Synthesis of a Novel ?-Glucosyl Ginsenoside F1 by Cyclodextrin Glucanotransferase and Its In Vitro Cosmetic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biotransformation
2.3. Glycosylation of Ginsenoside F1
2.4. Identification of Glycosylated Ginsenoside F1
2.5. Nuclear Magnetic Resonance Analysis
2.6. Cell Lines and Cell Culture
2.6.1. Ultraviolet Irradiation and Sample Treatment
2.6.2. Cytotoxicity Assay
2.6.3. In Vitro Tyrosinase Inhibition Activity
2.6.4. Assay for Inhibition of Matrix Metalloproteinase-1 Expression
3. Results and Discussion
3.1. Biotransformation of Minor Ginsenosides by Cyclodextrin glycosyltransferase (CGTase)
3.2. Specificity of Transglycosylation of Ginsenoside F1
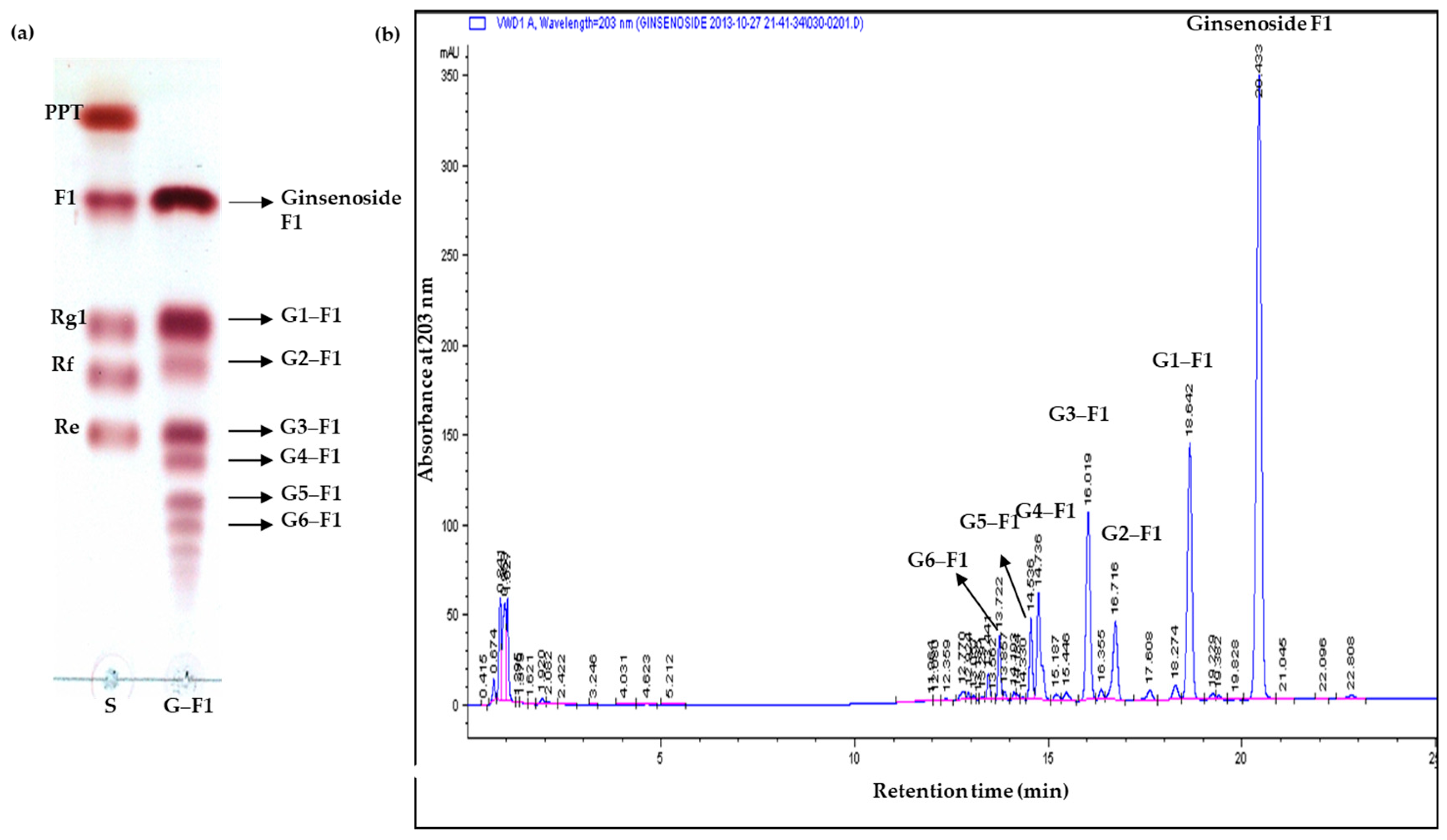
3.3. Transglycosylation Analysis of Ginsenoside F1
3.4. Characterization of Novel α-Glycosylated Ginsenoside F1
Water Solubility of Ginsenoside F1 and Novel α-Glycosylated Ginsenoside F1
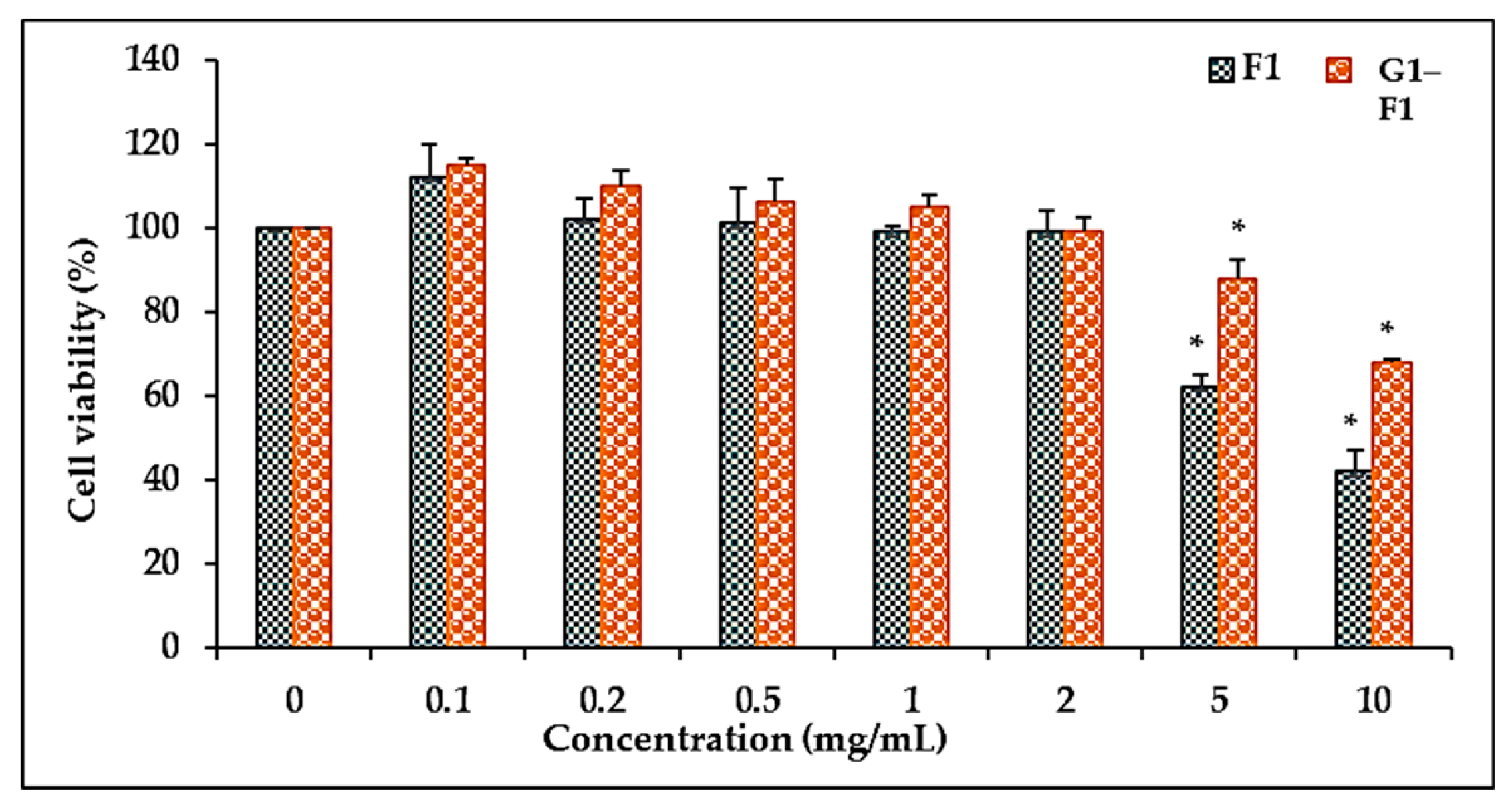
3.5. Cell Cytotoxicity
3.5.1. Comparison of Cell Viability of Ginsenoside F1 and Novel α-Glycosylated Ginsenoside F1 in Human Dermal Fibroblast Cells
3.5.2. Inhibition of Tyrosinase Activity by Ginsenoside F1 and G1–F1
3.5.3. Inhibition of Ultraviolet A (UVA)-Induced Matrix Metalloproteinase- (MMP-1) Expression of Ginsenoside F1 and G1–F1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, A.S.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, R.; Subramaniyam, S.; Kim, Y.J.; Kim, Y.C.; Yang, D.C. Ginsenoside compound K-bearing glycol chitosan conjugates: Synthesis, physicochemical characterization, and in vitro biological studies. Carbohydr. Polym. 2014, 112, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Siddiqi, M.H.; Noh, H.-Y.; Kim, Y.-J.; Kim, Y.-J.; Jin, C.-G.; Yang, D.-C. Anti-inflammatory activity of ginsenosides in LPS-stimulated RAW 264.7 cells. Sci. Bull. 2015, 60, 773–784. [Google Scholar] [CrossRef]
