A Hepatic Scaffold from Decellularized Liver Tissue: Food for Thought
Abstract
:1. Introduction
2. Method
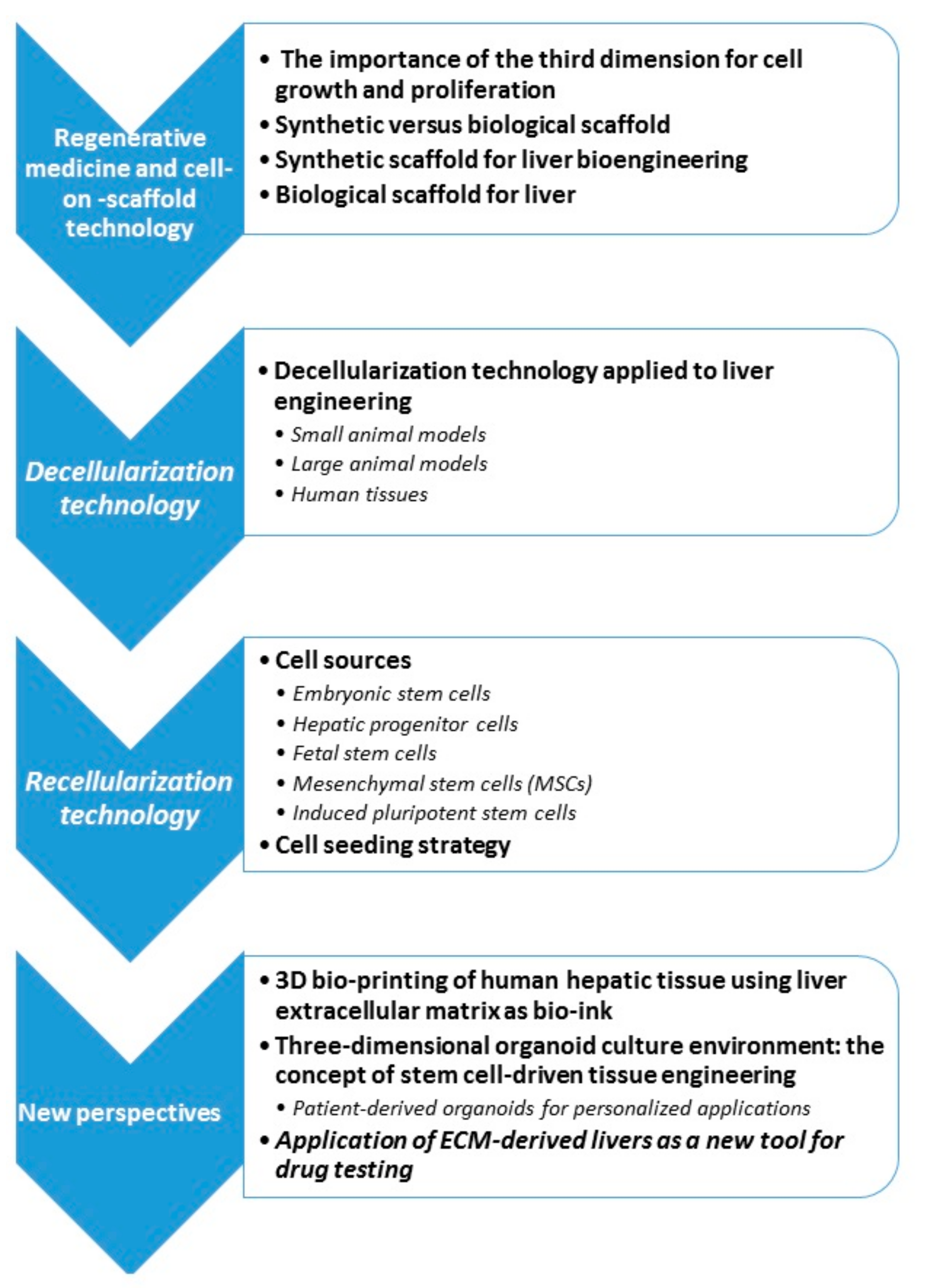
3. Regenerative Medicine and Cell-On-Scaffold Technology
3.1. The Importance of the Third Dimension for Cell Growth and Proliferation
3.2. Synthetic Versus Biological Scaffold
3.3. Synthetic Scaffold for Liver Bioengineering
3.4. Biological Scaffold for Liver
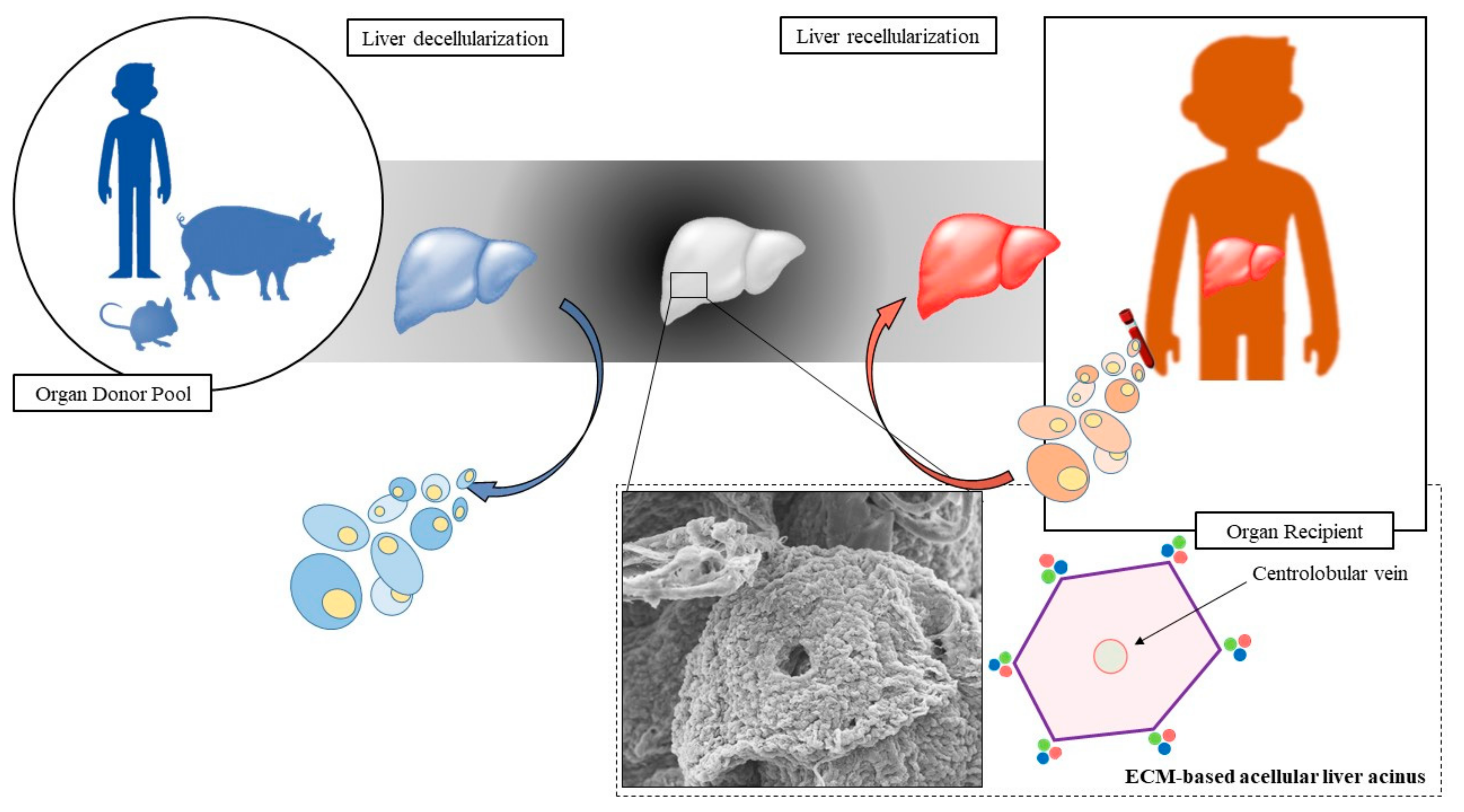
4. Decellularization Technology
4.1. Decellularization Technology Applied to Liver Engineering
4.1.1. Small Animal Models
4.1.2. Large Animal Model
4.1.3. Human Tissue
5. Recellularization Technology
5.1. Cell Sources
- Embryonic stem cells (ESCs)
- Hepatic progenitor cells (HPCs)
- Fetal stem cells
- Mesenchymal stem cells (MSCs)
- Induced pluripotent stem cells (iPSCs).
5.1.1. Embryonic Stem Cells
5.1.2. Hepatic Progenitor Cells
5.1.3. Fetal Stem Cells
5.1.4. Mesenchymal Stem Cells (MSCs)
5.1.5. Induced Pluripotent Stem Cells
5.2. Cell Seeding Strategy
6. New Perspectives
6.1. 3D Bio-Printing of Human Hepatic Tissue Using Liver Extracellular Matrix as Bio-Ink
6.2. Three-Dimensional Organoid Culture Environment: The Concept of Stem Cell-Driven Tissue Engineering
Patient-Derived Organoids for Personalized Applications
6.3. Application of ECM-Derived Livers as A New Tool for Drug Testing
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarin, S.K.; Choudhury, A. Acute-on-chronic liver failure: Terminology, mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute liver failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef]
- Boulter, L.; Lu, W.Y.; Forbes, S.J. Differentiation of progenitors in the liver: A matter of local choice. J. Clin. Invest. 2013, 123, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Briceno, J.; Padillo, J.; Rufián, S.; Solórzano, G.; Pera, C. Assignment of steatotic livers by the mayo model for end-stage liver disease. Transpl. Int. 2005, 18, 577–583. [Google Scholar] [CrossRef]
- Chen, A.A.; Thomas, D.K.; Ong, L.L.; Schwartz, R.E.; Golub, T.R.; Bhatia, S.N. Humanized mice with ectopic artificial liver tissues. Proc. Natl. Acad. Sci. USA 2011, 108, 11842–11847. [Google Scholar] [CrossRef]
- Dhawan, A.; Puppi, J.; Hughes, R.D.; Mitry, R.R. Human hepatocyte transplantation: Current experience and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 288–298. [Google Scholar] [CrossRef]
- Fox, I.J.; Roy-Chowdhury, J. Hepatocyte transplantation. J. Hepatol. 2004, 40, 878–886. [Google Scholar] [CrossRef]
- Lee, C.A.; Dhawan, A.; Smith, R.A.; Mitry, R.R.; Fitzpatrick, E. Instant Blood-Mediated Inflammatory Reaction in Hepatocyte Transplantation: Current Status and Future Perspectives. Cell Transplant. 2016, 25, 1227–1236. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Liang, L.; Li, J.; Demirci, U.; Wang, S. A decade of progress in liver regenerative medicine. Biomaterials 2018, 157, 161–176. [Google Scholar] [CrossRef]
- Stevens, K.R.; Scull, M.A.; Ramanan, V.; Fortin, C.L.; Chaturvedi, R.R.; Knouse, K.A.; Xiao, J.W.; Fung, C.; Mirabella, T.; Chen, A.X.; et al. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends. Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, D.; Hussain, A.; Yip, D.; Parekh, A.; Shrirao, A.; Cho, C.H. Long-term liver-specific functions of hepatocytes in electrospun chitosan nanofiber scaffolds coated with fibronectin. J. Biomed. Mater. Res. 2017, 105, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chae, S.; Kim, J.Y.; Han, W.; Kim, J.; Choi, Y.; Cho, D.W. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 2019. [Google Scholar] [CrossRef]
- Stevens, K.R.; Miller, J.S.; Blakely, B.L.; Chen, C.S.; Bhatia, S.N. Degradable hydrogels derived from PEG-diacrylamide for hepatic tissue engineering. J. Biomed. Mater. Res. 2015, 103, 3331–3338. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Peloso, A.; Dhal, A.; Zambon, J.P.; Li, P.; Orlando, G.; Atala, A.; Soker, S. Current achievements and future perspectives in whole-organ bioengineering. Stem Cell Res. Ther. 2015, 6, 107. [Google Scholar] [CrossRef]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef]
- Katari, R.; Peloso, A.; Orlando, G. Tissue engineering and regenerative medicine: Semantic considerations for an evolving paradigm. Front. Bioeng. Biotechnol. 2015, 2, 57. [Google Scholar] [CrossRef]
- Thomas, L. Notes of a biology-watcher. The planning of science. N. Engl. J. Med. 1973, 289, 89–90. [Google Scholar] [CrossRef]
