Structural Changes in the Acceptor Site of Photosystem II upon Ca2+/Sr2+ Exchange in the Mn4CaO5 Cluster Site and the Possible Long-Range Interactions
Abstract
:1. Introduction
2. Materials and Methods
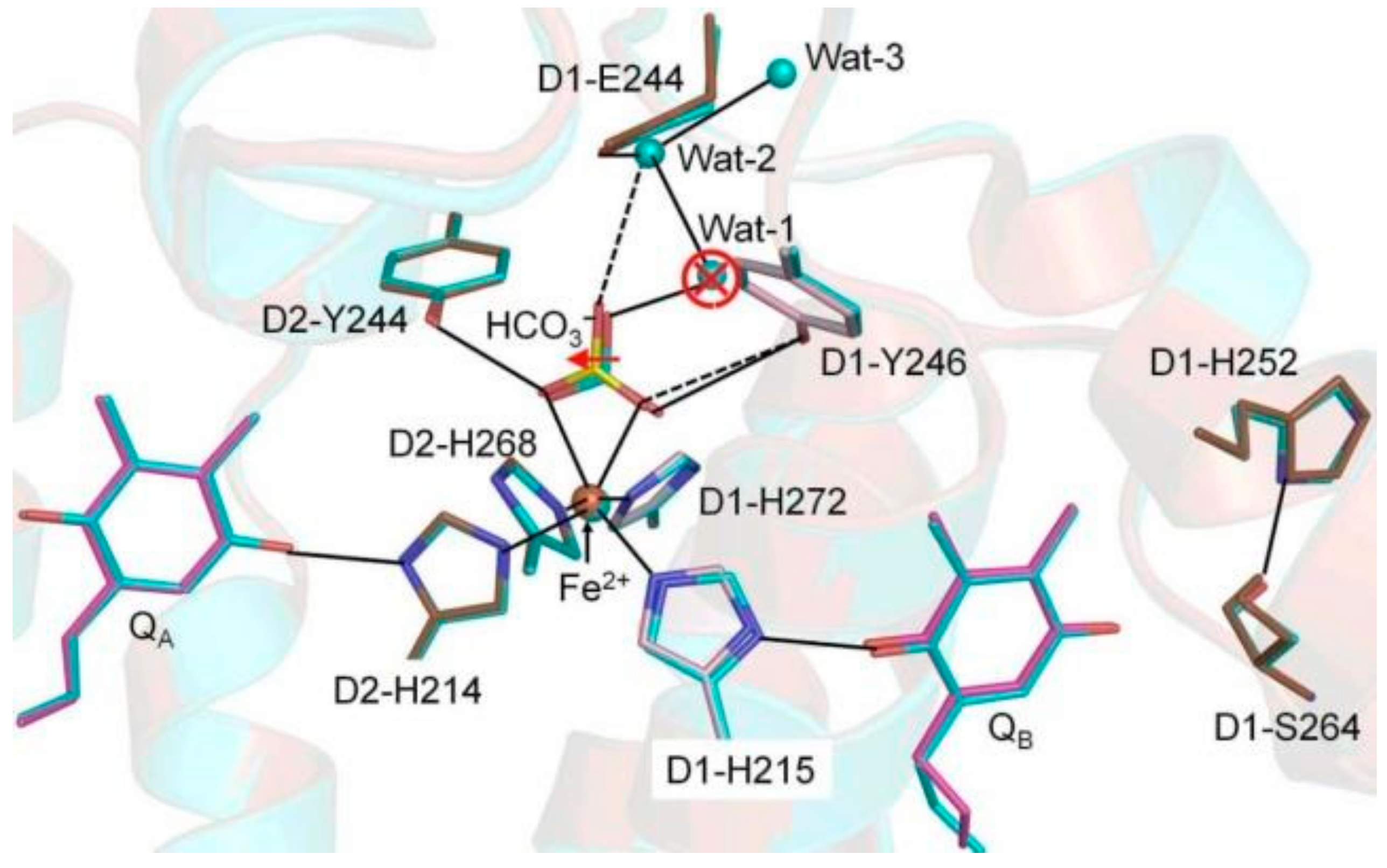
3. Results and Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, J.-R. The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Ann. Rev. Plant. Biol. 2015, 66, 23–48. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Koua, F.H.M.; Umena, Y.; Kawakami, K.; Shen, J.-R. Structure of Sr-substituted photosystem II at 2.1 Å resolution and its implications in the mechanism of water oxidation. Proc. Natl. Acad. Sci. USA 2003, 110, 3889–3894. [Google Scholar] [CrossRef]
- Kato, Y.; Noguchi, T. Long-Range Interaction between the Mn4CaO5 Cluster and the Non-heme Iron Center in Photosystem II as Revealed by FTIR Spectroelectrochemistry. Biochemistry 2014, 53, 4914–4923. [Google Scholar] [CrossRef]
- Kato, Y.; Ishii, R.; Noguchi, T. Comparative analysis of the interaction of the primary quinone QA in intact and Mn-depleted photosystem II membranes using light-induced ATR-FTIR spectroscopy. Biochemistry 2016, 55, 6355–6358. [Google Scholar] [CrossRef]
- Kato, Y.; Shibamoto, T.; Yamamoto, S.; Watanabi, T.; Ishida, N.; Sugiura, M.; Rappaport, F.; Boussac, A. Influence of the PsbA1/PsbA3, Ca2+/Sr2+ and Cl−/Br− exchanges on the redox potential of the primary quinone QA in Photosystem II from Thermosynechococcus elongatus as revealed by spectroelectrochemistry. Biochim. Biophys. Acta Bionenerg. 2012, 1817, 1998–2004. [Google Scholar] [CrossRef]
- Boussac, A.; Rappaport, F.; Carrier, P.; Verbavatz, J.M.; Gobin, R.; Kirilovsky, A.; Rutherford, A.W.; Sugiura, M. Biosynthetic Ca2+/Sr2+ Exchange in the Photosystem II Oxygen-evolving Enzyme of Thermosynechococcus elongatus. J. Biol. Chem. 2004, 279, 22809–22819. [Google Scholar] [CrossRef]
- Boussac, A.; Sugiura, M.; Rappaport, F. Probing the quinone binding site of photosystem II from Thermosynechococcus elongatus containing either PsbA1 or PsbA3 as the D1 protein through the binding characteristics of herbicides. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 119–129. [Google Scholar] [CrossRef]
