Link N Directly Targets IL-1β to Suppress Inflammation and Regulate Sensory Pain in Intervertebral Disc Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis
2.2. Antibodies
2.3. Human Nucleus Pulposus Cells
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Effect of LN on Cytokine Response
2.6. Peptide Docking
2.7. Immunoprecipitation
2.8. Competitive IP of IL1R1 with IL-1β
2.9. Ca2+-Mobilization
2.10. Histology
2.11. Statistical Analysis
3. Results
3.1. LN Modulates IL-1β-Induced Gene Expression in hNP Cells
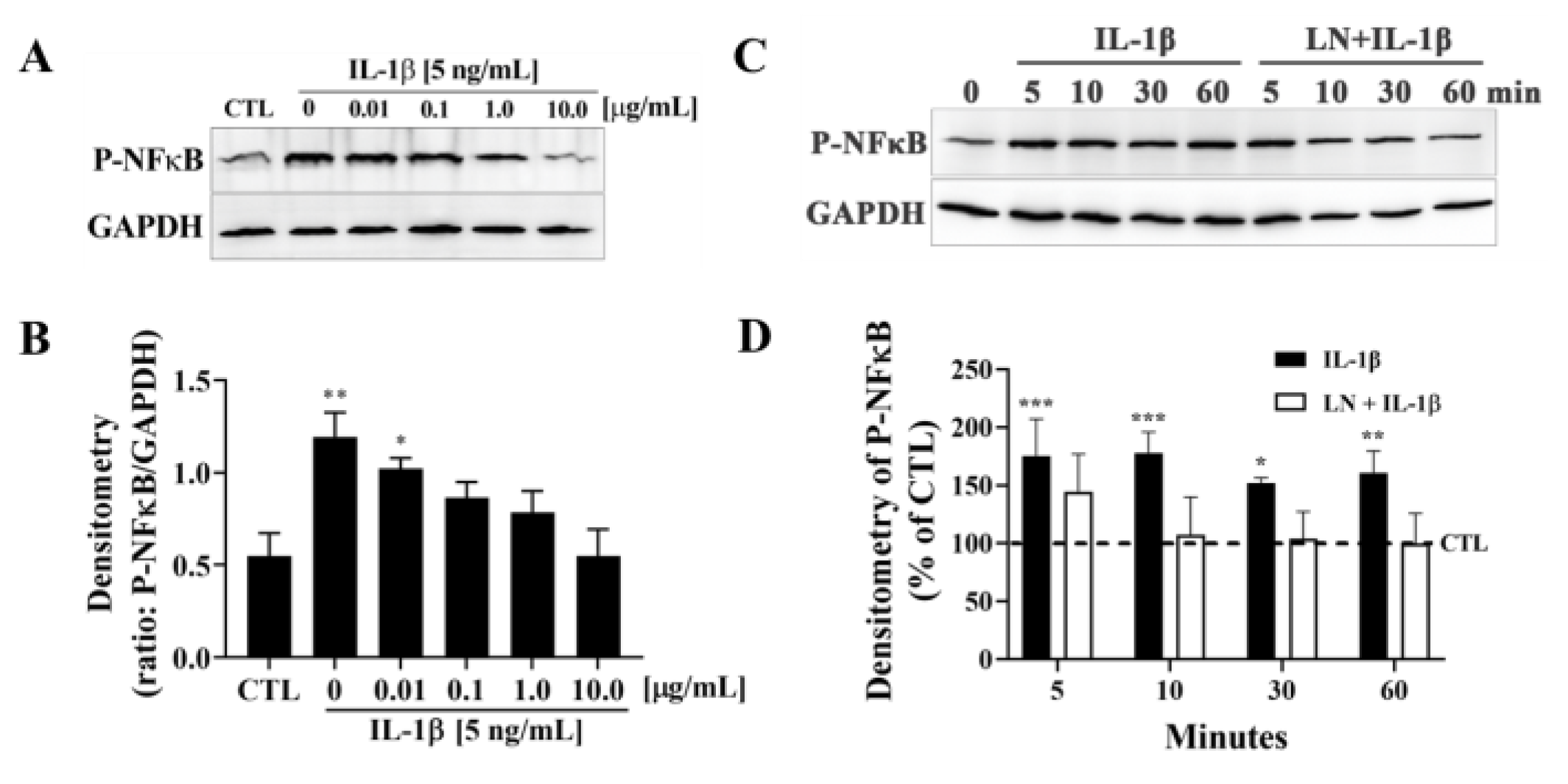
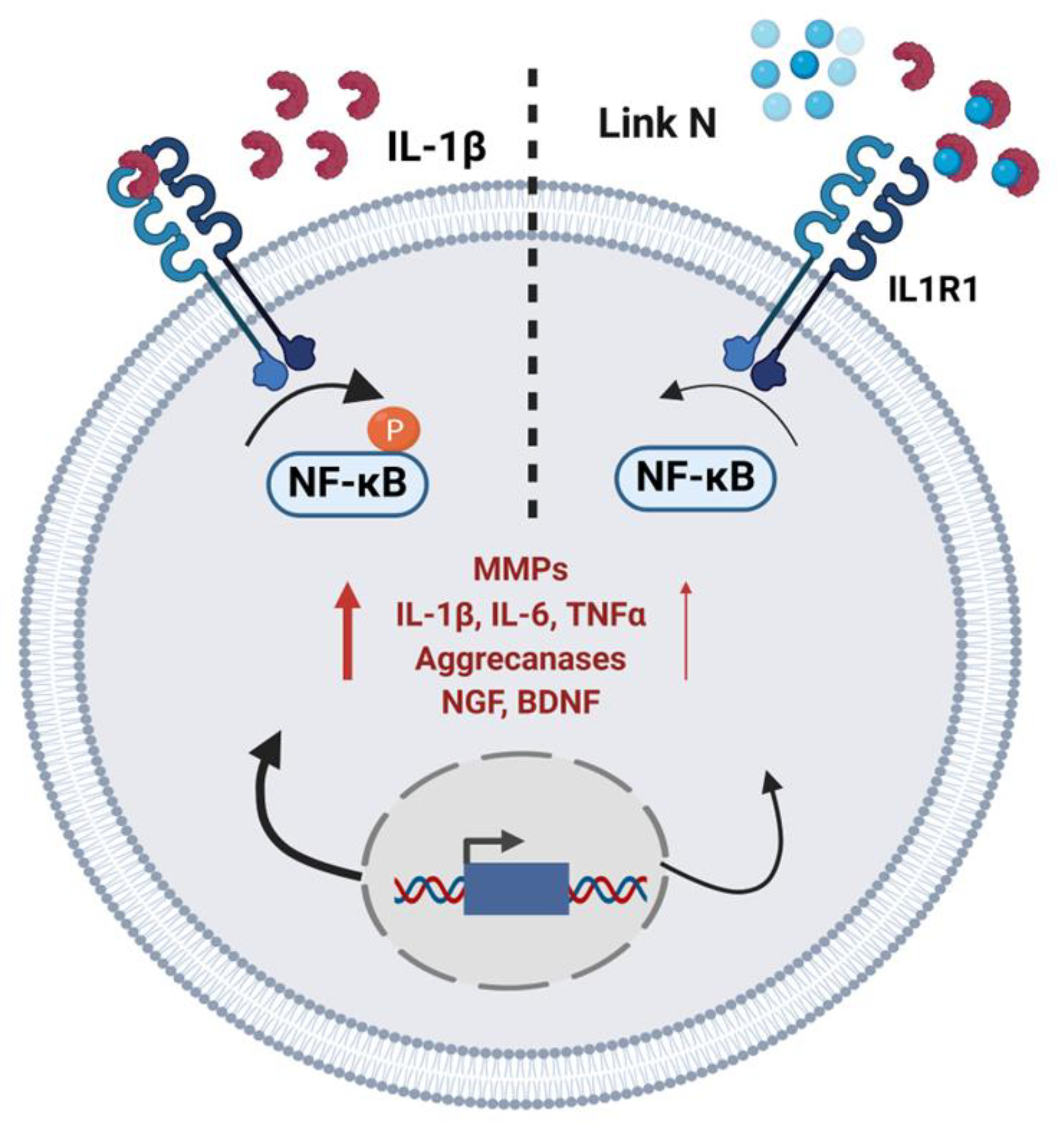
3.2. LN Suppresses IL-1β-Induced NF-κB Activation in hNP Cells
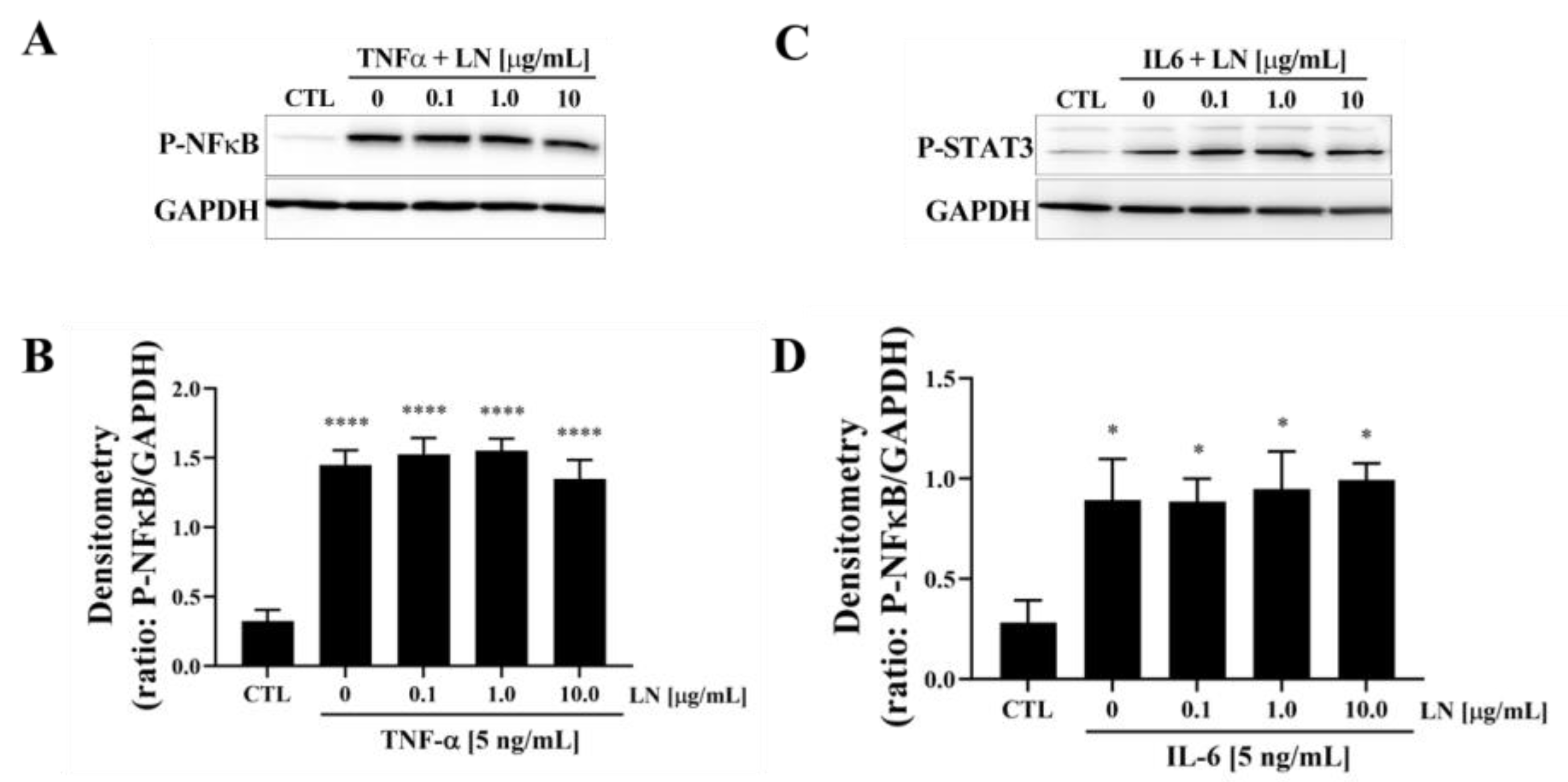
3.3. Cytokine-Targeted Specificity of LN in hNP Cells
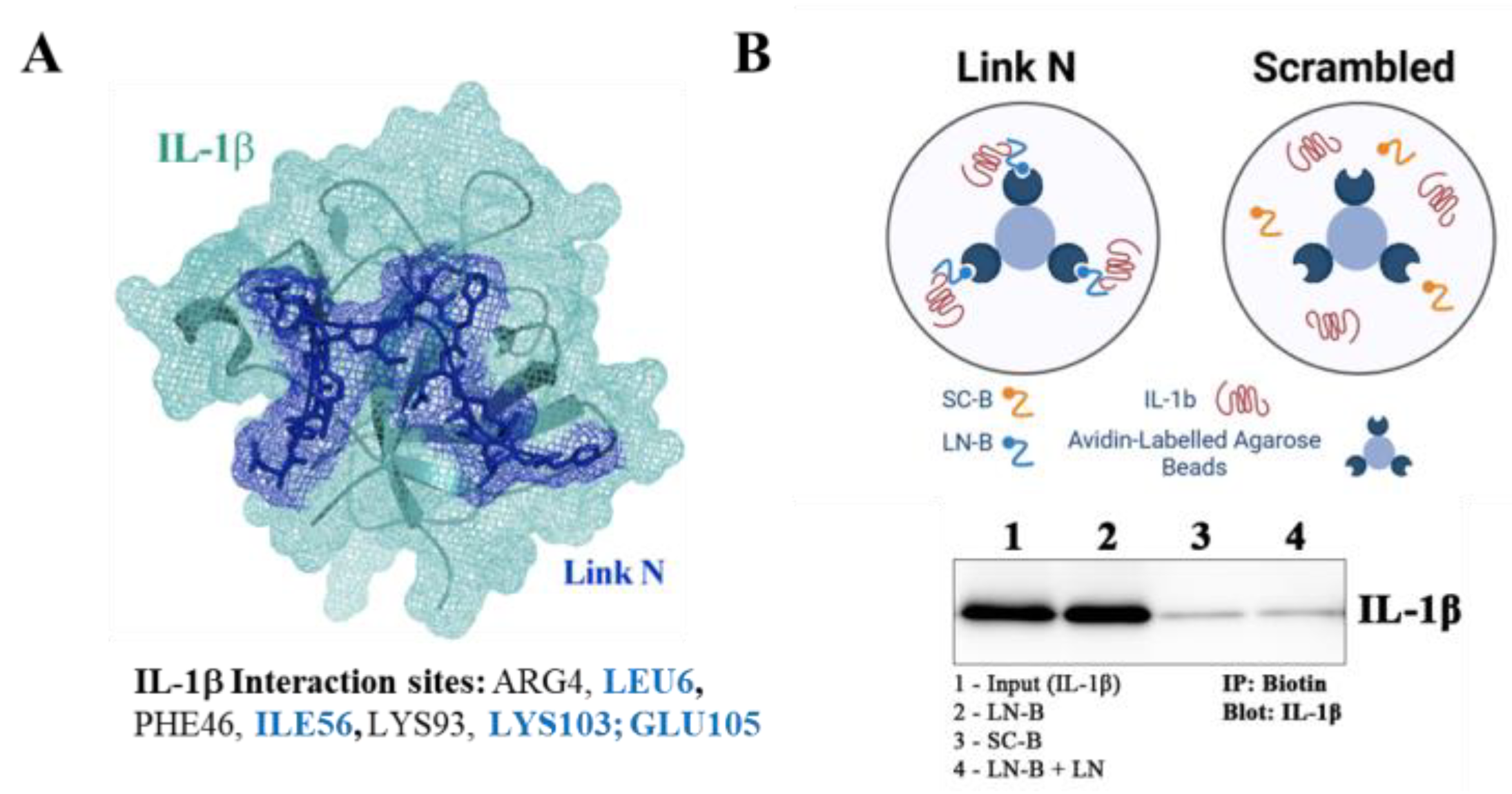
3.4. LN Interacts Specifically with IL-1β: Peptide Docking and Immunoprecipitation
3.5. LN Competitively Inhibits IL-1β Binding to IL-1 Receptor Type 1
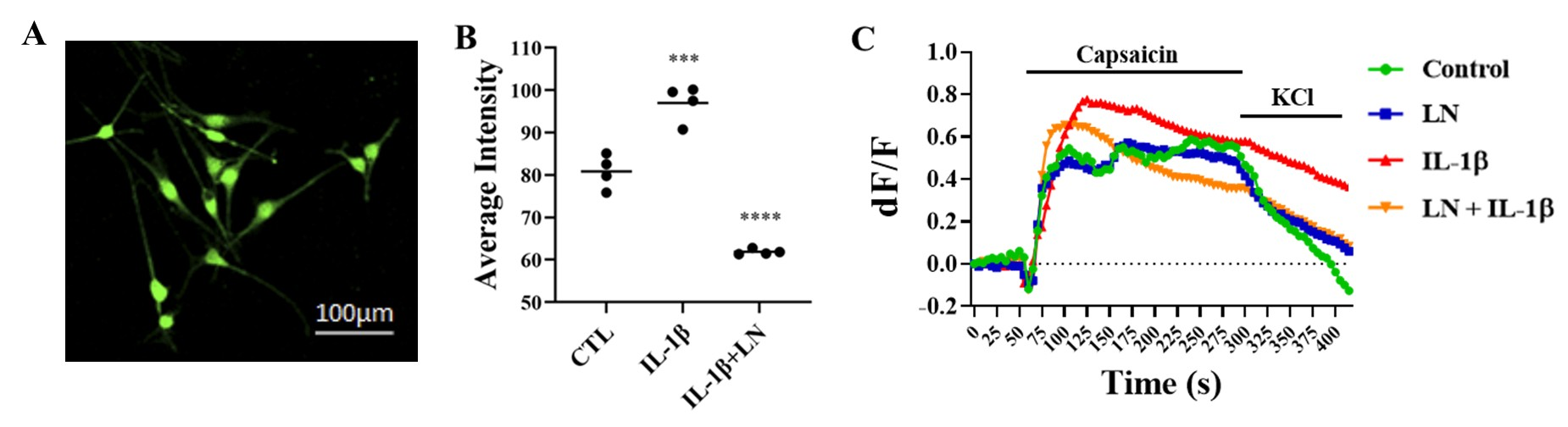
3.6. LN Modulates IL-1β-Induced DRG Hypersensitivity
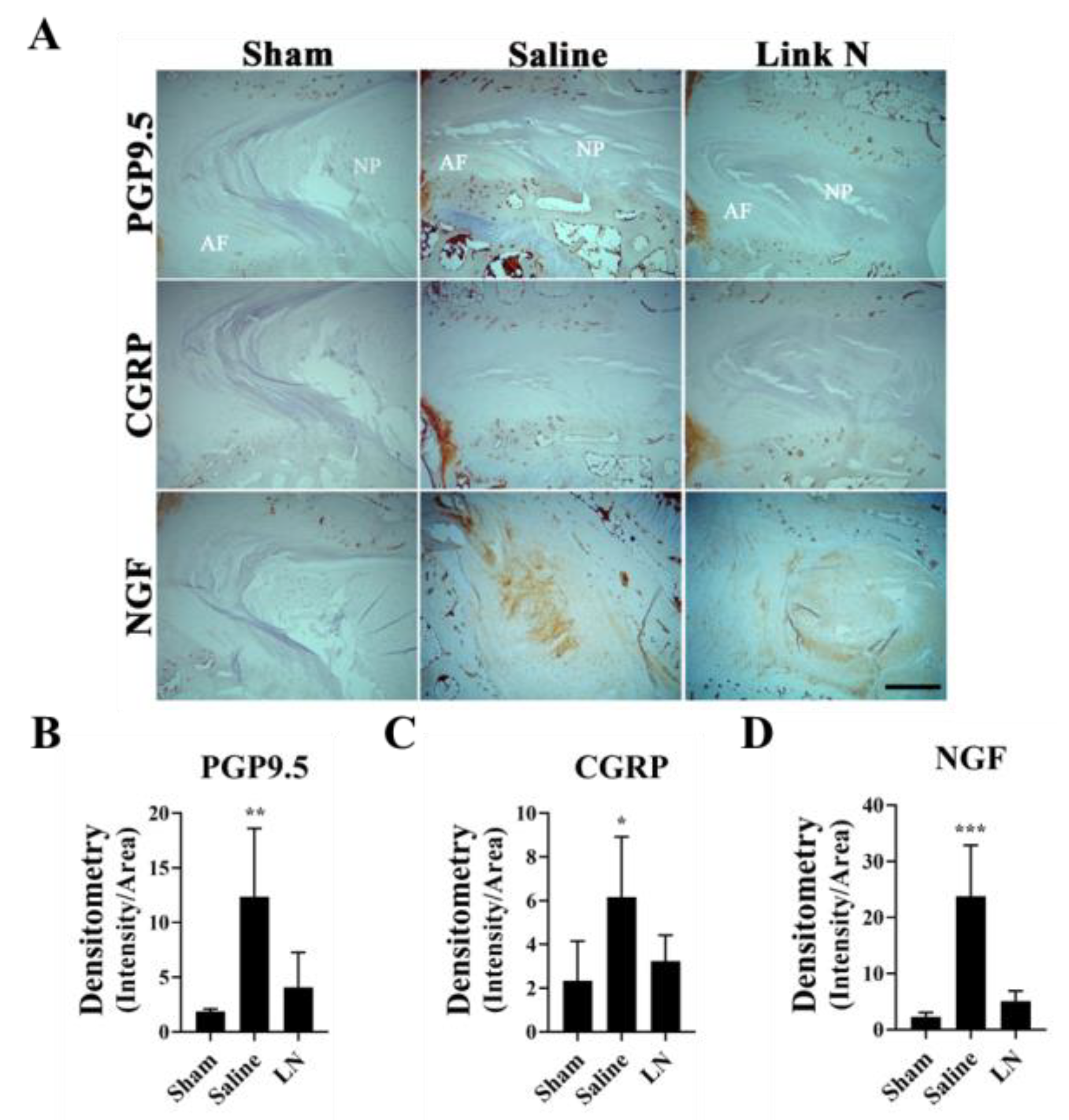
