Abstract
Cartilage regeneration is massive during tail regeneration in lizards but little is known about cartilage regeneration in other body regions of the skeleton. The recovery capability of injured epiphyses of femur and tibia of lizard knees has been studied by histology and 5BrdU immunohistochemistry in lizards kept at high environmental temperatures. Lizard epiphyses contain a secondary ossified center of variable extension surrounded peripherally by an articular cartilage and basally by columns of chondrocytes that form the mataphyseal or growth plate. After injury of the knee epiphyses, a broad degeneration of the articular cartilage during the first days post-injury is present. However a rapid regeneration of cartilaginous tissue is observed from 7 to 14 days post-injury and by 21 days post-lesions, a large part of the epiphyses are reformed by new cartilage. Labeling with 5BrdU indicates that the proliferating cells are derived from both the surface of the articular cartilage and from the metaphyseal plate, two chondrogenic regions that appear proliferating also in normal, uninjured knees. Chondroblasts proliferate by interstitial multiplication forming isogenous groups with only a scant extracellular matrix that later increases. The high regenerative power of lizard articular cartilage appears related to the permanence of growing cartilaginous centers in the epiphyses of long bones such as those of the knee during adulthood. It is likely that these regions contain resident stem cells that give rise to new chondroblasts of the articular and metaphyseal cartilage during most of the lizard’s lifetime, but can produce an excess of cartilaginous tissues when stimulated by the lesion.
1. Introduction
It is becoming more and more evident that the varying ability to regenerate tissues and organs among vertebrates is related to the permanence of a variable reserve of stem cells in different body regions, such as the skin, intestine and bone marrow, generally indicated as stem cell niches [1,2]. However, the extension and frequencies of these stem cell niches are likely variable among the adult forms of different species of vertebrates, and in particular between permanently growing species of heterothermic anamniotes such as fish and amphibians, or heterothermic amniotes such as extant reptiles, in comparison to those present in homeothermic amniotes such as birds and mammals where the process of growth ceases with sexual maturity. As far as is known, most turtles, crocodilians, lizards and snakes grow during most of their lifespan, albeit at different rates, and, although their growth slows down especially after reaching sexual maturity and in the later stages of life, it likely never stops completely [3].
The process of growth in reptiles, particularly the skeletal system whereby the lengthening of long and short bones proceeds at variable rates after sexual maturity, thereby suggests that cartilaginous cells are continuously produced in the growing centers to be later replaced by new bone tissue. Previous studies on the power of cartilage regeneration in lizards indicated extensive possibilities to form large masses of cartilaginous tissue after tail regeneration or vertebrae lesions (summarized in [4,5,6]). In particular, a large but continuous cartilaginous tube of over 0.2 mm in thickness and of over 4 cm in length can replace the vertebral column in the axial region of the regenerated tail in some lizards [7,8,9,10,11,12,13,14,15]. Little, however, is known about the regeneration ability of other skeletal elements in these reptiles.
In the present histological and immunocytochemical study we have analyzed chiefly the entity of the repairing process of bones forming the knee, femur and tibia, after injuries on the articular cartilages forming their epiphysis. It is known that, although in growing mammals some limited recovery of the articular cartilage is possible, this is not the case in adult mammals [16,17]. While during development and first months of post-natal life in mammals the articular cartilage of epiphyses is made of a non-organized cartilaginous tissues (“open type” of cartilage, see [16]), the mature and definitive articular cartilage is formed by 3−4 definite layers of chondrocytes resting on a continuous bone layer that delimits the secondary ossification center (“close type,” see [16]). It appears that the articular cartilage present in adults of anamniote vertebrates such as amphibians is similar to the “open type,” although most of the epiphyses are still made of cartilaginous tissue in adults [18,19,20,21,22]. While the general histological structure of the reptilian articular cartilage is known [18,23], it is unknown whether the articular cartilage can regenerate like the vertebrae of the tail and trunk after injury [5]. In the present study, we have addressed this problem and show that the epiphyses of long bones in adult lizards can produce a large mass of cartilaginous cells after injury. This is done through histology and immunocytochemistry on experimentally injured knees of lizards.