- Orlando, G.; Soker, S.; Stratta, R.J. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Ann. Surg. 2013, 258, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Bourlière, M.; Gordon, S.C.; Flamm, S.L.; Cooper, C.L.; Ramji, A.; Tong, M.; Ravendhran, N.; Vierling, J.M.; Tran, T.T.; Pianko, S.; et al. POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N. Engl. J. Med. 2017, 376, 2134–2146. [Google Scholar]
- Toso, C.; Pinto Marques, H.; Andres, A.; Castro Sousa, F.; Adam, R.; Kalil, A.; Clavien, P.A.; Furtado, E.; Barroso, E.; Bismuth, H. Compagnons Hépato-Biliaires Group. Liver transplantation for colorectal liver metastasis: Survival without recurrence can be achieved. Liver Transpl. 2017, 23, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Peloso, A.; Katari, R.; Zambon, J.P.; Orlando, G. Sisyphus, the Giffen’s paradox and the Holy Grail: Time for organ transplantation to transition toward a regenerative medicine-focused type of research. Expert Rev. Clin. Immunol. 2013, 9, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Arenas-Herrera, J.E.; Ko, I.K.; Atala, A.; Yoo, J.J. Decellularization for whole organ bioengineering. Biomed. Mater. 2013, 8. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 329, 1216–1219. [Google Scholar] [CrossRef]
- Watt, F.M.; Huck, W.T. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013, 14, 467–473. [Google Scholar] [CrossRef]
- March, S.; Ramanan, V.; Trehan, K.; Ng, S.; Galstian, A.; Gural, N.; Scull, M.A.; Shlomai, A.; Mota, M.M.; Khetani, S.R.; et al. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat. Protoc. 2015, 10, 2027–2053. [Google Scholar] [CrossRef]
- Haycock, J.W. 3D Cell Culture: A Review of Current Approaches and Techniques. Methods Mol. Biol. 2011, 695, 1–15. [Google Scholar]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Jones, J.R. Biomaterials Artificial Organs and Tissue Engineering, 1st ed.; Woodhead Publishing: Cambridge, UK, 2005. [Google Scholar]
- Kizawa, H.; Nagao, E.; Shimamura, M.; Zhang, G.; Torii, H. Scaffold-free 3D bio-printed human liver tissue stably maintains metabolic functions useful for drug discovery. Biochem. Biophys. Rep. 2017, 10, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lou, C.; Zhang, S.; Zhu, Z.; Xing, Q.; Wang, P.; Liu, T.; Liu, H.; Li, C.; Shi, W.; et al. Three-dimensional hydrogel culture conditions promote the differentiation of human induced pluripotent stem cells into hepatocytes. Cytotherapy 2018, 20, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Tapias, L.F.; Ott, H.C. Decellularized scaffolds as a platform for bioengineered organs. Curr. Opin. Organ Transplant. 2014, 19, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Petrosyan, A.; Orlando, G.; Peloso, A.; Wang, Z.; Farney, A.C.; Rogers, G.; Katari, R.; Da Sacco, S.; Sedrakyan, S.; De Filippo, R.E.; et al. Understanding the bioactivity of stem cells seeded on extracellular matrix scaffolds produced from discarded human kidneys: A critical step towards a new generation bio-artificial kidney. CellR4 2015, 3, e1401. [Google Scholar]
- Verstegen, M.M.A.; Willemse, J.; van den Hoek, S.; Kremers, G.J.; Luider, T.M.; van Huizen, N.A.; Willemssen, F.E.J.A.; Metselaar, H.J.; IJzermans, J.N.M.; van der Laan, L.J.W.; et al. Decellularization of Whole Human Liver Grafts Using Controlled Perfusion for Transplantable Organ Bioscaffolds. Stem Cells Dev. 2017, 26, 1304–1315. [Google Scholar] [CrossRef]
- Grant, R.; Hay, D.; Callanan, A. From scaffold to structure: The synthetic production of cell derived extracellular matrix for liver tissue engineering. Biomed. Phys. Eng. Express 2018, 4. [Google Scholar] [CrossRef]
- Török, E.; Lutgehetmann, M.; Bierwolf, J.; Melbeck, S.; Düllmann, J.; Nashan, B.; Ma, P.X.; Pollok, J.M. Primary human hepatocytes on biodegradable poly(l-lactic acid) matrices: A promising model for improving transplantation efficiency with tissue engineering. Liver Transplant. 2011, 17, 104–114. [Google Scholar] [CrossRef]
- Grant, R.; Hay, D.C.; Callanan, A.A. Drug-Induced Hybrid Electrospun Poly-Capro-Lactone: Cell-Derived Extracellular Matrix Scaffold for Liver Tissue Engineering. Tissue Eng. Part A. 2017, 23, 650–662. [Google Scholar] [CrossRef]
