Effects of Post Anthesis Foliar Application of Sodium Selenite to Soybeans (Glycine max): Lipid Composition and Oil Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Agronomic Experimental Design and Selenium Application
2.2. Height of Soybean Plants and Soybean Yield
2.3. Physical Characterization of Soybeans
2.4. Chemical Analyses of Soybeans
2.5. Oil Extraction
2.6. Physicochemical Characterization of Soybean Oil
2.7. Oxidation Induction Time (OIT)
2.8. Fatty Acids Composition
2.9. Tocopherols and Phytosterols in Soybean Oils
2.10. Statistical Analysis
3. Results
3.1. Agronomic Features of Soybean and Characterization during Seed Development
3.1.1. Height of Plants at Various Weeks after Sowing
3.1.2. Density, Thousand Seed Weight, and Yield per Hectare
3.1.3. Chemical Characterization of Selenized Soybeans
3.2. Soybean Oil General Characterization
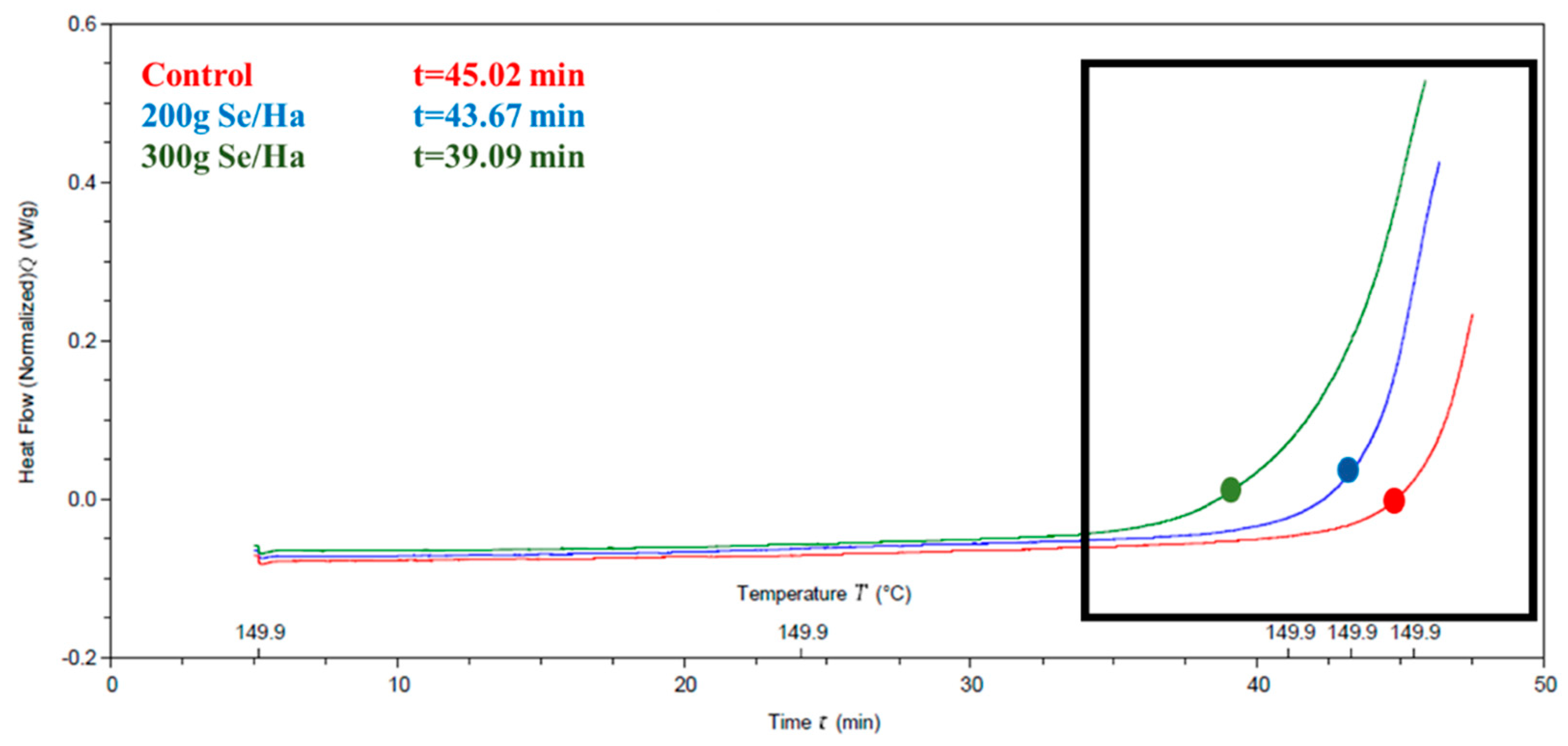
3.3. Oxidation Induction Time (OIT)
3.4. Fatty Acid Profiles
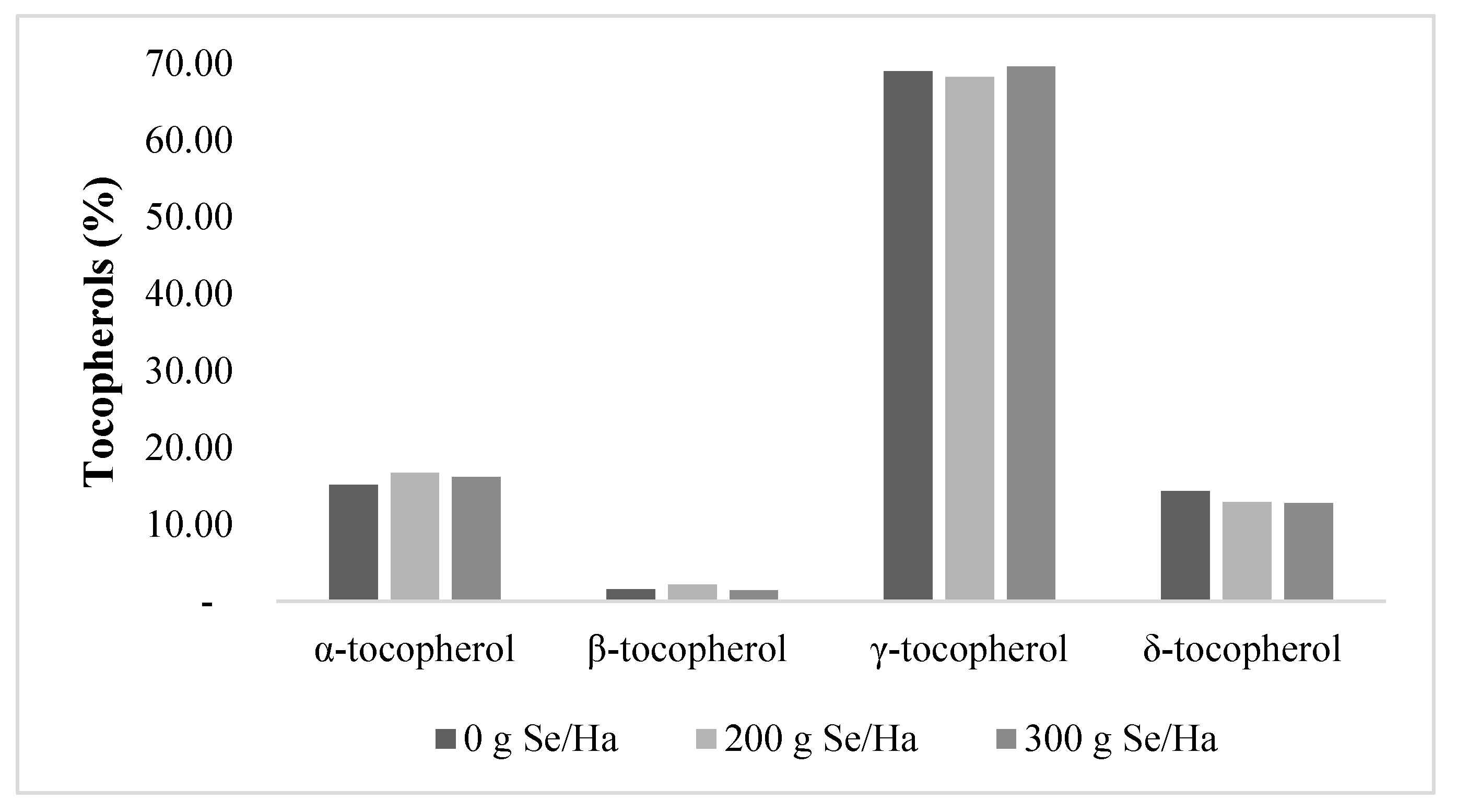
3.5. Tocopherol and Phytosterols Content in Soybean Oil
4. Discussion
4.1. Agronomic Attributes of Soybean and Characterization of the Seed
4.1.1. Height of Selenized Plants at Various Weeks after Sowing
4.1.2. Density, Thousand Weight, and Yield per Hectare
4.1.3. Chemical Characterization of Selenized Soybean Seeds
4.2. Soybean Oil General Characterization
4.3. Oxidation Induction Time (OIT)
4.4. Fatty Acid Profiles
4.5. Tocopherols and Phytosterols in Soybean Oil
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/ (accessed on 12 August 2019).