2. Materials and Methods
2.1. Experiments
In these experiments, adult individuals—males and females longer than 12 cm—of the Italian wall lizard Podarcis muralis were utilized. The husbandry and experimental manipulations followed the Italian guidelines for animal care and handling (Art. 5, DL 116/92). After ether-induced anesthesia, the skin of the right knee of lizards was cleaned with a Lugol iodinated-solution, the skin was cut open, the under-skin muscle and connective tissues sectioned in order to expose the epiphysis of knee bones, the apposed terminal regions of the femur and tibia. Using a thin scissor, the epiphysis of both femur and tibia were damaged under a stereomicroscope with 3 cuts each using a small scissor taking care not to section the femoral artery to avoid extensive bleeding and possible necrosis of the limb. A limited bleeding, however, derived from the cut of the tissues during surgery. After the lesion, the limb tissues were recomposed over the injured knee and the skin flaps reconstituted to cover the surgical incision. The incision was covered with an antiseptic and healing powder (Cicatrene, Welcome Italia, Pomezia-Rome) that also helped to hold the skin in place over the wound.
All the lizards (13 in total) recovered well after the operation, and regained an apparently normal movement of the operated hind-limbs, showing little walking impairment. The lizards were kept in cages at summer temperatures and sun exposition, reaching over 30 °C during most of the day, and remaining above 20 °C during the night. Two non-operated lizards served as normal controls. The operated lizards were sacrificed after 4 days (n = 2), 7 days (n = 3), 14 days (n = 3), and 21 days (n = 3) from the operation. Contralateral, non-injured knees served as further controls in each stage of recovery. Four hours before sacrifice, the lizards were injected with 60 mg/kg body weight with 5-Bromo-deoxyUridine (5BrdU) dissolved in Ringer. Two additional lizards at 21 days post-operation received 5BrdU about 48 h before sacrifice.
2.2. Tissue Preparation and Microscopic Techniques
The operated knees were collected at about half of the length of both femur and tibia, and immediately immersed in 4% paraformaldehyde at 0–4 °C for about 4 h. During this time care was taken to remove as much as possible non-bone tissue around the knee joint in order to allow a deep penetration of the fixative. The knees were than immersed for about 18 hours (with two changes) in a post-fixative decalcifying solution (5% formic acid, 15% formaldheyde 35%, and 80 distilled water in volumes) at room temperature. The tissues were dehydrated, clarified in xylene, and embedded in wax for the following histological and immunocytochemical study.
Using a microtome, sections along the longitudinal plane were obtained at 6–9 µm in thickness, and collected on series of 5–10 sections in glass slide pre-coated with albumin-chromoalum. After dewaxing and dehydration, some sections were stained with 1% methylene blue and 1% eosin, for the histological study. Other sections containing the knee articulation, mainly the femur and tibia but in some cases also the fibula, were instead utilized for the immunocytochemical detection.
Some sections were pre-incubated for 30 min with a 0.1 M Tris buffer solution at pH 7.6 containing 2% bovine serum albumine and 5% normal goat serum in order to saturate unspecific binding sites. Part of the sections were incubated with a mouse anti-5BrdU antibody at 1:100 dilution in the buffer for 5–6 h at room temperature and then rinsed in the buffer (in control sections the primary antibody was omitted from the solution). The 5BrdU-monoclonal antiserum (mouse G3G4) was developed by Kaufmann, S.J. of the University of Illinois at Urbana, USA, and was provided by the Developmental Studies Hybridoma Bank, maintained by the University of Iowa, Iowa City, USA. The sections were later incubated with a goat anti-mouse TRITC-conjugated secondary antibody (TRITC is tetramethyl rhodamine isotiocianate) diluted 1:100 in the above buffer solution. This immunodetection aimed to detect dividing cells. The immunoreacted sections were observed and photographed using an epifluorescence microscope equipped with a rhodamine filter.
3. Results
3.1. Histology of the Epiphyses in the Normal Knee
The normal epiphysis of the femur and tibia showed a similar histological aspect (Figure 1A). They consisted in a superficial layer of cartilaginous cells delimiting the epiphyseal outline and representing the articular cartilage that surrounded dorsally a marrow cavity of the secondary ossification center, made of bone trabeculae and blood marrow (Figure 1A–D). Between the two opposed articular cartilages, a meniscus comprised a cartilaginous tissues in its lateral part that was connected to the medial part interposed between the two articular surfaces, consisting in a fibrous and dense regular connective (Figure 1B).