- Linti, C.; Zipfel, A.; Schenk, M.; Dauner, M.; Doser, M.; Viebahn, R.; Becker, H.D.; Planck, H. Cultivation of porcine hepatocytes in polyurethane nonwovens as part of a biohybrid liver support system. Int. J. Artif. Organs. 2002, 25, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Narayan, R.; Maji, S.; Ghosh, S.K.; Maiti, T.K. Decellularized caprine liver extracellular matrix as a 2D substrate coating and 3D hydrogel platform for vascularized liver tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e1678–e1690. [Google Scholar] [CrossRef] [PubMed]
- Fasolino, I.; Guarino, V.; Guarino, V.; Marrese, M.; Cirillo, V.; Vallifuoco, M.; Tamma, M.L.; Vassallo, V.; Bracco, A.; Calise, F.; et al. HepG2 and human healthy hepatocyte in vitro culture and co-culture in PCL electrospun platforms. Biomed. Mater. 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Choi, Y.J.; Yong, W.J.; Pati, F.; Shim, J.H.; Kang, K.S.; Kang, I.H.; Park, J.; Cho, D.W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, A.C.; Chen, M.H.; Venkataraman, C.M.; Trubelja, A.; Rodell, C.B.; Dinh, P.V.; Hung, G.; MacArthur, J.W.; Soopan, R.V.; Burdick, J.A.; et al. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2015, 150, 1268–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaedi, M.; Soleimani, M.; Shabani, I.; Duan, Y.; Lotfi, A.S. Hepatic differentiation from human mesenchymal stem cells on a novel nanofiber scaffold. Cell Mol. Biol. Lett. 2012, 17, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Faulk, D.M.; Carruthers, C.A.; Warnerm, H.J.; Kramer, C.R.; Reing, J.E.; Zhang, L.; D’Amore, A.; Badylak, S.F. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014, 10, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.W.; Moran, E.C.; Baptista, P.M.; Soker, S.; Sparks, J.L. Scale-dependent mechanical properties of native and decellularized liver tissue. Biomech. Model. Mechanobiol. 2013, 12, 569–580. [Google Scholar] [CrossRef]
- Moran, E.C.; Baptista, P.M.; Evans, D.W.; Soker, S.; Sparks, J.L. Evaluation of parenchymal fluid pressure in native and decellularized liver tissue. Biomed. Sci. Instrum. 2012, 48, 303–309. [Google Scholar]
- Sánchez-Romero, N.; Sainz-Arnal, P.; Pla-Palacín, I.; Dachary, P.R.; Almeida, H.; Pastor, C.; Soto, D.R.; Rodriguez, M.C.; Arbizu, E.O.; Martinez, L.B.; et al. The role of extracellular matrix on liver stem cell fate: A dynamic relationship in health and disease. Differentiation 2019, 106, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.; Yeh, M.H.; Yarmush, M.L.; Uygun, B.E. Decellularized human liver extracellular matrix (hDLM)-mediated hepatic differentiation of human induced pluripotent stem cells (hIPSCs). J. Tissue Eng. Regen. Med. 2018, 12, e1962–e1973. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, W.; Uluer, M.C.; Langford, J.; Woodall, J.D.; Cimeno, A.; Dhru, U.; Werdesheim, A.; Harrison, J.; Rivera-Pratt, C.; Klepfer, S.; et al. Recellularization via the bile duct supports functional allogenic and xenogenic cell growth on a decellularized rat liver scaffold. Organogenesis 2017, 13, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pla-Palacín, I.; Sainz-Arnal, P.; Morini, S.; Almeida, M.; Baptista, P.M. Liver bioengineering using decellularized whole-liver scaffolds. Methods Mol. Biol. 2018, 1577, 293–305. [Google Scholar] [PubMed] [Green Version]
- Mazza, G.; Rombouts, K.; Rennie Hall, A.; Urbani, L.; Vinh Luong, T.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 13079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, C.B.; Yamauchi, M.; Miguez, P.; Roach, M.; Malavarca, R.; Costello, M.J.; Cardinale, V.; Wauthier, E.; Barbier, C.; et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology 2011, 53, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Shupe, T.; Williams, M.; Brown, A.; Willenberg, B.; Petersen, B.E. Method for the decellularization of intact rat liver. Organogenesis 2010, 6, 134–136. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cui, J.; Zhang, B.Q.; Zhang, H.; Bi, Y.; Kang, Q.; Wang, N.; Bie, P.; Yang, Z.; Wang, H.; et al. Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors. J. Biomed. Mater. Res. A. 2014, 102, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Lorvellec, M.; Scottoni, F.; Crowley, C.; Fiadeiro, R.; Maghsoudlou, P.; Pellegata, A.F.; Mazzacuva, F.; Gjinovci, A.; Lyne, A.M.; Zulini, J.; et al. Mouse decellularised liver scaffold improves human embryonic and induced pluripotent stem cells differentiation into hepatocyte-like cells. PLoS ONE 2017, 12, e0189586. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Xia, R.; Zhang, Y.; Zhang, H.; Bai, L. Decellularized liver scaffold for liver regeneration. Methods Mol. Biol. 2018, 1577, 11–23. [Google Scholar]
