Regeneration of Two-Walled Infrabony Periodontal Defects in Swine After Buccal Fat Pad-Derived Dedifferentiated Fat Cell Autologous Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Preparation of BFP-DFAT Cells
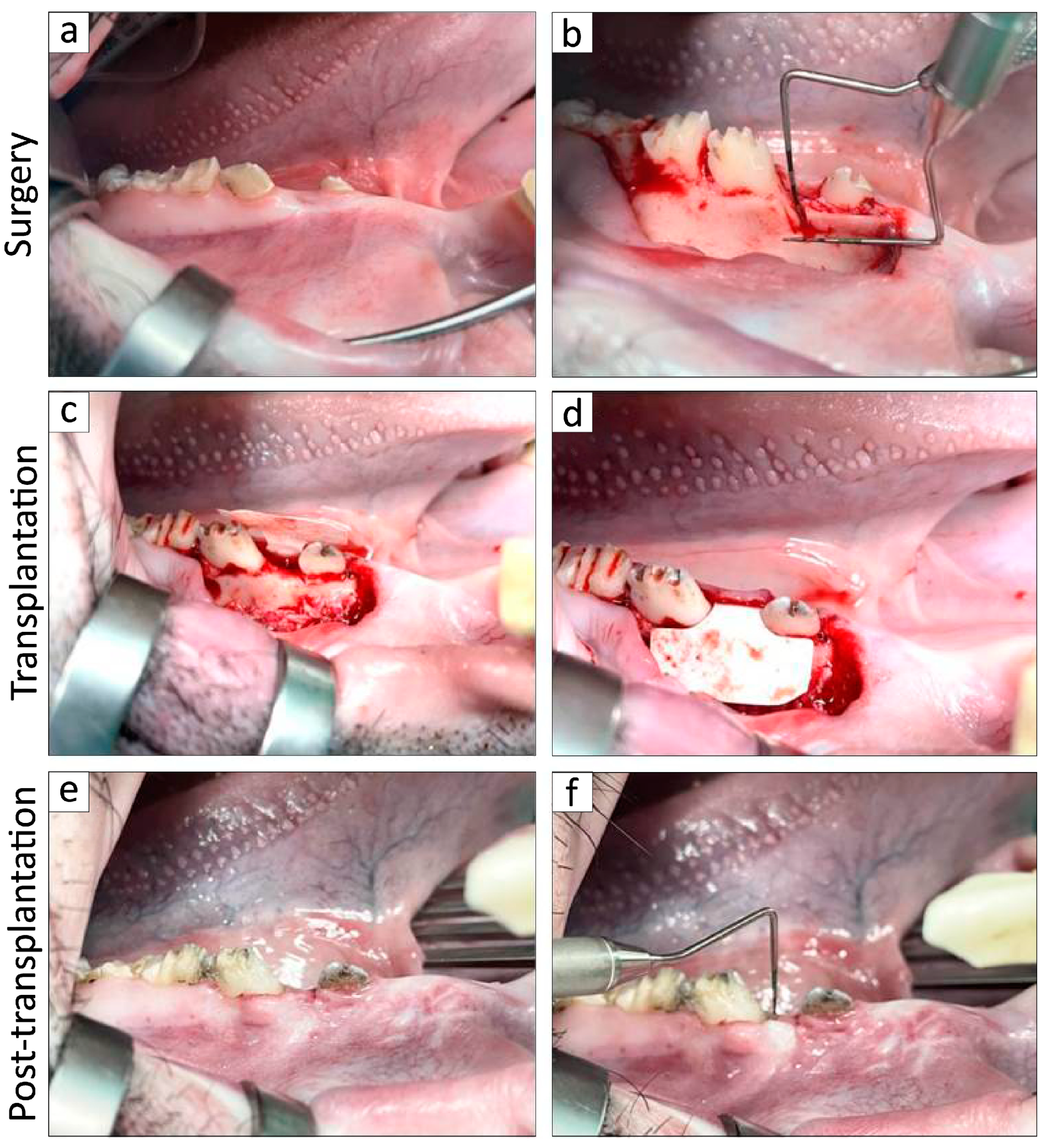
2.3. In Vivo Experimentation
2.4. Histological and Immunohistochemical Staining and Analysis
2.5. Statistical Analysis
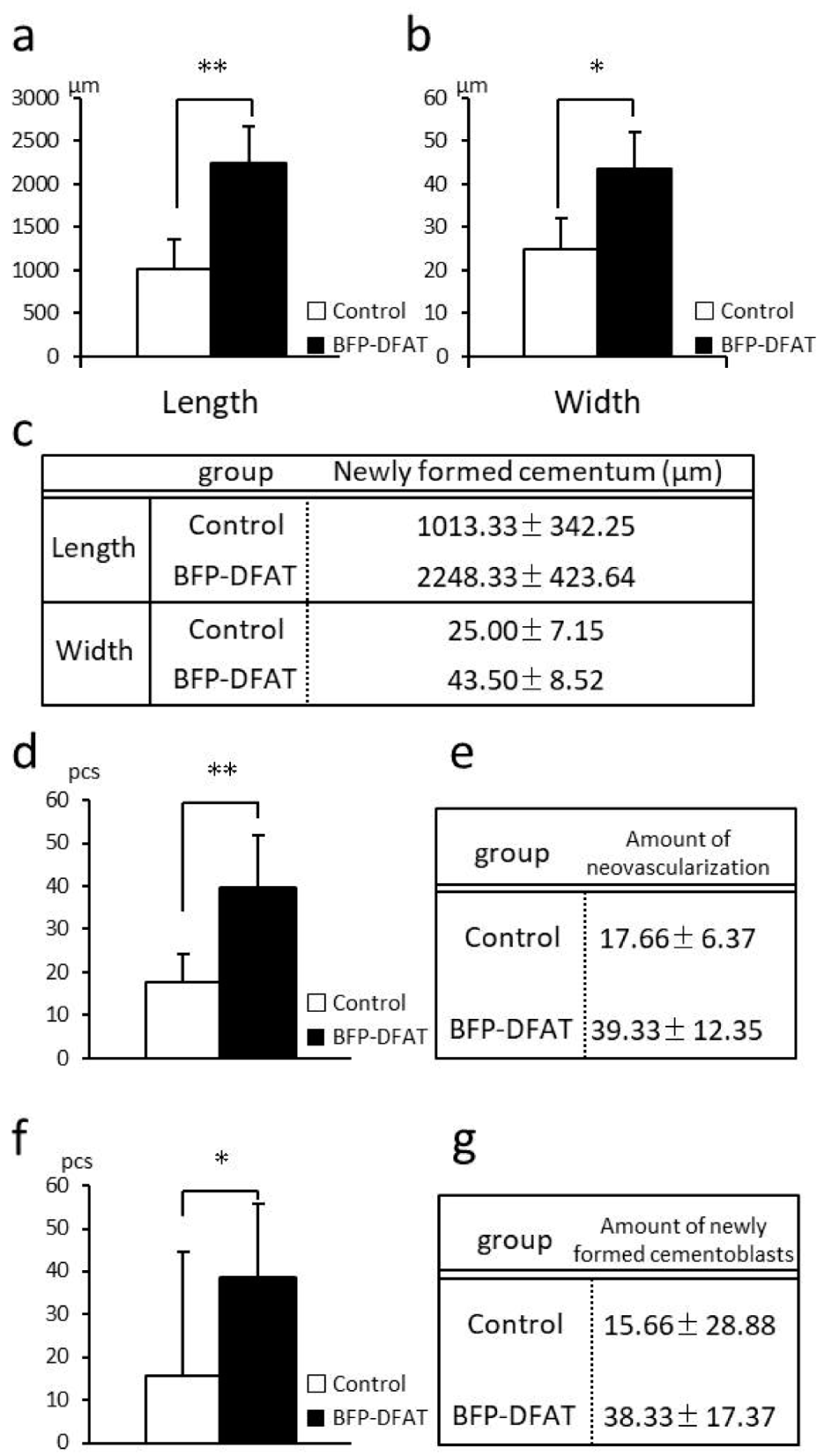
3. Results
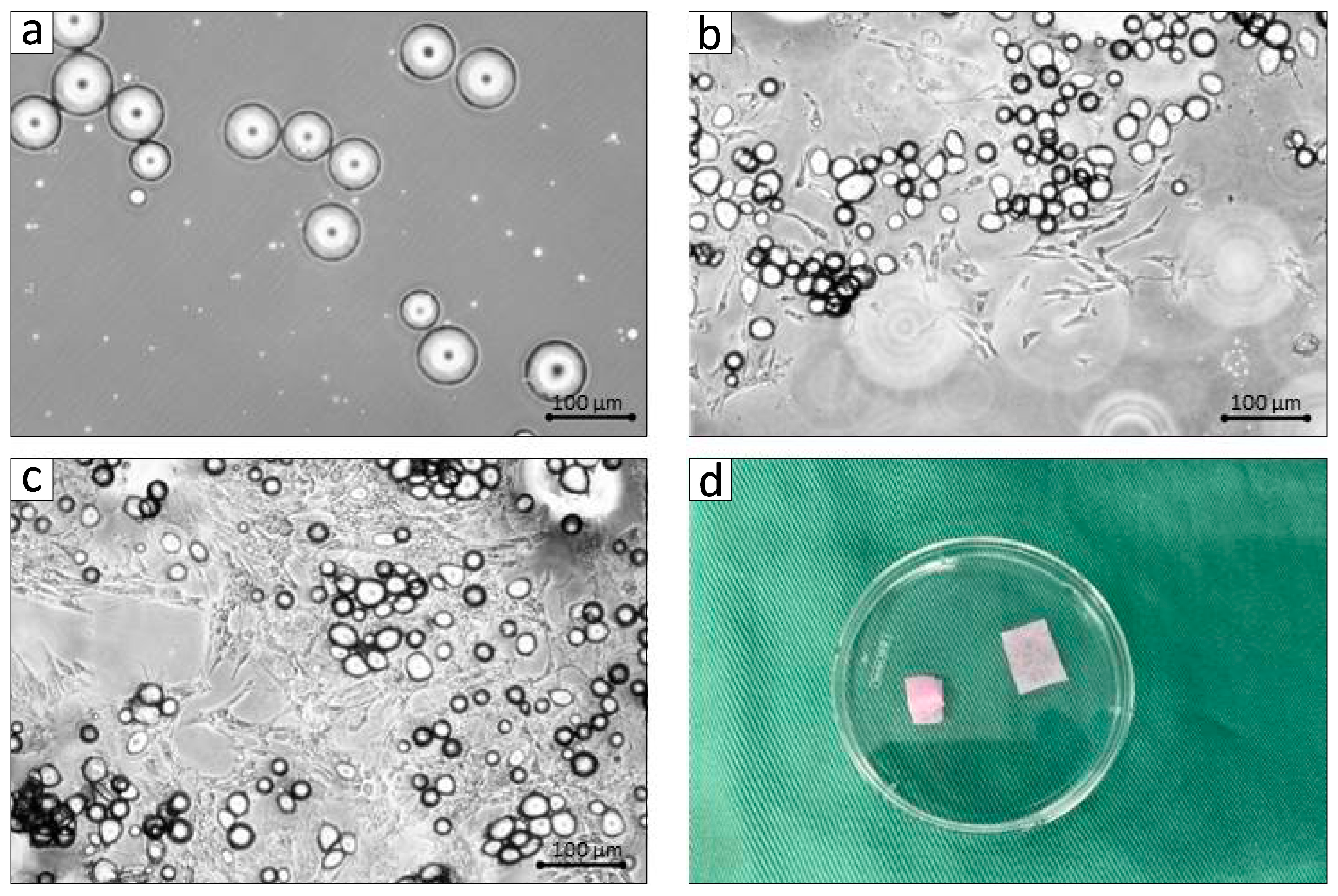
3.1. Establishment of BFP-Derived DFAT Cells and Clinical Assessments in the Adult MMPs
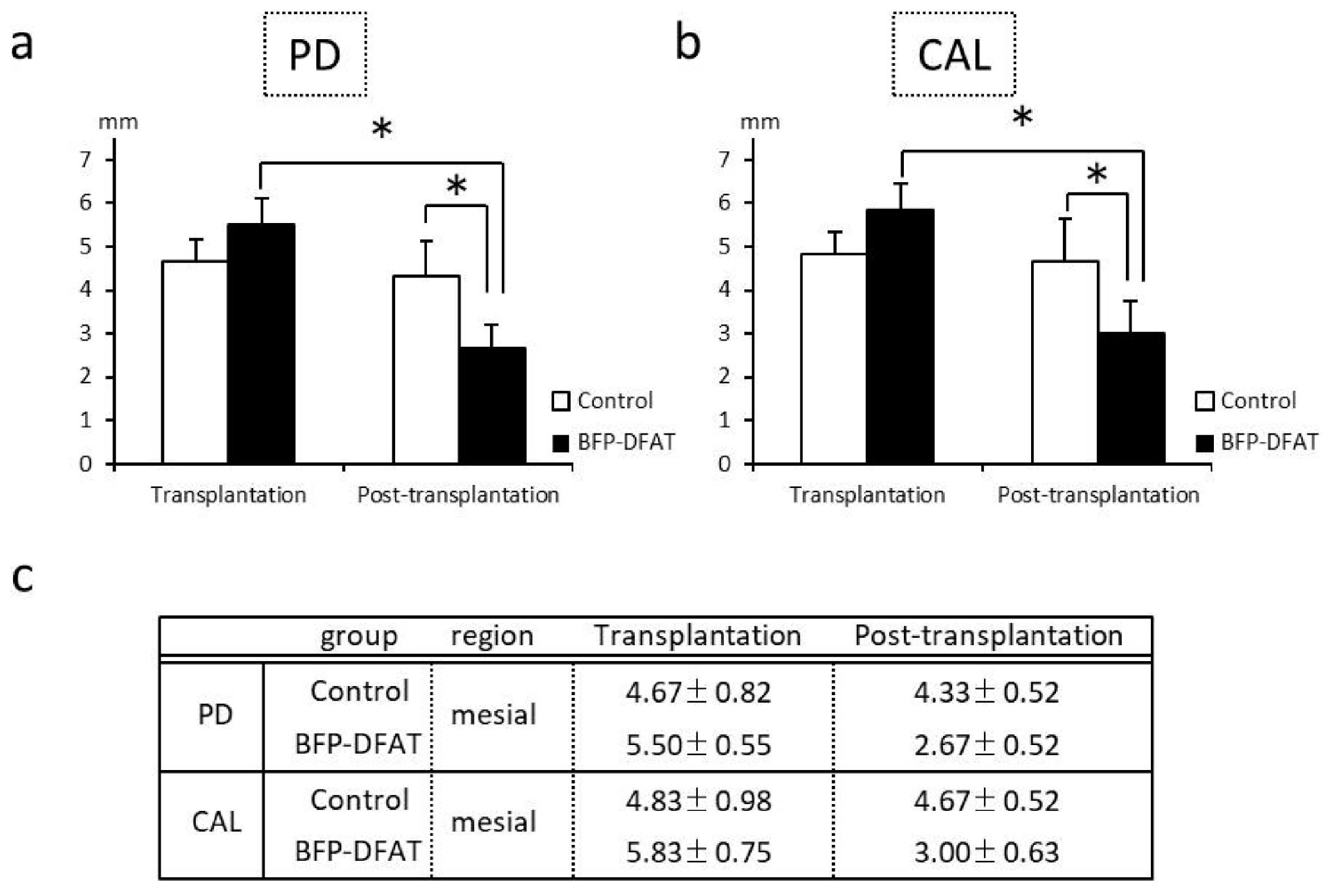
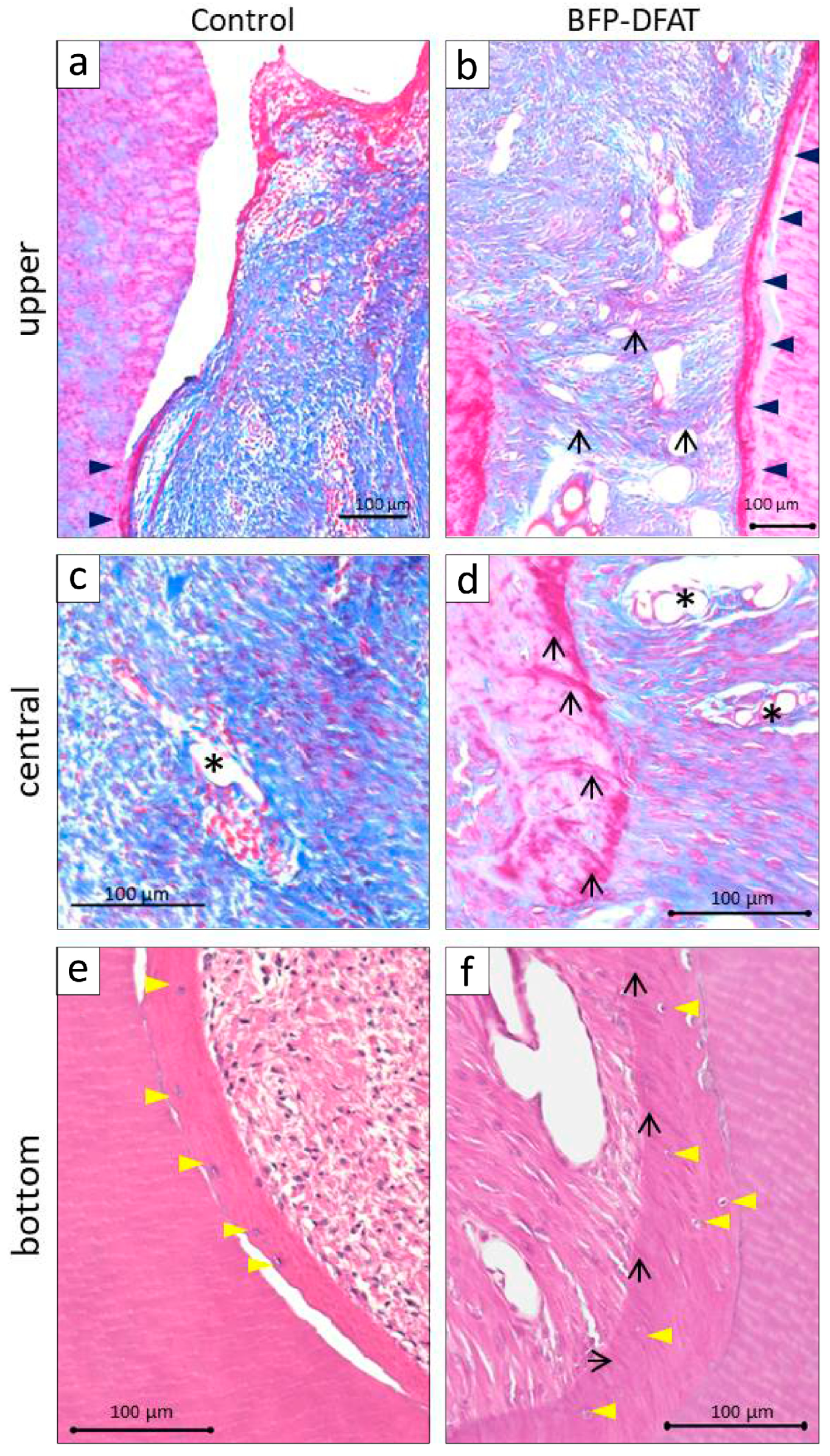
3.2. Transplantation of BFP-Derived DFAT Cells Enhanced Periodontal Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFAT | Dedifferentiated fat |
| BFP | Buccal fat pad |
| MMPs | Micro-mini pigs |
| MSCs | Mesenchymal stem cells |
| ASCs | Adipose derived stem/stromal cells |
| PBS | Phosphate-buffered saline |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
| EDTA | Ethylenediaminetetraacetic acid |
| PD | Probing depth |
| CAL | Clinical attachment level |
| SD | Standard deviation |
References
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontology 2017, 75, 152–188. [Google Scholar] [CrossRef] [PubMed]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal ligament stem cells: Regenerative potency in periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Shuo, Z.; Fuh, J.Y.H.; Lu, W.F. Design of three-dimensional scaffolds with tunable matrix stiffness for directing stem cell lineage specification: An in silico study. Bioengineering 2017, 4, 66. [Google Scholar] [CrossRef]