In the ventral part of the secondary ossification center, a metaphyseal cartilage (growth plate) was present, and it separated the secondary ossification cavity from the endochondral bone cavity of the diaphysis (Figure 1A,C). While the dorsal chondrocytes of the metaphyseal cartilage were irregularly arranged and discontinuous with the bone trabeculae (isotropic cartilage, see [16]), the ventral chondrocytes tended to pile as is typical for the growth plate (anisotropic cartilage, see [16]). However, the resting and proliferative zones were very limited, and the hypertrophic-piled chondrocytes rested on a discontinuous series of bony trabeculae (Figure 1C,E). The articular cartilage, however, appeared irregularly thickened around the secondary ossification center (Figure 1C) so that in some areas of the epiphysis this cartilage was actually merged with the metaphyseal cartilage (growth plate) located underneath, and the secondary ossification center appeared absent. The study at higher magnification of the articular cartilage showed the presence of flat and small chondrocytes on the superficial part. Beneath the superficial layer, chondrocytes assumed a rounded shape and enlarged forming isogenous groups in the deeper part that contacted the trabeculae of the secondary bone cavity. The latter formed an almost continuous bone lamina separating the articular cartilage from the bone marrow of the secondary ossification center (close-articular cartilage, see [16]). In conclusion, the secondary ossification center varied both in shape and extension in the epiphysis, so that the center appeared as a large cavity in the medial sections and became narrow to disappear in more peripheral (tangential) sections where the cartilaginous tissue was thicker.
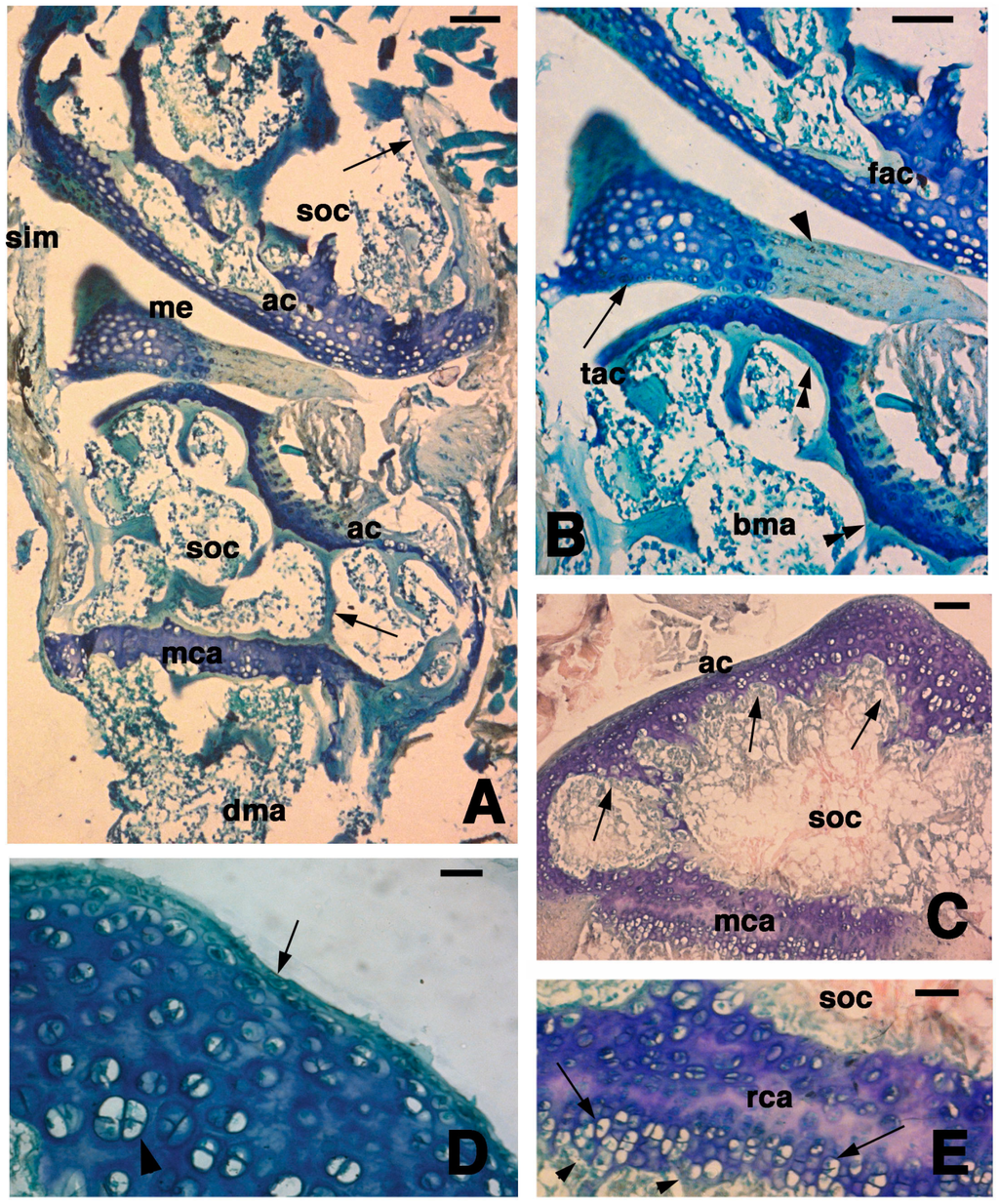
Figure 1.