3.7. LN Reduces Pain-Related Markers in a Rabbit Model of Disc Degeneration
3.8. Summary of LN Effects on NP Cellular Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IVD | Intervertebral Disc |
| NP | Nucleus Pulposus |
| AF | Annulus Fibrosis |
| IP | Immunoprecipitation |
| IHC | Immunohistochemistry |
| LN | Link N |
| DRG | Dorsal Root Ganglion |
| NGF | Nerve Growth Factor |
References
- Sliwa, K.; Global Burden of Disease Study 2013 Collaborators. Lancet, Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Emanuel, K.S.; Mader, K.; Peeters, M.; Kingma, I.; Rustenburg, C.; Vergroesen, P.-P.; Sammon, C.; Smit, T. Early changes in the extracellular matrix of the degenerating intervertebral disc, assessed by Fourier transform infrared imaging. Osteoarthr. Cartil. 2018, 26, 1400–1408. [Google Scholar] [CrossRef]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2023, 6, e1231. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.-J.; Cui, H.; Pan, H.; MC Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology 2009, 48, 5–10. [Google Scholar] [CrossRef]
- Meisel, H.J.; Agarwal, N.; Hsieh, P.C.; Skelly, A.; Park, J.-B.; Brodke, D.; Wang, J.C.; Yoon, S.T.; Buser, Z. Cell Therapy for Treatment of Intervertebral Disc Degeneration: A Systematic Review. Glob. Spine J. 2019, 9 (Suppl. 1), 39s–52s. [Google Scholar] [CrossRef]
- Richardson, S.M.; Doyle, P.; Minogue, B.M.; Gnanalingham, K.; A Hoyland, J. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res. Ther. 2009, 11, R126. [Google Scholar] [CrossRef]
- La Binch, A.; Cole, A.A.; Breakwell, L.M.; Michael, A.L.; Chiverton, N.; Cross, A.K.; Le Maitre, C.L. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res. Ther. 2014, 16, 416. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- García-Cosamalón, J.; Del Valle, M.E.; Calavia, M.G.; García-Suárez, O.; López-Muñiz, A.; Otero, J.; Vega, J.A. Intervertebral disc, sensory nerves and neurotrophins: Who is who in discogenic pain? J. Anat. 2010, 217, 1–15. [Google Scholar] [CrossRef]
