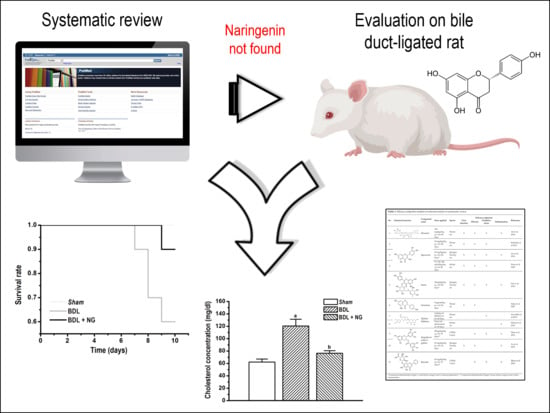
Analysis of Flavonoids Bioactivity for Cholestatic Liver Disease: Systematic Literature Search and Experimental Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review
2.1.1. Sources
2.1.2. Eligibility Criteria
2.1.3. Studies Selection
2.1.4. Meta-Analysis
2.1.5. Risk of Bias Assessment
2.2. Experimental Studies
2.2.1. Reagents and Materials
2.2.2. Sample Preparation
2.2.3. Animal Model
2.2.4. Biomarkers Quantification
2.2.5. Data Analysis and Statistics
3. Results
3.1. Systematic Review
3.2. Experimental Study
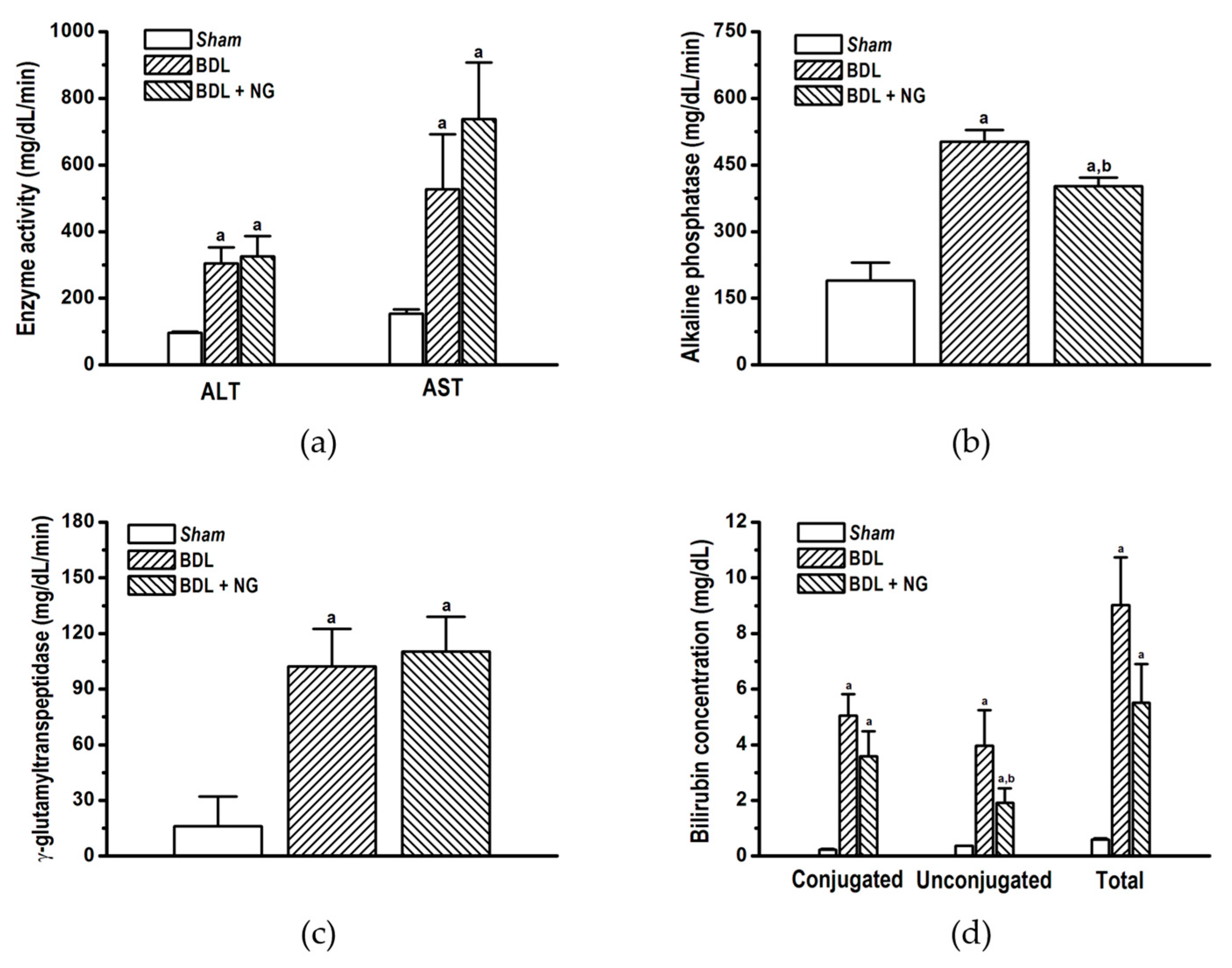
3.2.1. Signature of Obstructive Cholestasis in the Rat
3.2.2. Efficacy of NG in Obstructive Cholestasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, R. Global challenges in liver disease. Hepatology 2006, 44, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Araújo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018, 38, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Abshagen, K.; König, M.; Hoppe, A.; Müller, I.; Ebert, M.; Weng, H.; Holzhütter, H.-G.; Zanger, U.M.; Bode, J.; Vollmar, B.; et al. Pathobiochemical signatures of cholestatic liver disease in bile duct ligated mice. BMC Syst. Biol. 2015, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, E.J. Diagnosis and management of cholestatic liver disease. Clin. Gastroenterol. Hepatol. 2007, 5, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Bosoi, C.R.; Oliveira, M.M.; Ochoa-Sanchez, R.; Tremblay, M.; Ten Have, G.A.; Deutz, N.E.; Rose, C.F.; Bemeur, C. The bile duct ligated rat: A relevant model to study muscle mass loss in cirrhosis. Metab. Brain Dis. 2017, 32, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Vohra, B.P.S.; Zhang, Y.; Heuckeroth, R.O. Transcriptional profiling after bile duct ligation identifies PAI-1 as a contributor to cholestatic injury in mice. Hepatology 2005, 42, 1099–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudic, J.S.; Poropat, G.; Krstic, M.N.; Bjelakovic, G.; Gluud, C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst. Rev. 2012, 12, CD000551. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.N.; Cohen, J.; Blenkharn, J.I.; McConnell, J.S.; Barr, J.; Blumgart, L.H. A randomized clinical trial of oral ursodeoxycholic acid in obstructive jaundice. Br. J. Surg. 1986, 73, 634–636. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Crocenzi, F.A.; Roma, M.G. Silymarin as a new hepatoprotective agent in experimental cholestasis: New possibilities for an ancient medication. Curr. Med. Chem. 2006, 13, 1055–1074. [Google Scholar] [CrossRef]