- Yang, R.; Liu, Y.; Zhou, Z. Selenium and selenoproteins, from structure, function to food resource and nutrition. Food Sci. Technol. Res. 2017, 23, 363–373. [Google Scholar] [CrossRef]
- Hart, D.J.; Fairweather-Tait, S.J.; Broadley, M.R.; Dickinson, S.J.; Foot, I.; Knott, P.; McGrath, S.P.; Mowat, H.; Norman, K.; Scott, P.R. Selenium concentration and speciation in biofortified flour and bread: Retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem. 2011, 126, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Chumpitaz, C.C. El selenio, un elemento poco conocido con un rol biológico importante. Rev. Química 2013, 25, 29–33. [Google Scholar]
- Beckett, G.J.; Arthur, J.R. Selenium and endocrine systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef]
- Şlencu, B.G.; Ciobanu, C.; Cuciureanu, R. Selenium content in foodstuffs and its nutritional requirement for humans. Clujul Med. 2014, 85, 139–145. [Google Scholar]
- Combs, G.F.; Watts, J.C.; Jackson, M.I.; Johnson, L.K.; Zeng, H.; Scheett, A.J.; Uthus, E.O.; Schomburg, L.; Hoeg, A.; Hoefig, C.S.; et al. Determinants of selenium status in healthy adults. Nutr. J. 2011, 10, 75. [Google Scholar] [CrossRef]
- Park, K.; Rimm, E.; Siscovick, D.; Spiegelman, D.; Morris, J.S.; Mozaffarian, D. Demographic and lifestyle factors and selenium levels in men and women in the U.S. Nutr. Res. Pract. 2011, 5, 357–364. [Google Scholar] [CrossRef]
- Broadley, M.R.; Alcock, J.; Alford, J.; Cartwright, P.; Foot, I.; Fairweather-Tait, S.J.; Hart, D.J.; Hurst, R.; Knott, P.; McGrath, S.P.; et al. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 2010, 332, 5–18. [Google Scholar] [CrossRef]
- Gupta, U.C.; Winter, K.A.; Sanderson, J.B. Selenium content of barley as influenced by selenite and selenate-enriched fertilizers. Commun. Soil Sci. Plant Anal. 1993, 24, 1165–1170. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, J.; Pang, G. Effect of selenium on the yield and quality of green tea leaves harvested in early spring. J. Agric. Food Chem. 2003, 51, 3379–3381. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; De Feudis, M.; Hasuoka, P.E.; Regni, L.; Pacheco, P.H.; Onofri, A.; Businelli, D.; Proietti, P. The selenium supplementation influences olive tree production and oil stability against oxidation and can alleviate the water deficiency effects. Front. Plant Sci. 2018, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Guardado-Félix, D.; Antunes-Ricardo, M.; Rocha-Pizaña, M.R.; Martínez-Torres, A.-C.; Gutiérrez-Uribe, J.A.; Serna Saldivar, S.O. Chickpea (Cicer arietinum L.) sprouts containing supranutritional levels of selenium decrease tumor growth of colon cancer cells xenografted in immune-suppressed mice. J. Funct. Foods 2019, 53, 76–84. [Google Scholar] [CrossRef]
- Yang, F.; Chen, L.; Hu, Q.; Pan, G. Effect of the application of selenium on selenium content of soybean and its products. Biol. Trace Elem. Res. 2003, 93, 249–256. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 10th ed.; The Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Serna-Saldivar, S.O. Cereal Grains: Laboratory Reference and Procedures Manual, 1st ed.; CRC Press: Boca Raton FL, USA, 2012. [Google Scholar]