- Navarro-Tableros, V.; Herrera Sanchez, M.B.; Figliolini, F.; Romagnoli, R.; Tetta, C.; Camussi, G. Recellularization of Rat Liver Scaffolds by Human Liver Stem Cells. Tissue Eng. Part A. 2015, 21, 1929–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Jakus, A.E.; Baptista, P.M.; Soker, S.; Soto-Gutierrez, A.; Abecassis, M.M.; Shah, R.N.; Wertheim, J.A. Functional Maturation of Induced Pluripotent Stem Cell Hepatocytes in Extracellular Matrix-A Comparative Analysis of Bioartificial Liver Microenvironments. Stem Cells Transl. Med. 2016, 5, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Struecker, B.; Butter, A.; Hillebrandt, K.; Polenz, D.; Reutzel-Selke, A.; Tang, P.; Lippert, S.; Leder, A.; Rohn, S.; Geisel, D.; et al. Improved rat liver decellularization by arterial perfusion under oscillating pressure conditions. J. Tissue Eng. Regen. Med. 2017, 11, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Shi, X.; Tao, L.; Xiao, J.; Han, B.; Zhang, Y.; Yuan, X.; Ding, Y. Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold. Liver Int. 2013, 33, 448–458. [Google Scholar] [CrossRef]
- Mazza, G.; Al-Akkad, W.; Rombouts, K.; Pinzani, M. Liver tissue engineering: From implantable tissue to whole organ engineering. Hepatol. Commun. 2018, 2, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Mirmalek-Sani, S.H.; Sullivan, D.C.; Zimmerman, C.; Shupe, T.D.; Petersen, B.E. Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue. Am. J. Pathol. 2013, 183, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Yagi, H.; Fukumitsu, K.; Fukuda, K.; Kitago, M.; Shinoda, M.; Obara, H.; Itano, O.; Kawachi, S.; Tanabe, M.; Coudriet, G.M.; et al. Human-scale whole-organ bioengineering for liver transplantation: A regenerative medicine approach. Cell Transplant. 2013, 22, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Struecker, B.; Hillebrandt, K.H.; Voitl, R.; Butter, A.; Schmuck, R.B.; Reutzel-Selke, A.; Geisel, D.; Joehrens, K.; Pickerodt, P.A.; Raschzok, N.; et al. Porcine Liver Decellularization Under Oscillating Pressure Conditions: A Technical Refinement to Improve the Homogeneity of the Decellularization Process. Tissue Eng. Part C. Methods 2014, 21, 303–313. [Google Scholar] [CrossRef]
- Ko, I.K.; Peng, L.; Peloso, A.; Smith, C.J.; Dhal, A.; Deegan, D.B.; Zimmerman, C.; Clouse, C.; Zhao, W.; Shupe, T.D.; et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials 2015, 40, 72–79. [Google Scholar] [CrossRef]
- Barakat, O.; Abbasi, S.; Rodriguez, G.; Rios, J.; Wood, R.P.; Ozaki, C.; Holley, L.S.; Gauthier, P.K. Use of decellularized porcine liver for engineering humanized liver organ. J. Surg. Res. 2012, 173, e11–e25. [Google Scholar] [CrossRef]
- Gao, M.; Wang, Y.; He, Y.; Li, Y.; Wu, Q.; Yang, G.; Zhou, Y.; Wu, D.; Bao, J.; Bu, H. Comparative evaluation of decellularized porcine liver matrices crosslinked with different chemical and natural crosslinking agents. Xenotransplantation 2019, 26, e12470. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wu, Q.; Sun, J.; Zhou, Y.; Wang, Y.; Jiang, X.; Li, L.; Shi, Y.; Bu, H. Hemocompatibility improvement of perfusion-decellularized clinical-scale liver scaffold through heparin immobilization. Sci. Rep. 2015, 5, 10756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reflection paper on classification of advanced therapy medicinal products. EMA/CAT/600280/2010 Committee for Advanced Therapies. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/reflection-paper-classification-advanced-therapy-medicinal-products_en.pdf (accessed on 13 April 2012).
- Mazza, G.; Al-Akkad, W.; Telese, A.; Longato, L.; Urbani, L.; Robinson, B.; Hall, A.; Kong, K.; Frenguelli, L.; Marrone, G.; et al. Rapid production of human liver scaffolds for functional tissue engineering by high shear stress oscillation-decellularization. Sci. Rep. 2017, 7, 5534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Lessa, N.; Estrada, D.C.; Severson, E.B.; Lingala, S.; Zern, M.A.; Nolta, J.A.; Wu, J. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transplant. 2011, 17, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Kmieć, Z. Cooperation of Liver Cells in Health and Disease. Adv. Anat. Embryol. Cell Biol. 2001, 161, 1–151. [Google Scholar]
