lipid Res. 2010, 51, 900–906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, C.; Bradley, M.N.; Rong, X.; Wang, X.; Wagner, A.; Grijalva, V.; Castellani, L.W.; Salazar, J.; Realegeno, S.; Boyadjian, R.; et al. LXRα is uniquely required for maximal reverse cholesterol transport and atheroprotection in ApoE-deficient mice. J. Lipid Res. 2012, 53, 1126–1133. [Google Scholar] [CrossRef]
- Zhang, Y.; Breevoort, S.R.; Angdisen, J.; Fu, M.; Schmidt, D.R.; Holmstrom, S.R.; Kliewer, S.A.; Mangelsdorf, D.J.; Schulman, I.G. Liver LXRα expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J. Clin. Investig. 2012, 122, 1688–1699. [Google Scholar] [CrossRef]
- Nishida, T.; Ayaori, M.; Arakawa, J.; Suenaga, Y.; Shiotani, K.; Uto-Kondo, H.; Komatsu, T.; Nakaya, K.; Endo, Y.; Sasaki, M.; et al. Liver-specific Lxr inhibition represses reverse cholesterol transport in cholesterol-fed mice. Atherosclerosis 2024, 397, 117578. [Google Scholar] [CrossRef]
- Tangirala, R.K.; Bischoff, E.D.; Joseph, S.B.; Wagner, B.L.; Walczak, R.; Laffitte, B.A.; Daige, C.L.; Thomas, D.; Heyman, R.A.; Mangelsdorf, D.J.; et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. USA 2002, 99, 11896–11901. [Google Scholar] [CrossRef]
- Levin, N.; Bischoff, E.D.; Daige, C.L.; Thomas, D.; Vu, C.T.; Heyman, R.A.; Tangirala, R.K.; Schulman, I.G. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 135–142. [Google Scholar] [CrossRef]
- Endo-Umeda, K.; Kim, E.; Thomas, D.G.; Liu, W.; Dou, H.; Yalcinkaya, M.; Abramowicz, S.; Xiao, T.; Antonson, P.; Gustafsson, J.; et al. Myeloid LXR (Liver X Receptor) Deficiency Induces Inflammatory Gene Expression in Foamy Macrophages and Accelerates Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 719–731. [Google Scholar] [CrossRef]
- Feig, J.E.; Pineda-Torra, I.; Sanson, M.; Bradley, M.N.; Vengrenyuk, Y.; Bogunovic, D.; Gautier, E.L.; Rubinstein, D.; Hong, C.; Liu, J.; et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Investig. 2010, 120, 4415–4424. [Google Scholar] [CrossRef]
- Pourcet, B.; Feig, J.E.; Vengrenyuk, Y.; Hobbs, A.J.; Kepka-Lenhart, D.; Garabedian, M.J.; Morris, S.M.; Fisher, E.A.; Pineda-Torra, I. LXRα Regulates Macrophage Arginase 1 Through PU.1 and Interferon Regulatory Factor 8. Circ. Res. 2011, 109, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bradley, M.N.; Beaven, S.W.; Tontonoz, P. Phosphorylation of the liver X receptors. FEBS Lett. 2006, 580, 4835–4841. [Google Scholar] [CrossRef]
- Torra, I.P.; Ismaili, N.; Feig, J.E.; Xu, C.F.; Cavasotto, C.; Pancratov, R.; Rogatsky, I.; Neubert, T.A.; Fisher, E.A.; Garabedian, M.J. Phosphorylation of liver X receptor alpha selectively regulates target gene expression in macrophages. Mol. Cell Biol. 2008, 28, 2626–2636. [Google Scholar] [CrossRef]
- Hsieh, J.; Koseki, M.; Molusky, M.M.; Yakushiji, E.; Ichi, I.; Westerterp, M.; Iqbal, J.; Chan, R.B.; Abramowicz, S.; Tascau, L.; et al. TTC39B deficiency stabilizes LXR reducing both atherosclerosis and steatohepatitis. Nature 2016, 535, 303–307. [Google Scholar] [CrossRef]
- Kim, K.H.; Yoon, J.M.; Choi, A.H.; Kim, W.S.; Lee, G.Y.; Kim, J.B. Liver X receptor ligands suppress ubiquitination and degradation of LXRalpha by displacing BARD1/BRCA1. Mol. Endocrinol. 2009, 23, 466–474. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Blander, G.; Tse, J.G.; Krieger, M.; Guarente, L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 2007, 28, 91–106. [Google Scholar] [CrossRef]
- Anthonisen, E.H.; Berven, L.; Holm, S.; Nygård, M.; Nebb, H.I.; Grønning-Wang, L.M. Nuclear receptor liver X receptor is O-GlcNAc-modified in response to glucose. J. Biol. Chem. 2010, 285, 1607–1615. [Google Scholar] [CrossRef]
- Wu, C.; Hussein, M.A.; Shrestha, E.; Leone, S.; Aiyegbo, M.S.; Lambert, W.M.; Pourcet, B.; Cardozo, T.; Gustafson, J.A.; Fisher, E.A.; et al. Modulation of Macrophage Gene Expression via Liver X Receptor α Serine 198 Phosphorylation. Mol. Cell Biol. 2015, 35, 2024–2034. [Google Scholar] [CrossRef]
- Gage, M.C.; Bécares, N.; Louie, R.; Waddington, K.E.; Zhang, Y.; Tittanegro, T.H.; Rodríguez-Lorenzo, S.; Jathanna, A.; Pourcet, B.; Pello, O.M.; et al. Disrupting LXRα phosphorylation promotes FoxM1 expression and modulates atherosclerosis by inducing macrophage proliferation. Proc. Natl. Acad. Sci. USA 2018, 115, E6556–E6565. [Google Scholar] [CrossRef]
