Recent Stem-Cell-Based and Stem-Cell-Free Possibilities for the Therapeutic Management of the Osteonecrosis of the Jaw
Abstract
:1. Introduction
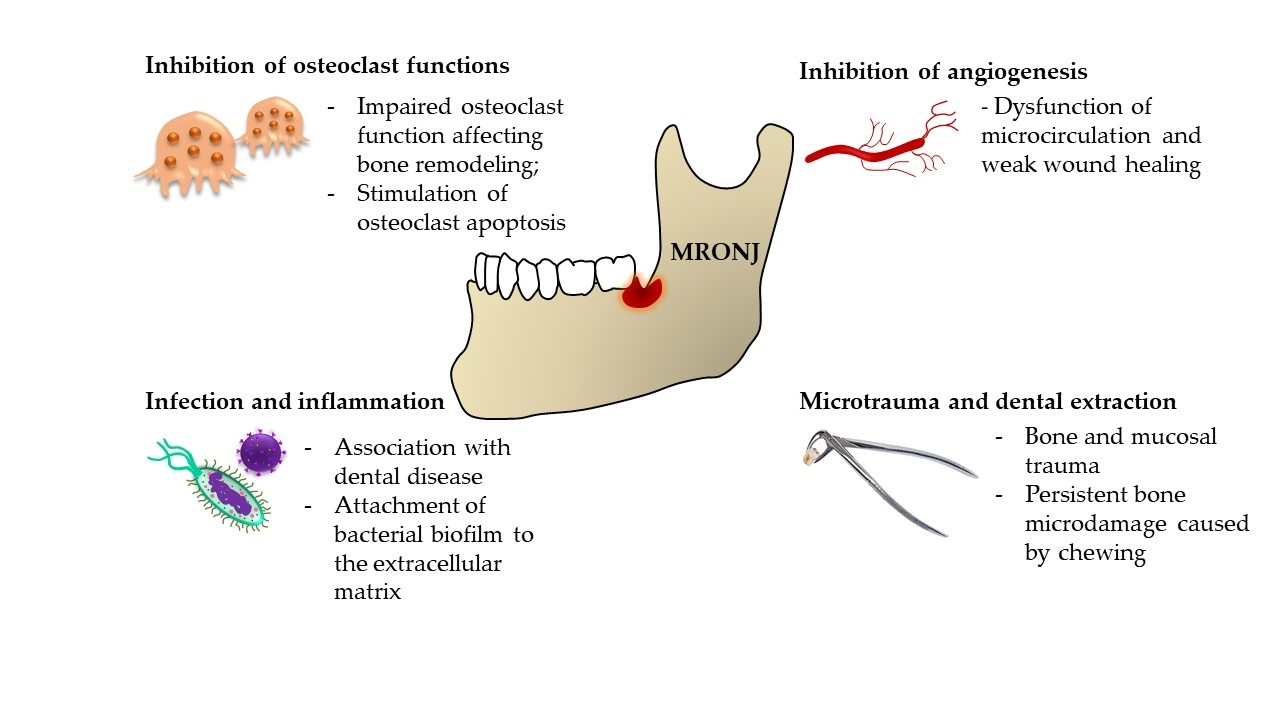
2. The Pathophysiology of MRONJ
3. MRONJ—Current Therapeutic Options
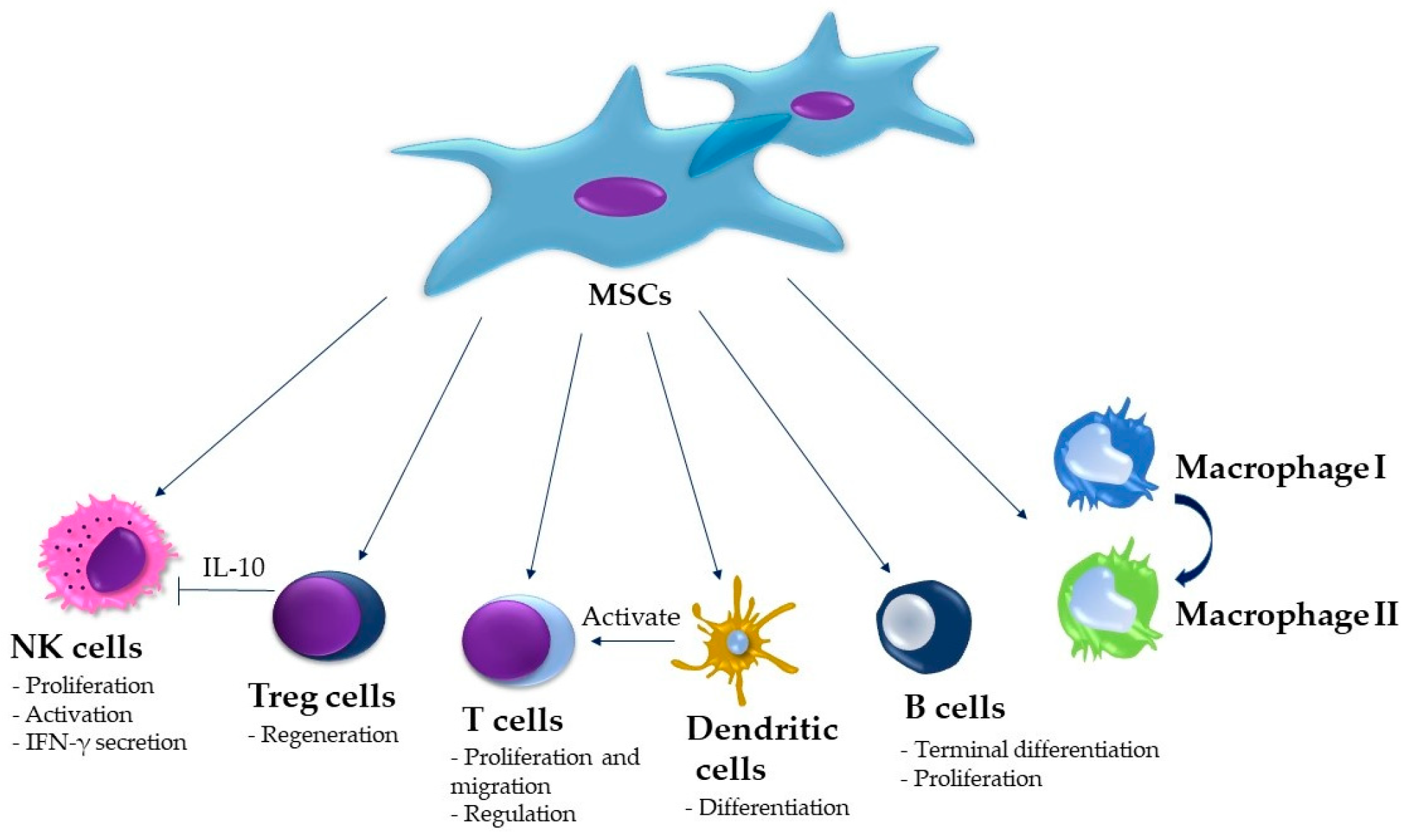
4. Stem-Cell-Based Therapy of the MRONJ
5. Stem-Cell-Free Therapy of MRONJ—Exosomes
6. Conclusions and Future Expectations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ONJ | Osteonecrosis of the jaw |
| MSC | Mesenchymal stem cells |
| BM | Bone marrow |
| RANK-L | Receptor activator of nuclear factor kappa-B ligand |
| MRONJ | Medication-related osteonecrosis of the jaw |
| BP | Bisphosphonate |
| ZA | Zoledronic acid |
| β-TCP | Β-tricalcium phosphate |
| IV | Intravenous infusion |
| miRNAs | MicroRNAs |
| lncRNAs | Long non-coding RNAs |
| ECM | Extracellular matrix |
| ADSC | Adipose tissue-derived stem cell |
References
- Lončar Brzak, B.; Horvat Aleksijević, L.; Vindiš, E.; Kordić, I.; Granić, M.; Juras, D.V.; Rogulj, A.A. Osteonecrosis of the Jaw. Dent. J. 2023, 11, 23. [Google Scholar] [CrossRef]
- Tetradis, S.; Allen, M.R.; Ruggiero, S.L. Pathophysiology of Medication-Related Osteonecrosis of the Jaw—A Minireview. JBMR Plus 2023, 7, e10785. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Kaneko, T.; Kamimoto, A.; Wada, M.; Takeuchi, Y.; Furuchi, M.; Iinuma, T. Medication-related osteonecrosis of the jaw after tooth extraction in patients receiving pharmaceutical treatment for osteoporosis: A retrospective cohort study. J. Dent. Sci. 2022, 17, 1619–1625. [Google Scholar] [CrossRef]
- Pichardo, S.E.C.; van der Hee, J.G.; Fiocco, M.; Appelman-Dijkstra, N.M.; van Merkesteyn, J.P.R. Dental implants as risk factors for patients with medication-related osteonecrosis of the jaws (MRONJ). Br. J. Oral Maxillofac. Surg. 2020, 58, 771–776. [Google Scholar] [CrossRef]
- Wick, A.; Bankosegger, P.; Otto, S.; Hohlweg-Majert, B.; Steiner, T.; Probst, F.; Ristow, O.; Pautke, C. Risk factors associated with onset of medication-related osteonecrosis of the jaw in patients treated with denosumab. Clin. Oral Investig. 2022, 26, 2839–2852. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, S.; Al-Omari, F.A.; Sasaki, M.; Sawase, T. Medication-related osteonecrosis of the jaw: A literature review and update. Genesis 2022, 60, e23500. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Arvandi, M.; Marth, C.; Egle, D.; Baumgart, F.; Emmelheinz, M.; Walch, B.; Lercher, J.; Iannetti, C.; Wöll, E.; et al. Incidence of Medication-Related Osteonecrosis of the Jaw in Patients With Breast Cancer During a 20-Year Follow-Up: A Population-Based Multicenter Retrospective Study. J. Clin. Oncol. 2025, 43, 180–188. [Google Scholar] [CrossRef]
- Boffano, P.; Agnone, A.M.; Neirotti, F.; Bonfiglio, R.; Brucoli, M.; Ruslin, M.; Durković, A.; Milosavljević, M.; Konstantinovic, V.; Rodríguez, J.C.V.; et al. Epidemiology, etiopathogenesis, and management of MRONJ: A European multicenter study. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101931. [Google Scholar] [CrossRef]