Histology of the articular epiphysis of the normal knee. (A) General aspect of the articular cartilage of the femur (top) and the tibia (bottom) with their secondary ossification centers (arrows indicate the bone trabeculae). Bar, 100 µm; (B) Detail of the articular surfaces of the femur and tibia with interposed meniscus made of a fibrous part (arrowhead) and a cartilaginous part (lunula, arrow). Bar, 50 µm; (C) Another section of a tibia showing the irregular thickness of the articular cartilage (arrows) surrounding the secondary ossification center. Bar, 50 µm; (D) Detail of the superficial region of the articular cartilage of a tibia showing flat cells (arrow) contacting the sinovial cavity and the relatively abundant extracellular ground substance among the isogenous groups (arrowhead) located within the cartilaginous mass. Bar, 20 µm; (E) Detail on the metaphyseal (growth) plate featuring serial resting cartilage bounding the hypertrophic cartilage (arrows) contacting the diaphyseal bone (arrowheads indicate bone trabeculae). Bar, 20 µm. Legends: ac, articular cartilage; bma, bone marrow; dma, bone marrow of the diaphysis; fac, femur articular cartilage; me, meniscus, mca, metaphyseal (growth) cartilaginous plate; rca, resting cartilage within the metaphyseal plate; sim, sinovial membrane; soc, secondary ossification center; tac, tibia articular cartilage.
3.2. Histological Observations at Four and Seven Days Post-Lesion
After 4 days from time of the lesion, the two articular cartilages that were injured showed that a large portion of the cartilage was degenerated and that the extracellular matrix lost its basophilia while few basophilic chondrocytes remained visible among an eosinophilic and irregular fibrous dense connective tissue (Figure 2A–D). The latter tissue likely resulted from the degeneration of chondrocytes and the disappearance of glycosominoglycans (GAGs) (evidenced by the loss of methylene blue stain), while the collagenous material remained in place, thereby conferring the prevalent eosinophilic stain. The signs of the lesion appeared as holes formed through the articular cartilage that penetrated into the secondary ossification center (Figure 2B,C). Conversely, the methaphyseal cartilage was generally unaffected from the injury and most basophilia and cellular organization was conserved (Figure 2C–E). The ligaments, meniscus and the surfaces of articular cartilages often appeared fragmented with irregularly displaced fibrous eosinophilic material, likely made of collagenous residual fibers while the proteoglycans are largely lost. The eosinophilic connective tissue replacing the cartilage contained sparse chondrocyte residual bodies, possibly representing pyknotic nuclei or condensed dead cells (see arrowheads in Figure 2E). The detailed histological observation at higher magnification of the metaphyseal cartilage (growth plate) showed that the degenerating hypertrophic chondrocytes were not completely replaced by bone so that trabecolae were discontinuous underneath the cartilage (double arrowheads in Figure 2E).
At 7 days post-lesion in some areas of the tibia and femur, there were regions of degenerated cartilage, just as there were at 4 days post-injury. Moreover, some areas with regenerating chondrocytes were seen (Figure 3A). The degenerated cartilage consisted in an outer eosinophilic tissue facing the sinovial cavity and in a still basophilic area containing groups of roundish chondrocytes (Figure 3B). The new cartilaginous tissue was formed by numerous roundish chondrocytes with scarce extracellular matrix and random disposition (Figure 3C). Chondrocytes tended to occupy most of the new articular cartilage, still largely covered by remnants of the external degenerated cartilage that were attached to the ligaments (Figure 3C,D). A line of flat chondrocytes, mimicking the superficial layer of a normal articular cartilage, was seen between the new basophilic cartilaginous cells and the degenerated eosinophilic cartilaginous tissue (arrows in Figure 3C). Also, the new cartilage formed numerous irregular layers of chondrocytes that tended to merge with the metaphyseal plate. In the latter only a thin pale layer, made of acidophilic or damaged chondrocytes with no basophilia, was visible between the new articular cartilage and the adjacent methaphyseal (growth) plate (Figure 3C,D). It also appeared that some cartilage regeneration also occurred in the meniscus.