- Mao, S.A.; Glorioso, J.M.; Nyberg, S.L. Liver regeneration. Translational Research: The Journal of Laboratory and Clinical Medicine. Transl. Res. 2014, 163, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Liu, L.; Shimada, M.; Wang, A.; Ruhnke, M.; Heeckt, P.; Muller, A.R.; Nussler, N.C.; Neuhaus, P.; Nussler, A. Induction, expression and maintenance of cytochrome P450 isoforms in long-term cultures of primary human hepatocytes. ALTEX 2004, 21, 3–11. [Google Scholar]
- Gupta, S.; Gorla, G.R.; Irani, A.N. Hepatocyte transplantation: Emerging insights into mechanisms of liver repopulation and their relevance to potential therapies. J. Hepatol. 1999, 30, 162–170. [Google Scholar] [CrossRef]
- Niwa, H. Mechanisms of Stem Cell Self-Renewal. In Essentials of Stem Cell Biology, 3rd ed.; Academic Press: London, UK, 2013; Volume 7, pp. 81–94. [Google Scholar]
- Banas, A.; Yamamoto, Y.; Teratani, T.; Ochiya, T. Stem cell plasticity: Learning from hepatogenic differentiation strategies. Dev. Dyn. 2007, 236, 3228–3241. [Google Scholar] [CrossRef]
- Takayama, K.; Nagamoto, Y.; Nagamoto, Y.; Kishimoto, K.; Tashiro, K.; Sakurai, F.; Tachibana, M.; Kanda, K.; Hayakawa, T.; Furue, M.K.; et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 2013, 34, 1781–1789. [Google Scholar] [CrossRef]
- Ramasamy, T.S.; Yu, J.S.L.; Selden, C.; Hodgson, H.; Cui, W. Application of Three-Dimensional Culture Conditions to Human Embryonic Stem Cell-Derived Definitive Endoderm Cells Enhances Hepatocyte Differentiation and Functionality. Tissue Eng. Part A. 2013, 19, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, K.; Raju, R.; Firpo, M.; O’Brien, T.D.; Verfaillie, C.M.; Hu, W.S. Spheroid Culture for Enhanced Differentiation of Human Embryonic Stem Cells to Hepatocyte-Like Cells. Stem Cells Dev. 2014, 23, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancho-Bru, P.; Najimi, M.; Caruso, M.; Pauwelyn, K.; Cantz, T.; Forbes, S.; Roskams, T.; Ott, M.; Gehling, U.; Sokal, E.; et al. Stem and progenitor cells for liver repopuletion: Can we standardise the process from bench to bedside? Gut 2009, 58, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [Green Version]
- Teramoto, K.; Asahina, K.; Kumashiro, Y.; Kakinuma, S.; Chinzei, R.; Shimizu-Saito, K.; Tanaka, Y.; Teraoka, H.; Arii, S. Hepatocyte differentiation from embryonic stem cells and umbilical cord blood cells. J. Hepato-Biliary-Pancreat. Surg. 2005, 12, 196–202. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, S.; Peng, G.; Liu, T.; Li, Y.; Xiang, D.; Wassler, M.J.; Shelat, H.S.; Geng, Y. Rotating Microgravity-Bioreactor Cultivation Enhances the Hepatic Differentiation of Mouse Embryonic Stem Cells on Biodegradable Polymer Scaffolds. Tissue Eng. Part A 2012, 18, 2376–2385. [Google Scholar] [CrossRef]
- Zhang, W.; Li, W.; Liu, B.; Wang, P.; Li, W.; Zhang, H. Efficient generation of functional hepatocyte-like cells from human fetal hepatic progenitor cells in vitro. J. Cell. Physiol. 2012, 227, 2051–2058. [Google Scholar] [CrossRef]
- Lees, J.G.; Lim, S.A.; Croll, T.; Williams, G.; Lui, S.; Cooper-White, J.; McQuade, L.R.; Mathiyalagan, B.; Tuch, B.E. Transplantation of 3D scaffolds seeded with human embryonic stem cells: Biological features of surrogate tissue and teratoma-forming potential. Regen. Med. 2007, 2, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Vestentoft, P.S. Development and molecular composition of the hepatic progenitor cell niche. Dan. Med J. 2013, 60, B4640. [Google Scholar]
- Khun, D.N.; Scheers, I.; Ehnert, S.; Jazouli, N.; Nyabi, O.; Buc-Calderon, P.; Meulemans, A.; Nussler, A.; Sokal, E.; Najimi, M. In vitro Differentiated adult human liver progenitor cells display mature hepatic metabolic functions: A potential tool for in vitro pharmacotoxicological testing. Cell Transplant. 2011, 20, 287–302. [Google Scholar]
- Strick-Marchand, H.; Weiss, M.C. Inducible differentiation and morphogenesis of bipotential liver cell lines from wild-type mouse embryos. Hepatology 2002, 36, 794–804. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, K.; Fisk, N.M. Fetal stem cells. Best Pr. Res. Clin. Obs. Gynaecol. 2004, 18, 853–875. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.M.; Siddiqui, M.M.; Lozier, G.; Rodriguez, S.R.; Atala, A.; Soker, S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011, 53, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Satija, N.K.; Singh, V.K.; Verma, Y.K.; Gupta, P.; Sharma, S.; Afrin, F.; Sharma, M.; Sharma, P.; Tripathi, R.P.; Gurudutta, G.U. Mesenchymal stem cell-based therapy: A new paradigm in regenerative medicine. J. Cell. Mol. Med. 2009, 13, 4385–4402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.; Zhang, N.; You, N.; Li, Q.; Liu, W.; Jiang, N.; Liu, J.; Zhang, H.; Wang, D.; Tao, K.; et al. The differentiation of MSCs into functional hepatocyte-like cells in a liver biomatrix scaffold and their transplantation into liver-fibrotic mice. Biomaterials 2012, 33, 8995–9008. [Google Scholar] [CrossRef]