- Tavakoli, H.; Rajabipour, A.; Mohtasebi, S.S. Moisture-dependent some engineering properties of soybean grains. Agric. Eng. Int. 2009, 11, 3–14. [Google Scholar]
- AOAC. AOAC Official Methods of Analysis; Association of Official Analytical Chemists. AOAC: Washington, DC, USA, 1992. [Google Scholar]
- Guardado-Félix, D.; Serna-Saldivar, S.O.; Cuevas-Rodríguez, E.O.; Jacobo-Velázquez, D.A.; Gutiérrez-Uribe, J.A. Effect of sodium selenite on isoflavonoid contents and antioxidant capacity of chickpea (Cicer arietinum L.) sprouts. Food Chem. 2017, 226, 69–74. [Google Scholar] [CrossRef]
- O’Brien, R.D. Fats and Oils: Formulating and Processing for Applications, 3rd ed.; CRC press: New York, NY, USA, 2009. [Google Scholar]
- Nielsen, S.S. Food Analysis; Springer: Boston, MA, USA, 2010. [Google Scholar]
- Dubaj, T.; Šimon, P. Validation of the estimation of oxidation induction time from non-isothermal DSC measurements. J. Therm. Anal. Calorim. 2014, 118, 919–923. [Google Scholar] [CrossRef]
- AOCS. Official and Tentative Methods of the American Oil Chemist’s Society; AOCS Press: Urbana, IL, USA, 2006. [Google Scholar]
- Kibar, H.; Öztürk, T. Physical and mechanical properties of soybean. Int. Agrophys. 2008, 22, 239–244. [Google Scholar]
- Isik, E. Some engineering properties of soybean grains. Am. J. Food Technol. 2007, 2, 115–125. [Google Scholar]
- Wandkar, S.V.; Ukey, P.D.; Pawar, D.A. Determination of physical properties of soybean at different moisture levels. Agric. Eng. Int. CIGR J. 2012, 14, 138–142. [Google Scholar]
- Davies, R.M.; El-Okene, A.M. Moisture-dependent physical properties of soybeans. Int. Agrophys. 2009, 23, 299–303. [Google Scholar]
- de Luna Jiménez, A. Composición y procesamiento de la soya para consumo humano. Investig. Cienc. 2007, 1, 35–44. [Google Scholar]
- Erickson, D.R. Practical Handbook of Soybean Processing and Utilization; Erickson, D.R., Ed.; Elsevier: St. Louis, MO, USA, 2015. [Google Scholar]
- Sarkar, A.; Golay, P.; Acquistapace, S.; Craft, B.D. Increasing the oxidative stability of soybean oil through fortification with antioxidants. Int. J. Food Sci. Technol. 2015, 50, 666–673. [Google Scholar] [CrossRef]
- D’Amato, R.; Proietti, P.; Nasini, L.; Del Buono, D.; Tedeschini, E.; Businelli, D. Increase in the selenium content of extra virgin olive oil: Quantitative and qualitative implications. Grasas Aceites 2014, 65. [Google Scholar] [CrossRef]
- Guardado-Félix, D.; Serna-Saldivar, O.S.; Gutiérrez-Uribe, A.J.; Chuck-Hernández, C. Selenium in Germinated Chickpea (Cicer arietinum L.) Increases the Stability of Its Oil Fraction. Plants 2019, 8, 113. [Google Scholar] [CrossRef]
- Nesaretnam, K.; Yew, W.W.; Wahid, M.B. Tocotrienols and cancer: Beyond antioxidant activity. Eur. J. Lipid Sci. Technol. 2007, 109, 445–452. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Demonty, I. Phytosterols: Natural compounds with established and emerging health benefits. OCL 2007, 14, 259–266. [Google Scholar] [CrossRef]
- Racette, S.B.; Lin, X.; Lefevre, M.; Spearie, C.A.; Most, M.M.; Ma, L.; Ostlund, R.E., Jr. Dose effects of dietary phytosterols on cholesterol metabolism: A controlled feeding study. Am. J. Clin. Nutr. 2010, 91, 32–38. [Google Scholar] [CrossRef]