3.3. Histology at Fourteen and Twenty-One Days Post-Lesion
At 14 days post-lesion, a large mass of regenerated, irregularly organized and small chondrocytes (isotropic cartilage) formed a large part of the epiphyses of both femur and tibia (Figure 4A). Most of these cells were rounded and the amount of extracellular matrix separating chondrocytes was more abundant that the previous stage (Figure 4B). In one case among those studied, some areas of the cartilaginous epiphyses showed chondrocytes directly contacting a mesenchymal tissue at the base of the epiphyses, possibly representing new bone marrow tissue (Figure 4C). The formation of a serial cartilage where chondrocytes form piles of cells was missing. Only the more superficial chondrocytes, contacting the synovial articular cavity, appeared flat and less basophilic than the inner hyperthrophic chondrocytes.
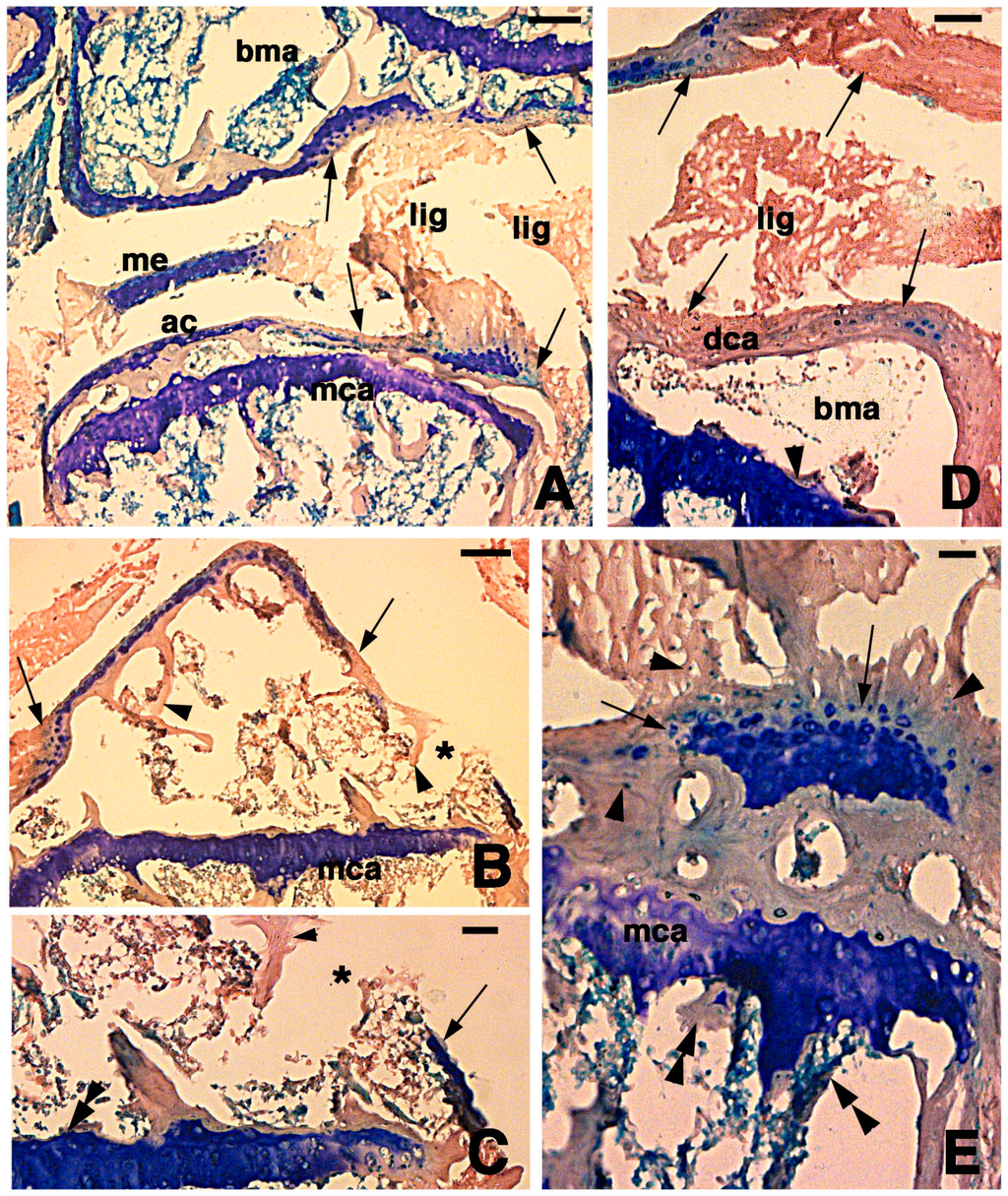
Figure 2.