- Wee, J.J.; Mee Park, K.; Chung, A.S. Biological activities of ginseng and its application to human health. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Sathishkumar, N.; Sathiyamoorthy, S.; Ramya, M.; Yang, D.U.; Lee, H.N.; Yang, D.C. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J. Enzyme Inhib. Med. Chem. 2012, 27, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Hu, Y.; Wu, W.Y.; Ye, M.; Guo, D.A. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry 2014, 106, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, C.; Murakami, K.; Hasegawa, H.; Murata, J.; Saiki, I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem. Biophys. Res. Commun. 1998, 246, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Tawab, M.A.; Bahr, U.; Karas, M.; Wurglics, M.; Schubert-Zsilavecz, M. Degradation of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, E.; Kim, E.; Yeom, M.H.; Kwon, O.; Yoon, T.H.; Lee, T.R.; Kim, K. Role of epidermal γδ T-cell-derived interleukin 13 in the skin-whitening effect of Ginsenoside F1. Exp. Dermatol. 2014, 23, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.S.; Rho, H.S.; Lee, Y.G.; Yeom, M.H.; Kim, D.H.; Lee, S.-J.; Hong, S.; Lee, J.; Cho, J.Y. Ginsenoside F1 modulates cellular responses of skin melanoma cells. J. Ginseng Res. 2011, 35, 86–91. [Google Scholar] [CrossRef]
- Lee, E.H.; Cho, S.Y.; Kim, S.J.; Shin, E.S.; Chang, H.K.; Kim, D.H.; Yeom, M.H.; Woe, K.S.; Lee, J.; Sim, Y.C.; et al. Ginsenoside F1 protects human HaCaT keratinocytes from ultraviolet-B-induced apoptosis by maintaining constant levels of Bcl-2. J. Investig. Dermatol. 2003, 121, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Nishimura, K.; Yasuyama, N.; Ebizuka, Y. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine max. FEBS Lett. 2010, 584, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, R.; Kim, Y.-H.; Kim, Y.; Kim, M.-K.; Kim, M.-J.; Yang, D. Enzymatic Formation of Novel Ginsenoside Rg1-α-Glucosides by Rat Intestinal Homogenates. Appl. Biochem. Biotechnol. 2015, 177, 1701–1715. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Kiso, T.; Okamoto, K.; Tomita, T.; Manan, M.B.; Kitahata, S. Synthesis of glycosyl glycerol by cyclodextrin glucanotransferases. J. Biosci. Bioeng. 2003, 95, 583–588. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Xiao, Q.Y.; Xia, Y. Transglycosylation of stevioside to improve the edulcorant quality by lower substitution using cornstarch hydrolyzate and CGTase. Food Chem. 2013, 138, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Kometani, T.; Terada, Y.; Nishimura, T.; Takii, H.; Okada, S. Transglycosylation to hesperidin by cyclodextrin glucanotransferase from an alkalophilic Bacillus species in alkaline pH and properties of hesperidin glycosides. Biosci. Biotechnol. Biochem. 1994, 58, 1990–1994. [Google Scholar] [CrossRef]
- Kamaruddin, K.; Illias, R.M.; Aziz, S.A.; Said, M.; Hassan, O. Effects of buffer properties on cyclodextrin glucanotransferase reactions and cyclodextrin production from raw sago (Cycas revoluta) starch. Biotechnol. Appl. Biochem. 2005, 41, 117–125. [Google Scholar] [PubMed]
- Suzuki, Y.; Suzuki, K. Enzymatic formation of 4G-α-D-glucopyranosyl-rutin. Agric. Biol. Chem. 1991, 55, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Feng, B.; Huang, H.Z.; Kang, L.P.; Cong, Y.; Zhou, W.B.; Zou, P.; Cong, Y.W.; Song, X.B.; Ma, B.P. Glucosylation of steroidal saponins by cyclodextrin glucanotransferase. Planta Med. 2010, 76, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Suzuki, Y. Enzymatic synthesis of a new derivative of thiamin, O-α-glucosylthiamin. Biosci. Biotechnol. Biochem. 1998, 62, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Jung, J.H.; Ha, S.J.; Cho, H.K.; Jung, D.H.; Kim, T.J.; Baek, N.I.; Yoo, S.H.; Park, C.S. High-yield enzymatic bioconversion of hydroquinone to α-arbutin, a powerful skin lightening agent, by amylosucrase. Appl. Microbiol. Biotechnol. 