Anticonvulsant Essential Oils and Their Relationship with Oxidative Stress in Epilepsy
Abstract
:1. Introduction
Relationship between Epilepsy and Oxidative Stress
2. Anticonvulsant Essential Oils
2.1. Bunium persicum (Boiss). B. Fedtsch.
2.2. Calamintha officinalis Moench
2.3. Cinnamosma madagascariensis Danguy
2.4. Citrus aurantium L. var. Amara
2.5. Dennettia tripetala G. Baker
2.6. Elettaria cardamomum L. Maton
2.7. Gardenia lucida Roxb.
2.8. Pimpinella anisum L.
2.9. Piper guineense Schum &Thonn
2.10. Smyrnium cordifolium Boiss.
2.11. Thymus vulgaris L.
2.12. Zataria multiflora Boiss.
2.13. Zhumeria majdae Rech.
2.14. Rosmarinus officinalis L., Ocimum basilicum L., Mentha pulegium L., M. spicata L., M. piperita L., Origanum dictamnus L. and Lavandula angustifolia Mill.
3. Chemical Constituents
3.1. Alpha-Asarone
| Species | Essential Oil | Major Components Reported in the Literature | Major Components of the Evaluated Essential Oil | Experimental Protocol | Anticonvulsant Activity and/or Mechanism | Animal Tests and/or Cell Line Reference |
|---|---|---|---|---|---|---|
| Bunium persicum (Boiss). B. Fedtsch | Seed | γ-Terpinene (46.1%), cuminaldehyde (15.5%), p-cymene (6.7%) and limonene (5.9%) [31] | - | PTZ induced seizure test Maximal electroshock test | Prolonged onset time of clonic and tonic seizures [36] | Male NMRI mice |
| Calamintha officinalis Moench | Leaf | - | Carvone (38.7%), neo-dihydrocarveol (9.9%), dihydrocarveol acetate (7.6%), dihydrocarveol (6.9%), 1,8-cineole (6.4%), cis-carvyl acetate (6.1%) [40] | PTZ induced seizure test | Protected against generalized tonic- clonic seizures Decreased the number and duration of convulsions Reduced mortality [40] | Adult male Wistar rats |
| Cinnamosma madagascariensis Danguy | Leaf | Linalool (30.1%), limonene (12.0%), myrcene (8.9%) and α-pinene (8.4%) [44] | - | PTZ induced seizure test | Increased the latency period Reduced the frequency and intensity of convulsions [45] | Adult male and female Wistar rats |
| Citrus aurantium L. var. amara | Blossoms | - | Linalool (28.5%), linalyl acetate (19.6%), nerolidol (9.1%) and farnesol (9.1%) [53] | PTZ induced seizure test Maximal electroshock test | Produced protection against clonic Exhibited inhibition of the tonic convulsion [53] | Male NMRI mice |
| Dennettia tripetala G. Baker | Seed | β-Phenyl nitroethane (87.4%), linalool (8.8%) [116] | - | PTZ induced seizure test strychnine induced seizure test | Offered protection against PTZ- induced convulsion Flumazenil blocked anticonvulsant effect [58] | Adult male and female albino mice |
| Elettaria cardamomum L. Maton | Seed | - | 1,8-Cineole (45.6%), α-terpinyl acetate (33.7%) [64] | PTZ induced seizure test Maximal electroshock test | Delayed onset of clonic seizures Increased onset time of tonic convulsions Reduced the percentage of hind limb tonic extension [64] | NMRI male mice |
| Gardenia lucida Roxb. | Apical buds and young shoots | - | α-Pinene (45%), spathulenol (31%) [67] | PTZ induced seizure test Maximal electroshock test | Protected against the intensity and frequency of convulsions, and mortality rate [67] | Male and female Swiss Albino mice |
| Pimpinella anisum L. | - | - | trans-Anethole (89.1%) | PTZ induced seizure test Electroencephalogram recordings | Prolonged time to appearance of seizures Decreased the frequency, amplitude, and duration of epileptiform burst discharges Showed neuroprotective effect [75] | Adult male Wistar rats |
| Piper guineense Schum & Thonn | Fruits | - | β-Sesquiphellandrene (20.9%), linalool (6.1%), limonene (5.8%), β-bisabolene (5.4%), α-pinene (5.3%) [82] | PTZ induced seizure test | Decreased mortality Reduced the Incidence of Convulsions [82] | Adult male and female albino mice |
| Smyrnium cordifolium Boiss | Plant | - | Curzerene (65.26%), δ-cadinene (14.39%), and γ-elemene (5.15%) [90] | PTZ induced seizure test | Prolonged onset time to seizure [90] | Mice |
| Thymus vulgaris L. | Fresh herb | - | Thymol (34.78%), p-cymene (14.18%), carvacrol (6.16%) and β-caryophyllene (5.46%) [94] | Maximal electroshock test | Protected against the convulsions | Male Swiss Albino mice |
| Zataria multiflora Boiss | - | - | - | PTZ induced seizure test Maximal electroshock test | Increased the onset time to clonic seizures Prevented tonic convulsions [102] | |
| Zhumeria majdae Rech | Aerial parts | - | - | PTZ induced seizure test Maximal electroshock test | Increased the onset time to tonic convulsions Prevented tonic Convulsions [109] | NMRI male mice |
| Rosmarinus officinalis L., Ocimum basilicum L., Mentha pulegium L., M. spicata L., M. piperita L., Origanum dictamnus L. and Lavandula angustifolia Mill. | - | - | - | PTZ induced seizure test | Increased seizure latency, decreased intensity, and differences in the quality of seizures, characteristics, from simple twitches to complete seizures [110] | Adult female white Balb-c mice |
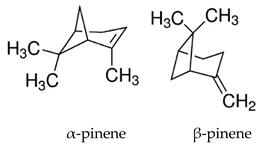
3.2. Alpha- and Beta-Pinene
3.3. (+)-ar-Turmerone
3.4. βeta-Caryophyllene
3.5. Borneol
3.6. Carvacrol
3.7. Carvacryl Acetate
3.8. Curcumol
3.9. Curzerene
3.10. Epoxy-Carvone
3.11. Eugenol
3.12. Gamma-Decanolactone
3.13. Linalool
3.14. Nerolidol
3.15. 1-Nitro-2-phenylethane
3.16. Terpinen-4-ol
3.17. Thymol
3.18. (-)-Verbenone
| Compounds | Experimental Protocol | Anticonvulsant Activity and/or Mechanism | Animal Tests and/or Cell Line Reference | Reference |
|---|---|---|---|---|
 alpha-asarone | PTZ induced seizure test Maximal electroshock test Pilocarpine-induced seizures test | Decreased the occurrence of tonic hind limb extension. Reduced the hind limb extensor phase of convulsion Increased latency to seizure | Male Swiss mice and male Wistar rats | [116] |
| Electrophysiological recording PTZ- and Kainate- induced seizure test | Enhanced tonic GABAergic inhibition Prolonged latency to clonic and tonic seizures | Rat hippocampal neurons and Male C57BL-6 mice | [117] | |
| Pilocarpine-induced status epilepticus rat model | Reduced learning and memory deficit Attenuated brain inflammation by inhibiting the NF-κB activation pathway in microglia | Adult male Sprague-Dawley rats Microglia cell culture | [119] | |
| Nicotine-induced seizure test | Prolonged onset time to seizure, but not prevented the occurrence Did not interact with nicotinic acetylcholine receptors | Male ICR mice | [118] | |
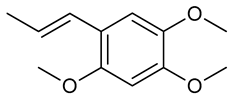
 | PTZ induced seizure test Neurochemical tests | Decreased the seizure intensity Reduced hippocampal nitrite level and striatal content of dopamine and norepinephrine | Male Swiss albino mice | [122] |
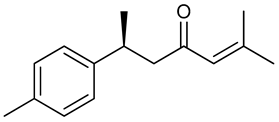 (+)-ar-Turmerone | 6-Hz psychomotor seizure mouse model PTZ infusion model | Displayed anticonvulsant properties Modulated the expression patterns of seizure-related genes | Male C57BI/6 mice Male NMRI mice AB adult zebra fish | [128] |
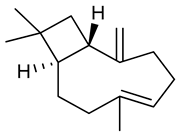 β-Caryophyllene | Kainic acid induced seizure test | Decreased the seizure intensity Reduced oxidative stress Reduced expression of TNF-α and IL-1β | Mice | [136] |
| PTZ induced seizure test | Increased latency to myoclonic jerks | Adult C57BL/6 mice of both genders | [143] | |
| Maximal electroshock test PTZ induced seizure test Kainate induced status epilepticus | Suppressed tonic-clonic seizures Decreased seizure scores Decreased lipid peroxidation | Male albino ICR mice | [144] | |
 Borneol | PTZ-induced kindling model | Suppressed the process of epileptogenesis Reduced oxidative stress Prevented neuronal damage | Male Swiss albino mice | [158] |
 Carvacrol | 6Hz psychomotor seizure test Maximal electroshock test PTZ induced seizure test Corneal kindling model Lithium-pilocarpine model | Prevented seizures in some tests. | Adult male CF No 1 albino mice | [173] |
| Induction of SE Electrophysiological recording Immunohistochemistry Rewarded alternating T-maze test | Prevented memory deficits following SE Inhibited TRPM7 channels | Male adult Sprague-Dawley rats | [174] | |
| Lipopolysaccharide-PTZ induced seizure test | Prevented the proconvulsant effect of LPS Increased hippocampal level COX-2 but not COX-1 | Adult male wistar rats | [175] | |
 Carvacryl acetate | Pilocarpine- PTZ- Picrotoxin- induced seizure test Determination of Na+, K+-ATPase activity Determination of d-ALA-D activity Evaluation of amino acids levels in mice hippocampus | Increased latency to first seizure Reduced percentage of seizures Improved Na+, K+-ATPase and d-aminolevulinic acid dehydratase activities Increased GABA levels | Male Swiss albino mice | [181] |
 Curcumol | Electrophysiological recording PTZ- and Kainate- induced seizure test | Suppressed epileptic activity Facilitated GABAergic inhibition | Male C57BL/6J mice | [186] |
 Curzerene | PTZ induced seizure test | Prolonged onset time to seizure and decreased the duration of seizure Effects on GABAergic and opioid systems | Mice | [90] |
 Epoxy-carvone | PTZ induced seizure test Maximal electroshock test Pilocarpine induced seizure test Strychnine Induced Seizure Test | Increased latency to seizure onset Prevented tonic seizures | Male Swiss albino mice | [196] |
| PTZ-induced kindling model | Decreased seizure scores Decreased proinflammatory Cytokines Showed neural protection | Male Swiss albino mice | [197] | |
 Eugenol | Intrahippocampal injection of kainic acid | Increased seizure threshold Reduced granule cell dispersion Suppressed mTORC1 hippocampal activation | Male C57BL/6 mice | [205] |
| Electrophysiological measurements Pilocarpine-induced epileptic seizures | Inhibited transient voltage-gated Na+ currents Reduced percentage of severe seizures | Neuronal cells (NG108-15) Adult Sprague–Dawley (SD) male rats | [206] | |
| Intracellular recording | Induced inhibitory and excitatory effects in a concentration-dependent manner | Neurons of land snails Caucasotachea atrolabiata | [207] | |
| Lithium-pilocarpine model | Decreased seizure stages Reversed oxidative stress Increased cell survival in hippocampal sub-regions | Male rats | [208] | |
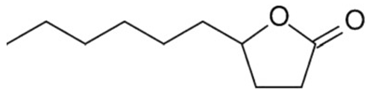 γ-Decanolactone | Isoniazid-, picrotoxin- and 4- aminopyridine- Induced Seizure Test | Prolong the latency to the first seizure Decreased the percentage of seizures | Male CF1 mice | [212] |
