Formulations of Curcumin Nanoparticles for Brain Diseases
Abstract
:1. Introduction
2. Curcumin Chemical Information
2.1. Thermal Analysis of Curcumin
2.2. Ultraviolet-Visible Spectrophotometric Analysis of Curcumin
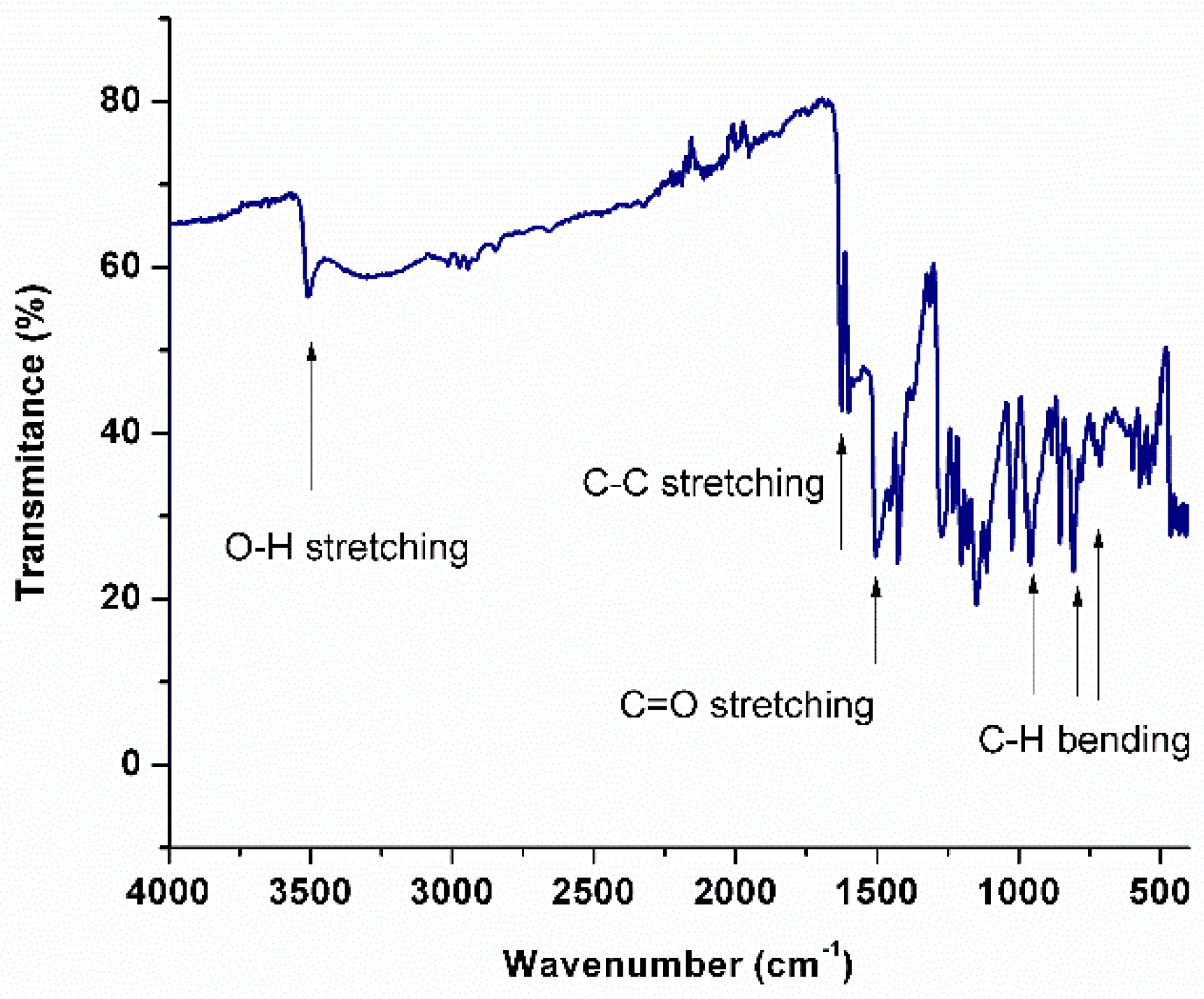
2.3. Fourier Transform Infrared Spectroscopy of Curcumin
2.4. Solubility
3. Biological Activity
3.1. Effect of Curcumin on Aggregation Protein
3.2. Effect of Curcumin in Neuroinflammation
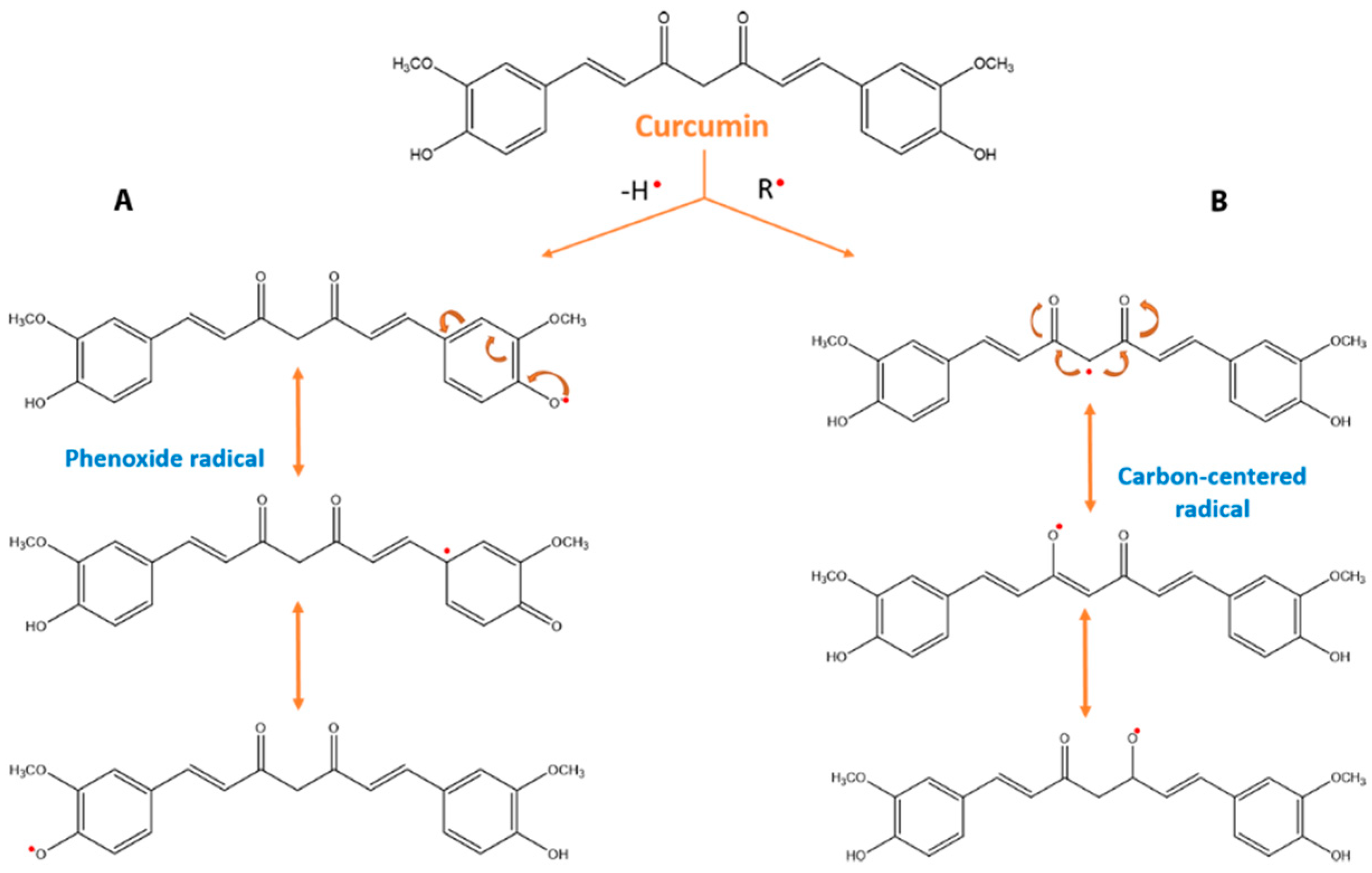
3.3. Effect of Curcumin on Oxidative Stress
4. Limitation of Chemical Properties and New Proposals
5. New Formulations of Curcumin
6. Formulations of Curcumin in Nanoparticles
6.1. Polymeric Nanoparticles
6.2. Lipid Nanoparticles
6.3. Liposomes
6.4. Cyclodextrins
6.5. Tools for Drug Targeting of Curcumin
7. Applications of Curcumin in Nanoparticles
7.1. Alzheimer’s Disease
7.2. Parkinson’s Disease
7.3. Huntington’s Disease
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer disease |
| AP-1 | activating protein-1 |
| APP | amyloid-β precursor protein |
| AR | androgen receptor |
| Arh-R | aryl hydrocarbon receptor |
| AS | α-synuclein |
| ASD | Amorphous solid dispersions |
| Aβ | amyloid-β |
| BACE1 | β-secretase enzyme |
| BBB | blood–brain barrier |
| BCS | Biopharmaceutics Classification System |
| cAK | autophosphorylation-activated protein kinase |
| CBP | CREB-binding protein |
| CDPK | Ca2+-dependent protein kinase cellular src kinase |
| CNS | central nervous system |
| COX-2 | cyclooxygenase-2 |
| cPK | protamine kinase |
| CTGF | connective tissue growth factor |
| DFF40 | DNA fragmentation factor; 40-kd subunit |
| DL | drug loading |
| DR-5 | death receptor-5 |
| EE | entrapment efficiency |
| EGF | epidermal growth Factor |
| EGF-R | EGF receptor |
| EGFRK | EGF receptor-kinase |
| Egr-1 | early growth response gene-1 |
| ELAM-1 | endothelial leukocyte adhesion molecule-1; Bcl-2, B-cell lymphoma protein 2 |
| EPC-R | endothelial protein C-receptor |
| EpRE | electrophile |
| ERK | extracellular receptor kinase |
| ER-α | estrogen receptor-α |
| FAK | focal adhesion kinase |
| Fas-R | Fas receptor |
| FDA | Food and Drug Administration |
| FGF | fibroblast growth factor |
| FPTase | farnesyl protein transferase |
| Gcl | Glutamate–cysteine ligase |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GST | glutathione-S-transferase |
| H2-R | histamine (2)-receptor |
| HGF | hepatocyte growth factor |
| HO | heme oxygenase |
| HSP-70 | heat shock protein 70 |
| IAP | inhibitory apoptosis protein |
| IARK | IL-1 receptor-associated kinase |
| ICAM-1 | intracellular adhesion molecule-1 |
| IFNγ | interferon gamma |
| IKK | IκB kinase |
| IL | interleukin |
| IL-8-R | interleukin-8-receptor |
| iNOS | inducible nitric oxide synthase |
| Inos | matrix inducible nitric oxide synthase |
| InsP3-R | inositol 1,4,5-triphosphate receptor |
| IR | Fourier transform infrared spectroscopy |
| IR | integrin receptor |
| JAK | janus kinase |
| JNK | c-jun N-terminal kinase |
| LDL-R | low-density lipoprotein-receptor |
| LOX | lipoxygenase |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MCP | monocyte chemoattractant protein |
| MDR | multidrug resistance |
| MIF | migration inhibition protein |
| MIP | macrophage inflammatory protein |
| MMP | matrix metalloproteinase |
| Mv | Millivolts |
| NAT | arylamine N-acetyltransferases |
| NF-κB | nuclear factor-κB |
| NGF | nerve growth factor |
| NMR | nuclear magnetic resonance |
| Nrf-2 | nuclear factor erythroid 2-related factor |
| PBCA | poly (butyl cyanoacrylate) |
| PDGF | platelet-derived growth factor |
| PhK | phosphorylase kinase |
| PKA | protein kinase A |
| PKB | protein kinase B |
| PKC | protein kinase C |
| PLGA | poly lactic-co-glycolic acid |
| pp60c-src | non-receptor protein tyrosine kinase c-Src |
| PPARγ | peroxisome proliferator-activated receptor-γ |
| PrP | prion protein |
| PrP-Res | prion protein resistance |
| PVA | polyvinyl alcohol |
| SHP-2 | Src homology 2 domain containing tyrosine phosphatase 2 |
| SMEDDS | Self-microemulsifying drug delivery systems |
| STAT | signal transducers and activators of transcription |
| TAT | transactivating–transduction peptide |
| TGF-β1 | transforming growth factor-β1 |
| TIMP | tissue inhibitor of metalloproteinase-3 |
| TK | protein tyrosine kinase |
| TNF | tumoral necrosis factor |
| TNF-α | tumor necrosis factor-α |