- Marks, P.; Gottlieb, S. Balancing safety and innovation for cell-based regenerative medicine. N. Engl. J. Med. 2018, 378, 954–959. [Google Scholar] [CrossRef]
- Heathman, T.R.J.; Nienow, A.W.; McCall, M.J.; Coopman, K.; Kara, B.; Hewitt, C.J. The translation of cell-based therapies: Clinical landscape and manufacturing challenges. Regen. Med. 2015, 10, 49–64. [Google Scholar] [CrossRef]
- Iwasaki, K.; Peng, Y.; Kanda, R.; Umeda, M.; Ishikawa, I. Stem cell transplantation and cell-free treatment for periodontal regeneration. Int. J. Mol. Sci. 2022, 23, 1011. [Google Scholar] [CrossRef]
- Cho, Y.D.; Kim, K.H.; Ryoo, H.M.; Lee, Y.M.; Ku, Y.; Seol, Y.J. recent advances of useful cell sources in the periodontal regeneration. Curr. Stem Cell Res. Ther. 2019, 14, 3–8. [Google Scholar] [CrossRef]
- Yamada, Y.; Ito, K.; Nakamura, S.; Ueda, M.; Nagasaka, T. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant. 2011, 20, 1003–1013. [Google Scholar] [CrossRef]
- Han, J.; Menicanin, D.; Marino, V.; Ge, S.; Mrozik, K.; Gronthos, S.; Bartold, P.M. Assessment of the regenerative potential of allogeneic periodontal ligament stem cells in a rodent periodontal defect model. J. Periodontal Res. 2014, 49, 333–345. [Google Scholar] [CrossRef]
- Suaid, F.F.; Ribeiro, F.V.; Rodrigues, T.L.; Silvério, K.G.; Carvalho, M.D.; Nociti, F.H.; Casati, M.Z.; Sallum, E.A. Autologous periodontal ligament cells in the treatment of class II furcation defects: A study in dogs. J. Clin. Periodontol. 2011, 38, 491–498. [Google Scholar] [CrossRef]
- Nuñez, J.; Sanz-Blasco, S.; Vignoletti, F.; Muñoz, F.; Arzate, H.; Villalobos, C.; Nuñez, L.; Caffesse, R.G.; Sanz, M. Periodontal regeneration following implantation of cementum and periodontal ligament-derived cells. J. Periodont. Res. 2012, 47, 33–44. [Google Scholar] [CrossRef]