- Rodan, G.A.; Reszka, A.A. Bisphosphonate mechanism of action. Curr. Mol. Med. 2002, 2, 571–577. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Teoh, L.; Moses, G.; Nguyen, A.P.; McCullough, M.J. Medication-related osteonecrosis of the jaw: Analysing the range of implicated drugs from the Australian database of adverse event notifications. Br. J. Clin. Pharmacol. 2021, 87, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral. Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Okawa, H.; Kondo, T.; Hokugo, A.; Cherian, P.; Campagna, J.J.; Lentini, N.A.; Sung, E.C.; Chiang, S.; Lin, Y.L.; Ebetino, F.H.; et al. Mechanism of bisphosphonate-related osteonecrosis of the jaw (BRONJ) revealed by targeted removal of legacy bisphosphonate from jawbone using competing inert hydroxymethylene diphosphonate. eLife 2022, 11, e76207. [Google Scholar] [CrossRef]
- Elsayed, R.; El-Awady, A.; Cutler, C.; Kurago, Z.; Elashiry, M.; Sun, C.; Bloomquist, R.; Meghil, M.M.; Elsalanty, M.E. Matrix-Bound Zolzoledronate Enhances the Biofilm Colonization of Hydroxyapatite: Effects on Osteonecrosis. Antibiotics 2021, 10, 1380. [Google Scholar] [CrossRef]
- Papadaki, M.E.; Lietman, S.A.; Levine, M.A.; Olsen, B.R.; Kaban, L.B.; Reichenberger, E.J. Cherubism: Best clinical practice. Orphanet J. Rare Dis. 2012, 7 (Suppl. S1), S6. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.D.; Shakir, M.K.M.; Hoang, T.D. Hyperparathyroidism-Jaw Tumor Syndrome. Case Rep. Oncol. 2021, 14, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Lombard, T.; Neirinckx, V.; Rogister, B.; Gilon, Y.; Wislet, S. Medication-Related Osteonecrosis of the Jaw: New Insights into Molecular Mechanisms and Cellular Therapeutic Approaches. Stem Cells Int. 2016, 2016, 8768162. [Google Scholar] [CrossRef]
- Elsayed, R.; Kurago, Z.; Cutler, C.W.; Arce, R.M.; Gerber, J.; Celis, E.; Sultan, H.; Elashiry, M.; Meghil, M.; Sun, C.; et al. Role of dendritic cell-mediated immune response in oral homeostasis: A new mechanism of osteonecrosis of the jaw. FASEB J. 2020, 34, 2595–2608. [Google Scholar] [CrossRef]
- Kuehn, S.; Scariot, R.; Elsalanty, M. Medication-Related Osteonecrosis: Why the Jawbone? Dent. J. 2023, 11, 109. [Google Scholar] [CrossRef]
- Roato, I.; Mauceri, R.; Notaro, V.; Genova, T.; Fusco, V.; Mussano, F. Immune Dysfunction in Medication-Related Osteonecrosis of the Jaw. Int. J. Mol. Sci. 2023, 24, 7948. [Google Scholar] [CrossRef]
- Soundia, A.; Elzakra, N.; Hadaya, D.; Gkouveris, I.; Bezouglaia, O.; Dry, S.; Aghaloo, T.; Tetradis, S. Macrophage Polarization during MRONJ Development in Mice. J. Dent. Res. 2024, 103, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.C.; Kanayama, K.; Kaur, K.; Park, S.H.; Park, S.; Kozlowska, A.; Sun, S.; McKenna, C.E.; Nishimura, I.; Jewett, A. Bisphosphonate-induced differential modulation of immune cell function in gingiva and bone marrow in vivo: Role in osteoclast-mediated NK cell activation. Oncotarget 2015, 6, 20002–20025. [Google Scholar] [CrossRef] [PubMed]