Histological aspects of the knee four days after the lesion. (A) The damaged ligaments, the meniscus, and the articular cartilages of the femur and tibia (arrows) are eosinophilic. Large part of the superficial articular cartilage has lost its basophilia to the methylene blue. The metaphyseal plates appear unchanged with respect to uninjured bones. Bar, 100 µm; (B) In this section, most of the basophilic articular cartilage has disappeared and is replaced by eosinophilic dense connective in continuation with bone trabeculae (arrowheads). The asterisk shows a hole in the degenerated cartilage corresponding to the point of lesion. Bar, 100 µm; (C) Detail on the hole (asterisk) bordered by completely eosinophilic degenerated cartilage (arrowhead) and a piece of cartilage showing some basophilia (arrow). The double arrowhead indicates the unchanged metaphyseal plate. Bar, 50 µm; (D) Detail showing the eosinophilic type of dense fibrous tissue (arrows) remained after cartilage degeneration on the femur (top arrows) and tibia (bottom arrows). Small regions containing basophilic chondrocytes remain. The arrowhead points to the basophilic metaphyseal plate. Bar, 50 µm; (E) Detail on the degenerated articular cartilage of a tibia showing an “island” of cartilaginous tissue (arrows) surrounded by small, possibly pyknotic cartilaginous cells (arrowheads). Double arrowheads indicate diaphyseal bone trabeculae. Bar, 25 µm. Legends: ac, articular cartilage; bma, bone marrow; lig, ligament; mca, metaphyseal (growth) plate.
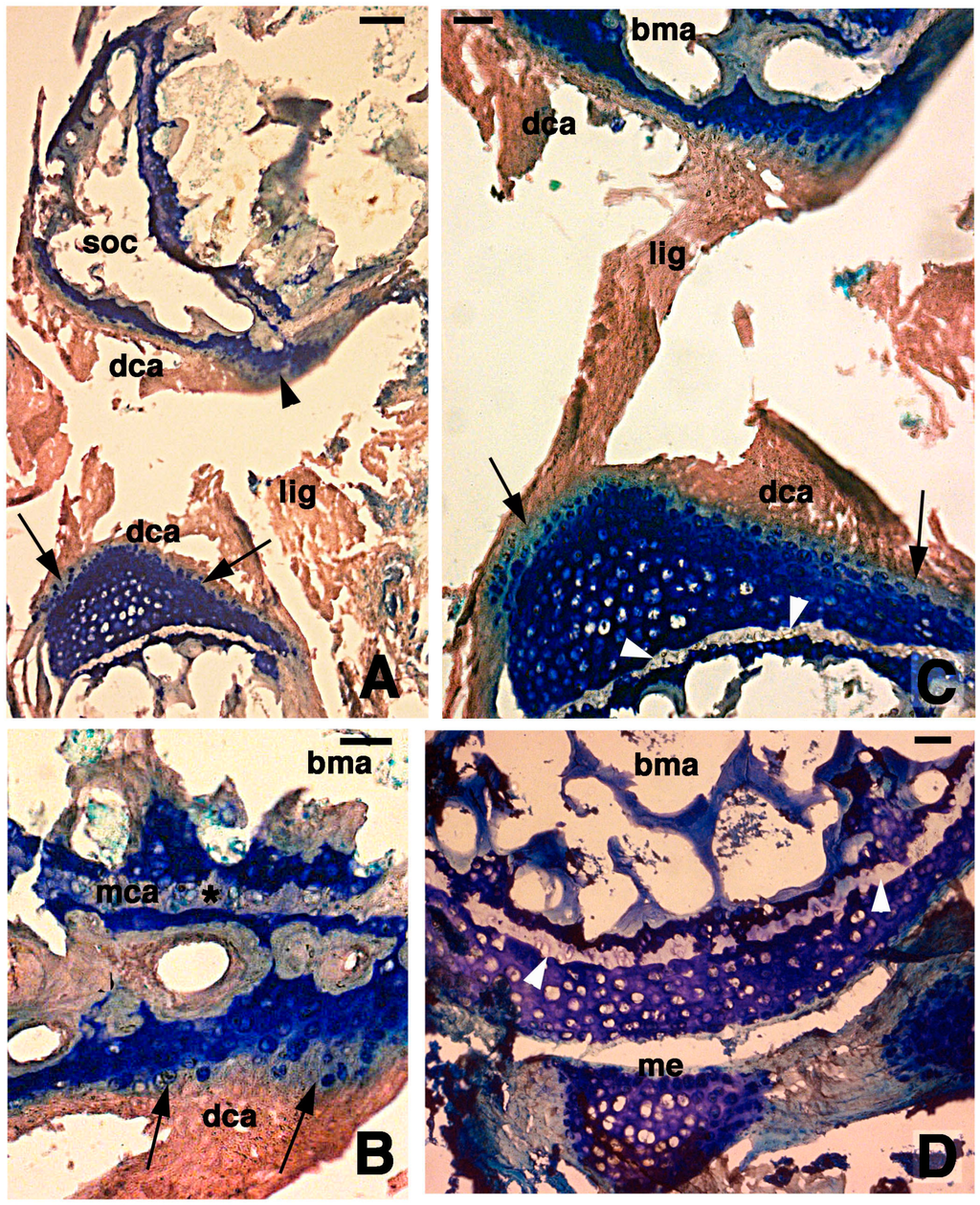
Figure 3.