| uPA | urokinase-type plasminogen activator |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
| Ψ | zeta potential |
References
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsamydai, A.; Jaber, N. Pharmacological aspects of curcumin: Review article. Int. J. Pharmacogn. 2018, 5, 313–326. [Google Scholar]
- Bengmark, S. Curcumin, an atoxic antioxidant and natural NFκB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: A shield against acute and chronic diseases. J. Parenter. Enter. Nutr. 2006, 30, 45–51. [Google Scholar] [CrossRef]
- Wang, S.L.; Ying, L.; Ying, W.; Chen, Y.F.; Na, L.X.; Li, S.T.; Sun, C.H. Curcumin, a potential inhibitor of Up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-κB and JNK pathway. Biomed. Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef]
- Guo, Y.Z.; He, P.; Feng, A.M. Effect of curcumin on expressions of NF-κBp65, TNF-α and IL-8 in placental tissue of premature birth of infected mice. Asian Pac. J. Trop. Med. 2017, 10, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Browne, A.; Child, D.; Tanzi, R.E. Curcumin decreases amyloid-β peptide levels by attenuating the maturation of amyloid-β precursor protein. J. Biol. Chem. 2010, 285, 28472–28480. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Gremião, M.P.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef]
- Li, J.; Sabliov, C. PLA/PLGA nanoparticles for delivery of drugs across the blood-brain barrier. Nanotechnol. Rev. 2013, 2, 241–257. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. In Natural Polymer Drug Delivery Systems; Nanoparticles, Plants, and Algae; Springer International Publishing: Cham, Switzerland, 2016; pp. 33–85. [Google Scholar]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Payton, F.; Sandusky, P.; Alworth, W.L. NMR study of the solution structure of curcumin. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, D.; Shirai, N.; Amatsubo, T.; Taguchi, H.; Hirao, K.; Urushitani, M.; Morikawa, S.; Inubushi, T.; Kato, M.; Kato, F.; et al. Relationship between the tautomeric structures of curcumin derivatives and their Aβ-binding activities in the context of therapies for Alzheimer’s disease. Biomaterials 2010, 31, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef] [PubMed]
- Subramani, P.A.; Panati, K.; Lebaka, V.R.; Redd, D.D.; Narala, V.R. Nanostructures for curcumin delivery: Posibilities and challenges. In Nano and -Micro Drug Delivery Systems; Andrew, W., Ed.; Elsevier Science Ltd Desing and Fabrication: Amsterdam, The Netherlands, 2017; pp. 393–418. [Google Scholar]
- Kharat, M.; Du, Z.; Zhang, G.; Mcclements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Agrawal, S.S.; Gupta, M. Development and validation of UV spectrophotometric method for the estimation of curcumin in bulk drug and pharmaceutical dosage forms. Int. J. Drug Dev. Res. 2012, 4, 375–380. [Google Scholar]
- Benassi, R.; Ferrari, E.; Lazzari, S.; Spagnolo, F.; Saladini, M. Theoretical study on Curcumin: A comparison of calculated spectroscopic properties with NMR, UV-vis and IR experimental data. J. Mol. Struct. 2008, 892, 168–176. [Google Scholar] [CrossRef]
- Athira, G.K.; Jyothi, A.N. Preparation and characterization of curcumin loaded cassava starch nanoparticles with improved cellular absorption. Int. J. Pharm. Pharm. Sci. 2014, 6, 171–176. [Google Scholar]
- O’Neil, M.J. (Ed.) The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th ed.; Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Hansen solubility parameters (HSP) for prescreening formulation of solid lipid nanoparticles (SLN): In Vitro testing of curcumin-loaded SLN in MCF-7 and BT-474 cell lines. Pharm. Dev. Technol. 2018, 23, 96–105. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griesser, M.; Pistis, V.; Suzuki, T.; Tejera, N.; Pratt, D.A.; Schneider, C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J. Biol. Chem. 2011, 286, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Haruyo, I. Curcumin: The Indian solid gold. In The Molecular Targets and Therapeutics Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Springer Science: Boston MA, USA, 2007; Volume 595, pp. 1–75. [Google Scholar]
- Reinke, A.A.; Gestwicki, J.E. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Lahiri, D.K. Neuroinflammation in Alzheimer’s disease: Different molecular targets and potential therapeutic agents including curcumin. Curr. Opin. Pharmacol. 2009, 9, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.F.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef]