- Wat, W.Z.M. Current Controversies on the Pathogenesis of Medication-Related Osteonecrosis of the Jaw. Dent. J. 2016, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- AlRowis, R.; Aldawood, A.; AlOtaibi, M.; Alnasser, E.; AlSaif, I.; Aljaber, A.; Natto, Z. Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review of Pathophysiology, Risk Factors, Preventive Measures and Treatment Strategies. Saudi Dent. J. 2022, 34, 202–210. [Google Scholar] [CrossRef]
- Goker, F.; Grecchi, E.; Grecchi, F.; Francetti, L.; Del Fabbro, M. Treatment of medication-related osteonecrosis of the jaw (MRONJ). A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2662–2673. [Google Scholar] [CrossRef]
- Ferreira, L.d.S.; Abreu, L.G.; Calderipe, C.B.; Martins, M.D.; Schuch, L.F.; Vasconcelos, A.C.U. Is teriparatide therapy effective for medication-related osteonecrosis of the jaw? A systematic review and meta-analysis. Osteoporos. Int. 2021, 32, 2449–2459. [Google Scholar] [CrossRef]
- Nowak, S.M.; Sacco, R.; Mitchell, F.L.; Patel, V.; Gurzawska-Comis, K. The effectiveness of autologous platelet concentrates in prevention and treatment of medication-related osteonecrosis of the jaws: A systematic review. J. Craniomaxillofac. Surg. 2024, 52, 671–691. [Google Scholar] [CrossRef]
- Ye, P.; Wei, T.; Lu, Z.Y.; Cai, Y.J. The Role of Autologous Platelet Concentrates in the Treatment of Medication-Related Osteonecrosis of the Jaw. J. Craniofac. Surg. 2021, 32, 621–625. [Google Scholar] [CrossRef]
- Sacco, R.; Akintola, O.; Sacco, N.; Acocella, A.; Calasans-Maia, M.D.; Maranzano, M.; Olate, S. The Use of Human Amniotic Membrane (hAM) as a Treatment Strategy of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review and Meta-Analysis of the Literature. Medicina 2023, 59, 968. [Google Scholar] [CrossRef]
- Momesso, G.A.C.; Lemos, C.A.A.; Santiago-Júnior, J.F.; Faverani, L.P.; Pellizzer, E.P. Laser surgery in management of medication-related osteonecrosis of the jaws: A meta-analysis. Oral Maxillofac. Surg. 2020, 24, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; Wu, C.B.; Sun, H.J.; Zhou, Q. Effectiveness of laser-assisted treatments for medication-related osteonecrosis of the jaw: A systematic review. Br. J. Oral Maxillofac. Surg. 2020, 58, 256–267. [Google Scholar] [CrossRef]
- Frutuoso, F.; Freitas, F.; Vilares, M.; Francisco, H.; Marques, D.; Caramês, J.; Moreira, A. Medication-Related Osteonecrosis of the Jaw: A Systematic Review of Case Reports and Case Series. Diseases 2024, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Bouland, C.L.; Javadian, R.; Gilis, S.; Yanni, A.; Le Clercq, M.; Mestrallet, P.; Kampouridis, S.; Bron, D.; Lalmand, M.; Vanden Eynden, X.; et al. Treatment of medication-related osteonecrosis of the jaw with cell therapy. Front Cell Dev. Biol. 2024, 12, 1338376. [Google Scholar] [CrossRef]