| Pilocarpine-Induced Seizure Test | Prolonged the latency to first clonic seizure and reduced oxidative stress | Male CF1 mice | [213] | |
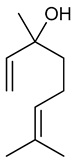 Linalool | PTZ induced seizure test | Suppressed action potentials at lower concentration. Excitatory effect in higher concentration. | Central neurons of snail Caucasotachea atrolabiata | [47] |
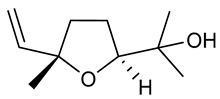 Linalool oxide | PTZ induced seizure test Maximal electroshock test | Increased latency to first seizure onset Reduced the duration of tonic seizures | Male Swiss albino mice | [225] |
 Nerolidol | PTZ-induced kindling test | Increased NE, DA, 5-HT in cortex and hippocampus Reduced oxidative stress | Male lake mice | [235] |
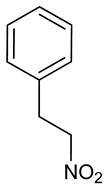 1-Nitro-2-phenylethane | PTZ induced seizure test strychnine induced seizure test | Offered protection against PTZ- strychnine-induced convulsion Flumazenil blocked anticonvulsant effect | Adult male and female albino mice | [58] |
 Terpinen-4-ol | PTZ induced seizure test 3-MP test Electroencephalogram recordings Dissociation and Patch-Clamp Recordings | Increased the latency to seizures Reduced the total time spent in generalized convulsions Reduced Na+ currents in a concentration-dependent manner | Adult male Swiss mice | [256] |
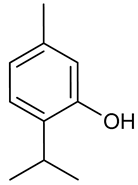 Thymol | 6 Hz psychomotor seizure test Maximal electroshock test PTZ induced seizure test Corneal kindling model Lithium-pilocarpine model | Prevented seizures in some tests. | Adult male CF No 1 albino mice | [173] |
| Maximal electroshock test PTZ-induced seizure test Strychnine-induced seizure test 4-Aminopyridine seizure test PTZ-induced kindling test | Reduced seizure scores Could block Na+ channels post GABAA receptor modulation | Male Wistar rats Male Swiss albino mice | [272] | |
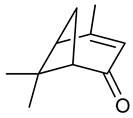 (-)-Verbenone | PTZ induced seizure test | Increased latency to onset of first seizure Reduced the percentage of tonic-clonic seizures Up regulated mRNA expression of BDNF and COX-2 Down regulated mRNA expression of c-fos | Male Swiss mice | [282] |
4. Discussion and Future Perspectives
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4TRP | Terpinen-4-ol |
| δ-ALA-D | δ-aminolevulinic acid dehydratase |
| ATP | Adenosine triphosphate |
| BDNF | Brain-derived neurotropic factor |
| BPEO | Bunium persicum essential oil |
| CAEO | Citrus aurantium essential oil |
| CAT | Catalase |
| CMEO | Cinnamosma madagascariensis essential oil |
| CNS | Central Nervous System |
| COEO | Calamintha officinalis essential oil |
| DTEO | Dennettia tripetala essential oil |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EC | Epoxy-carvone |
| ECEO | Elettaria cardamomum essential oil |
| EOs | Essential oils |
| ES | Epilepticus status |
| FRAP | Ferric reducing antioxidant power |
| GABA | δ-aminobutyric acid |
| GC/MS | Gas chromatography coupled to mass spectrometry |
| GLEO | Gardenia lucida essential oil |
| GSH | Glutathione reductase |
| GST | Glutathione S-transferase |
| GPx | Glutathione peroxidase |
| IL-1β | Interleukin 1β |
| iNOS | Inducible nitric oxide synthase |
| MDA | Malondialdehyde |
| MÊS | Maximal electroshock seizure |
| nAChRs | Nicotinic acetylcholine receptors |
| mTOR | mammalian target of rapamycin |
| NPH | 1-Nitro-2-phenylethane |
| OXL | Linalool oxide |
| PAEO | Pimpinella anisum essential oil |
| PGEO | Piper guineense essential oil |
| PTZ | Pentylenetetrazol |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygenated species |
| scMET | Metrazol |
| SE | Epilepticus status |
| SCEO | Smyrnium cordifolium essential oil |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor α |
| ZHMEO | Zhumeria majdae essential oil |
| ZMEO | Zataria multiflora essential oil |
References
- Beletsky, V.; Mirsattari, S.M. Epilepsy, Mental Health Disorder, or Both? Epilepsy Res. Treat. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; de Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Primers 2018, 4, 18024. [Google Scholar] [CrossRef] [PubMed]
- WHO. The World Health Report. In Epilepsy; WHO: Geneva, Switzerland, 2018; Available online: http://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 10 August 2018).
- Wilcox, K.S.; Dixon-Salazar, T.; Sills, G.J.; Ben-Menchem, E.; Steve White, H.; Porter, R.J.; Rogawski, M.A. Issues related to the development of new antiseizure treatments. Epilepsia 2013, 54, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, D.; Schachter, S.C. Drug treatment of epilepsy in adults. Br. Med. J. 2014, 348, g254. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.N.; Agra, M.D.F.; Maior, F.N.; de Sousa, D.P. Essential oils and their constituents: Anticonvulsant activity. Molecules 2011, 16, 2726–2742. [Google Scholar] [CrossRef]
- Komaki, A.; Hoseini, F.; Shahidi, S.; Baharlouei, N. Study of the effect of extract of Thymus vulgaris on anxiety in male rats. J. Tradit. Complement. Med. 2015, 6, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Pisochi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Cellular responses to oxidative stress: Adaptation, damage, repair, senscence and death. In Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007; p. 187. [Google Scholar]
- Winyard, P.G.; Moody, C.J.; Jacob, C. Oxidative activation of antioxidant defense. Trends Biochem. Sci. 2005, 30, 454–461. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef]
- Bellissimo, M.I.; Amado, D.; Abdalla, D.S.; Ferreira, E.C.; Cavalheiro, E.A.; da Graça, N.M.M. Superoxide dismutase, glutathione peroxidase activities and the hydroperoxide concentration are modified in the hippocampus of epileptic rats. Epilepsy Res. 2001, 46, 121–128. [Google Scholar] [CrossRef]
- Chuang, Y.C. Mitochondrial dysfunction and oxidative stress in seizure-induced neuronal cell death. Acta Neurol. Taiwanica 2010, 19, 3–15. [Google Scholar]
- Gluck, M.R.; Jayatilleke, E.; Shaw, S.; Rowan, A.J.; Haroutunian, V. CNS oxidative stress associated with the kainic acid rodent model of experimental epilepsy. Epilepsy Res. 2000, 39, 63–71. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinc, B.; Grabnar, I.; Vovk, T. Antioxidants as a preventive treatment for epileptic process: A review of the current status. Curr. Neuropharmacol. 2014, 12, 527–550. [Google Scholar] [CrossRef] [Green Version]
- Wong-ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.M.; Tieleman, D.P.; Monticelli, L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [Green Version]
- Blair, R.E.; Sombati, S.; Lawrence, D.C.; McCay, B.D.; DeLorenzo, R.J. Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J. Pharmacol. Exp. Ther. 2004, 310, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Pitkanen, A.; Lukasiuk, K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009, 14, 16–25. [Google Scholar] [CrossRef]
- Waldbaum, S.; Patel, M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res. 2010, 88, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Nasseh, I.E.; Amado, D.; Cavalheiro, E.A.; da Graça Naffah-Mazzacoratti, M.; Tengan, C.H. Investigation of mitochondrial involvement in the experimental model of epilepsy induced by pilocarpine. Epilepsy Res. 2006, 68, 229–239. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Liang, L.P.; Hellier, J.L.; Staley, K.J.; Patel, M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol. Dis. 2008, 30, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Jamme, I.; Petit, E.; Divoux, D.; Alain, G.; Maixent, J.M.; Nouvelot, A. Modulation of mouse cerebral Na+, K+-ATPase activity by oxygen free radicals. Neuroreport 1995, 7, 333–337. [Google Scholar] [PubMed]
- Fighera, M.R.; Royes, L.F.F.; Furian, A.F.; Oliveira, M.S.; Fiorenza, N.G.; Frussa-Filho, R.; Mello, C.F. GM1 ganglioside prevents seizures, Na+, K+-ATPase activity inhibition and oxidative stress induced by glutaric acid and pentylenetetrazole. Neurobiol. Dis. 2006, 22, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Henshall, D.C.; Murphy, B.M. Modulators of neuronal cell death in epilepsy. Curr. Opin. Pharm. 2008, 8, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Sutula, T.P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002, 1, 173–181. [Google Scholar] [CrossRef]
- Pauletti, A.; Terrone, G.; Shekh-Ahmad, T.; Salamone, A.; Ravizza, T.; Rizzi, M.; Balosso, S. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain 2019, 142, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Smith, J.; Patel, M. Metabolic dysfunction and oxidative stress in epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef] [Green Version]
- Rechinger, K.H. Compositae VII: Calendula; Flora Iranica No. 99-105; Akademische Druck-und Verlagsanstalt: Graz, Austria, 1989. [Google Scholar]
- Hassanzadazar, H.; Taami, B.; Aminzare, M.; Daneshamooz, S. Bunium persicum (Boiss.) B. Fedtsch: An overview on Phytochemistry, Therapeutic uses and its application in the food industry. J. Appl. Pharm. Sci. 2018, 8, 150–158. [Google Scholar]
- Tavakoli, K.A.; Keyhani, A.; Mahmoudvand, H.; Tavakoli, O.R.; Asadi, A.; Andishmand, M.; Azzizian, H.; Babaei, Z.; Zia-Ali, N. Efficacy of the Bunium persicum (Boiss) Essential Oil against Acute Toxoplasmosis in Mice Model. Iran. J. Parasitol. 2015, 10, 625–631. [Google Scholar]
- Shahsavari, N.; Barzegar, M.; Sahari, M.A.; Naghdibadi, H. Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant Foods Hum. Nutr. 2008, 63, 183–188. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Sajjadi, S.E.; Zomorodkia, M. Antinociceptive and anti-inflammatory activities of Bunium persicum essential oil, hydroalcoholic and polyphenolic extracts in animal models. Pharm. Biol. 2011, 49, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Nickavar, B.; Adeli, A.; Nickavar, A. Analyses of the essential oil from Bunium persicum fruit and its antioxidant constituents. J. Oleo Sci. 2014, 63, 741–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharafati, C.F.; Saholi, M.; Sharafati, C.R. Chemical composition, antioxidant and antibacterial activity of Bunium persicum, Eucalyptus globulus, and Rose Water on multidrug-resistant Listeria species. J. Evid.-Based Integr. Med. 2018, 23, 2515690X17751314. [Google Scholar]