- Raj, P.P. Intervertebral disc: Anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Jung, H.; Bhangoo, S.K.; White, F.A. Cytokine and chemokine regulation of sensory neuron function. Sens. Nerves 2009, 417–449. [Google Scholar]
- Miller, R.E.; Miller, R.J.; Malfait, A.M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Mandalia, S.; Raasch, J.; Knezevic, I.; Candido, K.D. Treatment of chronic low back pain-new approaches on the horizon. J. Pain Res. 2017, 10, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Weitzmann, M.N.; Sangadala, S.; Hutton, W.C.; Yoon, S.T. Link Protein N-terminal Peptide Binds to Bone Morphogenetic Protein (BMP) Type II Receptor and Drives Matrix Protein Expression in Rabbit Intervertebral Disc Cells*. J. Biol. Chem. 2013, 288, 28243–28253. [Google Scholar] [CrossRef]
- Mwale, F.; Masuda, K.; Grant, M.P.; Epure, L.M.; Kato, K.; Miyazaki, S.; Cheng, K.; Yamada, J.; Bae, W.C.; Muehleman, C.; et al. Short Link N promotes disc repair in a rabbit model of disc degeneration. Arthritis Res. Ther. 2018, 20, 201. [Google Scholar] [CrossRef]
- Alaqeel, M.; Grant, M.; Epure, L.; Salem, O.; AlShaer, A.; Huk, O.; Bergeron, S.; Zukor, D.; Kc, R.; Im, H.-J.; et al. Link N suppresses interleukin-1beta-induced biological effects on human osteoarthritic cartilage. Eur. Cell Mater. 2020, 39, 65–76. [Google Scholar] [CrossRef]
- Noorwali, H.; Grant, M.P.; Epure, L.M.; Madiraju, P.; Sampen, H.; Antoniou, J.; Mwale, F. Link N as a therapeutic agent for discogenic pain. JOR Spine 2018, 1, e1008. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sleigh, J.N.; West, S.J.; Schiavo, G. A video protocol for rapid dissection of mouse dorsal root ganglia from defined spinal levels. BMC Res. Notes 2020, 13, 302. [Google Scholar] [CrossRef]
- Bach, F.C.; Laagland, L.T.; Grant, M.P.; Creemers, L.B.; Ito, K.; Meij, B.P.; Mwale, F.; Tryfonidou, M.A. Link-N: The missing link towards intervertebral disc repair is species-specific. PLoS ONE 2017, 12, e0187831. [Google Scholar] [CrossRef]
- Wuertz, K.; Haglund, L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Glob. Spine J. 2013, 3, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Gonçalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20141191. [Google Scholar] [CrossRef] [PubMed]