- Mrozik, K.M.; Wada, N.; Marino, V.; Richter, W.; Shi, S.; Wheeler, D.L.; Gronthos, S.; Bartold, P.M. Regeneration of periodontal tissues using allogeneic periodontal ligament stem cells in an ovine model. Regen. Med. 2013, 8, 711–723. [Google Scholar] [CrossRef]
- Fu, X.; Jin, L.; Ma, P.; Fan, Z.; Wang, S. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine. J. Periodontol. 2014, 85, 845–851. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Z.; Xie, Y.; Hu, J.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.-T.; Wang, S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res. Ther. 2015, 6, 249. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, X.; Lin, M.; Doan, N.; Xiao, Y.; Yan, F. Application of autologous periosteal cells for the regeneration of class III furcation defects in Beagle dogs. Cytotechnology 2010, 62, 235–243. [Google Scholar] [CrossRef]
- Baer, P.C. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 2011, 20, 1805–1816. [Google Scholar] [CrossRef]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose tissue-derived stem cells in regenerative medicine. Transfus. Med. Hemother. Sin. 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Choi, J.W.; Park, E.J.; Shin, H.S.; Shin, I.S.; Ra, J.C.; Koh, K.S. In vivo differentiation of undifferentiated human adipose tissue-derived mesenchymal stem cells in critical-sized calvarial bone defects. Ann. Plast. Surg. 2014, 72, 225–233. [Google Scholar] [CrossRef]