- Mandegary, A.; Arab-Nozari, M.; Ramiar, H.; Sharififar, F. Anticonvulsant activity of the essential oil and methanolic extract of Bunium persicum (Boiss). B. Fedtsch. J. Ethnopharmacol. 2012, 140, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Maghrani, M.; Lemhadri, A.; Ouahidi, M.-L.; Jouad, H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 2002, 82, 97–103. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.A.; Morelli, I.; Cioni, P.L.; Bader, A.; Marino, A.; Alonzo, V. Preservative properties of Calamintha officinalis essential oil with and without EDTA. Lett. Appl. Microbiol. 2002, 35, 385–389. [Google Scholar] [CrossRef]
- Bouchra, C.; Achouri, M.; Hassani, L.M.I.; Hmamouchi, M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol. 2003, 89, 165–169. [Google Scholar] [CrossRef]
- Monforte, M.T.; Tzakou, O.; Nostro, A.; Zimbalatti, V.; Galati, E.M. Chemical composition and biological activities of Calamintha officinalis Moench essential oil. J. Med. Food 2011, 14, 297–303. [Google Scholar] [CrossRef]
- de Sousa, D.P.; de Farias, N.F.F.; de Almeida, R.N. Influence of the chirality of (R)-(−)- and (S)-(+)-carvone in the central nervous system: A comparative study. Chirality 2007, 19, 264–268. [Google Scholar] [CrossRef]
- Pernet, R.; Meyer, G. Pharmacopée de Madagascar; L’institut de Recherche Scientifique Tananarive-Tsimbazaza: Antananarivo, Madagascar, 1957; pp. 1–86. [Google Scholar]
- Beaujard, P. Plantes et medecine traditionnelle dans le Sud-Est de Madagascar. J. Ethnopharmacol. 1988, 23, 165–265. [Google Scholar] [CrossRef]
- Pavela, R.F.; Maggi, S.L.; Ngahang-Kamte, R.; Rakotosaona, P.; Rasoanaivo, M.; Nicoletti, A.; Canale, G.; Benelli, G. Chemical composition of Cinnamosma madagascariensis (Cannelaceae) essential oil and its larvicidal potential against the filariasis vector Culex quinquefasciatus Say. S. Afr. J. Bot. 2017, 108, 359–363. [Google Scholar] [CrossRef]
- Rakotosaona, R.; Randrianarivo, E.; Rasoanaivo, P.; Nicoletti, M.; Benelli, G.; Maggi, F. Effect of the Leaf Essential Oil from Cinnamosma madagascariensis Danguy on Pentylenetetrazol-induced Seizure in Rats. Chem. Biodivers. 2017, 14, e1700256. [Google Scholar] [CrossRef] [PubMed]
- Viana, G.S.; do Vale, T.G.; Silva, C.M.; Matos, F.J. Anticonvulsant activity of essential oils and active principles from chemotypes of Lippia alba (Mill.) N.E. Brown. Biol. Pharm. Bull. 2000, 23, 1314–1317. [Google Scholar] [PubMed] [Green Version]
- Vatanparast, J.; Bazleh, S.; Janahmadi, M. The effects of linalool on the excitability of central neurons of snail Caucasotachea atrolabiata. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 192, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Shabanian, G.; Rafieian-Kopaei, M.; Parvin, N.; Saadat, M.; Akhlaghi, M. Citrus aurantium blossom and preoperative anxiety. Rev. Bras. Anestesiol. 2011, 61, 702–712. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.Y.; Jiang, J.G.; Zhu, W.; Ou-Yang, Q. Anti-inflammatory Effect of Essential Oil from Citrus aurantium L. var. amara Engl. J. Agric. Food Chem. 2017, 65, 8586–8594. [Google Scholar] [CrossRef]
- Costa, C.A.; Cury, T.C.; Cassettari, B.O.; Takahira, R.K.; Flório, J.C.; Costa, M. Citrus aurantium L. essential oil exhibits anxiolytic-like activity mediated by 5-HT(1A)-receptors and reduces cholesterol after repeated oral treatment. BMC Complement. Altern. Med. 2013, 13, 42. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Sedaghat, M.M.; Vatandoost, H.; Abai, M.R. Chemical Compositions of the Peel Essential Oil of Citrus aurantium and Its Natural Larvicidal Activity against the Malaria Vector Anopheles stephensi (Diptera: Culicidae) in Comparison with Citrus paradisi. J. Arthropod-Borne Dis. 2016, 10, 577–585. [Google Scholar]
- Hsouna, B.A.; Gargouri, M.; Dhifi, W.; Saad, B.R.; Sayahi, N.; Mnif, W.; Saibi, W. Potential anti-inflammatory and antioxidant effects of Citrus aurantium essential oil against carbon tetrachloride-mediated hepatotoxicity: A biochemical, molecular and histopathological changes in adult rats. Environ. Toxicol. 2019, 34, 388–400. [Google Scholar] [CrossRef]
- Azanchi, T.; Shafaroodi, H.; Asgarpanah, J. Anticonvulsant activity of Citrus aurantium blossom essential oil (neroli): Involvment of the GABAergic system. Nat. Prod. Commun. 2014, 9, 1615–1618. [Google Scholar]
- Okwu, D.E.; Morah, F.N. Mineral and nutritive value of Dennettia tripetala fruits. Fruits 2004, 59, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Adjalian, E.; Sessou, P.; Fifa, T.D.; Dangou, B.J.; Odjo, T.; Figueredo, G. Chemical composition and bioefficacy of Dennettia tripetala and Uvariodendron angustifolium leaves essential oils against the angoumois grain moth, Sitotroga cerealella. Int. J. Biosci. 2014, 5, 161–172. [Google Scholar]
- Agbakwuru, E.O.P.; Osisiogu, I.U.; Rucker, G. Constituents of essential oil of Dennettia tripetala G. Baker (Annonaceae). Niger. J. Pharm. 1979, 10, 203–208. [Google Scholar]
- Okoh, S.O.; Iweriegbor, B.C.; Okoh, O.O.; Nwodo, U.U.I.; Okoh, A. Bactericidal and antioxidant properties of essential oils from the fruits Dennettia tripetala G. Baker. BMC Complement. Altern. Med. 2016, 16, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyemitan, I.A.; Elusiyan, C.A.; Akanmu, M.A.; Olugbade, T.A. Hypnotic, anticonvulsant and anxiolytic effects of 1-nitro-2-phenylethane isolated from the essential oil of Dennettia tripetala in mice. Phytomedicine 2013, 20, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Sharma, S.; Dua, A.; Mahajan, R. Antibacterial potential of polyphenol rich methanol extract of Cardamom (Amomum subulatum). J. Innov. Biol. 2016, 3, 271–275. [Google Scholar]
- Ajarem, J.S.; Ahmad, M. Effect of perinatal exposure of Cardamom (Elettaria cadrdamomum) on the post-natal development and social behaviour of mice offspring. J. King Saud Univ. Sci. 1991, 4, 34–57. [Google Scholar]
- Al-Zuhair, H.; el-Sayeh, B.; Ameen, H.A.; Al-Shoora, H. Pharmacological studies of cardamom oil in animals. Pharmacol. Res. 1996, 34, 79–82. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M. Cardamom (Elettaria cardamomum) perinatal exposure effects on the development, behavior and biochemical parameters in mice offspring. Saudi J. Biol. Sci. 2018, 25, 186–193. [Google Scholar] [CrossRef]
- Masoumi-Ardakani, Y.; Mahmoudvand, H.; Mirzaei, A.; Esmaeilpour, K.; Ghazvini, H.; Khalifeh, S.; Sepehri, G. The effect of Elettaria cardamomum extract on anxiety-like behavior in a rat model of post-traumatic stress disorder. Biomed. Pharmacother. 2017, 87, 489–495. [Google Scholar] [CrossRef]
- Masoumi-Ardakani, Y.; Mandegary, A.; Esmaeilpour, K.; Najafipour, H.; Sharififar, F.; Pakravanan, M.; Ghazvini, H. Chemical Composition, Anticonvulsant Activity, and Toxicity of Essential Oil and Methanolic Extract of Elettaria cardamomum. Planta Med. 2016, 82, 1482–1486. [Google Scholar] [CrossRef]
- Suryanarayana, L.; Mounica, P.; Prabhakar, A.; Sreekanth, G.; Rao, B.; Kumar, B.; Rao, A. Differentiating the gum resins of two closely related Indian Gardenia species G. gummifera and G. lucida, and establishing the source of Dikamali by TLC. JPC J. Planar Chromatogr. Mod. TLC 2012, 25, 363–367. [Google Scholar] [CrossRef]
- Chopra, R.N.; Nayar, S.L.; Chopra, C.L. Glossary of Indian Medicinal Plants Council of Scientific and Industrial Research; Council of Scientific & Industrial Research: New Delhi, India, 1956; p. 123. [Google Scholar]
- Shareef, M.Z.; Yellu, N.R.; Achanta, V.N. Neuropharmacological screening of essential oil from oleo gum resin of Gardenia lucida Roxb. J. Ethnopharmacol. 2013, 149, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Aghili, M.H. Makhzan Al-Adviah; Research Institute for Islamic and Complementary Medicine: Tehran, Iran, 2008; p. 239. [Google Scholar]
- Koch, C.; Reichling, J.; Schneele, J.; Schnitzler, P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine 2008, 15, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Asadollahpoor, A.; Abdollahi, M.; Rahimi, R. Pimpinella anisum L. fruit: Chemical composition and effect on rat model of nonalcoholic fatty liver disease. J. Res. Med. Sci. 2017, 15, 22–37. [Google Scholar]
- Samojlik, I.; Mijatović, V.; Petković, S.; Skrbić, B.; Božin, B. The influence of essential oil of aniseed (Pimpinella anisum, L.) on drug effects on the central nervous system. Fitoterapia 2012, 83, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Felšöciová, S.; Kačániová, M.; Horská, E.; Vukovič, N.; Hleba, L.; Petrová, J.; Rovná, K.; Stričík, M.; Hajduová, Z. Antifungal activity of essential oils against selected terverticillate penicillia. Ann. Agric. Environ. Med. 2015, 22, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Bardouki, H.; Pappa, A. Phytochemical profile and evaluation of the biological activities of essential oils derived from the Greek aromatic plant species Ocimum basilicum, Mentha spicata, Pimpinella anisum and Fortunella margarita. Molecules 2016, 21, 1069. [Google Scholar] [CrossRef] [Green Version]
- Tavallali, V.; Rahmati, S.; Bahmanzadegan, A. Antioxidant activity, polyphenolic contents and essential oil composition of Pimpinella anisum L. as affected by zinc fertilizer. J. Sci. Food Agric. 2017, 97, 4883–4889. [Google Scholar] [CrossRef]
- Karimzadeh, F.; Hosseini, M.; Mangeng, D.; Alavi, H.; Hassanzadeh, G.R.; Bayat, M.; Jafarian, M.; Kazemi, H.; Gorji, A. Anticonvulsant and neuroprotective effects of Pimpinella anisum in rat brain. BMC Complement. Altern. Med. 2012, 12, 76. [Google Scholar] [CrossRef] [Green Version]
- Mbongue, F.G.Y.; Kamtchouing, P.; Essame, O.J.L.; Yewah, P.M.; Dimo, T.; Lontsi, D. Effectoftheaqueousextractofdryfruitsof Piper guineense on the reproductivefunctionofadultmalerats. Indian J. Pharmacol. 2005, 7, 30–32. [Google Scholar] [CrossRef] [Green Version]
- Sumathykutty, M.A.; Rao, J.M.; Padmakumari, K.P.; Narayanan, C.S. Essential oil constituents of some piper species. Flavors Fragr. J. 1999, 14, 279–282. [Google Scholar] [CrossRef]
