Poly-(γ-glutamic acid) Production and Optimization from Agro-Industrial Bioresources as Renewable Substrates by Bacillus sp. FBL-2 through Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture and Inoculum Preparation
2.2. Poly-(γ-glutamic acid) Production
2.3. Optimization Studies Using RSM
2.4. Analytical Methods
2.5. Thin Layer Chromatograph (TLC)
3. Results and Discussion
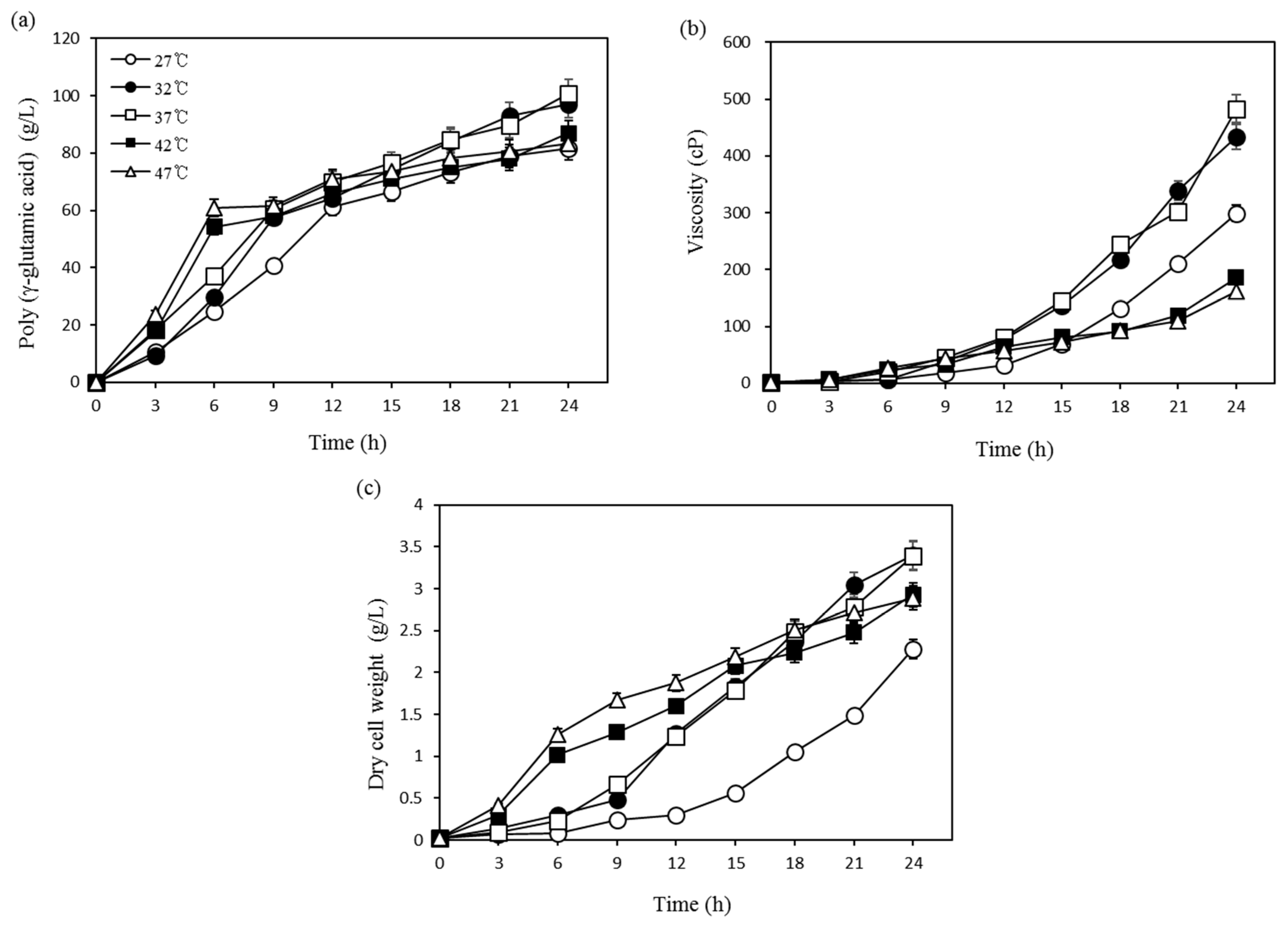
3.1. Optimization Using One-Factor-at-a-Time
3.2. Fed-Batch Fermentation
3.3. γ-PGA Production Using Agricultural Byproducts
3.4. Verification of the Optimal Model
3.5. Analysis of Hydrolysis Products of γ-PGA
Author Contributions
Funding
Conflicts of Interest
References
- Kubota, H.; Nambu, Y.; Endo, T. Convenient and quantitative esterification of poly(γ-glutamic acid) produced by microorganism. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2877–2878. [Google Scholar] [CrossRef]
- Shih, I.L.; Van, Y.T. The production of poly-(gamma-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–222. [Google Scholar] [CrossRef]
- Richard, A.; Margaritis, A. Rheology, oxygen transfer, and molecular weight characteristics of poly(glutamic acid) fermentation by Bacillus subtilis. Biotechnol. Bioeng. 2003, 82, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.H.; Huang, K.Y.; Kunene, S.C.; Lee, T.Y. Poly-γ-glutamic acid synthesis, gene regulation, phylogenetic relationships, and role in fermentation. Int. J. Mol. Sci. 2017, 18, 2644. [Google Scholar] [CrossRef]
- Kunioka, M. Biosynthesis of poly(γ-glutamic acid) from L-glutamine, citric acid, and ammonium sulfate in Bacillus subtilis IFO3335. Appl. Microbiol. Biotechnol. 1995, 44, 501–506. [Google Scholar] [CrossRef]
- Cao, M.; Geng, W.; Liu, L.; Song, C.; Xie, H.; Guo, W.; Jin, Y.; Wang, S. Glutamic acid independent production of poly-γ-glutamic acid by Bacillus amyloliquefaciens LL3 and cloning of pgsBCA genes. Bioresour. Technol. 2011, 102, 4251–4257. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-c-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Lei, P.; Xu, Z.; Jiang, Y.; Xu, Z.; Liang, J.; Feng, X.; Xu, H. Highly efficient rice straw utilization for poly-(γ-glutamic acid) production by Bacillus subtilis NX-2. Bioresour. Technol. 2015, 193, 370–376. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, X.; Zhou, Z.; Zhang, Y.; Xu, H. Economical production of poly(cglutamic acid) using untreated cane molasses and monosodium glutamate waste liquor by Bacillus subtilis NX-2. Bioresour. Technol. 2012, 114, 583–588. [Google Scholar] [CrossRef]
- Xiong, C.; Shouwen, C.; Ming, S.; Ziniu, Y. Medium optimization by response surface methodology for poly-γ-glutamic acid production using dairy manure as the basis of a solid substrate. Appl. Microbiol. Biotechnol. 2005, 69, 390–396. [Google Scholar] [CrossRef]
- Anju, A.J.; Bnod, P.; Pandey, A. Production and characterization of PGA from renewable resources. J. Exper. Biol. 2017, 55, 405–410. [Google Scholar]