- Krieger, A.; Rutherford, A.W.; Johnson, G.N. On the determination of redox midpoint potential of the primary quinone electron acceptor, QA, in photosystem II. Biochim. Biophys. Acta Bioenerg. 1995, 1229, 193–201. [Google Scholar] [CrossRef]
- Kargul, J.; Maghlaoui, K.; Murray, J.W.; Deak, Z.; Boussac, A.; Rutherford, A.W.; Vass, I.; Barber, J. Purification, crystallization and X-ray diffraction analyses of the T. elongatus PSII core dimer with strontium replacing calcium in the oxygen-evolving complex. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 404–413. [Google Scholar] [CrossRef]
- Saito, K.; Rutherford, A.W.; Ishikita, H. Mechanism of proton-coupled quinone reduction in photosystem II. Proc. Natl. Acad. Sci. USA 2013, 110, 954–959. [Google Scholar] [CrossRef]
- Chatterjee, R.; Milikisiyants, S.; Coates, C.S.; Koua, F.H.M.; Shen, J.R.; Lakshmi, K.V. The structure and activation of substrate water molecules in Sr2+-substituted photosystem II. Phys. Chem. Chem. Phys. 2014, 16, 20834–20843. [Google Scholar] [CrossRef]
- Cruickshank, D.W.J. Remarks about protein structure precision. Acta Cryst. D 1999, 55, 583–601. [Google Scholar] [CrossRef]
- Collaborative Computational Project, Number 4. The CCP4 suite: Programs for protein crystallography. Acta Cryst. D 1994, 50, 760–763. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Cryst. D 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Ishikita, H.; Knapp, E.W. Control of quinone redox potentials in photosystem II: Electron transfer and photoprotection. J. Am. Chem. Soc. 2005, 127, 14714–14720. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Liu, Y.; Zheng, Y.; Zhu, Q.; Han, G.; Shen, J.-R. Proton-coupled electron transfer of plastoquinone redox reactions in photosystem II: A pump-probe ultraviolet resonance Raman study. J. Phys. Chem. Lett. 2019, 10, 3240–3247. [Google Scholar] [CrossRef]
- Moore, G.R.; Pettigrew, G.W.; Rogers, N.K. Factors influencing redox potentials of electron transfer proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 4998–4999. [Google Scholar] [CrossRef]
- Zhou, H.X.; Pang, X. Electrostatic interactions in protein structure, folding, binding, and condensation. Chem. Rev. 2018, 118, 1691–1741. [Google Scholar] [CrossRef]
- Zhang, M.; Bommer, M.; Chatterjee, R.; Husseon, R.; Yano, J.; Dau, H.; Kern, J.; Dobbek, H.; Zouni, A. Structural insights into the light-driven auto-assembly process of the water-oxidizing Mn4CaO5-cluster in photosystem II. Elife 2017, 6, e2693. [Google Scholar]