- Akita, D.; Morokuma, M.; Saito, Y.; Yamanaka, K.; Akiyama, Y.; Sato, M.; Mashimo, T.; Toriumi, T.; Arai, Y.; Kaneko, T.; et al. Periodontal tissue regeneration by transplantation of rat adipose-derived stromal cells in combination with PLGA-based solid scaffolds. Biomed. Res. 2014, 35, 91–103. [Google Scholar] [CrossRef]
- Kunze, K.N.; Burnett, R.A.; Wright-Chisem, J.; Frank, R.M.; Chahla, J. Adipose-derived mesenchymal stem cell treatments and available formulations. Curr. Rev. Musculoskelet. Med. 2020, 13, 264–280. [Google Scholar] [CrossRef]
- Jumabay, M.; Boström, K.I. Dedifferentiated fat cells: A cell source for regenerative medicine. World J. Stem Cells. 2015, 7, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kano, K.; Kondo, D.; Fukuda, N.; Iribe, Y.; Tanaka, N.; Matsubara, Y.; Sakuma, T.; Satomi, A.; Otaki, M.; et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J. Cell. Physiol. 2008, 215, 210–222. [Google Scholar] [CrossRef]
- Wei, S.; Du, M.; Jiang, Z.; Duarte, M.S.; Fernyhough-Culver, M.; Albrecht, E.; Will, K.; Zan, L.; Hausman, G.J.; Elabd, E.M.Y.; et al. Bovine dedifferentiated adipose tissue (DFAT) cells: DFAT cell isolation. Adipocyte 2013, 2, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zan, L.; Hausman, G.J.; Rasmussen, T.P.; Bergen, W.G.; Dodson, M.V. Dedifferentiated adipocyte-derived progeny cells (DFAT cells): Potential stem cells of adipose tissue. Adipocyte 2013, 2, 122–127. [Google Scholar] [CrossRef]
- Kaku, M.; Akiba, Y.; Akiyama, K.; Akita, D.; Nishimura, M. Cell-based bone regeneration for alveolar ridge augmentation—Cell source, endogenous cell recruitment and immunomodulatory function. J. Prosthodont. Res. 2015, 59, 96–112. [Google Scholar] [CrossRef]