- Li, J.; Tao, R.; Wu, W.; Cao, H.; Xin, J.; Li, J.; Guo, J.; Jiang, L.; Gao, C.; Demetriou, A.A. 3D PLGA Scaffolds Improve Differentiation and Function of Bone Marrow Mesenchymal Stem Cell–Derived Hepatocytes. Stem Cells Dev. 2010, 19, 1427–1436. [Google Scholar] [CrossRef]
- Christ, B.; Dollinger, M.M. The generation of hepatocytes from mesenchymal stem cells and engraftment into the liver. Curr. Opin. Organ Transplant. 2011, 16, 69–75. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Chen, F.; Shi, Y.; Bao, J.; Bu, H. Construction of bioengineered hepatic tissue derived from human umbilical cord mesenchymal stem cells via aggregation culture in porcine decellularized liver scaffolds. Xenotransplantation 2017, 24, e12258. [Google Scholar] [CrossRef] [Green Version]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 19, 313–317. [Google Scholar] [CrossRef]
- Jia, F.; Wilson, K.D.; Sun, N.; Gupta, D.M.; Huang, M.; Li, Z.; Panetta, N.J.; Chen, Z.Y.; Robbins, R.C.; Kay, M.A.; et al. A nonviral minicircle vector for deriving human iPS cells. Nat. Methods 2010, 7, 197–199. [Google Scholar] [CrossRef] [Green Version]
- Subba Rao, M.; Sasikala, M.; Reddy, D.N. Thinking outside the liver: Induced pluripotent stem cells for hepatic applications. World J. Gastroenterol. 2013, 19, 3385–3396. [Google Scholar] [PubMed]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Blau, H.M. Nuclear reprogramming to a pluripotent state by three approaches. Nature 2010, 465, 704–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Soto-Gutierrez, A.; Tafaleng, E.; Kelly, V.; Roy-Chowdhury, J.; Fox, I.J. Modeling and therapy of human liver diseases using induced pluripotent stem cells: How far have we come? Hepatology 2011, 53, 708–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehtari, M.; Beiki, B.; Zeynali, B.; Hosseini, F.S.; Soleimanifar, F.; Kaabi, M.; Soleimani, M.; Enderami, S.E.; Kabiri, M.; Mahboudi, H. Decellularized Wharton’s jelly extracellular matrix as a promising scaffold for promoting hepatic differentiation of human induced pluripotent stem cells. J. Cell Biochem. 2019, 120, 6683–6697. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Mitry, R.R.; Dhawan, A. Human hepatocyte transplantation: State of the art. J. Intern. Med. 2009, 266, 339–357. [Google Scholar] [CrossRef]
- Soto-Gutierrez, A.; Zhang, L.; Medberry, C.; Fukumitsu, K.; Faulk, D.; Jiang, H.; Reing, J.; Gramignoli, R.; Komori, J.; Ross, M.; et al. A Whole-Organ Regenerative Medicine Approach for Liver Replacement. Tissue Eng. Part C. Methods 2011, 17, 677–686. [Google Scholar] [CrossRef]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef]
- Wang, S.; Qu, X.; Zhao, R.C. Clinical applications of mesenchymal stem cells. J. Hematol. Oncol. 2012, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Hussein, K.H.; Park, K.M.; Kang, K.S.; Woo, H.M. Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta Biomater. 2016, 1, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Shi, Y.; Sun, H.; Yin, X.; Yang, R.; Li, L.; Chen, X.; Bu, H. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant. 2011, 20, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Stern, M.M.; Smith, L.; Liu, Y.; Bharadwaj, S.; Liu, G.; Baptista, P.M.; Bergman, C.R.; Soker, S.; Yoo, J.J.; et al. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials 2011, 32, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Yagi, H.; Inomata, K.; Matsubara, K.; Hibi, T.; Abe, Y.; Kitago, M.; Shinoda, M.; Obara, H.; Itano, O.; et al. Mesenchymal stem cells support hepatocyte function in engineered liver grafts. Organogenesis 2014, 10, 268–277. [Google Scholar] [CrossRef]
- Jiang, W.C.; Cheng, Y.H.; Yen, M.H.; Chang, Y.; Yang, V.W.; Lee, O.K. Cryo-chemical decellularization of the whole liver for mesenchymal stem cells-based functional hepatic tissue engineering. Biomaterials 2014, 35, 3607–3617. [Google Scholar] [CrossRef]