- Angulo, P.; Patel, T.; Jorgensen, R.A.; Therneau, T.M.; Lindor, K.D. Silymarin in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology 2000, 32, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Schuler, J.; Hudson, M.L.; Schwartz, D.; Samudrala, R. A Systematic Review of Computational Drug Discovery, Development, and Repurposing for Ebola Virus Disease Treatment. Molecules 2017, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mukwaya, E.; Wong, M.-S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Sánchez-Salgado, J.C.; Ortiz-Andrade, R.R.; Aguirre-Crespo, F.; Vergara-Galicia, J.; León-Rivera, I.; Montes, S.; Villalobos-Molina, R.; Estrada-Soto, S. Hypoglycemic, vasorelaxant and hepatoprotective effects of Cochlospermum vitifolium (Willd) Sprengel: A potential agent for the treatment of metabolic syndrome. J. Ethnopharmacol. 2007, 109, 400–405. [Google Scholar]
- Sánchez-Salgado, J.C.; Castillo-España, P.; Ibarra-Barajas, M.; Villalobos-Molina, R.; Estrada-Soto, S. Cochlospermum vitifolium induces vasorelaxant and antihypertensive effects mainly by activation of NO/cGMP signaling pathway. J. Ethnopharmacol. 2010, 130, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, A.B.; Rios, M.Y. Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity. Molecules 2018, 23, 1952. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Rivera-Mancía, S.; Montes, S.; Méndez-Armenta, M.; Muriel, P.; Ríos, C. Morphological changes of rat astrocytes induced by liver damage but not by manganese chloride exposure. Metab. Brain. Dis. 2009, 24, 243–255. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wang, Y.-Y.; Chen, W.-Y.; Chuang, Y.-H.; Pan, P.-H.; Chen, C.-J. Beneficial effect of quercetin on cholestatic liver injury. J. Nutr. Biochem. 2014, 25, 1183–1195. [Google Scholar] [CrossRef]
- Pan, P.-H.; Lin, S.-Y.; Wang, Y.-Y.; Chen, W.-Y.; Chuang, Y.-H.; Wu, C.-C.; Chen, C.-J. Protective effects of rutin on liver injury induced by biliary obstruction in rats. Free Radic. Biol. Med. 2014, 73, 106–116. [Google Scholar] [CrossRef]