- Akita, D.; Kano, K.; Saito-Tamura, Y.; Mashimo, T.; Sato-Shionome, M.; Tsurumachi, N.; Yamanaka, K.; Kaneko, T.; Toriumi, T.; Arai, Y.; et al. Use of rat mature adipocyte-derived dedifferentiated fat cells as a cell source for periodontal tissue regeneration. Front. Physiol. 2016, 7, 50. [Google Scholar] [CrossRef]
- Akita, D.; Kazama, T.; Tsukimura, N.; Taniguchi, Y.; Takahashi, R.; Arai, Y.; Tsurumachi-Iwasaki, N.; Yasuda, H.; Okubo, T.; Kano, K.; et al. Transplantation of mature adipocyte-derived dedifferentiated fat cells facilitates periodontal tissue regeneration of class II furcation defects in miniature pigs. Materials 2022, 15, 1311. [Google Scholar] [CrossRef]
- Abad-Gallegos, M.; Figueiredo, R.; Rodríguez-Baeza, A.; Gay-Escoda, C. Use of Bichat’s buccal fat pad for the sealing of orosinusal communications: A presentation of 8 cases. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e215–e219. [Google Scholar] [CrossRef]
- Meyer, E.; Liebenberg, S.J.; Fagan, J.J. Buccal fat pad—A simple, underutilised flap. S. Afr. J. Surg. 2012, 50, 47–49. [Google Scholar]
- Cordero, G.B.; Ferrer, S.M.; Fernández, L. Odontogenic sinusitis, oro-antral fistula and surgical repair by Bichat’s fat pad: Literature review. Acta Otorrinolaringol. Esp. 2016, 67, 107–113. [Google Scholar] [CrossRef]
- Adekunle, A.A.; James, O.; Olanbiwonnu, A.O.; Adeyemo, W.L. A review of the use of buccal fat pad in cleft palate repair. Cleft Palate Craniofac. J. 2024, 61, 1116–1124. [Google Scholar] [CrossRef]
- Saravanan, K.; Narayanan, V. The use of buccal fat pad in the treatment of oral submucous fibrosis: A newer method. Int. J. Dent. 2012, 2012, 935135. [Google Scholar] [CrossRef]