2012, 94, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Syntheses of arbutin-α-glycosides and a comparison of their inhibitory effects with those of α-arbutin and arbutin on human tyrosinase. Chem. Pharm. Bull. 2003, 51, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.H.; Min, J.W.; Sathiyamoorthy, S.; Yang, D.U.; Kim, Y.J.; Yang, D.C. Biotransformation of ginsenosides Re and Rg1 into ginsenosides Rg2 and Rh1 by recombinant β-glucosidase. Biotechnol. Lett. 2012, 34, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Sim, G.-S.; Lee, B.-C.; Cho, H.; Lee, J.; Kim, J.-H.; Lee, D.-H.; Kim, J.-H.; Pyo, H.-B.; Moon, D.; Oh, K.-W.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, Y.H.; Song, G.S.; Suzuki, Y.; Kim, M.K. Enzymatic transglycosylation of ginsenoside Rg1 by rice seed α-glucosidase. Biosci. Biotechnol. Biochem. 2016, 80, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, Y.G.; Choi, K.J.; Uchida, K.; Suzuki, Y. Transglycosylation to ginseng saponins by cyclomaltodextrin glucanotransferases. Biosci. Biotechnol. Biochem. 2001, 65, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.B.; Feng, B.; Huang, H.Z.; Qin, Y.J.; Wang, Y.Z.; Kang, L.P.; Zhao, Y.; Wang, X.N.; Cai, Y.; Tan, D.W.; et al. Enzymatic synthesis of α-glucosyl-timosaponin BII catalyzed by the extremely thermophilic enzyme: Toruzyme 3.0L. Carbohydr. Res. 2010, 345, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Nakagawa, H.; Kurosu, J.; Yoshida, K.; Tsugane, T.; Shimura, S.; Kirimura, K.; Kino, K.; Usami, S. α-anomer-selective glucosylation of (+)-catechin by the crude enzyme, showing glucosyl transfer activity, of Xanthomonas campestris WU-9701. J. Biosci. Bioeng. 2000, 90, 625–630. [Google Scholar] [CrossRef]
- Kligman, A.M. Early destructive effect of sunlight on human skin. JAMA 1969, 210, 2377–2380. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, J.W.; Ai, C.Z.; Xiang, N.; Liu, H.X.; Yang, L. Anti-androgen-independent prostate cancer effects of ginsenoside metabolites in vitro: Mechanism and possible structure-activity relationship investigation. Arch. Pharm. Res. 2009, 32, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.L.; Dang, L.Z.; Zhang, K.Q.; Liang, L.M.; Li, G.H. Cloning and heterologous expression of UDP-glycosyltransferase genes from Bacillus subtilis and its application in the glycosylation of ginsenoside Rh1. Lett. Appl. Microbiol. 