- Aguzzi, A.; O’Connor, T. Protein aggregation diseases: Pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 2010, 9, 237–248. [Google Scholar] [CrossRef]
- Hafner-Bratkovič, I.; Gašperšič, J.; Šmid, L.M.; Bresjanac, M.; Jerala, R. Curcumin binds to the α-helical intermediate and to the amyloid form of prion protein -A new mechanism for the inhibition of PrPSc accumulation. J. Neurochem. 2008, 104, 1553–1564. [Google Scholar] [CrossRef]
- Caughey, B.; Raymond, L.D.; Raymond, G.J.; Maxson, L.; Silveira, J.; Baron, G.S. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J. Virol. 2003, 77, 5499–5502. [Google Scholar] [CrossRef]
- Pandey, N.; Strider, J.; Nolan, W.C.; Yan, S.X.; Galvin, J.E. Curcumin inhibits aggregation of α-synuclein. Acta Neuropathol. 2008, 115, 479–489. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Sharma, N.; Punnepalli, S.; D’Souza, A.; Raghothama, S.; Atreya, H.S. Curcumin nanoconjugate Inhibits aggregation of N-terminal region (Aβ-16) of an amyloid beta peptide. New J. Chem. 2018, 42, 19881–19892. [Google Scholar] [CrossRef]
- Mithu, V.S.; Sarkar, B.; Bhowmik, D.; Das, A.K.; Chandrakesan, M. Curcumin Alters the Salt Bridge-containing Turn Region in amyloid β(1-42) aggregates. J. Biol. Chem. 2014, 289, 11122–11131. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Atwood, Æ.C.S.; Moir, Æ.R.D.; Hartshorn, M.A.; Tanzi, Æ.R.E.; Bush, A.I. Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer’s Aβ peptides. J. Biol. Inorg. Chem. 2004, 9, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuropretective effects of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.J., Shishodia, S., Eds.; Springer: Boston, MA, USA, 2007; Volume 595, pp. 197–212. [Google Scholar]
- Vassar, R. Bace 1 The β-secretase enzyme in alzheimer’s disease. J. Mol. Neurosci. 2004, 23, 105–113. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil is in the Details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlstetter, M.; Lippe, E.; Walczak, Y.; Moehle, C.; Aslanidis, A.; Mirza, M.; Langmann, T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflammation 2011, 8, 1–12. [Google Scholar] [CrossRef]
- Tizabi, Y.; Hurley, L.L.; Qualls, Z.; Akinfiresoye, L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules 2014, 19, 20864–20879. [Google Scholar] [CrossRef]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef]
- Jurrmann, N.; Brigelius-Flohe, R.; Bol, G.F. Curcumin blocks interleukin-1 (IL-1) signaling by inhibiting the recruitment of the IL-1 receptor-associated kinase IRAK in murine thymoma EL-4 cells. J. Nutr. 2005, 135, 1859–1864. [Google Scholar] [CrossRef]
- Devi, Y.S.; DeVine, M.; DeKuiper, J.; Ferguson, S.; Fazleabas, A.T. Inhibition of IL-6 signaling pathway by curcumin in uterine decidual cells. PLoS ONE 2015, 10, e0125627. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Cicero, A.F.G.; Simental-Mendía, L.E.; Aggarwal, B.B.; Gupta, S.C. Curcumin downregulates human tumor necrosis factor-α levels: A systematic review and meta-analysis ofrandomized controlled trials. Pharmacol. Res. 2016, 107, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-κ B is suppressed by curcumin (diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [PubMed]
- Howe, C.L.; LaFrance-Corey, R.G.; Goddery, E.N.; Johnson, R.K.; Mirchia, K. Neuronal CCL2 expression drives inflammatory monocyte infiltration into the brain during acute virus infection. J. Neuroinflamm. 2017, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Zhao, L.-X.; Cao, D.-L.; Zhang, X.; Gao, Y.-J.; Xia, C. Curcumin Inhibits LPS-Induced CCL2 Expression via JNK Pathway in C6 Rat Astrocytoma Cells. Cell. Mol. Neurobiol. 2012, 32, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Stadelman, H.L.; Roselli, C.E. Curcumin blocks CCL2-induced adhesion, motility and invasion, in part, through down-regulation of CCL2 expression and proteolytic activity. Int. J. Oncol. 2009, 34, 1319–1327. [Google Scholar] [PubMed]
- Midura-Kiela, M.T.; Radhakrishnan, V.; Kiela, P. Curcumin inhibits interferon-γ signaling in colonic epithelial cells. Am. J. Physiol. Liver Phisiol. 2012, 302, G86–G96. [Google Scholar] [CrossRef] [PubMed]