- Kabiru, A.Y.; Ibikunle, G.F.; Innalegwu, D.A.; Bola, B.M.; Madaki, F.M. In Vivo Antiplasmodial and Analgesic Effect of Crude Ethanol Extract of Piper guineense Leaf Extract in Albino Mice. Scientifica 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyinloye, B.E.; Osunsanmi, F.O.; Ajiboye, B.O.; Ojo, O.A.; Kappo, A.P. Modulatory Effect of Methanol Extract of Piper guineense in CCl₄-Induced Hepatotoxicity in Male Rats. Int. J. Environ. Res. Public Health 2017, 14, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukwandu, N.C.; Odaibo, A.B.; Okorie, T.G.; Nmorsi, O.P. Molluscicidal effect of Piper guineense. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 447–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tankam, J.M.; Ito, M. Inhalation of the essential oil of Piper guineense from Cameroon shows sedative and anxiolytic-like effects in mice. Biol. Pharm. Bull. 2013, 36, 1608–1614. [Google Scholar] [CrossRef] [Green Version]
- Oyemitan, I.A.; Olayera, O.A.; Alabi, A.; Abass, L.A.; Elusiyan, C.A.; Oyedeji, A.O.; Akanmu, M.A. Psychoneuropharmacological activities and chemical composition of essential oil of fresh fruits of Piper guineense (Piperaceae) in mice. J. Ethnopharmacol. 2015, 166, 240–249. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Odubanjo, O.V.; Akinbola, I.A. Antioxidative properties and inhibition of key enzymes relevant to type-2 diabetes and hypertension by essential oils from black pepper. Adv. Pharmacol. Sci. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Elisabetsky, E.; Brum, L.F.; Souza, D.O. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine 1999, 6, 107–113. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- Mehrabi, Y.; Mehrabi, N. Determination of feed nutritive value of Smyrnium cordifolium Boiss in animal nutrition. J. Sci. Res. 2011, 10, 659–663. [Google Scholar]
- Khanahmadi, M.; Rezazadeh, S.H.; Taran, M. In Vitro Antimicrobial and Antioxidant Properties of Smyrnium cordifolium Boiss. (Umbelliferae) Extract. Asian J. Plant Sci. 2010, 9, 99–103. [Google Scholar] [CrossRef]
- Tabaraki, R.; Ghadiri, F. In Vitro antioxidant activities of aqueous and ethanolic extracts of Smyrnium cordifolium Boiss and Sinapis arvensis L. Int. Food Res. J. 2013, 20, 2111–2115. [Google Scholar]
- Abbasi, N.; Mohammadyari, E.; Asadollahi, K.; Tahmasebi, M.; Ghobad, A.; Taherikalani, M. Medicinal characteristics of Smyrnium cordifolium Boiss. plant extract in rats. J. Med. Plant Res. 2014, 8, 395–400. [Google Scholar]
- Abbasi, N.; Mohammadpour, S.; Karimi, E.; Aidy, A.; Karimi, P.; Azizi, M.; Asadollah, K. Protective effects of Smyrnium cordifolium boiss essential oil on pentylenetetrazol-induced seizures in mice: Involvement of benzodiazepine and opioid antagonists. J. Biol. Regul. Homeost. Agents 2017, 31, 683–689. [Google Scholar] [PubMed]
- Fani, M.; Kohanteb, J. In Vitro antimicrobial activity of thymus vulgaris essential oil against major oral pathogens. J. Evid. Based Complement. Altern. Med. 2017, 22, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.E.; Benincasa, B.I.; Fachin, A.L.; Franca, S.C.; Contini, S.S.; Chagas, A.C.; Beleboni, R.O. Thymus vulgaris L. essential oil and its main component thymol: Anthelmintic effects against Haemonchus contortus from sheep. Vet. Parasitol. 2016, 228, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Abdelli, W.; Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, E.; Jirovetz, L. Chemical composition and anti-inflammatory activity of algerian thymus vulgaris essential oil. Nat. Prod. Commun. 2017, 12, 611–614. [Google Scholar] [CrossRef] [Green Version]
- Skalicka-Woźniak, K.; Walasek, M.; Aljarba, T.M.; Stapleton, P.; Gibbons, S.; Xiao, J.; Łuszczki, J.J. The anticonvulsant and anti-plasmid conjugation potential of Thymus vulgaris chemistry: An in vivo murine and in vitro study. Food Chem. Toxicol. 2018, 120, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Saei-Dehkordi, S.S.; Tajik, H.; Moradi, M.; Khalighi-Sigaroodi, F. Chemical composition of essential oils in Zataria multiflora Boiss. From different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem. Toxicol. 2010, 48, 1562–1567. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B.; Tajik, H.; Aliakbarlu, J. Physicochemical and antioxidative characteristics of Iranian pomegranate (Punica granatum L. cv. Rabbab-e-Neyriz) juice and comparison of its antioxidative activity with Zataria multiflora Boiss essential oil. Vet. Res. Forum 2014, 5, 313–318. [Google Scholar]
- Saedi, E.D.; Mahmoudvand, H.; Sharififar, F.; Fallahi, S.; Monzote, L.; Ezatkhah, F. Chemical composition along with anti-leishmanial and cytotoxic activity of Zataria multiflora. Pharm. Biol. 2016, 54, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Raeisi, M.; Tajik, H.; Razavi-Rohani, S.M.; Tepe, B.; Kiani, H.; Khoshbakht, R.; Shirzad, A.H.; Tadrisi, H. Inhibitory effect of Zataria multiflora Boiss. essential oil, alone and in combination with monolaurin, on Listeria monocytogenes. Vet. Res. Forum 2016, 7, 7–11. [Google Scholar] [PubMed]
- Majlessi, N.; Choopani, S.; Kamalinejad, M.; Azizi, Z. Amelioration of amyloid β-induced cognitive deficits by Zataria multiflora Boiss. essential oil in a rat model of Alzheimer’s disease. CNS Neurosci. Ther. 2012, 18, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kavoosi, G.; da Silva, J.A.T. Inhibitory effects of Zataria multiflora essential oil and its main components on nitric oxide and hydrogen peroxide production in glucose-stimulated human monocyte. Food Chem. Toxicol. 2012, 50, 3079–3085. [Google Scholar] [CrossRef] [PubMed]
- Karimian, P.; Kavoosi, G.; Saharkhiz, M.J. Antioxidant, nitric oxide scavenging and malondialdehyde scavenging activities of essential oils from different chemotypes of Zataria multiflora. Nat. Prod. Res. 2012, 26, 2144–2147. [Google Scholar]
- Mandegary, A.; Sharififar, F.; Abdar, M. Anticonvulsant effect of the essential oil and methanolic extracts of Zataria multiflora Boiss. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 93–97. [Google Scholar] [CrossRef]
- Aynehchi, Y. Pharmacognosy and Medicinal Plants of Iran; Tehran University Press: Tehran, Iran, 1986. [Google Scholar]
- Davari, M.; Ezazi, R. Chemical composition and antifungal activity of the essential oil of Zhumeria majdae, Heracleum persicum and Eucalyptus sp. against some important phytopathogenic fungi. J. Mycol. Med. 2017, 27, 463–468. [Google Scholar] [CrossRef]
- Valifard, M.; Omidpanah, N.; Farshad, S.; Shahidi, M.A.; Mohsenzadeh, S.; Farshad, O. Antibacterial activity of extracts and essential oils of two Iranian medicinal plants, Salvia mirzayanii and Zhumeria majdae, against Helicobacter pylori. Middle East J. Sci. Res. 2014, 19, 1001–1006. [Google Scholar]
- Hosseinzadeh, H.; Ramezani, M.; Fadishei, M.; Mahmoudi, M. Antinociceptive, anti-inflammatory and acute toxicity effects of Zhumeria majdae extracts in mice and rats. Phytomedicine 2002, 9, 135–141. [Google Scholar] [CrossRef]
- Moein, M.R.; Pawar, R.S.; Khan, S.I.; Tekwani, B.L.; Khan, I.A. Antileishmanial, antiplasmodial and cytotoxic activities of 12,16-dideoxy aegyptinone B from Zhumeria majdae Rech. f. & Wendelbo. Phytother. Res. 2008, 22, 283–285. [Google Scholar]
- Moein, S.; Moein, M.R. Relationship between antioxidant properties and phenolics in Zhumeria majdae. J. Med. Plants Res. 2010, 2010, 517–521. [Google Scholar]
- Mandegary, A.; Sharififar, F.; Abdar, M.; Arab-Nozari, M. Anticonvulsant activity and toxicity of essential oil and methanolic extract of Zhumeria majdae Rech, a unique Iranian plant in mice. Neurochem. Res. 2012, 37, 2725–2730. [Google Scholar] [CrossRef] [PubMed]
- Koutroumanidou, E.; Kimbaris, A.; Kortsaris, A.; Bezirtzoglou, E.; Polissiou, M.; Charalabopoulos, K.; Pagonopoulou, O. Increased seizure latency and decreased severity of pentylenetetrazol-induced seizures in mice after essential oil administration. Epilepsy Res. Treat. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Koppula, S.; Kumar, H.; Park, J.Y.; Kim, I.W.; More, S.V.; Kim, I.S.; Han, S.D.; Kim, S.K.; Yoon, S.H.; et al. Alpha-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioural deficits in a mouse model of Parkinson’s disease. Neuropharmacology 2015, 97, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Cheong, Y.J.; Koo, Y.M.; Kim, S.; Noh, C.K.; Son, Y.H.; Kang, C.; Sohn, N.W. α-Asarone Ameliorates Memory Deficit in Lipopolysaccharide-Treated Mice via Suppression of Pro-Inflammatory Cytokines and Microglial Activation. Biomol. Ther. 2014, 22, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Jo, M.J.; Kumar, H.; Joshi, H.P.; Choi, H.; Ko, W.K.; Kim, J.M.; Hwang, S.S.S.; Park, S.Y.; Sohn, S.; Bello, A.B.; et al. Oral Administration of α-Asarone Promotes Functional Recovery in Rats with Spinal Cord Injury. Front. Pharmacol. 2018, 7, 445. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Tian, Z.; Qin, S.L.; Zhao, P.Y.; Jiang, X.; Tian, Z. Anxiolytic-like effects of α-asarone in a mouse model of chronic pain. Metab. Brain Dis. 2017, 32, 2119–2129. [Google Scholar] [CrossRef]
- Chen, Q.X.; Miao, J.K.; Li, C.; Li, X.W.; Wu, X.M.; Zhang, X.P. Anticonvulsant activity of acute and chronic treatment with a-asarone from Acorus gramineus in seizure models. Biol. Pharm. Bull. 2013, 36, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Oyemitan, I.A.; Elusiyan, C.A.; Akinkunmi, E.O.; Obuotor, E.M.; Akanmu, M.A.; Olugbade, T.A. Memory enhancing, anticholinesterase and antimicrobial activities of β-phenylnitroethane and essential oil of Dennettia tripetala Baker f. J. Ethnopharmacol. 2019, 229, 256–261. [Google Scholar] [CrossRef]
- Huang, C.; Li, W.G.; Zhang, X.B.; Wang, L.; Xu, T.L.; Wu, D.; Li, Y. α-Asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Chellian, R.; Pandy, V. Protective effect of α-asarone against nicotine-induced seizures in mice, but not by its interaction with nicotinic acetylcholine receptors. Biomed. Pharmacother. 2018, 108, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Lai, X.; Xu, Y.; Miao, J.K.; Li, C.; Liu, J.Y.; Hua, Y.Y.; Ma, Q.; Chen, Q. α-Asarone Attenuates Cognitive Deficit in a Pilocarpine-Induced Status Epilepticus Rat Model via a Decrease in the Nuclear Factor-κB Activation and Reduction in Microglia Neuroinflammation. Front. Neurol. 2017, 8, 661. [Google Scholar] [CrossRef] [PubMed]
- Sundaramahalingam, M.; Ramasundaram, S.; Rathinasamy, S.D.; Natarajan, R.P.; Somasundaram, T. Role of Acorus calamus and alpha-asarone on hippocampal dependent memory in noise stress exposed rats. Pak. J. Biol. Sci. 2013, 16, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Yao, P.; Wang, H.; Duan, R.; Dong, T.T.; Tsim, K.W. Asarone from Acori Tatarinowii Rhizome prevents oxidative stress-induced cell injury in cultured astrocytes: A signaling triggered by Akt activation. PLoS ONE 2017, 12, e0179077. [Google Scholar] [CrossRef] [Green Version]