- Juliano, B.O.; Hicks, P.A. Rice functional properties and rice food products. Food Rev. Int. 1996, 12, 71–103. [Google Scholar] [CrossRef]
- Cicero, A.F.; Gaddi, A. rice bran oil and gamma-oryzanol in the treatment of hyperlipoproteinaemias and other conditions. Phytother. Res. 2001, 15, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Wheat in Milling and Baking. In Cereal Science and Technology; Aberdeen University Press: Aberdeen, UK, 1989; pp. 367–407. [Google Scholar]
- Kaewjumpola, G.; Oruna-Conchab, M.J.; Niranjanb, K.; Thawornchinsombuta, S. The production of hydrolysates from industrially defatted rice bran and its surface image changes during extraction. J. Sci. Food Agric. 2017, 98, 3290–3298. [Google Scholar] [CrossRef]
- Kunioka, M.; Goto, A. Biosynthesis of poly(γ-glutamic acid) from L-glutamic acid, citric acid, and ammonium sulfate in Bacillus subtilis IFO3335. Appl. Microbiol. Biotechnol. 1994, 40, 867–872. [Google Scholar] [CrossRef]
- Shin, I.L.; Van, V.T.; Yeh, L.C.; Lin, H.G.; Chan, Y.N. Production of a bio-polymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour. Technol. 2001, 79, 267–272. [Google Scholar]
- Cheng, C.; Asada, Y.; Aaida, T. Production of γ-polyglutamic acid by Bacillus subtilis A35 under denitrifying conditions. Agric. Biol. Chem. 1989, 53, 2369–2375. [Google Scholar]
- Kubota, H.; Matsunobu, T.; Uotani, K.; Takebe, H.; Satoh, A.; Tanaka, T.; Taniguchi, M. Production of poly(γ-glutamic acid) by Bacillus subtilis F-2-01. Biosci. Biotech. Biochem. 1993, 57, 1212–1213. [Google Scholar] [CrossRef]
- Cromwick, A.M.; Birrer, G.A.; Gross, R.A. Effects of pH and aeration on gamma-poly(glutamic acid) formation by Bacillus licheniformis in controlled batch fermentor cultures. Biotechnol. Bioeng. 1996, 50, 222–227. [Google Scholar] [CrossRef]
- Zanuy, D.; Aleman, C.; MunozGuerra, S. On the helical conformation of unionized poly(γ-D-glutamic acid). Int. J. Biol. Macromol. 1998, 23, 175–184. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Singhal, R.S. Effect of Aeration and Agitation on Synthesis of Poly (γ-glutamic acid) in Batch Cultures of Bacillus licheniformis NCIM 2324. Biotechnol. Bioproc. Eng. 2010, 15, 635–640. [Google Scholar] [CrossRef]
- Yoon, S.H.; Do, J.H.; Lee, S.Y.; Chang, H.N. Production of poly-γ-glutamic acid by fed-batch culture of Bacillus licheniformis. Biotechnol. Lett. 2000, 22, 585–588. [Google Scholar] [CrossRef]
- Jeong, G.T.; Kim, J.N.; Ryu, H.W.; Wee, Y.J. Improved production of poly(γ-glutamic acid) by Bacillus subtilis RKY3 and its recovery from viscous fermentation broth as a biodegradable polymer. J. Chem. Technol. Biotechnol. 2014, 89, 728–734. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Lele, S.S.; Singhal, R.S. A statistical approach to optimization of fermentative production of poly(γ-glutamic acid) from Bacillus licheniformis NCIM 2324. Bioresour. Technol. 2009, 100, 826–832. [Google Scholar] [CrossRef]