- Ogadimma, I.; Uzairu, A.; Eyije, S.; Ola, S. Evaluation of the antioxidant properties of curcumin derivatives by genetic function algorithm. J. Adv. Res. 2018, 12, 47–54. [Google Scholar]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; Hady, S. El Antioxidant and structure—Activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflamatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Springer International Publishing: Cham, Switzerland, 2007; pp. 105–126. [Google Scholar]
- Chen, Y.; Xiao, H.; Zheng, J.; Liang, G. Structure-Thermodynamics-Antioxidant Activity Relationships of Selected Natural Phenolic Acids and Derivatives: An Experimental and Theoretical Evaluation. PLoS ONE 2015, 10, e0121276. [Google Scholar] [CrossRef] [PubMed]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Okezie, I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic. Res. 2009, 39, 1119–1125. [Google Scholar] [CrossRef]
- Maiti, P.; Dunbar, G.L. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-movahedi, A.A. Intracellular ROS Protection Efficiency and Free Radical- Scavenging Activity of Curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef]
- González-Reyes, S.; Guzmán-Beltrán, S.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Curcumin Pretreatment Induces Nrf2 and an Antioxidant Response and Prevents Hemin-Induced Toxicity in Primary Cultures of Cerebellar Granule Neurons of Rats. Oxid. Med. Cell. Longev. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Haryuna, T.; Munir, D.; Maria, A.; Bashiruddin, J. The Antioxidant Effect of Curcumin on Cochlear Fibroblasts in Rat Models of Diabetes Mellitus. Iran J. Otorhinolaryngol. 2017, 29, 197–202. [Google Scholar] [PubMed]
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282. [Google Scholar] [CrossRef]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef]
- Sharma, R.A. Phase I Clinical Trial of Oral Curcumin: Biomarkers of Systemic Activity and Compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [Green Version]
- Gangurde, A.B.; Kundaikar, H.S.; Javeer, S.D.; Jaiswar, D.R.; Degani, M.S.; Amin, P.D. Enhanced solubility and dissolution of curcumin by a hydrophilic polymer solid dispersion and its in silico molecular modeling studies. J. Drug Deliv. Sci. Technol. 2015, 29, 226–237. [Google Scholar] [CrossRef]
- Chuah, A.M.; Jacob, B.; Jie, Z.; Ramesh, S.; Mandal, S.; Puthan, J.K.; Deshpande, P.; Vaidyanathan, V.V.; Gelling, R.W.; Patel, G.; et al. Enhanced bioavailability and bioefficacy of an amorphous solid dispersion of curcumin. Food Chem. 2014, 156, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Zhao, J.; Ding, Y.; Li, L. Journal of Colloid and Interface Science A cost-effective method to prepare curcumin nanosuspensions with enhanced oral bioavailability. J. Colloid Interface Sci. 2017, 485, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, H.; Zhang, C.; Chen, W.; Cheng, W.; Chen, X.; Ye, X. Preparation and in-vitro/in-vivo evaluation of curcumin nanosuspension with solubility enhancement. J. Pharm. Pharmacol. 2016, 68, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)—Challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, J.; Huang, X.; Wen, C. Self-microemulsifying drug delivery system improves curcumin dissolution and bioavailability. Drug Dev. Ind. Pharm. 2011, 37, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jaisamut, P.; Wiwattanawongsa, K.; Graidist, P.; Sangsen, Y.; Wiwattanapatapee, R. Enhanced Oral Bioavailability of Curcumin Using a Supersaturatable Self-Microemulsifying System Incorporating a Hydrophilic Polymer; In Vitro and In Vivo Investigations. AAPS PharmSciTech 2018, 19, 730–740. [Google Scholar] [CrossRef]
- Bele, M.H.; Shaikh, A.A.; Paralkar, S.G. To enhance the solubility of curcumin by solid self-microemulsifying drug delivery system (SMEDDS). Indo Am. J. Pharm. Res. 2017, 7, 8587–8607. [Google Scholar]
- Cui, J.; Yu, B.; Zhao, Y.; Zhu, W.; Li, H.; Lou, H.; Zhai, G. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int. J. Pharm. 2009, 371, 148–155. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Sun, M.; Su, X.; Ding, B.; He, X.; Liu, X.; Yu, A.; Lou, H.; Zhai, G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine 2012, 7, 1085–1100. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticles-a historical perspective. Int. J. Pharm. 2007, 331, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leson, A. There is plenty of room at the bottom. Vak. Forsch. und Prax. 2005, 17, 123. [Google Scholar] [CrossRef]