- Le Maitre, C.L.; Hoyland, J.A.; Freemont, A.J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res. Ther. 2007, 9, R77. [Google Scholar] [CrossRef]
- Pezet, S.; McMahon, S.B. Neurotrophins: Mediators and modulators of pain. Annu. Rev. Neurosci. 2006, 29, 507–538. [Google Scholar] [CrossRef]
- Burke, J.G.; Watson, R.W.G.; McCormack, D.; Dowling, F.E.; Walsh, M.G.; Fitzpatrick, J.M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Jt. Surg. Br. 2002, 84, 196–201. [Google Scholar] [CrossRef]
- Kamali, A.; Ziadlou, R.; Lang, G.; Pfannkuche, J.; Cui, S.; Li, Z.; Richards, R.G.; Alini, M.; Grad, S. Small molecule-based treatment approaches for intervertebral disc degeneration: Current options and future directions. Theranostics 2021, 11, 27–47. [Google Scholar] [CrossRef]
- Fujita, D.; Matsuoka, Y.; Yamakita, S.; Horii, Y.; Ishikawa, D.; Kushimoto, K.; Amino, H.; Amaya, F. Rapid cleavage of IL-1β in DRG neurons produces tissue injury-induced pain hypersensitivity. Mol. Pain 2024, 20, 