- Bruinsma, B.G.; Kim, Y.; Berendsen, T.A.; Ozer, S.; Yarmush, M.L.; Uygun, B.E. Layer-by-layer heparinization of decellularized liver matrices to reduce thrombogenicity of tissue engineered grafts. J. Clin. Transl. Res. 2015, 1, 48–56. [Google Scholar]
- Zhou, P.; Huang, Y.; Guo, Y.; Wang, L.; Ling, C.; Guo, Q.; Wang, Y.; Zhu, S.; Fan, X.; Zhu, M.; et al. Decellularization and Recellularization of Rat Livers With Hepatocytes and Endothelial Progenitor Cells. Artif. Organs. 2016, 40, E25–E38. [Google Scholar] [CrossRef]
- Park, K.M.; Hussein, K.H.; Hong, S.H.; Ahn, C.; Yang, S.R.; Park, S.M.; Kweon, O.K.; Kim, B.M.; Woo, H.M. Decellularized Liver Extracellular Matrix as Promising Tools for Transplantable Bioengineered Liver Promotes Hepatic Lineage Commitments of Induced Pluripotent Stem Cells. Tissue Eng. Part A. 2016, 22, 449–460. [Google Scholar] [CrossRef]
- Ogiso, S.; Yasuchika, K.; Fukumitsu, K.; Ishii, T.; Kojima, H.; Miyauchi, Y.; Yamaoka, R.; Komori, J.; Katayama, H.; Kawai, T.; et al. Efficient recellularisation of decellularised whole-liver grafts using biliary tree and foetal hepatocytes. Sci. Rep. 2016, 6, 35887. [Google Scholar] [CrossRef]
- Wen, X.; Huan, H.; Wang, X.; Chen, X.; Wu, L.; Zhang, Y.; Liu, W.; Bie, P.; Xia, F. Sympathetic neurotransmitters promote the process of recellularization in decellularized liver matrix via activating the IL-6/Stat3 pathway. Biomed. Mater. 2016, 11, 065007. [Google Scholar] [CrossRef]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J Plast. Reconstr. Aesthet. Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Algzlan, H.; Varada, S. Three-Dimensional Printing of the Skin. JAMA Dermatol. 2015, 151, 207. [Google Scholar] [CrossRef]
- Owens, C.M.; Marga, F.; Forgacs, G.; Heesch, C.M. Biofabrication and testing of a fully cellular nerve graft. Biofabrication 2013, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonzo, M.; AnilKumar, S.; Roman, B.; Tasnim, N.; Joddar, B. 3D Bioprinting of cardiac tissue and cardiac stem cell therapy. Transl. Res. 2019, 211, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Ayan, B.; Ozbolat, I.T. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. 2017, 51, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, P.; Ozbolat, V.; Ayan, B.; Dhawan, A.; Ozbolat, I.T. Bone tissue bioprinting for craniofacial reconstruction. Biotechnol. Bioeng. 2017, 114, 2424–2431. [Google Scholar] [CrossRef]
- Kabirian, F.; Mozafari, M. Decellularized ECM-derived bioinks: Prospects for the future. Methods 2019, in press. [Google Scholar] [CrossRef]
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-Derived Decellularized Extracellular Matrix: A Game Changer for Bioink Manufacturing? Trends Biotechnol. 2018, 36, 787–805. [Google Scholar] [CrossRef]
- Lee, H.; Han, W.; Kim, H.; Ha, D.H.; Jang, J.; Kim, B.S.; Cho, D.W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 10, 1229–1237. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huch, M.; Koo, B.K. Modeling mouse and human development using organoid cultures. Development 2015, 142, 3113–3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 15, 299–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar]
- Hindley, C.J.; Cordero-Espinoza, L.; Huch, M. Organoids from adult liver and pancreas: Stem cell biology and biomedical utility. Dev. Biol. 2016, 15, 251–261. [Google Scholar] [CrossRef]
- Nantasanti, S.; de Bruin, A.; Rothuizen, J.; Penning, L.C.; Schotanus, B.A. Concise Review: Organoids Are a Powerful Tool for the Study of Liver Disease and Personalized Treatment Design in Humans and Animals. Stem Cells Transl. Med. 2016, 5, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Miyao, M.; Kotani, H.; Ishida, T.; Kawai, C.; Manabe, S.; Abiru, H.; Tamaki, K. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Lab. Invest. 2015, 95, 1130–1144. [Google Scholar] [CrossRef] [Green Version]
- LeCluyse, E.L. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur. J. Pharm. Sci. 2001, 13, 343–368. [Google Scholar] [CrossRef]
- Mußbach, F.; Settmacher, U.; Dirsch, O.; Xie, C.; Dahmen, U. Bioengineered Livers: A New Tool for Drug Testing and a Promising Solution to Meet the Growing Demand for Donor Organs. Eur. Surg. Res. 2016, 57, 224–239. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Li, J. Stem cells with decellularized liver scaffolds in liver regeneration and their potential clinical applications. Liver Int. 2015, 35, 687–694. [Google Scholar] [CrossRef]