- Surej, K.L.K.; Kurien, N.M.; Sakkir, N. Buccal fat pad reconstruction for oral submucous fibrosis. Natl. J. Maxillofac. Surg. 2010, 1, 164–167. [Google Scholar] [CrossRef]
- Jolly, S.S.; Singh, A.; Rattan, V. The buccal fat pad as a primary flap for the reconstruction of intraoral defect after resection of oral cavity malignant tumors: A retrospective study. J. Craniofac. Surg. 2024, 35, e109–e111. [Google Scholar] [CrossRef]
- Bardazzi, A.; Beltramini, G.A.; Autelitano, L.; Bazzacchi, R.; Rabbiosi, D.; Pedrazzoli, M.; Tewfik, K.; Rezzonico, A.; Biglioli, F. Use of buccinator myomucosal flap in tongue reconstruction. J. Craniofac. Surg. 2017, 28, 1084–1087. [Google Scholar] [CrossRef]
- Broccaioli, E.; Niada, S.; Rasperini, G.; Ferreira, L.M.; Arrigoni, E.; Yenagi, V.; Brini, A.T. Mesenchymal stem cells from Bichat’s fat pad: In vitro comparison with adipose-derived stem cells from subcutaneous tissue. Biores. Open Access 2013, 2, 107–117. [Google Scholar] [CrossRef]
- Shiraishi, T.; Sumita, Y.; Wakamastu, Y.; Nagai, K.; Asahina, I. Formation of engineered bone with adipose stromal cells from buccal fat pad. J. Dent. Res. 2012, 91, 592–597. [Google Scholar] [CrossRef]
- Kishimoto, N.; Momota, Y.; Hashimoto, Y.; Tatsumi, S.; Ando, K.; Omasa, T.; Kotani, J. The osteoblastic differentiation ability of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad. Clin. Oral Investig. 2014, 18, 1893–1901. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Paris, S.; Becker, S.T.; Neuschl, M.; De Buhr, W.; Sälzer, S.; Wulff, A.; Elrefai, M.; Darhous, M.S.; El-Masry, M.; et al. Periodontal regeneration employing gingival margin-derived stem/progenitor cells: An animal study. J. Clin. Periodontol. 