- Nifosì, G.; Nifosì, L.; Nifosì, A.F. Mesenchymal stem cells in the treatment of osteonecrosis of the jaw. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 65–75. [Google Scholar] [CrossRef]
- Ning, H.; Wu, X.; Wu, Q.; Yu, W.; Wang, H.; Zheng, S.; Chen, Y.; Li, Y.; Su, J. Microfiber-Reinforced Composite Hydrogels Loaded with Rat Adipose-Derived Stem Cells and BMP-2 for the Treatment of Medication-Related Osteonecrosis of the Jaw in a Rat Model. ACS Biomater. Sci. Eng. 2019, 5, 2430–2443. [Google Scholar] [CrossRef]
- Kaibuchi, N.; Iwata, T.; Yamato, M.; Okano, T.; Ando, T. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 2016, 42, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, S.; He, Y.; Zhang, Y. Prevention of medication-related osteonecrosis of the jaw in mice by adipose-derived stem cells associated with activated autophagic flux. J. Dent. Sci. 2024, 19, 2106–2113. [Google Scholar] [CrossRef]
- Zang, X.; He, L.; Zhao, L.; He, Y.; Xiao, E.; Zhang, Y. Adipose-derived stem cells prevent the onset of bisphosphonate-related osteonecrosis of the jaw through transforming growth factor β-1-mediated gingival wound healing. Stem Cell Res. Ther. 2019, 10, 169. [Google Scholar] [CrossRef]
- Kikuiri, T.; Kim, I.; Yamaza, T.; Akiyama, K.; Zhang, Q.; Li, Y.; Chen, C.; Chen, W.; Wang, S.; Le, A.D.; et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J. Bone Miner. Res. 2010, 25, 1668–1679. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Mao, L.; Liu, Y.; Gao, R.; Zheng, Z.; Chen, W.; Le, A.; Shi, S.; Wang, S. Allogeneic mesenchymal stem cell therapy for bisphosphonate-related jaw osteonecrosis in Swine. Stem Cells Dev. 2013, 22, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Barba-Recreo, P.; Del Castillo Pardo de Vera, J.L.; Georgiev-Hristov, T.; Ruiz Bravo-Burguillos, E.; Abarrategi, A.; Burgueño, M.; García-Arranz, M. Adipose-derived stem cells and platelet-rich plasma for preventive treatment of bisphosphonate-related osteonecrosis of the jaw in a murine model. J. Craniomaxillofac. Surg. 2015, 43, 1161–1168. [Google Scholar] [CrossRef]
- Kuroshima, S.; Sasaki, M.; Nakajima, K.; Tamaki, S.; Hayano, H.; Sawase, T. Transplantation of Noncultured Stromal Vascular Fraction Cells of Adipose Tissue Ameliorates Osteonecrosis of the Jaw-Like Lesions in Mice. J. Bone Miner. Res. 2018, 33, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Rodriguez, E.; González-Martín-Moro, J.; Cebrián-Carretero, J.L.; Del Castillo, J.L.; Pozo-Kreilinger, J.J.; Ruiz-Bravo, E.; García-Arranz, M.; Hernández-Godoy, J.; Burgueño, M. Bisphosphonate-related osteonecrosis. Application of adipose-derived stem cells in an experimental murine model. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e529–e536. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.J.; Oñate-Sánchez, R.; Gonzálvez-García, M.; Vallés-Bergadá, M.; Martínez, C.M.; Revilla-Nuin, B.; Guerrero-Gironés, J.; Moraleda, J.M.; García-Bernal, D. Allogeneic Bone Marrow Mesenchymal Stem Cell Transplantation in Tooth Extractions Sites Ameliorates the Incidence of Osteonecrotic Jaw-Like Lesions in Zoledronic Acid-Treated Rats. J. Clin. Med. 2020, 9, 1649. [Google Scholar] [CrossRef]