2015, 60, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Jin, Y.; Wang, C.; Kim, Y.J.; Perez, Z.E.J.; Baek, N.I.; Mathiyalagan, R.; Markus, J.; Yang, D.C. Rare ginsenoside Ia synthesized from F1 by cloning and overexpression of the UDP-glycosyltransferase gene from Bacillus subtilis: Synthesis, characterization, and in vitro melanogenesis inhibition activity in BL6B16 cells. J. Ginseng Res. 2018, 42, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, R.; Yang, D.C. Ginseng nanoparticles: A budding tool for cancer treatment. Nanomedicine 2017, 12, 1091–1094. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.S.; Lee, H.J.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.U.; Lee, D.Y.; Min, J.W.; Jimenez, Z.; Yang, D.C. Synthesis of a Novel ?-Glucosyl Ginsenoside F1 by Cyclodextrin Glucanotransferase and Its In Vitro Cosmetic Applications. Biomolecules 2018, 8, 142. https://doi.org/10.3390/biom8040142
Moon SS, Lee HJ, Mathiyalagan R, Kim YJ, Yang DU, Lee DY, Min JW, Jimenez Z, Yang DC. Synthesis of a Novel ?-Glucosyl Ginsenoside F1 by Cyclodextrin Glucanotransferase and Its In Vitro Cosmetic Applications. Biomolecules. 2018; 8(4):142. https://doi.org/10.3390/biom8040142
Chicago/Turabian StyleMoon, Seong Soo, Hye Jin Lee, Ramya Mathiyalagan, Yu Jin Kim, Dong Uk Yang, Dae Young Lee, Jin Woo Min, Zuly Jimenez, and Deok Chun Yang. 2018. "Synthesis of a Novel ?-Glucosyl Ginsenoside F1 by Cyclodextrin Glucanotransferase and Its In Vitro Cosmetic Applications" Biomolecules 8, no. 4: 142. https://doi.org/10.3390/biom8040142
APA StyleMoon, S. S., Lee, H. J., Mathiyalagan, R., Kim, Y. J., Yang, D. U., Lee, D. Y., Min, J. W., Jimenez, Z., & Yang, D. C. (2018). Synthesis of a Novel ?-Glucosyl Ginsenoside F1 by Cyclodextrin Glucanotransferase and Its In Vitro Cosmetic Applications. Biomolecules, 8(4), 142. https://doi.org/10.3390/biom8040142