Histological aspects of the knee 7 days after the lesion. (A) Degenerating articular cartilage surface of the femur (arrowhead) while a region of regenerating cartilage is seen in the tibia (arrows) localized beneath the degenerated and eosinophilic articular cartilage with fragments of ligaments/meniscus. Bar, 100 µm; (B) Detail of the degenerated femur eosinophilic cartilage, in continuation with degenerating (pyknotic) chondrocytes (arrows). Also part of the metaphyseal plate appears to be degenerating (asterisk). Bar, 50 µm; (C) Detail on the ligament connection between the regenerated tibia cartilage (arrows) and the degenerating cartilage in the femur. Bar, 25 µm; (D) Case showing regeneration in the articular cartilage of the femur although a region of degenerated and chromophobic cartilage is still present in part of the metaphyseal plate. Bar, 25 µm. Legends: bma, bone marrow; dca, degenerated cartilage; lig, ligament; me, meniscus; mca, metaphyseal plate; soc, secondary ossification center.
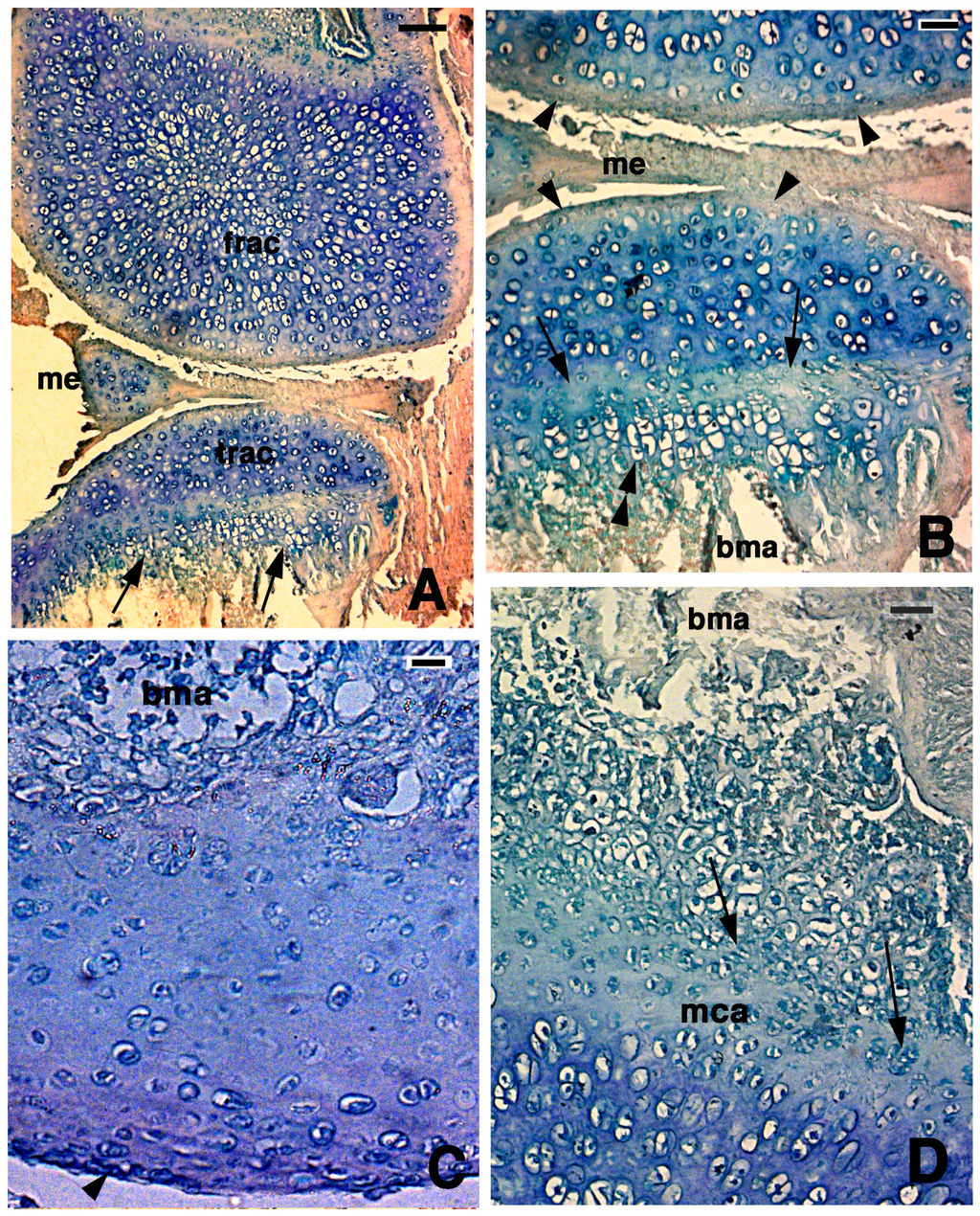
Figure 4.
Histological aspects of the knee 14 days after the lesion. (A) In this case, the two apposed articular surfaces, femur at the top and tibia at the bottom, appear made of a highly cellular cartilaginous tissue. In the tibia, the beginning of a serial cartilage is present (arrows). Bar, 50 µm; (B) Close-up of the apposed articular cartilaginous surfaces where flat sinovial layers are present (arrowheads). The arrows indicate the presence of a likely resting and proliferating cartilaginous region where chondroblasts start to pile and form the hypertophic chondrocytes (double arrowheads). Bar, 20 µm; (C) Detail on the regenerated articular cartilage of the femur featuring flat superficial chondrocytes (arrowhead) and absence of piled condrocytes in contact with the bone marrow where the metaphyseal plate will reform later. Bar, 10 