- Nishimaki, K.; Kaibuchi, N.; Washio, K.; Yamato, M. Application of mesenchymal stromal cell sheets to prevent medication-related osteonecrosis of the jaw with titanium implants in rats. Odontology 2024, 112, 938–949. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Lin, Q.; Shi, T.; Liu, G. Exosome-loaded hydrogels for craniofacial bone tissue regeneration. Biomed. Mater. 2024, 19, 052002. [Google Scholar] [CrossRef]
- Zou, J.; Yang, W.; Cui, W.; Li, C.; Ma, C.; Ji, X.; Hong, J.; Qu, Z.; Chen, J.; Liu, A.; et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J. Nanobiotechnology 2023, 21, 14. [Google Scholar] [CrossRef]
- Huber, J.; Griffin, M.F.; Longaker, M.T.; Quarto, N. Exosomes: A Tool for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 101–113. [Google Scholar] [CrossRef]
- Cooper, L.F.; Ravindran, S.; Huang, C.C.; Kang, M. A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration. Front. Physiol. 2019, 10, 1569. [Google Scholar] [CrossRef]
- Coppin, L.; Sokal, E.; Stéphenne, X. Thrombogenic Risk Induced by Intravascular Mesenchymal Stem Cell Therapy: Current Status and Future Perspectives. Cells 2019, 8, 1160. [Google Scholar] [CrossRef]
- Hoang, V.T.; Le, D.S.; Hoang, D.M.; Phan, T.T.K.; Ngo, L.A.T.; Nguyen, T.K.; Bui, V.A.; Nguyen Thanh, L. Impact of tissue factor expression and administration routes on thrombosis development induced by mesenchymal stem/stromal cell infusions: Re-evaluating the dogma. Stem Cell Res. Ther. 2024, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Kaibuchi, N.; Iwata, T.; Koga, Y.K.; Okamoto, T. Novel Cell Therapy Using Mesenchymal Stromal Cell Sheets for Medication-Related Osteonecrosis of the Jaw. Front. Bioeng. Biotechnol. 2022, 10, 902349. [Google Scholar] [CrossRef]
- Jun, E.K.; Zhang, Q.; Yoon, B.S.; Moon, J.H.; Lee, G.; Park, G.; Kang, P.J.; Lee, J.H.; Kim, A.; You, S. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int. J. Mol. Sci. 2014, 15, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zelisko, N.; Lesyk, R.; Stoika, R. Structure, unique biological properties, and mechanisms of action of transforming growth factor β. Bioorg. Chem. 2024, 150, 107611. [Google Scholar] [CrossRef]
- Otto, S.; Schnoedt, E.M.; Troeltzsch, M.; Kaeppler, G.; Aljohani, S.; Liebermann, A.; Fliefel, R. Clinical and Radiographic Outcomes of Dental Implants in Patients Treated With Antiresorptive Drugs: A Consecutive Case Series. J. Oral Implantol. 2023, 49, 39–45. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N.; Burgos-Alonso, N. Stromal vascular fraction technologies and clinical applications. Expert Opin. Biol. Ther. 2019, 19, 1289–1305. [Google Scholar] [CrossRef]