- Zahran, M.H.; Hussein, A.M.; Barakat, N.; Awadalla, A.; Khater, S.; Harraz, A.; Shokeir, A.A. Sildenafil activates antioxidant and antiapoptotic genes and inhibits proinflammatory cytokine genes in a rat model of renal ischemia/reperfusion injury. Int. Urol. Nephrol. 2015, 47, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, A.; Kumar, B.; Singh, S.K.; Bhatia, A.; Gulati, M.; Prakash, T.; Bawa, P.; Malik, A.H. Protective effect of co-administration of curcumin and sildenafil in alcohol induced neuropathy in rats. Eur. J. Pharmacol. 2017, 805, 58–66. [Google Scholar] [CrossRef]
- Ali, F.E.M.; Azouz, A.A.; Bakr, A.G.; Abo-Youssef, A.M.; Hemeida, R.A.M. Hepatoprotective effects of diosmin and/or sildenafil against cholestatic liver cirrhosis: The role of Keap-1/Nrf-2 and P38-MAPK/NF-κB/iNOS signaling pathway. Food Chem. Toxicol. 2018, 120, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, Y.A.; Steiner, J.M. Laboratory Evaluation of the Liver. Vet. Clin. North Am. Small Anim. Pract. 2017, 47, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.L.; Ocampo, G.; Fariña, G.G.; Reyes-Esparza, J.; Rodríguez-Fragoso, L. Genistein decreases liver fibrosis and cholestasis induced by prolonged biliary obstruction in the rat. Ann. Hepatol. 2007, 6, 41–47. [Google Scholar]
- Peres, W.; Tuñón, M.J.; Collado, P.S.; Herrmann, S.; Marroni, N.; González-Gallego, J. The flavonoid quercetin ameliorates liver damage in rats with biliary obstruction. J. Hepatol. 2000, 33, 742–750. [Google Scholar] [CrossRef]
- Stedman, C.; Liddle, C.; Coulter, S.; Sonoda, J.; Alvarez, J.G.; Evans, R.M.; Downes, M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc. Natl. Acad. Sci. USA 2006, 103, 11323–11328. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, H.; Gores, G.J. Bile acid regulation of hepatic physiology: IV. Bile acids and death receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G734–G738. [Google Scholar]
- Ivey, K.L.; Jensen, M.K.; Hodgson, J.M.; Eliassen, A.H.; Cassidy, A.; Rimm, E.B. Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. Br. J. Nutr. 2017, 117, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- Agra, L.C.; Ferro, J.N.S.; Barbosa, F.T.; Barreto, E. Triterpenes with healing activity: A systematic review. J. Dermatolog. Treat. 2015, 26, 465–470. [Google Scholar] [CrossRef]