17448069241285357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hutton, W.C.; Yoon, S.T. ISSLS Prize winner: Effect of link protein peptide on human intervertebral disc cells. Spine 2013, 38, 1501–1507. [Google Scholar] [CrossRef]
- Bobick, B.E.; Chen, F.H.; Le, A.M.; Tuan, R.S. Regulation of the chondrogenic phenotype in culture. Birth Defects Res. C Embryo Today 2009, 87, 351–371. [Google Scholar] [CrossRef] [PubMed]


| Human Genes | Primer Sequence | Size (bp) |
|---|---|---|
| h-IL1β | F (390–410): 5′-ACCTATCTTCTTCGACACATG-3′R (537–557): 5′-ACCACTTGTTGCTCCATATCC-3′ | 148 |
| h-TNFα | F (310–326): 5′-ACC ACG CTC TTC TGC CT-3′R (436–456): 5′-TAC AAC ATG GGC TAC AGG CTT-3′ | 127 |
| h-NGF | F (302–321): 5′-TCA GCA TTC CCT TGA CAC TG-3′R (521–540): 5′-TGC TCC TGT GAG TCC TGT TG-3′ | 220 |
| h-BDNF | F (481–501):5′-GAC ATC ATT GGC TGA CAC TTT-3′R (586–605): 5′-TAC TGA GCA TCA CCC TGG AC-3′ | 106 |
| h-GAPDH | F (113–133): 5′-TGA AGG TCG GAG TCA ACG GAT-3′R (273–293): 5′-TTC TCA GCC TTG ACG GTG CCA-3′ | 181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grant, M.P.; Alad, M.; Yousef, F.; Epure, L.M.; Antoniou, J.; Mwale, F. Link N Directly Targets IL-1β to Suppress Inflammation and Regulate Sensory Pain in Intervertebral Disc Degeneration. Biomolecules 2025, 15, 603. https://doi.org/10.3390/biom15040603
Grant MP, Alad M, Yousef F, Epure LM, Antoniou J, Mwale F. Link N Directly Targets IL-1β to Suppress Inflammation and Regulate Sensory Pain in Intervertebral Disc Degeneration. Biomolecules. 2025; 15(4):603. https://doi.org/10.3390/biom15040603
Chicago/Turabian StyleGrant, Michael P., Muskan Alad, Fajer Yousef, Laura M. Epure, John Antoniou, and Fackson Mwale. 2025. "Link N Directly Targets IL-1β to Suppress Inflammation and Regulate Sensory Pain in Intervertebral Disc Degeneration" Biomolecules 15, no. 4: 603. https://doi.org/10.3390/biom15040603
APA StyleGrant, M. P., Alad, M., Yousef, F., Epure, L. M., Antoniou, J., & Mwale, F. (2025). Link N Directly Targets IL-1β to Suppress Inflammation and Regulate Sensory Pain in Intervertebral Disc Degeneration. Biomolecules, 15(4), 603. https://doi.org/10.3390/biom15040603