- Kulsum, M.U.; Baque, M.A.; Karim, M.A. Effects of different nitrogen levels on the morphology and yield of blackgram. J. Agron. 2007, 6, 125. [Google Scholar] [CrossRef][Green Version]
- Soares, M.M.; Oliveira, G.L.; Soriano, P.E.; Sekita, M.C.; Sediyama, T. Performance of soybean plants as function of seed size: II. Nutritional stress. J. Seed Sci. 2013, 35, 419–427. [Google Scholar] [CrossRef]
- Yin, Z.; Qi, H.; Chen, Q.; Zhang, Z.; Jiang, H.; Zhu, R.; Hu, Z.; Wu, X.; Li, C.; Zhang, Y. Soybean plant height QTL mapping and meta-analysis for mining candidate genes. Plant Breed. 2017, 136, 688–698. [Google Scholar] [CrossRef]
- Chakraverty, A.; Mujumdar, A.S.; Ramaswamy, H.S. Handbook of Postharvest Technology: Cereals, Fruits, Vegetables, Tea, and Spices; CRC press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Mauricio, R.A.S.; Figueroa, J.D.C.; Taba, S.; Reyes, M.L.V.; Rincón, F.S.; Mendoza, A.G. Characterization of maize accessions by grain and tortilla quality traits. Rev. Fitotec. Mex 2004, 27, 213–222. [Google Scholar]
- Padua, G.P.; Zito, R.K.; Arantes, N.E.; França-Neto, J.B. Influência do tamanho da semente na qualidade fisiológica e na produtividade da cultura da soja. Embrapa Soja-Artigo Periódico Indexado 2010, 19, 188. [Google Scholar] [CrossRef]
- dos Santos, P.M.; Reis, M.S.; Sediyama, T.; Araújo, E.F.; Cecon, P.R.; dos Santos, M.R. Efeito da classificação por tamanho da semente de soja na sua qualidade fisiológica durante o armazenamento. Acta Sci. Agron. 2005, 27, 395–402. [Google Scholar] [CrossRef]
- Maldonado, M.N.; Ascencio, L.G.; Espinosa, V.G.; de los Peña, R.M.Á. Estrategias Tecnológicas Para Contrarrestar La Sequía En La Producción de Soya En El Sur De Tamaulipas; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP): Ciudad de México, México, 2013; Available online: http://biblioteca.inifap.gob.mx:8080/jspui/handle/123456789/3955 (accessed on 22 November 2019).
- USDA Basic Report: 16108, Soybeans, Mature Seeds, Raw. Available online: https://fdc.nal.usda.gov/ (accessed on 6 October 2019).
- Lazo-Vélez, M.A.; Guardado-Félix, D.; Avilés-González, J.; Romo-López, I.; Serna-Saldívar, S.O. Effect of germination with sodium selenite on the isoflavones and cellular antioxidant activity of soybean (Glycine max). LWT 2018, 93, 64–70. [Google Scholar] [CrossRef]
- Formo, M.W.; Jungermann, E.; Norris, F.A.; Sonntag, N.O.V. Bailey’s Industrial Oil and Fat Products, 4th ed.; Swern, D., Ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1982. [Google Scholar]
- de la Luz Mora, M.; Pinilla, L.; Rosas, A.; Cartes, P. Selenium uptake and its influence on the antioxidative system of white clover as affected by lime and phosphorus fertilization. Plant Soil 2008, 303, 139–149. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Nowak, J.; Kaklewski, K.; Ligocki, M. Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol. Biochem. 2004, 36, 1553–1558. [Google Scholar] [CrossRef]
- Tan, C.P.; Man, Y.B.C.; Selamat, J.; Yusoff, M.S.A. Comparative studies of oxidative stability of edible oils by differential scanning calorimetry and oxidative stability index methods. Food Chem. 2002, 76, 385–389. [Google Scholar] [CrossRef]