- Khanna, S.C.; Soliva, M.; Speiser, P. Epoxy resin beads as a pharmaceutical dosage form II: Dissolution studies of epoxy-amine beads and release of drug. J. Pharm. Sci. 1969, 58, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Alyautdin, R.N.; Kharkevich, D.A.; Ivanov, A.A. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1995, 674, 171–174. [Google Scholar] [CrossRef]
- Müller, R.H.; Maaßen, S.; Weyhers, H.; Mehnert, W. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. J. Drug Target. 1996, 4, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Wang, M.; Jing, G.; Zhu, R.; Wang, S. Antidepressant effects of curcumin and HU-211 coencapsulated solid lipid nanoparticles against corticosterone-induced cellular and animal models of major depression. Int. J. Nanomed. 2016, 11, 4975–4990. [Google Scholar] [CrossRef]
- Kakkar, V.; Muppu, S.K.; Chopra, K.; Kaur, I.P. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013, 85, 339–345. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromol. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Das, M.; Tripathy, K.; Sahoo, S.K. Delivery of Dual Drug Loaded Lipid Based Nanoparticles across the Blood-Brain Barrier Impart Enhanced Neuroprotection in a Rotenone Induced Mouse Model of Parkinson’s Disease. ACS Chem. Neurosci. 2016, 7, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P.; Ko, Y.T. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015, 32, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Kaur, I.P. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011, 49, 2906–2913. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, M.S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Sharif Zadeh, M.; Akbari Javar, H.; Hamidi, M. Brain Delivery of Curcumin Using Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Preparation, Optimization, and Pharmacokinetic Evaluation. ACS Chem. Neurosci. 2018, 10, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Madane, R.G.; Mahajan, H.S. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: Design, characterization, and in vivo study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar] [PubMed]
- Fang, M.; Jin, Y.; Bao, W.; Gao, H.; Xu, M.; Wang, D.; Wang, X.; Yao, P.; Liu, L. In vitro characterization and in vivo evaluation of nanostructured lipid curcumin carriers for intragastric administration. Int. J. Nanomed. 2012, 7, 5395–5404. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Asghar, S.; Gao, S.; Su, Z.; Song, J.; Huo, M.; Meng, W.; Ping, Q.; Xiao, Y. A novel LDL-mimic nanocarrier for the targeted delivery of curcumin into the brain to treat Alzheimer’s disease. Colloids Surfaces B Biointerfaces 2015, 134, 88–97. [Google Scholar] [CrossRef]
- Meng, F.; Asghar, S.; Xu, Y.; Wang, J.; Jin, X.; Wang, Z.; Wang, J.; Ping, Q.; Zhou, J.; Xiao, Y. Design and evaluation of lipoprotein resembling curcumin-encapsulated protein-free nanostructured lipid carrier for brain targeting. Int. J. Pharm. 2016, 506, 46–56. [Google Scholar] [CrossRef]
- Ameruoso, A.; Palomba, R.; Palange, A.L.; Cervadoro, A.; Lee, A.; Di Mascolo, D.; Decuzzi, P. Ameliorating amyloid-β fibrils triggered inflammation via curcumin-loaded polymeric nanoconstructs. Front. Immunol. 2017, 8, 1411. [Google Scholar] [CrossRef]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. ApoE3 Mediated Poly(butyl) Cyanoacrylate Nanoparticles Containing Curcumin: Study of Enhanced Activity of Curcumin against Beta Amyloid Induced Cytotoxicity Using In Vitro Cell Culture Model. Mol. Pharm. 2010, 7, 815–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. ApoE3 mediated polymeric nanoparticles containing curcumin: Apoptosis induced in vitro anticancer activity against neuroblastoma cells. Int. J. Pharm. 2012, 437, 29–41. [Google Scholar] [CrossRef]
- Szymusiak, M.; Hu, X.; Leon Plata, P.A.; Ciupinski, P.; Wang, Z.J.; Liu, Y. Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin. Int. J. Pharm. 2016, 511, 415–423. [Google Scholar] [CrossRef]
- Tsai, Y.M.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, F.; Szymusiak, M.; Liu, Y.; Wang, Z.J. Curcumin Attenuates Opioid Tolerance and Dependence by Inhibiting Ca2+/Calmodulin-Dependent Protein Kinase II α Activity. J. Pharmacol. Exp. Ther. 2015, 352, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Lomash, V.; Samim, M.; Flora, S.J.S. Curcumin encapsulated in chitosan nanoparticles: A novel strategy for the treatment of arsenic toxicity. Chem. Biol. Interact. 2012, 199, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, M.; Anisian, D.; Ghasemi-Kasman, M.; Akbari, A.; Khalili-Fomeshi, M.; Ghasemi, S.; Ahmadi, F.; Moghadamnia, A.A.; Ebrahimpour, A. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 462–471. [Google Scholar] [CrossRef]