- Felipe, C.F.B.; Albuquerque, A.M.S.; de Pontes, J.L.X.; de Melo, J.Í.V.; Rodrigues, T.C.M.L.; de Sousa, A.M.P.; de Almeida, R.N. Comparative study of alpha-and beta-pinene effect on PTZ-induced convulsions in mice. Fund. Clin. Pharmacol. 2019, 33, 181–190. [Google Scholar] [CrossRef]
- Kim, D.; Suh, Y.; Lee, H.; Lee, Y. Immune activation and antitumor response of ar-turmerone on P388D1 lymphoblast cell implanted tumors. Int. J. Mol. Med. 2013, 31, 386–392. [Google Scholar] [CrossRef]
- Ali, A.; Wang, Y.H.; Khan, I.A. Larvicidal and Biting Deterrent Activity of Essential Oils of Curcuma longa, Ar-turmerone, and Curcuminoids Against Aedes aegypti and Anopheles quadrimaculatus (Culicidae: Diptera). J. Med. Entomol. 2015, 52, 979–986. [Google Scholar] [CrossRef]
- Jankasem, M.; Wuthi-Udomlert, M.; Gritsanapan, W. Antidermatophytic Properties of Ar-Turmerone, Turmeric Oil, and Curcuma longa Preparations. ISRN Dermatol. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Han, A.R.; Park, H.R.; Jang, E.J.; Kim, H.K.; Jeong, M.G.; Song, H.; Park, G.H.; Seo, E.K.; Hwang, E.S. Suppression of Inflammatory cytokine production by ar-Turmerone isolated from Curcuma phaeocaulis. Chem. Biodivers. 2014, 11, 1034–1041. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa. L. Indian J. Pharmacol. 2011, 43, 526. [Google Scholar]
- Orellana-Paucar, A.M.; Afrikanova, T.; Thomas, J.; Aibuldinov, Y.K.; Dehaen, W.; de Witte, P.A.; Esguerra, C.V. Insights from zebrafish and mouse models on the activity and safety of ar-turmerone as a potential drug candidate for the treatment of epilepsy. PLoS ONE 2013, 8, e81634. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Tintino, C.D.M.; Pessoa, R.T.; Fernandes, M.N.M.; Alcântara, I.S.; da Silva, B.A.F.; de Oliveira, M.R.C.; Martins, A.O.B.P.B.; da Silva, M.D.S.; Tintino, S.R.; Rodrigues, F.F.G.; et al. Anti-inflammatory and anti-edematogenic action of the Croton campestris A. St.-Hil (Euphorbiaceae) essential oil and the compound β-caryophyllene in in vivo models. Phytomedicine 2018, 41, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.L.B.; Silva, R.C.; da Silva, J.K.R.; Suemitsu, C.; Mourão, R.H.V.; Maia, J.G.S. Chemical variability in the essential oil of leaves of Araçá (Psidium guineense Sw.), with occurrence in the Amazon. Chem. Cent. J. 2018, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Wang, W.; Zhu, L. Larvicidal activity of Zanthoxylum acanthopodium essential oil against the malaria mosquitoes, Anopheles anthropophagus and Anopheles sinensis. Malar. J. 2018, 17, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, D.R.; Silva, D.M.; Florentino, I.F.; de Brito, A.; Fajemiroye, J.O.; Silva, D.P.B.; da Rocha, F.; Costa, E.A.; De Carvalho, P.G. Monoamine Involvement in the Antidepressant-Like Effect of β-Caryophyllene. CNS Neurol. Disord. Drug Targets 2018, 17, 309–320. [Google Scholar] [CrossRef]
- Oliveira, G.L.D.S.; Machado, K.C.; Machado, K.C.; da Silva, A.P.D.S.C.L.; Feitosa, C.M.; de Castro Almeida, F.R. Non-clinical toxicity of β-caryophyllene, a dietary cannabinoid: Absence of adverse effects in female Swiss mice. Regul. Toxicol. Pharmacol. 2018, 92, 338–346. [Google Scholar] [CrossRef]
- Liu, H.; Song, Z.; Liao, D.; Zhang, T.; Liu, F.; Zhuang, K.; Luo, K.; Yang, L. Neuroprotective effects of trans-caryophyllene against kainic acid induced seizure activity and oxidative stress in mice. Neurochem. Res. 2015, 40, 118–123. [Google Scholar] [CrossRef]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ma, W.; Du, J. β-Caryophyllene (BCP) ameliorates MPP+ induced cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Askari, V.R.; Shafiee-Nick, R. Promising neuroprotective effects of β-caryophyllene against LPS-induced oligodendrocyte toxicity: A mechanistic study. Biochem. Pharmacol. 2019, 159, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Yamamoto, M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- de Oliveira, C.C.; de Oliveira, C.V.; Grigoletto, J.; Ribeiro, L.R.; Funck, V.R.; Grauncke, A.C.; de Souza, T.L.; Souto, N.S.; Furian, A.F.; Menezes, I.R.; et al. Anticonvulsant activity of β-caryophyllene against pentylenetetrazol-induced seizures. Epilepsy Behav. 2016, 56, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Tchekalarova, J.; da Conceição Machado, K.; Júnior, A.L.G.; de Carvalho Melo Cavalcante, A.A.; Momchilova, A.; Tzoneva, R. Pharmacological characterization of the cannabinoid receptor 2 agonist, β-caryophyllene on seizure models in mice. Seizure 2018, 57, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Hattori, A. Camphor in the Edo era-camphor and borneol for medicines. Yakushigaku Zasshi 1999, 35, 49–54. [Google Scholar]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) Essential Oil on Acute Inflammatory Response. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jafari, A.; Rasmi, Y.; Hajaghazadeh, M.; Karimipour, M. Hepatoprotective effect of thymol against subchronic toxicity of titanium dioxide nanoparticles: Biochemical and histological evidences. Environ. Toxicol. Pharmacol. 2018, 58, 29–36. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Rahimmalek, M.; Sabzalian, M.R. Variations in Essential Oil Composition and Antioxidant Activity in Perovskia abrotanoides Kar. Collected from Different Regions in Iran. Chem. Biodivers. 2018, 15, e1700565. [Google Scholar] [CrossRef] [PubMed]
- Noroozisharaf, A.; Kaviani, M. Effect of soil application of humic acid on nutrients uptake, essential oil and chemical compositions of garden thyme (Thymus vulgaris L.) under greenhouse conditions. Physiol. Mol. Biol. Plants 2018, 24, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Estarrón, M.; Fuentes, V. Essential oil of rosemary (Rosmarinus officinalis L.) from Cuba. J. Essent. Oil Res. 1998, 10, 111–112. [Google Scholar] [CrossRef]
- Lima, C.F.; Carvalho, F.; Fernandes, E.; Bastos, M.D.L.; Santos-Gomes, P.C.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Evaluation of toxic/protective effects of the essential oil of Salvia officinalis on freshly isolated rat hepatocytes. Toxicol. In Vitro 2004, 18, 457–465. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, Y.Y.; Li, J.; Lin, Y.H.; Luo, C.X.; Zhu, D.Y. (+)-Borneol alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. Eur. J. Pharmacol. 2015, 757, 53–58. [Google Scholar] [CrossRef]
- Zhou, H.H.; Zhang, L.; Zhou, Q.G.; Fang, Y.; Ge, W.H. (+)-Borneol attenuates oxaliplatin-induced neuropathic hyperalgesia in mice. Neuroreport 2016, 27, 160–165. [Google Scholar] [CrossRef]
- Cao, B.; Ni, H.Y.; Li, J.; Zhou, Y.; Bian, X.L.; Tao, Y.; Cai, C.Y.; Qin, C.; Wu, H.Y.; Chang, L.; et al. (+)-Borneol suppresses conditioned fear recall and anxiety-like behaviors in mice. Biochem. Biophys. Res. Commun. 2018, 495, 1588–1593. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, A.; Lu, H.; Cheng, Q. The Role and Mechanism of Borneol to Open the Blood-Brain Barrier. Integr. Cancer Ther. 2018, 17, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.L.; Fu, B.M.; Zhang, Z.J. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood-brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Yin, C.Y.; Wu, H.Y.; Tian, B.B.; Zhu, Y.; Luo, C.X.; Zhu, D.Y. (+)-Borneol is neuroprotective against permanent cerebral ischemia in rats by suppressing production of proinflammatory cytokines. J. Biomed. Res. 2017, 31, 306–314. [Google Scholar]
- Tambe, R.; Jain, P.; Patil, S.; Ghumatkar, P.; Sathaye, S. Antiepileptogenic effects of borneol in pentylenetetrazole-induced kindling in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, L.; Lan, X.; Li, L.; Zhang, T.T.; Sun, J.H.; Du, G.H. Protection by borneol on cortical neurons against oxygen-glucose deprivation/reperfusion: Involvement of anti-oxidation and anti-inflammation through nuclear transcription factor κappaB signaling pathway. Neuroscience 2011, 176, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, S.; Wang, L. Purification and identification of carvacrol from the root of Stellera chamaejasme and research on its insecticidal activity. Nat. Prod. Res. 2011, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Choi, Y.; Jang, J.; Park, T. Carvacrol Protects against Hepatic Steatosis in Mice Fed a High-Fat Diet by Enhancing SIRT1-AMPK Signaling. Evid. Based Complement Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura de Vasconcelos, S.S.; Caleffi-Ferracioli, K.R.; Hegeto, L.A.; Baldin, V.P.; Nakamura, C.V.; Stefanello, T.F.; Freitas, G.G.; Yamazaki, D.A.; Scodro, R.B.; Siqueira, V.L.; et al. Carvacrol activity & morphological changes in Mycobacterium tuberculosis. Future Microbiol. 2018, 13, 877–888. [Google Scholar] [PubMed]
- Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anticancer Drugs 2015, 26, 813–823. [Google Scholar] [CrossRef]
- Alavinezhad, A.; Khazdair, M.; Boskabady, M.H. Possible therapeutic effect of carvacrol on asthmatic patients: A randomized, double blind, placebo-controlled, Phase II clinical trial. Phytother. Res. 2018, 32, 151–159. [Google Scholar] [CrossRef]
- Silva, J.C.; Almeida, J.R.G.S.; Quintans, J.S.S.; Gopalsamy, R.G.; Shanmugam, S.; Serafini, M.R.; Oliveira, M.R.C.; Silva, B.A.F.; Martins, A.O.B.P.B.; Castro, F.F.; et al. Enhancement of orofacial antinociceptive effect of carvacrol, a monoterpene present in oregano and thyme oils, by β-cyclodextrin inclusion complex in mice. Biomed. Pharmacother. 2016, 84, 454–461. [Google Scholar] [CrossRef]
- Nóbrega, R.O.; Teixeira, A.P.; Oliveira, W.A.; Lima, E.O.; Lima, I.O. Investigation of the antifungal activity of carvacrol against strains of Cryptococcus neoformans. Pharm. Biol. 2016, 54, 2591–2596. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ba, L.; Huang, W.; Liu, Y.; Pan, H.; Mingyao, E.; Shi, P.; Wang, Y.; Li, S.; Qi, H.; et al. Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur. J. Pharmacol. 2017, 796, 90–100. [Google Scholar] [CrossRef]