- Aguilera Fuentes, P.H.; Pinheiro do Prado, A.C.; Ogliari, P.; Deschamps, F.C.; Barrera Arellano, D.; Bolini, H.M.A.; Block, J.M. Evaluation of physico-chemical and sensory quality during storage of soybean and canola oils packaged in PET bottles. J. Am. Oil Chem. Soc. 2013, 90, 619–629. [Google Scholar] [CrossRef]
- Bailey, A.E. Flavor reversion in edible fats. J. Am. Oil Chem. Soc. 1946, 23, 55–58. [Google Scholar] [CrossRef]
- Dutton, H.J.; Lancaster, C.R.; Evans, C.D.; Cowan, J.C. The flavor problem of soybean oil. VIII. Linolenic acid. J. Am. Oil Chem. Soc. 1951, 28, 115–118. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O.; Perez-Carrillo, E.; Heredia-Olea, E. Soybean-Fortified Wheat Flour Tortillas. In Flour and Breads and Their Fortification in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2019; pp. 291–306. [Google Scholar]
- Kumar, V.; Rani, A.; Jha, P.; Hussain, L.; Pal, V.; Petwal, V.; Kumar, P.; Dwivedi, J. Lipoxygenase and tocopherol profiling of soybean genotypes exposed to electron beam irradiation. J. Am. Oil Chem. Soc. 2017, 94, 457–463. [Google Scholar] [CrossRef]
- Stein, H.H.; Berger, L.L.; Drackley, J.K.; Fahey, G.C.; Hernot, D.C.; Parsons, C.M. Soybeans, Chemistry, Production, Processing, and Utilization; AOCS Press: Urbana, IL, USA, 2008; pp. 39–71. [Google Scholar]
- Carrera, C.S.; Seguin, P. Factors affecting tocopherol concentrations in soybean seeds. J. Agric. Food Chem. 2016, 64, 9465–9474. [Google Scholar] [CrossRef] [PubMed]
- Abramovič, H.; Butinar, B.; Nikolič, V. Changes occurring in phenolic content, tocopherol composition and oxidative stability of Camelina sativa oil during storage. Food Chem. 2007, 104, 903–909. [Google Scholar] [CrossRef]
- Anwar, F.; Kamal, G.M.; Nadeem, F.; Shabir, G. Variations of quality characteristics among oils of different soybean varieties. J. King Saud Univ. 2016, 28, 332–338. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.O.; Min, D.B. Effects and prooxidant mechanisms of oxidized α-tocopherol on the oxidative stability of soybean oil. J. Food Sci. 2007, 72, 223–230. [Google Scholar] [CrossRef]
- Maras, J.E.; Bermudez, O.I.; Qiao, N.; Bakun, P.J.; Boody-Alter, E.L.; Tucker, K.L. Intake of α-tocopherol is limited among US adults. J. Am. Diet. Assoc. 2004, 104, 567–575. [Google Scholar] [CrossRef]
- Dixit, A.K.; Antony, J.I.X.; Sharma, N.K.; Tiwari, R.K. Soybean constituents and their functional benefits. In Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry; Tiwari, V.K., Mishra, B.B., Eds.; Research Signpost: Kerala, India, 2011; Volume 2, pp. 367–383. [Google Scholar]
- Kalogeropoulos, N.; Grigorakis, D.; Mylona, A.; Falirea, A.; Andrikopoulos, N.K. Dietary Evaluation of Vegetables Pan-Fried in Virgin Olive Oil Following the Greek Traditional Culinary Practice. Ecol. Food Nutr. 2006, 45, 105–123. [Google Scholar] [CrossRef]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef]
- Johnson, L.A.; White, P.J.; Galloway, R. Soybeans: Chemistry, Production, Processing, and Utilization; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Oehrl, L.L.; Hansen, A.P.; Rohrer, C.A.; Fenner, G.P.; Boyd, L.C. Oxidation of phytosterols in a test food system. J. Am. Oil Chem. Soc. 2001, 78, 1073–1078. [Google Scholar] [CrossRef]