- Elad, S.; Czerninski, R.; Avgil, M.; Or, R. Hematopoietic stem cells local transplantation for the treatment of osteonecrosis of the jaws. Support Care Cancer 2005, 13, 455. [Google Scholar]
- Cella, L.; Oppici, A.; Arbasi, M.; Moretto, M.; Piepoli, M.; Vallisa, D.; Zangrandi, A.; Di Nunzio, C.; Cavanna, L. Autologous bone marrow stem cell intralesional transplantation repairing bisphosphonate related osteonecrosis of the jaw. Head Face Med. 2011, 7, 16. [Google Scholar] [CrossRef]
- Voss, P.J.; Matsumoto, A.; Alvarado, E.; Schmelzeisen, R.; Duttenhöfer, F.; Poxleitner, P. Treatment of stage II medication-related osteonecrosis of the jaw with necrosectomy and autologous bone marrow mesenchymal stem cells. Odontology 2017, 105, 484–493. [Google Scholar] [CrossRef] [PubMed]
- De Santis, G.C.; de Macedo, L.D.; Orellana, M.D.; Innocentini, L.; Ferrari, T.C.; Ricz, H.M.A.; Caruso, S.R.; Fernandes, T.R.; Covas, D.T. Mesenchymal stromal cells administration for osteonecrosis of the jaw caused by bisphosphonate: Report of two cases. Acta Oncol. 2020, 59, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Bouland, C.; Meuleman, N.; Widelec, J.; Keiani-Mothlagh, K.; Voisin, C.; Lagneaux, L.; Philippart, P. Case reports of medication-related osteonecrosis of the jaw (MRONJ) treated with uncultured stromal vascular fraction and L-PRF. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Safarova, Y.; Umbayev, B.; Hortelano, G.; Askarova, S. Mesenchymal stem cells modifications for enhanced bone targeting and bone regeneration. Regen. Med. 2020, 15, 1579–1594. [Google Scholar] [CrossRef]
- Lau, C.S.; Park, S.Y.; Ethiraj, L.P.; Singh, P.; Raj, G.; Quek, J.; Prasadh, S.; Choo, Y.; Goh, B.T. Role of Adipose-Derived Mesenchymal Stem Cells in Bone Regeneration. Int. J. Mol. Sci. 2024, 25, 6805. [Google Scholar] [CrossRef]
- Brozovich, A.; Sinicrope, B.J.; Bauza, G.; Niclot, F.B.; Lintner, D.; Taraballi, F.; McCulloch, P.C. High Variability of Mesenchymal Stem Cells Obtained via Bone Marrow Aspirate Concentrate Compared With Traditional Bone Marrow Aspiration Technique. Orthop. J. Sports Med. 2021, 9, 23259671211058459. [Google Scholar] [CrossRef]
- Joo, H.S.; Suh, J.H.; Lee, H.J.; Bang, E.S.; Lee, J.M. Current Knowledge and Future Perspectives on Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Agent. Int. J. Mol. Sci. 2020, 21, 727. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Loganathan, T.; Doss C, G.P. Non-coding RNAs in human health and disease: Potential function as biomarkers and therapeutic targets. Funct. Integr. Genom. 2023, 23, 33. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Hánělová, K.; Raudenská, M.; Masařík, M.; Balvan, J. Protein cargo in extracellular vesicles as the key mediator in the progression of cancer. Cell Commun. Signal. 2024, 22, 25. [Google Scholar] [CrossRef]
- Dong, X.; Shen, L.H.; Yi, Z.; He, L.H.; Yi, Z. Exosomes from Adipose-Derived Stem Cells Can Prevent Medication-Related Osteonecrosis of the Jaw. Med. Sci. Monit. 2021, 27, e929684. [Google Scholar] [CrossRef]