- Dati, L.M.; Ulrich, H.; Real, C.C.; Feng, Z.P.; Sun, H.S.; Britto, L.R. Carvacrol promotes neuroprotection in the mouse hemiparkinsonian model. Neuroscience 2017, 356, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, H.; Rajaei, Z.; Alaei, H.; Shahidani, S. Chronic treatment with carvacrol improves passive avoidance memory in a rat model of Parkinson’s disease. Arq. Neuropsiquiatr. 2018, 76, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Celik, F.; Gocmez, C.; Bozkurt, M.; Kaplan, I.; Kamasak, K.; Akil, E.; Uzar, E. Neuroprotective effects of carvacrol and pomegranate against methotrexate-induced toxicity in rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2988–2993. [Google Scholar] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F.; Borji, A. Protective effects of carvacrol against oxidative stress induced by chronic stress in rat’s brain, liver, and kidney. Biochem. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Elhady, M.A.; Khalaf, A.A.A.; Kamel, M.M.; Noshy, P.A. Carvacrol ameliorates behavioral disturbances and DNA damage in the brain of rats exposed to propiconazole. Neurotoxicology 2019, 70, 19–25. [Google Scholar] [CrossRef]
- Mishra, R.K.; Baker, M.T. Seizure prevention by the naturally occurring phenols, carvacrol and thymol in a partial seizure-psychomotor model. Bioorg. Med. Chem. Lett. 2014, 24, 5446–5449. [Google Scholar] [CrossRef]
- Khalil, A.; Kovac, S.; Morris, G.; Walker, M.C. Carvacrol after status epilepticus (SE) prevents recurrent SE, early seizures, cell death, and cognitive decline. Epilepsia 2017, 58, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Sadegh, M.; Sakhaie, M.H. Carvacrol mitigates proconvulsive effects of lipopolysaccharide, possibly through the hippocampal cyclooxygenase-2 inhibition. Metab. Brain Dis. 2018, 33, 2045–2050. [Google Scholar] [CrossRef]
- Moraes, J.; Carvalho, A.A.; Nakano, E.; de Almeida, A.A.; Marques, T.H.; Andrade, L.N.; de Freitas, R.M.; de Sousa, D.P. Anthelmintic activity of carvacryl acetate against Schistosoma mansoni. Parasitol. Res. 2013, 112, 603–610. [Google Scholar] [CrossRef]
- Damasceno, S.R.; Oliveira, F.R.; Carvalho, N.S.; Brito, C.F.; Silva, I.S.; Sousa, F.B.; Silva, R.O.; Sousa, D.P.; Barbosa, A.L.; Freitas, R.M.; et al. Carvacryl acetate, a derivative of carvacrol, reduces nociceptive and inflammatory response in mice. Life Sci. 2014, 94, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, E.M.; Sousa, N.A.; de Araújo, S.; Júnior, J.L.P.; Araújo, A.R.; Iles, B.; Pacífico, D.M.; Brito, G.A.C.; Souza, E.P.; Sousa, D.P.; et al. Carvacryl acetate, a novel semisynthetic monoterpene ester, binds to the TRPA1 receptor and is effective in attenuating irinotecan-induced intestinal mucositis in mice. J. Pharm. Pharmacol. 2017, 69, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.F.; Costa, L.M.; Silva, O.A.; de Almeida, A.A.; Cerqueira, G.S.; de Sousa, D.P.; de Freitas, R.M. Anxiolytic-like effects of carvacryl acetate, a derivative of carvacrol, in mice. Pharmacol. Biochem. Behav. 2013, 112, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, L.F.; Costa, L.M.; de Almeida, A.A.; Silva, O.A.; Cerqueira, G.S.; de Sousa, D.P.; de Freitas, R.M. Is there a correlation between in vitro antioxidant potential and in vivo effect of carvacryl acetate against oxidative stress in mice hippocampus? Neurochem. Res. 2014, 39, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.F.; Costa, L.M.; de Almeida, A.A.; Silva, O.A.; Santos-Cerqueira, G.; de Sousa, D.P.; Pires, R.M.; Satyal, P.; de Freitas, R.M. Neuropharmacological effects of carvacryl acetate on δ-aminolevulinic dehydratase, Na+, K+-ATPase activities and amino acids levels in mice hippocampus after seizures. Chem. Biol. Interact. 2015, 226, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.F.; Hoffmann, M.S.; Gerbatin, R.R.; Fiorin, F.S.; Dobrachinski, F.; Mota, B.C.; Wouters, A.T.; Pavarini, S.P.; Soares, F.A.; Fighera, M.R.; et al. Treadmill exercise protects against pentylenetetrazol-induced seizures and oxidative stress after traumatic brain injury. J. Neurotrauma 2013, 30, 1278–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Su, G.; Tang, Z.; Wang, L.; Fu, W.; Zhao, S.; Ba, Y.; Bai, B.; Yue, P.; Lin, Y.; et al. Curcumol Exerts Anticancer Effect in Cholangiocarcinoma Cells via Down-Regulating CDKL3. Front. Physiol. 2018, 9, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zong, C.; Gao, Y.; Cai, R.; Fang, L.; Lu, J.; Liu, F.; Qi, Y. Curcumol exhibits anti-inflammatory properties by interfering with the JNK-mediated AP-1 pathway in lipopolysaccharide-activated RAW264.7 cells. Eur. J. Pharmacol. 2014, 723, 339–345. [Google Scholar] [CrossRef]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Wang, J.J.; Huang, C.; Wang, L.; Deng, S.; Xu, T.L.; Ge, W.H.; Li, W.G.; Li, F. Curcumol from Rhizoma Curcumae suppresses epileptic seizure by facilitation of GABA(A) receptors. Neuropharmacology 2014, 81, 244–255. [Google Scholar] [CrossRef]
- Liu, Y.M.; Fan, H.R.; Ding, J.; Huang, C.; Deng, S.; Zhu, T.; Xu, T.L.; Ge, W.H.; Li, W.G.; Li, F. Curcumol allosterically modulates GABA(A) receptors in a manner distinct from benzodiazepines. Sci. Rep. 2017, 7, 46654. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, J.; Guo, J.; Wang, Q.; Zhu, S.; Gao, S.; Yang, C.; Wei, M.; Pan, X.; Zhu, W.; et al. Cytotoxic and Antitumor Effects of Curzerene from Curcuma longa. Planta Med. 2017, 83, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindarajan, M.; Rajeswary, M.; Senthilmurugan, S.; Vijayan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Curzerene, trans-β-elemenone, and γ-elemene as effective larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus: Toxicity on non-target aquatic predators. Environ. Sci. Pollut. Res. Int. 2018, 25, 10272–10282. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R. New or uncommon volatile components in the most diverse natural scents. Riv. Ital. EPPOS 1997, 18, 18–47. [Google Scholar]
- Jirovetz, L.; Buchbauer, G.; Shafi, P.M.; Abraham, G.T. Analysis of the essential oil of the roots of the medicinal plant Kaempferia galanga L. (Zingiberaceae) from South India. Acta Pharm. Turc. 2001, 43, 107–110. [Google Scholar]
- da Rocha, M.L.; Oliveira, L.E.; Patrício, C.C.; de Sousa, D.P.; de Almeida, R.N.; Araújo, D.A. Antinociceptive and anti-inflammatory effects of the monoterpene α,β-epoxy-carvone in mice. J. Nat. Med. 2013, 67, 743–749. [Google Scholar] [CrossRef]
- Siqueira, B.P.J.; Menezes, C.T.; Silva, J.P.; Sousa, D.P.; Batista, J.S. Antiulcer effect of epoxy-carvone. Rev. Bras. Farmacogn. 2012, 22, 144–149. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, D.P.; Júnior, G.A.S.; Andrade, L.N.; Calasans, F.R.; Nunes, X.P.; Barbosa-Filho, J.M.; Batista, J.S. Structural Relationships and Spasmolytic Activity of Monoterpene Analogues Found in Many Aromatic Plants. Z. Naturforsch. C 2008, 63, 808–812. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, D.P.; Nóbrega, F.F.F.; Claudino, F.S.; Almeida, R.N.; Leite, J.R.; Mattei, R. Pharmacological effects of the monoterpene α,β-epoxy-carvone in mice. Braz. J. Pharm. 2007, 17, 170–175. [Google Scholar] [CrossRef]
- Salgado, P.R.; Da Fonsêca, D.V.; Braga, R.M.; De Melo, C.G.; Andrade, L.N.; De Almeida, R.N.; De Sousa, D.P. Comparative Anticonvulsant Study of Epoxycarvone Stereoisomers. Molecules 2015, 20, 19660–19673. [Google Scholar] [CrossRef] [Green Version]
- Salgado, P.R.R.; da Fonsêca, D.V.; de Melo, C.G.F.; Leite, F.C.; Alves, A.F.; Ferreira, P.B.; de Almeida, R.N. Comparison of behavioral, neuroprotective, and proinflammatory cytokine modulating effects exercised by (+)-cis-EC and (−)-cis-EC stereoisomers in a PTZ-induced kindling test in mice. Fundam. Clin. Pharmacol. 2018, 32, 507–515. [Google Scholar] [CrossRef]
- Wu, B.N.; Hwang, T.L.; Liao, C.F.; Chen, I.J. Vaninolol: A new selective beta 1-adrenoceptor antagonist derived from vanillin. Biochem. Pharmacol. 1994, 48, 101–109. [Google Scholar] [PubMed]
- Senanayake, U.M.; Wills, R.B.H.; Lee, T.H. Biosynthesis of eugenol and cinnamic aldehyde in Cinnamomum zeylanicum. Phytochemistry 1977, 16, 2032–2033. [Google Scholar] [CrossRef]
- Burgoyne, C.C.; Giglio, J.A.; Reese, S.E.; Sima, A.P.; Laskin, D.M. The efficacy of a topical anesthetic gel in the relief of pain associated with localized alveolar osteitis. J. Oral Maxillofac. Surg. 2010, 68, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Jayaramaiah, R.H.; Agawane, S.B.; Vannuruswamy, G.; Korwar, A.M.; Anand, A.; Dhaygude, V.S.; Shaikh, M.L.; Joshi, R.S.; Boppana, R.; et al. Potential Dual Role of Eugenol in Inhibiting Advanced Glycation End Products in Diabetes: Proteomic and Mechanistic Insights. Sci. Rep. 2016, 6, 18798–18811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, N.; Liu, X.W.; Yang, Y.J.; Li, J.Y.; Mohamed, I.; Liu, G.R.; Zhang, J.Y. Preventive Effect of Aspirin Eugenol Ester on Thrombosis in κ-Carrageenan-Induced Rat Tail Thrombosis Model. PLoS ONE 2015, 10, e0133125. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—From the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- Jeong, K.H.; Lee, D.S.; Kim, S.R. Effects of eugenol on granule cell dispersion in a mouse model of temporal lobe epilepsy. Epilepsy Res. 2015, 115, 73–76. [Google Scholar] [CrossRef]
- Huang, C.W.; Chow, J.C.; Tsai, J.J.; Wu, S.N. Characterizing the effects of Eugenol on neuronal ionic currents and hyperexcitability. Psychopharmacology 2012, 221, 575–587. [Google Scholar] [CrossRef]
- Vatanparast, J.; Khalili, S.; Naseh, M. Dual effects of eugenol on the neuronal excitability: An in vitro study. Neurotoxicology 2017, 58, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Joushi, S.; Salmani, M.E. Effect of eugenol on lithium-pilocarpine model of epilepsy: Behavioral, histological, and molecular changes. Iran. J. Basic Med. Sci. 2017, 20, 745–752. [Google Scholar] [PubMed]
- Said, M.M.; Abd Rabo, M.M. Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain. Arh. Hig. Rada Toksikol. 2017, 68, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yogalakshmi, B.; Viswanathan, P.; Anuradha, C.V. Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 2010, 268, 204–212. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.A.; Lino, F.L.; Cappelari, S.E.; da Silva Brum, L.F.; Picada, J.N.; Pereira, P. Effects of gamma-decanolactone on seizures induced by PTZ-kindling in mice. Exp. Brain Res. 2008, 187, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, P.; Coelho, V.R.; Regner, G.G.; da Silva, L.L.; Martinez, K.; Fonseca, A.; Pereira, P. Neuropharmacological Profile of Gamma-Decanolactone on hemically-induced Seizure in Mice. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 222–227. [Google Scholar] [CrossRef]