- Breton, J.; Boullais, C.; Burie, J.R.; Nabedryk, E.; Mioskowski, C. Binding sites of quinones in photosynthetic bacterial reaction centers investigated by light-induced FTIR difference spectroscopy: Symmetry of the carbonyl interactions and close equivalence of the QB vibrations in Rhodobacter sphaeroides and Rhodopseudomonas viridis probed by isotope labelling. Biochemistry 1994, 33, 14378–14386. [Google Scholar]
- Brudler, R.; de Groot, H.J.M.; van Liemt, W.B.S.; Steggerda, W.F.; Esmeijer, R.; Gast, P.; Hoff, A.J.; Lugtenburg, J.; Gerwert, K. Asymmetric binding of the 1- and 4-C=O groups of QA in Rhodobacter sphaeroides R26 reaction centers monitored by Fourier transform infra-red spectroscopy using site-specific isotopically labelled ubiquinone-10. EMBO J. 1994, 13, 5523–5530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gunner, M.R. Energetics of quinone-dependent electron and proton transfers in Rhodobacter sphaeroides reaction centres. Biochemistry 2005, 44, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Ermler, U.; Fritzsch, G.; Buchanan, S.K.; Michel, H. Structure of the photosynthetic reaction center from Rhodopbacter sphaeroides at 2.65 Å resolution: Cofactors and protein-cofactor interactions. Structure 1994, 2, 935–936. [Google Scholar] [CrossRef]
- Ashizawa, R.; Noguchi, T. Effects of hydrogen bonding interactions on the redox potential and molecular vibrations of plastoquinone as studied using density functional theory calculations. Phys. Chem. Chem. Phys. 2014, 16, 11864–11876. [Google Scholar] [CrossRef] [PubMed]
- Shevela, D.; Eaton-Rye, J.J.; Shen, J.-R.; Govindjee. Photosystem II and the unique role of bicarbonate: A historical perspective. Biochim. Biophs. Acta Bioenerg. 2012, 1817, 1134–1151. [Google Scholar] [CrossRef] [Green Version]
- Brinkert, K.; De Causmaecker, S.; Kriger-Liszkay, A.; Fantuzzi, A.; Rutherford, A.W. Bicarbonate-induced redox tuning in photosystem II for regulation and protection. Proc. Natl. Acad. Sci. USA 2016, 113, 1244–12149. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Fukushima, Y.; Kamiya, N. Two different structures of the oxygen-evolving complex in the same polypeptide frameworks of photosystem II. J. Am. Chem. Soc. 2017, 139, 1718–1721. [Google Scholar] [CrossRef]