2012, 39, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Liu, S.S.; Ma, J.L.; Wang, D.S.; E, L.L.; Liu, H.C. Enhancement of periodontal tissue regeneration by transplantation of osteoprotegerin-engineered periodontal ligament stem cells. Stem Cells Res. Ther. 2015, 6, 22. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Fang, D.; Shi, S. The miniature pig: A useful large animal model for dental and orofacial research. Oral Dis. 2007, 13, 530–537. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008, 26, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Lovell-Badge, R.; Anthony, E.; Barker, R.A.; Bubela, T.; Brivanlou, A.; Carpenter, M.; Charo, R.A.; Clark, A.; Clyton, E.; Cong, Y.; et al. ISSCR guidelines for stem cell research and clinical translation: The 2021 update. Stem Cell Rep. 2021, 16, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Animal models for periodontal regeneration and peri-implant responses. Periodontol 2000 2015, 68, 66–82. [Google Scholar] [CrossRef]
- Fu, Y.; Karbaat, L.; Wu, L.; Leijten, J.; Both, S.K.; Karperien, M. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng. Part B Rev. 2017, 23, 515–528. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, R.; Hou, Y.; Wang, H.; Li, Y.; Zhu, J.; Fu, C. Application of mesenchymal stem cell-derived exosomes from different sources in intervertebral disc degeneration. Front. Bioeng. Biotechnol. 2022, 10, 1019437. [Google Scholar] [CrossRef]