- Natale, A.; Vanmol, K.; Arslan, A.; Van Vlierberghe, S.; Dubruel, P.; Van Erps, J.; Thienpont, H.; Buzgo, M.; Boeckmans, J.; De Kock, J. Technological advancements for the development of stem cell-based models for hepatotoxicity testing. Arch. Toxicol. 2019, 93, 1789–1805. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.K.; Bardia, A.; Lakkireddy, C.R.; Nagarapu, R.; Habeeb, M.A.; Khan, A.A. Bioengineered humanized livers as better three-dimensional drug testing model system. World J. Hepatol. 2018, 27, 22–33. [Google Scholar] [CrossRef] [PubMed]

| Synthetic Scaffolds | Biological Scaffolds | |
|---|---|---|
| Advantages | -sterilizable -economical -easy to synthesize -do not require organ donors -no pathogenicity | -sterilizable -native organ structure -active molecules useful for cell growth (peptides and ECM-specific proteins) -not immunogenic |
| Disadvantages | -cell apoptosis in the absence of a vasculature system -difficulty to reproduce organ complexity -biocompatibility | -organ donors -standardization of optimal decellularization conditions |
| Scaffold Origin | Decellularization Technique and Agents | Cell Source | Recellularization Technique and Culture Time (days) | Ref. | |
|---|---|---|---|---|---|
| Rat | PV-p | SDS | Rat hepatocytes | PV-i (7) | [52] |
| Rat | PV-p | 1%, 0.5%, 0.25% SDS + 1% Triton X-100 | Rat hepatocytes | PV-i (0.25) | [115] |
| Rat | IVC-p | 3% Triton X-100/0.5% EGTA | Mouse hepatocytes | DI/PV-p (7) | [111] |
| Ferret | PV-p | 1% Triton X-100/0.1% NH4OH | h-fetal liver cells + h-UVEC | PV-i (7) | [96] |
| Rat | PV-p | 1% Triton X-100 + 0.05% NaOH vs. 1% SDS | Rat hepatocytes | PV-i (7) | [116] |
| Pig | PV-p | 0.25%, 0.5% SDS | h-fetal stellate cells + h-fetal hepatocytes | PV-i (13) | [71] |
| Mouse | PV-p | 1% SDS + Triton X-100 | h-iPCS | PV-i (14) | [59] |
| Pig | PV-p | 0.01%, 0.1%, 1% SDS + 1% Triton X-100 | Porcine hepatocytes | PV-i (28) | [68] |
| Rat | SVC-p | Trypsin, Triton X-100 + EGTA | Rat hepatocytes + rat BM-MSCs | PV-i (6) | [117] |
| Mouse | PV-p | 1% Triton X-100 + 0.1% NH4OH | Mouse BM-MSCs | PV-i (28) | [118] |
| Rat | PV-p | Triton X-100 + 0.1% SDS | h-liver stem cells | PV, IVC, SVC + CBD-i (21) | [62] |
| Pig | PV-p | 1% Triton X-100/0.1% NH4OH | Mouse vascular endothelial cells | PV-i (3) | [70] |
| Human | IVC-p | 3% Triton X-100 + 1% SDS | h-hepatic stellate cells/HepG2/Sk-hep-1 | DI (21) | [56] |
| Rat | PV-p | 0.01%,0.1%, 0.2% SDS + 0.1% Triton X-100 | Adult rat hepatocytes | DI (5) | [119] |
| Rat | PV-p | 1% Triton X-100 + 0.1% NH4OH | h-iPSCs hepatocytes | DI (14) | [63] |
| Rat | PV-p | 1% Triton X-100/0.1% NH4OH | Rat liver cell line + h-endothelial cell line | PV-i + DI (7) | [120] |
| Pig | PV-p | 0.1% SDS | Porcine iPSC-heps | PV-i (5) | [121] |
| Pig | PV-p | 0.1% SDS | Hep-G2 + h-endothelial cell line | PV-i, PV-i + HA-i (10) | [114] |
| Rat | PV-p | 0.02% Trypsin/0.05% EGTA + 1% Triton X-100/0.05% EGTA | Mouse fetal hepatocytes | CBD-i (7) | [122] |
| Mouse | PV-p | 1% SDS + 1% Triton X-100 | Mouse hepatocytes | PV-i (7) | [123] |
| Mouse | PV-p | 4% SDC +2000 kU DNAse-I | h-ESCS and iPSCs | DI (13) | [60] |
| Human | a | SDS, Triton X-100, SDC, DNAse | h-hepatic stellate cells/HepG2/hepatocytes | SS/p (14) | [75] |
| Human | PV-p + HA-p | 4% Triton X-100/1% NH4OH | h-UVECs | SS (5) | [38] |
| Pig | PV-p | 1% Triton X-100/0.1% NH4OH | Pig UVECs/MSCs/hepatoblasts | PV-i + HA-i (21) | [55] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, S.; Peloso, A.; Zoro, T.; Avanzini, M.A.; Cobianchi, L. A Hepatic Scaffold from Decellularized Liver Tissue: Food for Thought. Biomolecules 2019, 9, 813. https://doi.org/10.3390/biom9120813
Croce S, Peloso A, Zoro T, Avanzini MA, Cobianchi L. A Hepatic Scaffold from Decellularized Liver Tissue: Food for Thought. Biomolecules. 2019; 9(12):813. https://doi.org/10.3390/biom9120813
Chicago/Turabian StyleCroce, Stefania, Andrea Peloso, Tamara Zoro, Maria Antonietta Avanzini, and Lorenzo Cobianchi. 2019. "A Hepatic Scaffold from Decellularized Liver Tissue: Food for Thought" Biomolecules 9, no. 12: 813. https://doi.org/10.3390/biom9120813
APA StyleCroce, S., Peloso, A., Zoro, T., Avanzini, M. A., & Cobianchi, L. (2019). A Hepatic Scaffold from Decellularized Liver Tissue: Food for Thought. Biomolecules, 9(12), 813. https://doi.org/10.3390/biom9120813