- Yang, R.; Zheng, Y.; Wang, Q.; Zhao, L. Curcumin-loaded chitosan–bovine serum albumin nanoparticles potentially enhanced Aβ 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res. Lett. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef]
- Pieretti, S.; Ranjan, A.P.; Di Giannuario, A.; Mukerjee, A.; Marzoli, F.; Di Giovannandrea, R.; Vishwanatha, J.K. Curcumin-loaded Poly (D, L-lactide-co-glycolide) nanovesicles induce antinociceptive effects and reduce pronociceptive cytokine and BDNF release in spinal cord after acute administration in mice. Colloids Surf. B Biointerfaces 2017, 158, 379–386. [Google Scholar] [CrossRef]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Hua, H.; Wang, A.; Liu, W.; Li, Y.; Fu, F.; Shi, Y.; Sun, K. Cyclic hexapeptide-conjugated nanoparticles enhance curcumin delivery to glioma tumor cells and tissue. Int. J. Nanomed. 2017, 12, 5717–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef] [PubMed]
- Orunoğlu, M.; Kaffashi, A.; Pehlivan, S.B.; Şahin, S.; Söylemezoğlu, F.; Karlı-Oğuz, K.; Mut, M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater. Sci. Eng. C 2017, 78, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jiang, M.; Fang, J.; Yang, M.F.; Zhang, S.; Yin, Y.X.; Li, D.W.; Mao, L.L.; Fu, X.Y.; Hou, Y.; et al. Enhanced Therapeutic Potential of Nano-Curcumin Against Subarachnoid Hemorrhage-Induced Blood–Brain Barrier Disruption Through Inhibition of Inflammatory Response and Oxidative Stress. Mol. Neurobiol. 2017, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.L.; Baum, L. Highly Stabilized Curcumin Nanoparticles Tested in an In Vitro Blood–Brain Barrier Model and in Alzheimer’s Disease Tg2576 Mice. AAPS J. 2013, 15, 324–336. [Google Scholar] [CrossRef]
- Yang, L.; Gao, S.; Asghar, S.; Liu, G.; Song, J.; Wang, X.; Ping, Q.; Zhang, C.; Xiao, Y. Hyaluronic acid/chitosan nanoparticles for delivery of curcuminoid and its in vitro evaluation in glioma cells. Int. J. Biol. Macromol. 2015, 72, 1391–1401. [Google Scholar] [CrossRef]
- Mirgani, M.T.; Isacchi, B.; Sadeghizadeh, M.; Marra, F.; Bilia, A.R.; Mowla, S.J.; Najafi, F.; Babaei, E. Dendrosomal curcumin nanoformulation downregulates pluripotency genes via miR-145 activation in U87MG glioblastoma cells. Int. J. Nanomed. 2014, 9, 403–417. [Google Scholar]
- Kumar, A.; Ahuja, A.; Ali, J.; Baboota, S. Curcumin-loaded lipid nanocarrier for improving bioavailability, stability and cytotoxicity against malignant glioma cells. Drug Deliv. 2016, 23, 214–229. [Google Scholar] [CrossRef]
- Nasr, M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016, 23, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Vaz, G.R.; Hädrich, G.; Bidone, J.; Rodrigues, J.L.; Falkembach, M.C.; Putaux, J.L.; Hort, M.A.; Monserrat, J.M.; Varela Junior, A.S.; Teixeira, H.F.; et al. Development of Nasal Lipid Nanocarriers Containing Curcumin for Brain Targeting. J. Alzheimer’s Dis. 2017, 59, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jiang, Y.; Gupta, S.; Younus, M.; Ramzan, M. Anti-Inflammatory Potency of Nano-Formulated Puerarin and Curcumin in Rats Subjected to the Lipopolysaccharide-Induced Inflammation. J. Med. Food 2013, 16, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wáng, Y.X.J.; Chow, A.H.L.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Bigdeli, B.; Jalili-baleh, L.; Baharifar, H.; Akrami, M.; Dehghani, S.; Goliaei, B.; Amani, A.; Lotfabadi, A.; Rashedi, H.; et al. Curcumin-lipoic acid conjugate as a promising anticancer agent on the surface of gold-iron oxide nanocomposites: A pH-sensitive targeted drug delivery system for brain cancer theranostics. Eur. J. Pharm. Sci. 2018, 114, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Naserzadeh, P.; Hafez, A.A.; Abdorahim, M.; Abdollahifar, M.A.; Shabani, R.; Peirovi, H.; Simchi, A.; Ashtari, K. Curcumin loading potentiates the neuroprotective efficacy of Fe3O4 magnetic nanoparticles in cerebellum cells of schizophrenic rats. Biomed. Pharmacother. 2018, 108, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.H.; Chiu, T.L.; Huang, W.C.; Lai, Y.H.; Hu, S.H.; Chen, Y.Y.; Chen, S.Y. Dual-Targeting Lactoferrin-Conjugated Polymerized Magnetic Polydiacetylene-Assembled Nanocarriers with Self-Responsive Fluorescence/Magnetic Resonance Imaging for in vivo Brain Tumor Therapy. Adv. Healthc. Mater. 2016, 5, 688–695. [Google Scholar] [CrossRef]