- Takedachi, M.; Kawasaki, K.; Sawada, K.; Sakura, K.; Murata, M.; Shimomura, J.; Kawakami, K.; Morimoto, C.; Miki, K.; Takeshita, N.; et al. Periodontal Tissue Regeneration by Transplantation of Autologous Adipose Tissue-Derived Multi-Lineage Progenitor Cells With Carbonate Apatite. Cell Transplant. 2023, 32, 09636897231198296. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akita, D.; Tsukimura, N.; Kazama, T.; Takahashi, R.; Taniguchi, Y.; Inoue, J.; Suzuki, A.; Tanabe, N.; Seki, K.; Arai, Y.; et al. Regeneration of Two-Walled Infrabony Periodontal Defects in Swine After Buccal Fat Pad-Derived Dedifferentiated Fat Cell Autologous Transplantation. Biomolecules 2025, 15, 604. https://doi.org/10.3390/biom15040604
Akita D, Tsukimura N, Kazama T, Takahashi R, Taniguchi Y, Inoue J, Suzuki A, Tanabe N, Seki K, Arai Y, et al. Regeneration of Two-Walled Infrabony Periodontal Defects in Swine After Buccal Fat Pad-Derived Dedifferentiated Fat Cell Autologous Transplantation. Biomolecules. 2025; 15(4):604. https://doi.org/10.3390/biom15040604
Chicago/Turabian StyleAkita, Daisuke, Naoki Tsukimura, Tomohiko Kazama, Rie Takahashi, Yoshiki Taniguchi, Jin Inoue, Ayana Suzuki, Nodoka Tanabe, Keisuke Seki, Yoshinori Arai, and et al. 2025. "Regeneration of Two-Walled Infrabony Periodontal Defects in Swine After Buccal Fat Pad-Derived Dedifferentiated Fat Cell Autologous Transplantation" Biomolecules 15, no. 4: 604. https://doi.org/10.3390/biom15040604
APA StyleAkita, D., Tsukimura, N., Kazama, T., Takahashi, R., Taniguchi, Y., Inoue, J., Suzuki, A., Tanabe, N., Seki, K., Arai, Y., Asano, M., Sato, S., Hagiwara, Y., Kano, K., Honda, M., & Matsumoto, T. (2025). Regeneration of Two-Walled Infrabony Periodontal Defects in Swine After Buccal Fat Pad-Derived Dedifferentiated Fat Cell Autologous Transplantation. Biomolecules, 15(4), 604. https://doi.org/10.3390/biom15040604