- Pfluger, P.; Regner, G.G.; Coelho, V.R.; da Silva, L.L.; Nascimento, L.; Viau, C.M.; Pereira, P. Gamma-Decanolactone Improves Biochemical Parameters Associated with Pilocarpine-Induced Seizures in Male Mice. Curr. Mol. Pharmacol. 2018, 11, 162–169. [Google Scholar] [CrossRef]
- Linck, V.M.; da Silva, A.L.; Figueiro, M.; Caramao, E.B.; Moreno, P.R.; Elisabetsky, E. Effects of inhaled linalool in anxiety, social interaction and aggressive behavior inmice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- Batista, P.A.; Werner, M.F.; Oliveira, E.C.; Burgos, L.; Pereira, P.; Brum, L.F.; Story, G.M.; Santos, A.R. The antinociceptive effect of (−)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J. Pain 2010, 11, 1222–1229. [Google Scholar] [CrossRef]
- Magnard, J.L.; Bony, A.R.; Bettini, F.; Campanaro, A.; Blerot, B.; Baudino, S.; Jullien, F. Linalool and linalool nerolidol synthases in roses, several genes for little scent. Plant Physiol. Biochem. 2018, 127, 74–87. [Google Scholar] [CrossRef]
- Dos Santos, É.R.Q.; Maia, C.S.F.; Fontes-Junior, E.A.; Melo, A.S.; Pinheiro, B.G.; Maia, J.G.S. Linalool-rich essential oils from the Amazon display antidepressant-type effect in rodents. J. Ethnopharmacol. 2018, 212, 43–49. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Posada-Duque, R.; Cortes, N.C.; Arias-Londoño, J.D.; Cardona-Gómez, G.P. Changes in the hippocampal and peripheral phospholipid profiles are associated with neurodegeneration hallmarks in a long-term global cerebral ischemia model: Attenuation by Linalool. Neuropharmacology 2018, 135, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Jung, A.N.; Jung, Y.S. Linalool Ameliorates Memory Loss and Behavioral Impairment Induced by REM-Sleep Deprivation through the Serotonergic Pathway. Biomol. Ther. 2018, 26, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Sci. 2017, 174, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mehri, S.; Meshki, M.A.; Hosseinzadeh, H. Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wistar rats. Drug Chem. Toxicol. 2015, 38, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Demyttenaere, J.C.R.; Willemen, H.M. Biotransformation of linalool to furanoid and pyranoid linalool oxides by Aspergillus niger. Phytochemistry 1998, 47, 1029–1036. [Google Scholar] [CrossRef]
- Hilmer, J.M.; Gatfield, I.L. Process for the Preparation of Linalool Oxide or Linalool Oxide-Containing Mixtures. U.S. Patent US 6703, 9 March 2004. [Google Scholar]
- Souto-Maior, F.N.; de Carvalho, F.L.; de Morais, L.C.; Netto, S.M.; de Sousa, D.P.; de Almeida, R.N. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol. Biochem. Behav. 2011, 100, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Souto-Maior, F.N.; Fonsêca, D.V.; Salgado, P.R.; Monte, L.O.; de Sousa, D.P.; de Almeida, R.N. Antinociceptive and anticonvulsant effects of the monoterpene linalool oxide. Pharm. Biol. 2017, 55, 63–67. [Google Scholar] [CrossRef]
- Pacifico, S.; D’Abrosca, B.; Golino, A.; Mastellone, C.; Piccolella, S.; Fiorentino, A.; Monaco, P. Antioxidant evaluation of polyhydroxylated nerolidols from redroot pigweed (Amaranthus retroflexus) leaves. LWT Food Sci. Technol. 2008, 41, 1665–1671. [Google Scholar] [CrossRef]
- M’sou, S.; Alifriqui, M.; Romane, A. Phytochemical study and biological effects of the essential oil of Fraxinus dimorpha Coss & Durieu. Nat. Prod. Res. 2017, 31, 2797–2800. [Google Scholar]
- Ko, Y.J.; Ahn, G.; Ham, Y.M.; Song, S.M.; Ko, E.Y.; Cho, S.H.; Yoon, W.J.; Kim, K.N. Anti-inflammatory effect and mechanism of action of Lindera erythrocarpa essential oil in lipopolysaccharide-stimulated RAW264.7 cells. EXCLI J. 2017, 16, 1103–1113. [Google Scholar]
- Scalvenzi, L.; Grandini, A.; Spagnoletti, A.; Tacchini, M.; Neill, D.; Ballesteros, J.L.; Sacchetti, G.; Guerrini, A. Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile. Molecules 2017, 22, 1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceole, L.F.; Cardoso, M.D.G.; Soares, M.J. Nerolidol, the main constituent of Piper aduncum essential oil, has anti-Leishmania braziliensis activity. Parasitology 2017, 144, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.K.; Kaur, D.; Pahwa, P. Assessment of anxiolytic effect of nerolidol in mice. Indian J. Pharmacol. 2016, 48, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Krist, S.; Banovac, D.; Tabanca, N.; Wedge, D.E.; Gochev, V.K.; Wanner, J.; Schmidt, E.; Jirovetz, L. Antimicrobial activity of nerolidol and its derivatives against airborne microbes and further biological activities. Nat. Prod. Commun. 2015, 10, 143–148. [Google Scholar] [CrossRef]
- Fonsêca, D.V.; Salgado, P.R.; de Carvalho, F.L.; Salvadori, M.G.; Penha, A.R.; Leite, F.C.; Borges, C.J.; Piuvezam, M.R.; Pordeus, L.C.; Sousa, D.P.; et al. Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABAergic system and proinflammatory cytokines. Fundam. Clin. Pharmacol. 2016, 30, 14–22. [Google Scholar] [CrossRef]
- Melekoglu, R.; Ciftci, O.; Eraslan, S.; Cetin, A.; Basak, N. The beneficial effects of nerolidol and hesperidin on surgically induced endometriosis in a rat model. Gynecol. Endocrinol. 2018, 34, 975–980. [Google Scholar] [CrossRef]
- Kaur, D.; Pahwa, P.; Goel, R.K. Protective Effect of Nerolidol Against Pentylenetetrazol-Induced Kindling, Oxidative Stress and Associated Behavioral Comorbidities in Mice. Neurochem. Res. 2016, 41, 2859–2867. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Khair, S.B.A.; Ojha, S.; Haque, M.E. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef] [Green Version]
- Neto, J.D.N.; de Almeida, A.A.C.; da Silva, O.J.; dos Santos, P.S.; de Sousa, D.P.; de Freitas, R.M. Antioxidant effects of nerolidol in mice hippocampus after open field test. Neurochem. Res. 2013, 38, 1861–1870. [Google Scholar] [CrossRef]
- Gottlieb, O.R.; Magalhães, M.T. Occurrence of 1-nitro-2-phenylethane in Ocotea pretiosa and Aniba canelilla. J. Org. Chem. 1959, 24, 2070. [Google Scholar] [CrossRef]
- Gottlieb, O.R. Chemosystematics on the Lauraceae. Phytochemistry 1972, 5, 1537–1570. [Google Scholar] [CrossRef]
- Vale, J.K.; Lima, A.B.; Pinheiro, B.G.; Cardoso, A.S.; Silva, J.K.; Maia, J.G.; de Sousa, G.E.; da Silva, A.B.; Sousa, P.J.; Borges, R.S. Evaluation and theoretical study on the anti-inflammatory mechanism of 1-nitro-2-phenylethane. Planta Med. 2013, 79, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.B.; Santana, M.B.; Cardoso, A.S.; Silva, J.K.R.; Maia, J.G.S.; Carvalho, J.C.T.; Sousa, P.J.C. Antinociceptive activity of 1-nitro-2-phenylethane, the main component of Aniba canelilla essential oil. Phytomedicine 2009, 16, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Giongo, J.L.; Vaucher, R.A.; Da Silva, A.S.; Oliveira, C.B.; de Mattos, C.B.; Baldissera, M.D.; Sagrillo, M.R.; Monteiro, S.G.; Custódio, D.L.; Souza, M.; et al. Trypanocidal activity of the compounds present in Aniba canelilla oil against Trypanosoma evansi and its effects on viability of lymphocytes. Microb. Pathog. 2017, 103, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Cosker, F.; Lima, F.J.; Lahlou, S.; Magalhães, P.J. Cytoprotective effect of 1-nitro-2-phenylethane in mice pancreatic acinar cells subjected to taurocholate: Putative role of guanylyl cyclase-derived 8-nitro-cyclic-GMP. Biochem. Pharmacol. 2014, 91, 191–201. [Google Scholar] [CrossRef]
- Brito, T.S.; Lima, F.J.; Aragão, K.S.; de Siqueira, R.J.; Sousa, P.J.; Maia, J.G.; Filho, J.D.; Lahlou, S.; Magalhães, P.J. The vasorelaxant effects of 1-nitro-2-phenylethane involve stimulation of the soluble guanylate cyclase-cGMP pathway. Biochem. Pharmacol. 2013, 85, 780–788. [Google Scholar] [CrossRef] [Green Version]
- de Siqueira, R.J.; Macedo, F.I.; Interaminense, L.F.; Duarte, G.P.; Magalhães, P.J.; Brito, T.S.; da Silva, J.K.; Maia, J.G.; Sousa, P.J.; Leal-Cardoso, J.H.; et al. 1-Nitro-2-phenylethane, the main constituent of the essential oil of Aniba canelilla, elicits a vago-vagal bradycardiac and depressor reflex in normotensive rats. Eur. J. Pharmacol. 2010, 638, 90–98. [Google Scholar] [CrossRef]
- Wang, Y.; You, C.X.; Yang, K.; Wu, Y.; Chen, R.; Zhang, W.J.; Liu, Z.L.; Du, S.S.; Deng, Z.W.; Geng, Z.F.; et al. Bioactivity of Essential Oil of Zingiber purpureum Rhizomes and Its Main Compounds against Two Stored Product Insects. J. Econ. Entomol. 2015, 108, 925–932. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Ballero, M.; Sanna, C.; Donno, Y.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Tirillini, B.; et al. Essential oil composition and biological activity from Artemisia caerulescens subsp. densiflora (Viv.) Gamisans ex Kerguélen & Lambinon (Asteraceae), an endemic species in the habitat of La Maddalena Archipelago. Nat. Prod. Res. 2016, 30, 1802–1809. [Google Scholar]
- Ray, A.; Jena, S.; Kar, B.; Patnaik, J.; Panda, P.C.; Nayak, S. Chemical composition and antioxidant activities of essential oil of Hedychium greenii and Hedychium gracile from India. Nat. Prod. Res. 2017, 33, 1482–1485. [Google Scholar] [CrossRef]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Salvatore, G.; Mondello, F.; Arancia, G.; Molinari, A. Terpinen-4-ol, the main component of Melaleuca alternifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells. J. Investig. Dermatol. 2004, 122, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial Mechanism of Terpinen-4-ol Against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Murata, S.; Ito, H.; Iwasaki, K.; Villareal, M.O.; Zheng, Y.W.; Matsui, H.; Isoda, H.; Ohkohchi, N. Terpinen-4-ol inhibits colorectal cancer growth via reactive oxygen species. Oncol. Lett. 2017, 14, 2015–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morcia, C.; Malnati, M.; Terzi, V. In Vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A 2012, 29, 415–422. [Google Scholar]