- Lazar, A.N.; Mourtas, S.; Youssef, I.; Parizot, C.; Dauphin, A.; Delatour, B.; Antimisiaris, S.G.; Duyckaerts, C. Curcumin-conjugated nanoliposomes with high affinity for Aβ deposits: Possible applications to Alzheimer disease. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 712–721. [Google Scholar] [CrossRef]
- Li, W.; Zhou, M.; Xu, N.; Hu, Y.; Wang, C.; Li, D.; Liu, L.; Li, D. Comparative analysis of protective effects of curcumin, curcumin-β-cyclodextrin nanoparticle and nanoliposomal curcumin on unsymmetrical dimethyl hydrazine poisoning in mice. Bioengineered 2016, 7, 334–341. [Google Scholar] [CrossRef]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.-Y.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Djiokeng Paka, G.; Doggui, S.; Zaghmi, A.; Safar, R.; Dao, L.; Reisch, A.; Klymchenko, A.; Roullin, V.G.; Joubert, O.; Ramassamy, C. Neuronal Uptake and Neuroprotective Properties of Curcumin-Loaded Nanoparticles on SK-N-SH Cell Line: Role of Poly(lactide-co-glycolide) Polymeric Matrix Composition. Mol. Pharm. 2016, 13, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Aβ aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Lu, S.; Liu, X.-G.; Zhu, J.; Wang, Y.-J.; Liu, R.-T. PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer’s disease mice. Oncotarget 2017, 8, 81001–81013. [Google Scholar] [PubMed]
- Siddique, Y.H.; Khan, W.; Singh, B.R.; Naqvi, A.H. Synthesis of Alginate-Curcumin Nanocomposite and Its Protective Role in Transgenic Drosophila Model of Parkinson’s Disease. ISRN Pharmacol. 2013, 2013, 794582. [Google Scholar] [CrossRef] [PubMed]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy. Theranostics 2018, 8, 2264–2277. [Google Scholar] [CrossRef] [PubMed]








| Carrier | Composition | Ligand/Stabilizer | Size, PI and Ψ (mV) | % of EE and DL | Model of Evaluation | Reference |
|---|---|---|---|---|---|---|
| Solid lipid nanoparticles | Polyoxyethylene stearate, stearic acid | Lecithin | 60, Ψ = −21.7 | DL = 21.61 | Major depression (in vitro and in vivo models) | [85] |
| Compritol 888 ATO (Gattefossé, Saint-Priest, France) | Tween 80, soya lecithin | 136 | 81.9, 92.3 | Cerebral ischemic injury (in vivo model) | [86] | |
| Stearic acid | Lecithin, taurocholate | 148 | EE = 93.2 | Huntington’s disease (in vivo model) | [87] | |
| Glyceryl monooleate | Pluronic F-68, vitamin E TPGS | 93, Ψ = −30.9 | EE = 65 | Rotenone-induced mouse model of Parkinson’s disease (in vitro and in vivo models) | [88] | |
| Palmitic acid, cholesterol | N-trimethyl Chitosan vitamin E TPGS | 412, 0.26, Ψ = 35.7 | 93, 4 | Biodistribution (in vitro and in vivo models) | [89] | |
| Compritol 888 ATO | Tween 80, soya lecithin | 136 | 81.9, 92.3 | Aluminum-induced behavioral (in vivo model) | [90] | |
| Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) | Cetyl palmitate (SLN), cetyl palmitate + oleic acid (NLC) | Tween 80 | 204.7, 0.194 and 117.36, 0.188 | SLN = 83.98, 4.54 NLC = 82.60, 4.67 | Pharmacokinetic (in vivo model) | [91] |
| Nanostructured lipid carriers | Precirol, capmul MCM | Tween 80, soya lecithin | 146, 0.18, Ψ = −21.4 | EE = 90.86 | Astrocytoma-glioblastoma (in vitro and in vivo models) | [92] |
| Glyceryl monostearate, soy lecithin, medium chain triglycerides | Poloxamer 188 | 129, 0.25 Ψ = −27.8 | 95.9, 4.21 | Pharmacokinetic and biodistribution (in vivo model) | [93] | |
| Phosphatidyl choline, cholesterol oleate, glycerol trioleate | Lactoferrin | 103.8, PI = 0.15, Ψ = −5.80 | 96.51, 2.60 | Alzheimer´s disease (in vitro and in vivo models) | [94] | |
| PC, cholesterol oleate, glycerol trioleate | Polysorbate 80 | 90.5, 0.14, Ψ = −20.3 | EE = 94.39, DL = 3.29 | Biodistribution (in vitro and in vivo models) | [95] | |
| Polymeric nanoparticles | PLGA | Lipid monolayer | 193.4, PI=0.115, Ψ = −43.8 | 13.23, 2.31 | Inflammation model (in vitro model) | [96] |
| Poly(butyl) cyanoacrylate | Apolipoprotein E3 | 197, 0.18, Ψ = −22.44 | - | Beta amyloid induced cytotoxicity in neuroblastoma cells (in vitro model) | [97] | |
| Poly(butyl) cyanoacrylate | Apolipoprotein E3 | 197, 0.18, Ψ = −22.44 | EE = 77.85 | Anticancer activity in neuroblastoma cells (in vitro model) | [98] | |