| Independent Variables | Coded Symbols | Levels | ||||
|---|---|---|---|---|---|---|
| −1.414 | −1 | 0 | 1 | 1.414 | ||
| Bran (g/L) | X1 | 1.7 | 10 | 30 | 50 | 58.3 |
| H2SO4 (%, v/v) | X2 | 0.017 | 0.1 | 0.3 | 0.5 | 0.583 |
| Run No. | Coded Variable Level | |
|---|---|---|
| X1 | X2 | |
| 1 | −1 | −1 |
| 2 | 1 | −1 |
| 3 | −1 | 1 |
| 4 | 1 | 1 |
| 5 | −1.414 | 0 |
| 6 | 1.414 | 0 |
| 7 | 0 | −1.414 |
| 8 | 0 | 1.414 |
| 9 | 0 | 0 |
| 10 | 0 | 0 |
| 11 | 0 | 0 |
| 12 | 0 | 0 |
| 13 | 0 | 0 |
| 14 | 0 | 0 |
| Optimization Step | Dry Cell Weight (g/L) | Viscosity (cP) | Poly-(γ-glutamic acid) Production (g/L) | Poly-(γ-glutamic acid) Production (Fold) |
|---|---|---|---|---|
| Before optimization * | 0.2 | 6.7 | 27.1 | 1.0 |
| After fermentor optimization ** | 3.4 | 483.2 | 100.7 | 3.7 |
| After fed-batch fermentation *** | 3.1 | 160.9 | 175.9 | 6.5 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| (a) | Model | 375.7881 | 5 | 75.15762 | 37.56641 | < 0.0001 |
| Residual | 16.00528 | 8 | 2.00066 | |||
| Lack of Fit | 13.1064 | 3 | 4.3688 | 7.53532 | 0.0265 | |
| Pure Error | 2.898882 | 5 | 0.579776 | |||
| Corrected Total | 391.7934 | 13 | ||||
| R2 = 0.9591; adjusted R2 = 0.9336; adequately precision = 17.625; CV = 8.11%. | ||||||
| (b) | Model | 346.0666 | 5 | 69.213397 | 29.51582 | <0.0001 |
| Residual | 18.75965 | 8 | 2.344356691 | |||
| Lack of Fit | 17.97731 | 3 | 5.992436649 | 38.29799 | 0.0007 | |
| Pure Error | 0.782344 | 5 | 0.156468716 | |||
| Corrected Total | 364.8563 | 13 | ||||
| R2 = 0.9485; adjusted R2 = 0.9164; adequately precision = 15.053; CV = 15.33%. | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.-Y.; Reddy, L.V.; Charalampopoulos, D.; Wee, Y.-J. Poly-(γ-glutamic acid) Production and Optimization from Agro-Industrial Bioresources as Renewable Substrates by Bacillus sp. FBL-2 through Response Surface Methodology. Biomolecules 2019, 9, 754. https://doi.org/10.3390/biom9120754
Song D-Y, Reddy LV, Charalampopoulos D, Wee Y-J. Poly-(γ-glutamic acid) Production and Optimization from Agro-Industrial Bioresources as Renewable Substrates by Bacillus sp. FBL-2 through Response Surface Methodology. Biomolecules. 2019; 9(12):754. https://doi.org/10.3390/biom9120754
Chicago/Turabian StyleSong, Da-Young, Lebaka Veeranjaneya Reddy, Dimitris Charalampopoulos, and Young-Jung Wee. 2019. "Poly-(γ-glutamic acid) Production and Optimization from Agro-Industrial Bioresources as Renewable Substrates by Bacillus sp. FBL-2 through Response Surface Methodology" Biomolecules 9, no. 12: 754. https://doi.org/10.3390/biom9120754
APA StyleSong, D.-Y., Reddy, L. V., Charalampopoulos, D., & Wee, Y.-J. (2019). Poly-(γ-glutamic acid) Production and Optimization from Agro-Industrial Bioresources as Renewable Substrates by Bacillus sp. FBL-2 through Response Surface Methodology. Biomolecules, 9(12), 754. https://doi.org/10.3390/biom9120754