- Watanabe, J.; Sakai, K.; Urata, Y.; Toyama, N.; Nakamichi, E.; Hibi, H. Extracellular Vesicles of Stem Cells to Prevent BRONJ. J. Dent. Res. 2020, 99, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; He, Y.; Chen, S.; He, L.; Zhang, Y. Exosomes from Adipose-Derived Mesenchymal Stromal Cells Prevent Medication-Related Osteonecrosis of the Jaw by Inhibiting Macrophage M1 Polarization and Pyroptosis. Int. J. Nanomed. 2024, 19, 12675–12693. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381. [Google Scholar] [CrossRef]
- Minami, S.; Fujii, Y.; Yoshioka, Y.; Hatori, A.; Kaneko, K.; Ochiya, T.; Chikazu, D. Extracellular vesicles from mouse bone marrow macrophages-derived osteoclasts treated with zoledronic acid contain miR-146a-5p and miR-322-3p, which inhibit osteoclast function. Bone 2025, 190, 117323. [Google Scholar] [CrossRef]
- Zheng, Y.; Dong, X.; Wang, X.; Wang, J.; Chen, S.; He, Y.; An, J.; He, L.; Zhang, Y. Exosomes Derived from Adipose Tissue-Derived Mesenchymal Stromal Cells Prevent Medication-Related Osteonecrosis of the Jaw through IL-1RA. Int. J. Mol. Sci. 2023, 24, 8694. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Tian, W. Small Extracellular Vesicles Derived from Adipose Tissue Prevent Bisphosphonate-Related Osteonecrosis of the Jaw by Promoting Angiogenesis. Int. J. Nanomed. 2021, 16, 3161–3172. [Google Scholar] [CrossRef]
- Xing, X.; Han, S.; Li, Z.; Li, Z. Emerging role of exosomes in craniofacial and dental applications. Theranostics 2020, 10, 8648–8664. [Google Scholar] [CrossRef]
- Chaudhary, D.; Trivedi, R.N.; Kathuria, A.; Goswami, T.K.; Khandia, R.; Munjal, A. In vitro And In vivo Immunomodulating Properties of Mesenchymal Stem Cells. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, M.; Zhang, Y.; Jiang, X.; Xu, L. Comparison of Biological Properties and Clinical Application of Mesenchymal Stem Cells from the Mesoderm and Ectoderm. Stem Cells Int. 2023, 2023, 4547875. [Google Scholar] [CrossRef] [PubMed]
| Animal Model | Pre-Treatment | Type of MSCs | Administration | Outcome | Ref. |
|---|---|---|---|---|---|
| Mouse | IV ZA and dexamethasone | BM-MSCs from C57BL/6J mice | Infusion via tail vein | - Complete mucosal healing and bone regeneration- The inhibition of Th17 and restoration of Treg levels | [40] |
| Minipig | IV ZA | Allogeneic BM-MSCs | Intravenous infusion | - Mucosal healing- Bone reconstruction- Decrease of proinflammatory IL-17 levels and elevation of Tregs | [41] |
| Rat | IV ZA | Allogenic ADSCs/PRP | Direct placement into the socket | - No bone exposure- Vascularization- Decreased osteonecrosis and increased osteoclast density | [42] |
| Mouse | Subcutaneous administration of ZA and intraperitoneal injections of CY | Noncultured SVF | Tail-vein injection | - Reduced MRONJ lesion- Increased osteoblast and osteocytes number- Decreased necrotic bone area | [43] |