| PLGA | - | 100 | 94.7, 47.3 | Bioavailability in the CNS (in vivo model) | [99] | |
| PLGA | PVA | 163, 0.053, Ψ = −12.5 | EE = 46.9 | Pharmacokinetic (in vivo model) | [100] | |
| PLGA | - | - | - | Opioid tolerance and dependence (in vivo model) | [101] | |
| Chitosan | Tween 80 | 10, Ψ = −16.8 | - | Arsenic toxicity (in vivo model) | [102] | |
| Chitosan-alginate | - | 50 | - | Epilepsy (in vivo model) | [103] | |
| Chitosan | Bovine serum albumin | 143.5, 0.021, Ψ = −10.8 | EE = 95.4 | Phagocytosis of the Aβ peptide (in vitro model) | [104] | |
| PLGA | PVA | 200, Ψ = −19 | EE = 77 | Neurogenesis (in vitro and in vivo models) | [105] | |
| PLGA | PVA | 153, 0.15 | 90, 9.5 | Pain (in vivo model) | [106] | |
| PLGA | Tet-1 | 150-200, Ψ = −30 to −20 | - | Amyloid aggregates (in vitro model) | [107] | |
| PLGA | PEG-B6 peptide | 150, Ψ = 3.8 | DL = 15.6 | Alzheimer transgenic mice (in vitro and in vivo models) | [108] | |
| PLGA | PEG, cyclic hexapeptide | 97.3, 0.16 | EE = 80.5 | Glioma tumor cells (in vitro and in vivo models) | [109] | |
| PLGA | PEG, transferrin receptor-binding peptide T7 | 130, Ψ = −15.9 | EE = 18 | Brain tumor (in vitro and in vivo models) | [110] | |
| PLGA | 1,2-distearoyl-glycerol-3-phospho-ethanolamine- N-[methoxy (polyethylene glycol)-2000 | 169, 0.22 | EE = 35 | Glioblastoma (in vitro and in vivo models) | [111] | |
| PLGA | PVA | 220, Ψ = −20.6 | 81.7, 16.3 | Subarachnoid hemorrhage-induced BBB disruption (in vivo model) | [112] | |
| PLA–PEG | PVP | 55, 0.09, Ψ = −0.29 | EE = 99 | Alzheimer’s Disease Tg2576 Mice (in vitro and in vivo models) | [113] | |
| Hyaluronic acid/chitosan | - | 207, Ψ = 25.3 | 89.9, 6.5 | Glioma cells (in vitro model) | [114] | |
| Polymeric micelle | Oleoyl chloride, polyethylene glycol 400 | - | 142, 0.4, Ψ = −7 | EE = 87 | Glioblastoma cells (in vitro model) | [115] |
| Nanoemulsion | Labrafac Lipophile WL 1349, Solutol HS 15, Transcutol HP | Tween 80, Tween 20 | 67, 0.137, Ψ = −37 | - | Malignant glioma cells (in vitro model) | [116] |
| Labrafac Lipophile | Cremophor RH40 | 114, 0.25, Ψ = −21.8 | - | Biodistribution (ex vivo and in vivo models) | [117] | |
| Castor oil | Soybean lecithin, PEG 660-stereate | 20.7, 0.19, Ψ = −9.7 | EE ≥ 99 | Permeation in Franz cells (ex vivo model) | [118] | |
| Metallic nanoparticles | Au | PEG | - | - | Lipopolysaccharide-induced inflammation (in vitro and in vivo models) | [119] |
| Magnetic nanoparticles | Iron (II) sulfate heptahydrate | PEG-PLA | 94, 0.14, Ψ = −0.01 | EE = 99 | Detection of amyloid plaques in Alzheimer’s (in vitro and in vivo models) | [120] |
| Gold-iron oxide | Glutathione | 40, 0.185, Ψ = −16 | EE = 70, 0.7 | Brain cancer (in vitro model) | [121] | |
| Fe3O4 | - | 185, Ψ = −37.5, | EE = 75 | Schizophrenic rats (in vivo model) | [122] | |
| Iron oxide. SPIO nanoparticles, 10,12-pentacosadiynoic acid | PVA, Lactoferrin | 100 | EE = 90.3 | Orthotopic Brain Tumor-Bearing Rat (in vitro and in vivo models) | [123] | |
| Liposomes | 1,2-dipalmitoyl-sn-glycerol-3-phosphatidylcholine, cholesterol | - | 207, 0.25, Ψ = −10.5 | - | Amyloid peptide plaques (in vivo model) | [124] |
| β-cyclodextrin (BCD), nanoliposome (NL) | β-cyclodextrin, phosphatidylcholine:cholesterol (5:1) | Tween 80 for liposome | 133.49, −31.76 and 121.81, −7.91 | BCD = 76.6, 19.73 NL = 88.2, 4.13 | Dimethylhydrazine induced poison (in vivo model) | [125] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. https://doi.org/10.3390/biom9020056
Del Prado-Audelo ML, Caballero-Florán IH, Meza-Toledo JA, Mendoza-Muñoz N, González-Torres M, Florán B, Cortés H, Leyva-Gómez G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules. 2019; 9(2):56. https://doi.org/10.3390/biom9020056
Chicago/Turabian StyleDel Prado-Audelo, María L., Isaac H. Caballero-Florán, Jorge A. Meza-Toledo, Néstor Mendoza-Muñoz, Maykel González-Torres, Benjamín Florán, Hernán Cortés, and Gerardo Leyva-Gómez. 2019. "Formulations of Curcumin Nanoparticles for Brain Diseases" Biomolecules 9, no. 2: 56. https://doi.org/10.3390/biom9020056
APA StyleDel Prado-Audelo, M. L., Caballero-Florán, I. H., Meza-Toledo, J. A., Mendoza-Muñoz, N., González-Torres, M., Florán, B., Cortés, H., & Leyva-Gómez, G. (2019). Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules, 9(2), 56. https://doi.org/10.3390/biom9020056