| Treatment | Yield, | Bulk Density, | True Density, | Thousand Seed |
|---|---|---|---|---|
| kg/Ha | kg/m3 | kg/m3 | Weight, g | |
| Control | 1179.8 ± 80.1 a | 693.3 ± 5.8 a | 1242.1 ± 2.2 a | 122 ± 3.9 a |
| 200 g/Ha | 1268.1 ± 167.5 a | 700.0 ± 10.0 a | 1240.5 ± 0.8 a | 120 ± 8.3 a |
| 300 g/Ha | 1209.2 ± 206.5 a | 700.0 ± 10.0 a | 1240.0 ± 0.9 a | 115 ± 1.3 a |
| Treatment | Crude Fat 1 | Crude Fiber 1 | Ash 1 | Crude Protein 1 | Nitrogen Free Extract 1,2 | Selenium Content µg/g seed |
|---|---|---|---|---|---|---|
| Control | 20.8 ± 0.1 a | 9.3 ± 1.4 a | 7.1 ± 0.0 a | 37.6 ± 0.5 a | 25.2 ± 1.6 a | 1.01 ± 0.14 a |
| 200 g/Ha | 22.0 ± 0.1 a | 9.0 ± 0.5 a | 7.2 ± 0.1 a | 37.5 ± 0.2 a | 24.3±0.0 a | 18.94 ± 0.73 b |
| 300 g/Ha | 22.1 ± 1.0 a | 8.4 ± 1.1 a | 7.2 ± 0.1 a | 35.9 ± 0.3 b | 26.4±0.8 a | 23.35 ± 0.25 c |
| Treatment | Density, g/mL | Refractive Index, RI | Iodine Index, % | Saponification Value, mg KOH/g Sample |
|---|---|---|---|---|
| Control | 0.9029 ± 0.003 a | 1.4709 ± 0.003 a | 118.07 a | 192.42 a |
| 200g Se/Ha | 0.9097 ± 0.005 a | 1.4685 ± 0.003 a | 118.60 a | 193.18 a |
| 300g Se/Ha | 0.9045 ± 0.004 a | 1.4718 ± 0.001 a | 119.73 a | 193.39 a |
| Fatty Acid (% w/w) | Control | 200g Se/Ha | 300g Se/Ha |
|---|---|---|---|
| C12:0 Lauric | 0.27 | 0.14 | 0.12 |
| C14:0 Myristic | 0.30 | 0.17 | 0.18 |
| C16:0 Palmitic | 11.81 | 11.93 | 11.70 |
| C18:0 Stearic | 3.42 | 3.89 | 4.18 |
| C20:0 Arachidic | 0.51 | 0.53 | 0.52 |
| C22:0 Behenic | 0.65 | 0.64 | 0.67 |
| C24:0 Lignoceric | 0.31 | 0.33 | 0.32 |
| Saturated | 17.27 | 17.63 | 17.69 |
| C16:1 Palmitoleic | 0.12 | 0.15 | 0.11 |
| C18:1 Oleic | 33.85 | 32.26 | 31.31 |
| C20:1 Eicosenoic | 0.33 | 0.34 | 0.40 |
| Monounsaturated | 34.30 | 32.75 | 31.82 |
| C18:2 Linoleic | 42.70 | 44.17 | 44.43 |
| C18:3 Linolenic | 5.53 | 5.25 | 5.84 |
| Polyunsaturated | 48.23 | 49.42 | 50.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalante-Valdez, M.J.; Guardado-Félix, D.; Serna-Saldívar, S.O.; Barrera-Arellano, D.; Chuck-Hernández, C. Effects of Post Anthesis Foliar Application of Sodium Selenite to Soybeans (Glycine max): Lipid Composition and Oil Stability. Biomolecules 2019, 9, 772. https://doi.org/10.3390/biom9120772
Escalante-Valdez MJ, Guardado-Félix D, Serna-Saldívar SO, Barrera-Arellano D, Chuck-Hernández C. Effects of Post Anthesis Foliar Application of Sodium Selenite to Soybeans (Glycine max): Lipid Composition and Oil Stability. Biomolecules. 2019; 9(12):772. https://doi.org/10.3390/biom9120772
Chicago/Turabian StyleEscalante-Valdez, María José, Daniela Guardado-Félix, Sergio O. Serna-Saldívar, Daniel Barrera-Arellano, and Cristina Chuck-Hernández. 2019. "Effects of Post Anthesis Foliar Application of Sodium Selenite to Soybeans (Glycine max): Lipid Composition and Oil Stability" Biomolecules 9, no. 12: 772. https://doi.org/10.3390/biom9120772
APA StyleEscalante-Valdez, M. J., Guardado-Félix, D., Serna-Saldívar, S. O., Barrera-Arellano, D., & Chuck-Hernández, C. (2019). Effects of Post Anthesis Foliar Application of Sodium Selenite to Soybeans (Glycine max): Lipid Composition and Oil Stability. Biomolecules, 9(12), 772. https://doi.org/10.3390/biom9120772