| Rat | Subcutaneous administration of ZA and dexamethasone | Allogenic BM-MSCs | Tissue-engineered cell sheet | - Active neoangiogenesis- Complete wound healing | [37] |
| Rabbit | IV ZA and dexamethasone | ADSCs | Direct placement into the extraction socket | - Gingival wound healing- Bone remodeling- Higher expression of TGF-β1 and fibronectin | [39] |
| Rat | Intraperitoneal ZA injection | ADSCs | Absorbable hemostatic gelatin sponge | - New bone formation- Intense vascularization | [44] |
| Rat | IV ZA | Allogenic BM-MSCs | Synthetic β-tricalcium phosphate (β-TCP) construct | - New bone formation, increased number of osteoclasts and osteoblasts | [45] |
| Mouse | Intraperitoneal ZA injection | ADSCs | Intravenous infusion | - Faster gingival epithelium healing, less bone exposure- New bone formation- Activation of autophagic flux | [38] |
| Rat | Subcutaneous administration of zoledronate and dexamethasone | Allogeneic BM-MSCs | Tissue-engineered cell sheet | - New bone laminar formation | [46] |
| Patient’s Gender/Age | Primary Disease | Medication | Type of Stem Cells | Carrier of MSCs | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Female/75 | Severe osteoporosis | Alendronate and pamidronate | Autologous BM-MSCs | Fibrine sponge | - Bone healing; concentric ossification- Complete MRONJ healing | [60] |
| 5 Females/54–771 Male/60 | Breast cancer; osteoporosis | Oral alendronate and IV ZA | Autologous BM-MSCs | Collagen membrane | - Complete mucosal healing- No signs of MRONJ recurrences | [61] |
| Male/68 | Multiple myeloma and bone disease | Cyclophosphamide, bortezomib, and dexamethasone; iv ZA | Autologous BM-MSCs | Bone substitute (Geistlich Bio-OssVR Collagen) | - Complete wound healing and bone regeneration- No pain or infection | [62] |
| Female/66 | Metastatic breast cancer | IV ZA | Autologous BM-MSCs | Direct injection | - Almost complete bone regeneration- No pain or swelling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazreku, M.; Danišovič, L.; Klein, M.; Kleinová, M. Recent Stem-Cell-Based and Stem-Cell-Free Possibilities for the Therapeutic Management of the Osteonecrosis of the Jaw. Biomolecules 2025, 15, 595. https://doi.org/10.3390/biom15040595
Mazreku M, Danišovič L, Klein M, Kleinová M. Recent Stem-Cell-Based and Stem-Cell-Free Possibilities for the Therapeutic Management of the Osteonecrosis of the Jaw. Biomolecules. 2025; 15(4):595. https://doi.org/10.3390/biom15040595
Chicago/Turabian StyleMazreku, Merita, L’uboš Danišovič, Martin Klein, and Mária Kleinová. 2025. "Recent Stem-Cell-Based and Stem-Cell-Free Possibilities for the Therapeutic Management of the Osteonecrosis of the Jaw" Biomolecules 15, no. 4: 595. https://doi.org/10.3390/biom15040595
APA StyleMazreku, M., Danišovič, L., Klein, M., & Kleinová, M. (2025). Recent Stem-Cell-Based and Stem-Cell-Free Possibilities for the Therapeutic Management of the Osteonecrosis of the Jaw. Biomolecules, 15(4), 595. https://doi.org/10.3390/biom15040595