- Stanca, E.; Serviddio, G.; Bellanti, F.; Vendemiale, G.; Siculella, L.; Giudetti, A.M. Down-regulation of LPCAT expression increases platelet-activating factor level in cirrhotic rat liver: potential antiinflammatory effect of silybin. Biochim. Biophys. Acta. 2013, 1832, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2015, 5, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Menżyk, T.; Bator, M.; Derra, A.; Kierach, R.; Kukla, M. The role of metabolic disorders in the pathogenesis of intrahepatic cholestasis of pregnancy. Clin. Exp. Hepatol. 2018, 4, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Orlandini, B.; Tenori, L.; Biagini, M.R.; Milani, S.; Renzi, D.; Luchinat, C.; Calabrò, A.S. The metabolic signature of Primary Biliary Cholangitis and its comparison with Coeliac Disease. J. Proteome Res. 2018, 18, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.E.; Meyer, D.J.; Campbell, M.; McMurphy, R. Serum lipid and lipoprotein changes in ponies with experimentally induced liver disease. Am. J. Vet. Res. 1990, 51, 1380–1384. [Google Scholar]
- Jessen, N.; Buhl, E.S.; Schmitz, O.; Lund, S. Impaired insulin action despite upregulation of proximal insulin signaling: novel insights into skeletal muscle insulin resistance in liver cirrhosis. J. Hepatol. 2006, 45, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Sheu, W.H.-H.; Chen, W.-Y.; Lee, F.-Y.; Huang, C.-J. Stimulated resistin expression in white adipose of rats with bile duct ligation-induced liver cirrhosis: relationship to cirrhotic hyperinsulinemia and increased tumor necrosis factor-alpha. Mol. Cell. Endocrinol. 2005, 232, 1–8. [Google Scholar] [CrossRef]
- Enochsson, L.; Isaksson, B.; Strömmer, L.; Erlanson-Albertsson, C.; Permert, J. Bile duct obstruction is associated with early postoperative upregulation of liver uncoupling protein-2 and reduced circulating glucose concentration in the rat. Nutrition 2010, 26, 405–410. [Google Scholar] [CrossRef]
- Catala, J.; Daumas, M.; Chanh, A.P.; Lasserre, B.; Hollande, E. Insulin and glucagon impairments in relation with islet cells morphological modifications following long term pancreatic duct ligation in the rabbit--a model of non-insulin-dependent diabetes. Int. J. Exp. Diabetes Res. 2001, 2, 101–112. [Google Scholar] [CrossRef]
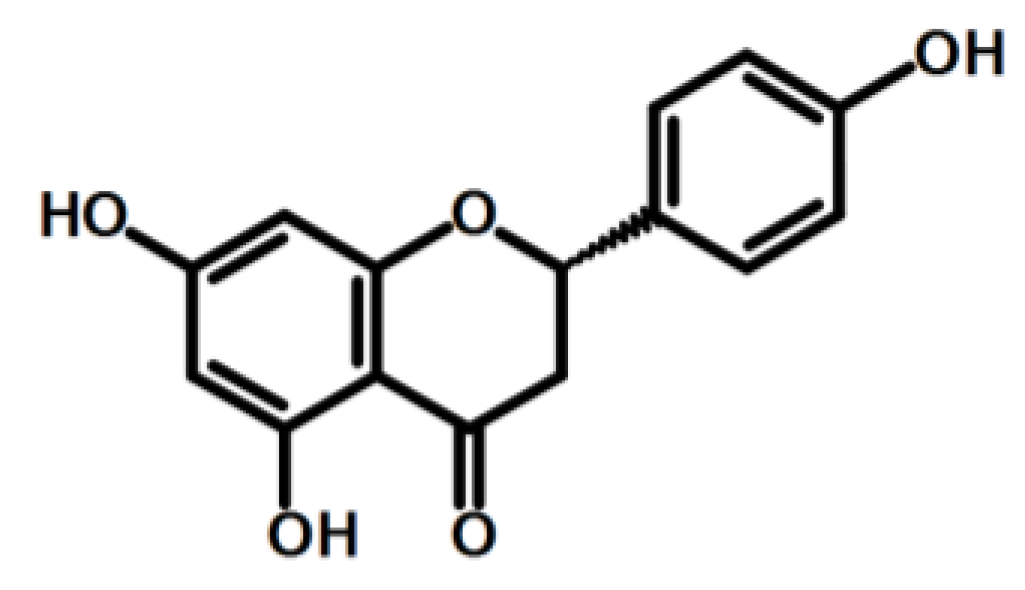