| Ligand | Subunit | Sr-PSII (Å) | Ca-PSII (Å) | B-factor | Sr-PSII (Å2) | Ca-PSII (Å2) |
|---|---|---|---|---|---|---|
| Fe(II) | ||||||
| NE2-His215 NE2-His214 NE2-His268 NE2-His272 O1-BCT O2-BCT | psbA/D1 psbD/D2 psbD/D2 psbA/D1 psbD/D2 psbD/D2 | 2.06(0.02) 2.10(0.01) 2.20(0.02) 2.26(0.04) 2.30(0.02) 2.39(0.02) | 2.16(0.03) 2.17(0.07) 2.28(0.02) 2.26(0.01) 2.33(0.00) 2.29(0.05) | Fe(II) NE2-His215 NE2-His214 NE2-His268 NE2-His272 O1-BCT O2-BCT | 29.61(0.42) 28.48(0.17) 27.24(1.97) 27.22(1.31) 29.67(0.94) 39.41(0.77) 39.74(0.68) | 27.41(0.52) 25.75(0.79) 23.72(0.20) 24.37(0.30) 28.25(0.26) 31.23(0.24) 34.04(0.23) |
| Quinone B | ||||||
| O1/ND1-His215 O2/OG-Ser264 O2/N-Phe265 O2/O2-Phe265 | psbA/D1 psbA/D1 psbA/D1 psbA/D1 | 2.50(0.00) 2.76(0.06) 2.82(0.09) 3.15(0.19) | 2.48(0.06) 2.74(0.02) 2.95(0.05) 3.09(0.08) | O1/QB O2/QB OG-Ser264 N-Phe265 O2-Phe265 ND1-His215 | 52.37(0.55) 52.73(0.53) 48.57(0.46) 47.07(0.51) 49.90(0.49) 30.16(1.94) | 60.45(0.20) 74.23(0.19) 63.62(0.17) 57.01(0.19) 66.33(0.18) 27.23(0.16) |
| Quinone A | ||||||
| O2/ND1-His214 O1/N-Phe261 | psbD/D2 psbD/D2 | 2.79(0.00) 3.02(0.00) | 2.66(0.06) 2.95(0.03) | O2/QA O1/QA ND1-His214 N-Phe261 | 28.47(0.58) 28.60(0.62) 27.32(0.47) 26.74(0.45) | 25.13(0.21) 25.60(0.22) 22.80(0.44) 23.36(0.17) |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koua, F.H.M. Structural Changes in the Acceptor Site of Photosystem II upon Ca2+/Sr2+ Exchange in the Mn4CaO5 Cluster Site and the Possible Long-Range Interactions. Biomolecules 2019, 9, 371. https://doi.org/10.3390/biom9080371
Koua FHM. Structural Changes in the Acceptor Site of Photosystem II upon Ca2+/Sr2+ Exchange in the Mn4CaO5 Cluster Site and the Possible Long-Range Interactions. Biomolecules. 2019; 9(8):371. https://doi.org/10.3390/biom9080371
Chicago/Turabian StyleKoua, Faisal Hammad Mekky. 2019. "Structural Changes in the Acceptor Site of Photosystem II upon Ca2+/Sr2+ Exchange in the Mn4CaO5 Cluster Site and the Possible Long-Range Interactions" Biomolecules 9, no. 8: 371. https://doi.org/10.3390/biom9080371
APA StyleKoua, F. H. M. (2019). Structural Changes in the Acceptor Site of Photosystem II upon Ca2+/Sr2+ Exchange in the Mn4CaO5 Cluster Site and the Possible Long-Range Interactions. Biomolecules, 9(8), 371. https://doi.org/10.3390/biom9080371