- Lahlou, S.; Interaminense, L.F.; Leal-Cardoso, J.H.; Duarte, G.P. Antihypertensive effects of the essential oil of Alpinia zerumbet and its main constituent, terpinen-4-ol, in DOCA-salt hypertensive conscious rats. Fundam. Clin. Pharmacol. 2003, 17, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Hayama, K.; Ishijima, S.A.; Maruyama, N.; Irie, H.; Kurihara, J.; Abe, S. Suppression of inflammatory reactions by terpinen-4-ol, a main constituent of tea tree oil, in a murine model of oral candidiasis and its suppressive activity to cytokine production of macrophages in vitro. Biol. Pharm. Bull. 2013, 36, 838–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Tagami, H. A toxicologic and dermatologic assessment of cyclic acetates when used as fragrance ingredients. Food Chem. Toxicol. 2008, 46, S1–S27. [Google Scholar] [CrossRef]
- Nóbrega, F.F.; Salvadori, M.G.; Masson, C.J.; Mello, C.F.; Nascimento, T.S.; Leal-Cardoso, J.H.; de Sousa, D.P.; Almeida, R.N. Monoterpenoid terpinen-4-ol exhibits anticonvulsant activity in behavioural and electrophysiological studies. Oxid. Med. Cell. Longev. 2014, 2014, 703848. [Google Scholar] [CrossRef]
- Santos-Nascimento, T.; Veras, K.M.; Cruz, J.S.; Leal-Cardoso, J.H. Inhibitory effect of terpinen-4-ol on voltage-dependent potassium currents in rat small sensory neurons. J. Nat. Prod. 2015, 78, 173–180. [Google Scholar] [CrossRef]
- Parente, M.S.R.; Custódio, F.R.; Cardoso, N.A.; Lima, M.J.A.; Melo, T.S.; Linhares, M.I.; Siqueira, R.M.P.; Nascimento, A.Á.D.; Catunda-Júnior, F.E.A.; Melo, C.T.V. Antidepressant-Like Effect of Lippia sidoides CHAM (Verbenaceae) Essential Oil and Its Major Compound Thymol in Mice. Sci. Pharm. 2018, 86, 27. [Google Scholar] [CrossRef] [Green Version]
- Torres-Martínez, R.; García-Rodríguez, Y.M.; Ríos-Chávez, P.; Saavedra-Molina, A.; López-Meza, J.E.; Ochoa-Zarzosa, A.; Garciglia, R.S. Antioxidant Activity of the Essential Oil and its Major Terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn. Mag. 2018, 13, S875–S880. [Google Scholar] [PubMed]
- Silva, L.A.; Milhomem, M.N.; Santos, M.O.; Arruda, A.C.P.; de Castro, J.A.M.; Fernandes, Y.M.L.; Maia, J.G.S.; Costa-Junior, L.M. Seasonal analysis and acaricidal activity of the thymol-type essential oil of Ocimum gratissimum and its major constituents against Rhipicephalus microplus (Acari: Ixodidae). Parasitol. Res. 2018, 117, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.R.; Ansari, H.S. Anti-Obesity Effect of Arq Zeera and Its Main Components Thymol and Cuminaldehyde in High Fat Diet Induced Obese Rats. Drug Res. 2018, 68, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- André, W.P.P.; Cavalcante, G.S.; Ribeiro, W.L.C.; Santos, J.M.L.D.; Macedo, T.F.; Paula, H.C.B.; Morais, S.M.; Melo, J.V.; Bevilaqua, C.M.L. Anthelmintic effect of thymol and thymol acetate on sheep gastrointestinal nematodes and their toxicity in mice. Rev. Bras. Parasitol. Vet. 2017, 26, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, F.; Xu, Y.; Zhang, J.; Qi, J.; Liu, X.; Wang, R. Thymol alleviates lipopolysaccharide-stimulated inflammatory response via downregulation of RhoA-mediated NF-κB signalling pathway in human peritoneal mesothelial cells. Eur. J. Pharmacol. 2018, 6, 210–220. [Google Scholar] [CrossRef]
- Geyikoglu, F.; Yilmaz, E.G.; Serkan, E.H.; Koc, K.; Cerig, S.; Simsek, O.N.; Aysin, F. Hepatoprotective Role of Thymol in Drug-Induced Gastric Ulcer Model. Ann. Hepatol. 2019, 17, 980–991. [Google Scholar] [CrossRef]
- Santos, S.R.L.; Melo, M.A.; Cardoso, A.V.; Santos, R.L.C.; de Sousa, D.P.; Cavalcanti, S.C.H. Structure-activity relationships of larvicidal monoterpenes and derivatives against Aedes aegypti Linn. Chemosphere 2011, 84, 150–153. [Google Scholar] [CrossRef]
- Aydın, E.; Turkez, H.; Tasdemir, S.; Hacımuftuoglu, F. Anticancer, Antioxidant and Cytotoxic Potential of Thymol in vitro Brain Tumor Cell Model. Cent. Nerv. Syst. Agents Med. Chem. 2017, 17, 116–122. [Google Scholar] [CrossRef]
- Venu, S.; Naik, D.B.; Sarkar, S.K.; Aravind, U.K.; Nijamudheen, A.; Aravindakumar, C.T. Oxidation reactions of thymol: A pulse radiolysis and theoretical study. Am. J. Phys. Chem. 2013, 117, 291–299. [Google Scholar] [CrossRef]
- Nagoor, M.M.F.; Stanely, M.P.P. Protective effects of thymol on altered plasma lipid peroxidation and nonenzymic antioxidants in isoproterenol-induced myocardial infarcted rats. J. Biochem. Mol. Toxicol. 2012, 26, 368–373. [Google Scholar] [CrossRef]
- Delgado-Marín, L.; Sánchez-Borzone, M.; García, D.A. Neuroprotective effects of gabaergic phenols correlated with their pharmacological and antioxidant properties. Life Sci. 2017, 175, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Asadbegi, M.; Komaki, A.; Salehi, I.; Yaghmaei, P.; Ebrahim-Habibi, A.; Shahidi, S.; Golipoor, Z. Effects of thymol on amyloid-β-induced impairments in hippocampal synaptic plasticity in rats fed a high-fat diet. Brain Res. Bull. 2018, 137, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, A.E.; Garlet, Q.; da Cunha, J.A.; Bandeira, G.J.; Brusque, I.C.M.; Salbego, J.; Heinzmann, B.M.; Baldisserotto, B. Monoterpenoids (thymol, carvacrol and S-(+)-linalool) with anesthetic activity in silver catfish (Rhamdia quelen): Evaluation of acetylcholinesterase and GABAergic activity. Braz. J. Med. Biol. Res. 2017, 50, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancheti, J.; Shaikh, M.F.; Chaudhari, R.; Somani, G.; Patil, S.; Jain, P.; Sathaye, S. Characterization of anticonvulsant and antiepileptogenic potential of thymol in various experimental models. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 59–66. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef]
- Evtuguin, D.V.; Tomás, J.L.; Silva, A.M.S.; Neto, C.P. Characterization of an acetylated heteroxylan from Eucalyptus globulus Labill. Carbohydr. Res. 2003, 338, 597–604. [Google Scholar] [CrossRef]
- Lima, D.K.; Ballico, L.J.; Lapa, F.R.; Gonçalves, H.P.; de Souza, L.M.; Iacomini, M.; Werner, M.F.; Baggio, C.H.; Pereira, I.T.; da Silva, L.M.; et al. Evaluation of the antinociceptive, anti-inflammatory and gastric antiulcer activities of the essential oil from Piper aleyreanum C.DC in rodents. J. Ethnopharmacol. 2012, 142, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Lou, Q.; Cheng, C.; Lu, M.; Sun, J. Gut-Associated Bacteria of Dendroctonus valens and their Involvement in Verbenone Production. Microb. Ecol. 2015, 70, 1012–1023. [Google Scholar] [CrossRef]
- Kuo, C.F.; Su, J.D.; Chiu, C.H.; Peng, C.C.; Chang, C.H.; Sung, T.Y.; Chyau, C.C. Anti-inflammatory effects of supercritical carbon dioxide extract and its isolated carnosic acid from Rosmarinus officinalis leaves. J. Agric. Food Chem. 2011, 59, 3674–3685. [Google Scholar] [CrossRef]
- Vegezzi, D.; Switzerland, M. Method for the Preparation of Verbenone, Myrtenal and Pinocarveol and Their Therapeutical Use. U.S. Patent US 4,190,675, 26 February 1980. [Google Scholar]
- Hu, Q.; Lin, G.S.; Duan, W.G.; Huang, M.; Lei, F.H. Synthesis and Biological Activity of Novel (Z)- and (E)-Verbenone Oxime Esters. Molecules 2017, 22, 1678. [Google Scholar] [CrossRef] [Green Version]
- Patrusheva, O.S.; Zarubaev, V.V.; Shtro, A.A.; Orshanskaya, Y.R.; Boldyrev, S.A.; Ilyina, I.V.; Kurbakova, S.Y.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F. Anti-influenza activity of monoterpene-derived substituted hexahydro-2H-chromenes. Bioorg. Med. Chem. 2016, 24, 5158–5161. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Song, S.; Hwang, S.; Kim, C.; Kim, M.; Gu, J.; Oh, Y.K.; Lee, K.; Kwon, J.; Lee, K.; et al. Discovery of novel (1S)-(−)-verbenone derivatives with anti-oxidant and anti-ischemic effects. Bioorg. Med. Chem. Lett. 2013, 23, 5421–5425. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.G.F.; Salgado, P.R.R.; da Fonsêca, D.V.; Braga, R.M.; Filho, M.R.D.C.; de Farias, I.E.V.; de Luna, F.H.; Lima, E.M.; do Amaral, I.P.G.; de Sousa, D.P.; et al. Anticonvulsive activity of (1S)-(−)-verbenone involving RNA expression of BDNF, COX-2, and c-fos. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Takemiya, T.; Sugiura, H.; Yamagata, K. Role of inflammatory mediators in the pathogenesis of epilepsy. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Zhen, J.L.; Chang, Y.N.; Qu, Z.Z.; Fu, T.; Liu, J.Q.; Wang, W.P. Luteolin rescues pentylenetetrazole-induced cognitive impairment in epileptic rats by reducing oxidative stress and activating PKA/CREB/ BDNF signaling. Epilepsy Behav. 2016, 57, 177–184. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Raphael, E.; Brocksom, U.; Brocksom, T.J. Sedative effect of monoterpene alcohols in mice: A preliminary screening. Z. Naturforsch. C 2007, 62, 563–566. [Google Scholar] [CrossRef]
- Woolf, A. Essential oil poisoning. J. Toxicol. Clin. Toxicol. 1999, 37, 721–727. [Google Scholar] [CrossRef]
- Mathew, T.; Kamath, V.; Kumar, R.S.; Srinivas, M.; Hareesh, P.; Jadav, R.; Swamy, S. Eucalyptus oil inhalation-induced seizure: A novel, underrecognized, preventable cause of acute symptomatic seizure. Epilepsia Open 2017, 2, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Steinmetz, M.D.; Vial, M.; Millet, Y. Actions of essential oils of rosemary and certain of its constituents (eucalyptol and camphor) on the cerebral cortex of the rat in vitro. J. Toxicol. Clin. Exp. 1987, 7, 259. [Google Scholar]
- Ćulić, M.; Keković, G.; Grbić, G.; Martać, L.; Soković, M.; Podgorac, J.; Sekulić, S. Wavelet and fractal analysis of rat brain activity in seizures evoked by camphor essential oil and 1,8-cineole. Gen. Physiol. Biophys. 2009, 28, 33–40. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonsêca, D.V.; da Silva Maia Bezerra Filho, C.; Lima, T.C.; de Almeida, R.N.; de Sousa, D.P. Anticonvulsant Essential Oils and Their Relationship with Oxidative Stress in Epilepsy. Biomolecules 2019, 9, 835. https://doi.org/10.3390/biom9120835
da Fonsêca DV, da Silva Maia Bezerra Filho C, Lima TC, de Almeida RN, de Sousa DP. Anticonvulsant Essential Oils and Their Relationship with Oxidative Stress in Epilepsy. Biomolecules. 2019; 9(12):835. https://doi.org/10.3390/biom9120835
Chicago/Turabian Styleda Fonsêca, Diogo Vilar, Carlos da Silva Maia Bezerra Filho, Tamires Cardoso Lima, Reinaldo Nóbrega de Almeida, and Damião Pergentino de Sousa. 2019. "Anticonvulsant Essential Oils and Their Relationship with Oxidative Stress in Epilepsy" Biomolecules 9, no. 12: 835. https://doi.org/10.3390/biom9120835
APA Styleda Fonsêca, D. V., da Silva Maia Bezerra Filho, C., Lima, T. C., de Almeida, R. N., & de Sousa, D. P. (2019). Anticonvulsant Essential Oils and Their Relationship with Oxidative Stress in Epilepsy. Biomolecules, 9(12), 835. https://doi.org/10.3390/biom9120835