- Voisin, M.; Shrestha, E.; Rollet, C.; Nikain, C.A.; Josefs, T.; Mahé, M.; Barrett, T.J.; Chang, H.R.; Ruoff, R.; Schneider, J.A.; et al. Inhibiting LXRα phosphorylation in hematopoietic cells reduces inflammation and attenuates atherosclerosis and obesity in mice. Commun. Biol. 2021, 4, 420. [Google Scholar] [CrossRef] [PubMed]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M. Nuclear receptors as targets for drug development: Regulation of cholesterol and bile acid metabolism by nuclear receptors. J. Pharmacol. Sci. 2005, 97, 177–183. [Google Scholar] [CrossRef]
- Hartman, H.B.; Gardell, S.J.; Petucci, C.J.; Wang, S.; Krueger, J.A.; Evans, M.J. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR-/- and apoE-/- mice. J. Lipid Res. 2009, 50, 1090–1100. [Google Scholar] [CrossRef]
- Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H272–H281. [Google Scholar] [CrossRef]
- Hanniman, E.A.; Lambert, G.; McCarthy, T.C.; Sinal, C.J. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J. Lipid Res. 2005, 46, 2595–2604. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Vales, C.; Lee, F.Y.; Lee, H.; Lusis, A.J.; Edwards, P.A. FXR deficiency causes reduced atherosclerosis in Ldlr-/- mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2316–2321. [Google Scholar] [CrossRef]
- Guo, G.L.; Santamarina-Fojo, S.; Akiyama, T.E.; Amar, M.J.; Paigen, B.J.; Brewer, B., Jr.; Gonzalez, F.J. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta 2006, 1761, 1401–1409. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, L.; Hu, X.; Wang, X.; Xu, F.; Chen, B.; Liang, X.; Xia, J.; Wang, P.; Aibara, D.; et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Investig. 2021, 131, e142865. [Google Scholar] [CrossRef]
- Yamazaki, T.; Cable, E.E.; Schnabl, B. Peroxisome proliferator–activated receptor delta and liver diseases. Hepatol. Commun. 2025, 9, e0646. [Google Scholar] [CrossRef]
- Babaev, V.R.; Ishiguro, H.; Ding, L.; Yancey, P.G.; Dove, D.E.; Kovacs, W.J.; Semenkovich, C.F.; Fazio, S.; Linton, M.F. Macrophage expression of peroxisome proliferator-activated receptor-alpha reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2007, 116, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Barish, G.D.; Atkins, A.R.; Downes, M.; Olson, P.; Chong, L.W.; Nelson, M.; Zou, Y.; Hwang, H.; Kang, H.; Curtiss, L.; et al. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc. Natl. Acad. Sci. USA 2008, 105, 4271–4276. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Boisvert, W.A.; Lee, C.H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K.; et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- Zheng, Y.; Shao, M.; Zheng, Y.; Sun, W.; Qin, S.; Sun, Z.; Zhu, L.; Guan, Y.; Wang, Q.; Wang, Y.; et al. PPARs in atherosclerosis: The spatial and temporal features from mechanism to druggable targets. J. Adv. Res. 2025, 69, 225–244. [Google Scholar] [CrossRef]
- Lockhart, S.M.; Muso, M.; Zvetkova, I.; Lam, B.Y.H.; Ferrari, A.; Schoenmakers, E.; Duckett, K.; Leslie, J.; Collins, A.; Romartínez-Alonso, B.; et al. Damaging mutations in liver X receptor-α are hepatotoxic and implicate cholesterol sensing in liver health. Nat. Metab. 2024, 6, 1922–1938. [Google Scholar] [CrossRef]
- Clark, A.T.; Russo-Savage, L.; Ashton, L.A.; Haghshenas, N.; Amselle, N.A.; Schulman, I.G. A mutation in LXRα uncovers a role for cholesterol sensing in limiting metabolic dysfunction-associated steatohepatitis. Nat. Commun. 2025, 16, 1102. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo-Umeda, K.; Makishima, M. Exploring the Roles of Liver X Receptors in Lipid Metabolism and Immunity in Atherosclerosis. Biomolecules 2025, 15, 579. https://doi.org/10.3390/biom15040579
Endo-Umeda K, Makishima M. Exploring the Roles of Liver X Receptors in Lipid Metabolism and Immunity in Atherosclerosis. Biomolecules. 2025; 15(4):579. https://doi.org/10.3390/biom15040579
Chicago/Turabian StyleEndo-Umeda, Kaori, and Makoto Makishima. 2025. "Exploring the Roles of Liver X Receptors in Lipid Metabolism and Immunity in Atherosclerosis" Biomolecules 15, no. 4: 579. https://doi.org/10.3390/biom15040579
APA StyleEndo-Umeda, K., & Makishima, M. (2025). Exploring the Roles of Liver X Receptors in Lipid Metabolism and Immunity in Atherosclerosis. Biomolecules, 15(4), 579. https://doi.org/10.3390/biom15040579