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Allister, E.M.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Edwards, J.Y.; Markle, J.M.; Hegele, R.A.; Huff, M.W. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes 2009, 58, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Sicklick, J.K.; Ma, Q.; Yang, L.; Huang, J.; Qi, Y.; Chen, W.; Li, Y.-X.; Goldschmidt-Clermont, P.J.; Diehl, A.M. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology 2006, 44, 1267–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabirifar, R.; Ghoreshi, Z.-A.-S.; Safari, F.; Karimollah, A.; Moradi, A.; Eskandari-Nasab, E. Quercetin protects liver injury induced by bile duct ligation via attenuation of Rac1 and NADPH oxidase1 expression in rats. HBPD INT 2017, 16, 88–95. [Google Scholar] [CrossRef]



| No | Chemical Structure | Compound Name | Dose Applied | Species | Efficacy Endpoints | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Liver Function | Fibrosis | Oxidative Stress | Inflammation | ||||||
| 1 |  | Diosmin | 100 mg/kg/day, p.o. for 28 days | Wistar rat | X | X | X | X | Ali et al 2018 |
| 2 | 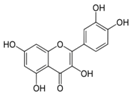 | Quercetin | 30 mg/kg/day p.o. for 28 days | Wistar rat | X | X | X | Kabirifar et al 2017 | |
| 3 | 25 mg/kg/day p.o. for 28 days * | Sprague Dawley rat | X | X | X | X | Lin et al 2014 | ||
| 4 | 75, 150, 300 μmol/kg/day i.p. for 28 days | Wistar rat | X | X | X | Peres et al 2000 | |||
| 5 |  | Rutin | 25mg/kg/day p.o. for 28 days * | Sprague Dawley rat | X | X | X | X | Pan et al 2014 |
| 6 |  | Genistein | 5 μg/rat/day p.o. for 56 days | Wistar rat | X | X | Salas et al 2007 | ||
| 7 | 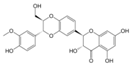 | Silybin/Silibinin | 0.4g/kg ad libitum p.o. for 28 days | Wistar rat | X | Serviddio et al 2014 | |||
| 8 | Dose not determined for 28 days | Wistar rat | X | Stanca et al 2013 | |||||
| 9 | 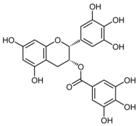 | Epigallo- catechin 3-gallate | 30mg/kg/day i.p. for 14 days ** | C57BL/6 mice | X | X | X | Shen et al 2015 | |
| 10 | 25 mg/kg/day p.o. for 14 days | Sprague Dawley rat | X | X | X | Yu et al 2015 | |||
| 11 |  | Baicalin | 50 mg/kg/day i.p. for 14 days ** | C57BL/6 mice | X | X | X | Shen et al 2017 | |
| No | Reference | Compound | Change % | |||||
|---|---|---|---|---|---|---|---|---|
| ALT | AST | AP | GGT | Total Bilirubin | Mortality | |||
| 1 | Ali et al 2018 | Diosmin | 48.77 | 48.32 | 50.53 | 46.37 | 56.77 | 20% |
| 2 | Kabirifar et al 2017 | Quercetin | 32.89 | 34.32 | 44.44 | ND | ND | ND |
| 3 | Lin et al 2014 | Quercetin | 30.05 | 35.98 | ND | 55.87 | 36.26 | ND |
| 4 | Peres et al 2000 * | Quercetin | 55.81 | 78.57 | 49.86 | ND | ND | ND |
| 5 | Pan et al 2014 | Rutin | 37.16 | 44.55 | ND | 63.49 | 40.66 | ND |
| 6 | Salas et al 2007 | Genistein | 51.53 | ND | 59.88 | 73.68 | 68.97 | ND |
| 7 | Serviddio et al 2014 | Silybin/Silibinin | ND | ND | ND | ND | ND | ND |
| 8 | Stanca et al 2013 | Silybin/Silibinin | 30.76 | 23.68 | 27.78 | ND | 39.76 | ND |
| 9 | Shen et al 2015 | Epigallocatechin 3-Gallate | ND | ND | ND | ND | ND | ND |
| 10 | Yu et al 2015 | Epigallocatechin 3-Gallate | −29.77 | 3.66 | ND | ND | -4.64 | ND |
| 11 | Shen et al 2017 | Baicalin | ND | ND | ND | ND | ND | ND |
| 12 | Current article | NG | −6.79 | −40.02 | 19.78 | −7.82 | 38.91 | 30% |
| No | Reference | Compound | Change % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Glucose | Cholesterol | Triglycerides | Insulin | VLDL | LDL | HDL | |||
| 3 | Lin et al 2014 | Quercetin | ND | 14.78 | 26.47 | ND | ND | ND | ND |
| 5 | Pan et al 2014 | Rutin | ND | 19.13 | 25.00 | ND | ND | ND | ND |
| 10 | Yu et al 2015 | Epigallocatechin 3-Gallate | ND | 0.69 | ND | ND | ND | 0.74 | −18.52 |
| 12 | Current article | NG | −2.51 | 36.33 | 29.43 | 44.44 | 29.43 | 58.44 | −48.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Salgado, J.C.; Estrada-Soto, S.; García-Jiménez, S.; Montes, S.; Gómez-Zamudio, J.; Villalobos-Molina, R. Analysis of Flavonoids Bioactivity for Cholestatic Liver Disease: Systematic Literature Search and Experimental Approaches. Biomolecules 2019, 9, 102. https://doi.org/10.3390/biom9030102
Sánchez-Salgado JC, Estrada-Soto S, García-Jiménez S, Montes S, Gómez-Zamudio J, Villalobos-Molina R. Analysis of Flavonoids Bioactivity for Cholestatic Liver Disease: Systematic Literature Search and Experimental Approaches. Biomolecules. 2019; 9(3):102. https://doi.org/10.3390/biom9030102
Chicago/Turabian StyleSánchez-Salgado, Juan Carlos, Samuel Estrada-Soto, Sara García-Jiménez, Sergio Montes, Jaime Gómez-Zamudio, and Rafael Villalobos-Molina. 2019. "Analysis of Flavonoids Bioactivity for Cholestatic Liver Disease: Systematic Literature Search and Experimental Approaches" Biomolecules 9, no. 3: 102. https://doi.org/10.3390/biom9030102
APA StyleSánchez-Salgado, J. C., Estrada-Soto, S., García-Jiménez, S., Montes, S., Gómez-Zamudio, J., & Villalobos-Molina, R. (2019). Analysis of Flavonoids Bioactivity for Cholestatic Liver Disease: Systematic Literature Search and Experimental Approaches. Biomolecules, 9(3), 102. https://doi.org/10.3390/biom9030102