µm; (D) Detail of the more basophilic regenerated femur cartilage that confines with the proliferating region of a forming metaphyseal plate (arrows on some piled chondroblasts). Bar, 20 µm. Legends: bma, bone marrow; frac, femur regenerated articular cartilage; mca, metaphyseal plate; me, meniscus; trac, tibia regenerated articular cartilage.
In the other cases, the lowermost part of the regenerated cartilage, confining with the bone marrow cavity of the diaphysis, tended to form a new metaphyseal plate, as indicated by the association of chondroblasts in form of piles of cells (double arrowhead in Figure 4B,D). The extracellular matrix surrounding the flat chondrocytes of the germinative area in the new metaphyseal plate was less basophilic than the new cartilage forming the remaining epiphyses, but the basophilia increased again in hypertrophic serial chondrocytes before calcification started.
Finally, at 21 days after lesion, the epiphyses of both femur and tibia appeared largely made of maturing cartilage (Figure 5A). In one case, chondrocytes were irregularly distributed and numerous cells were hypertrophic and surrounded by an abundant and very basophilic matrix in the central part of these cartilaginous epiphyses, while they formed long piled metaphyseal plates in continuation with the bone marrow cavities of the diaphyses (Figure 5B). In the other cases, the cartilage appeared more cellular, but chondrocytes tended to form piles of cells in the basal area of the epiphyses forming parallel isogenous groups (Figure 5C). Superficial cells bordering the synovial articular cavity were typically flat while numerous recent isogenous groups were seen in the inner region of the articular cartilage (arrowheads in Figure 5D).
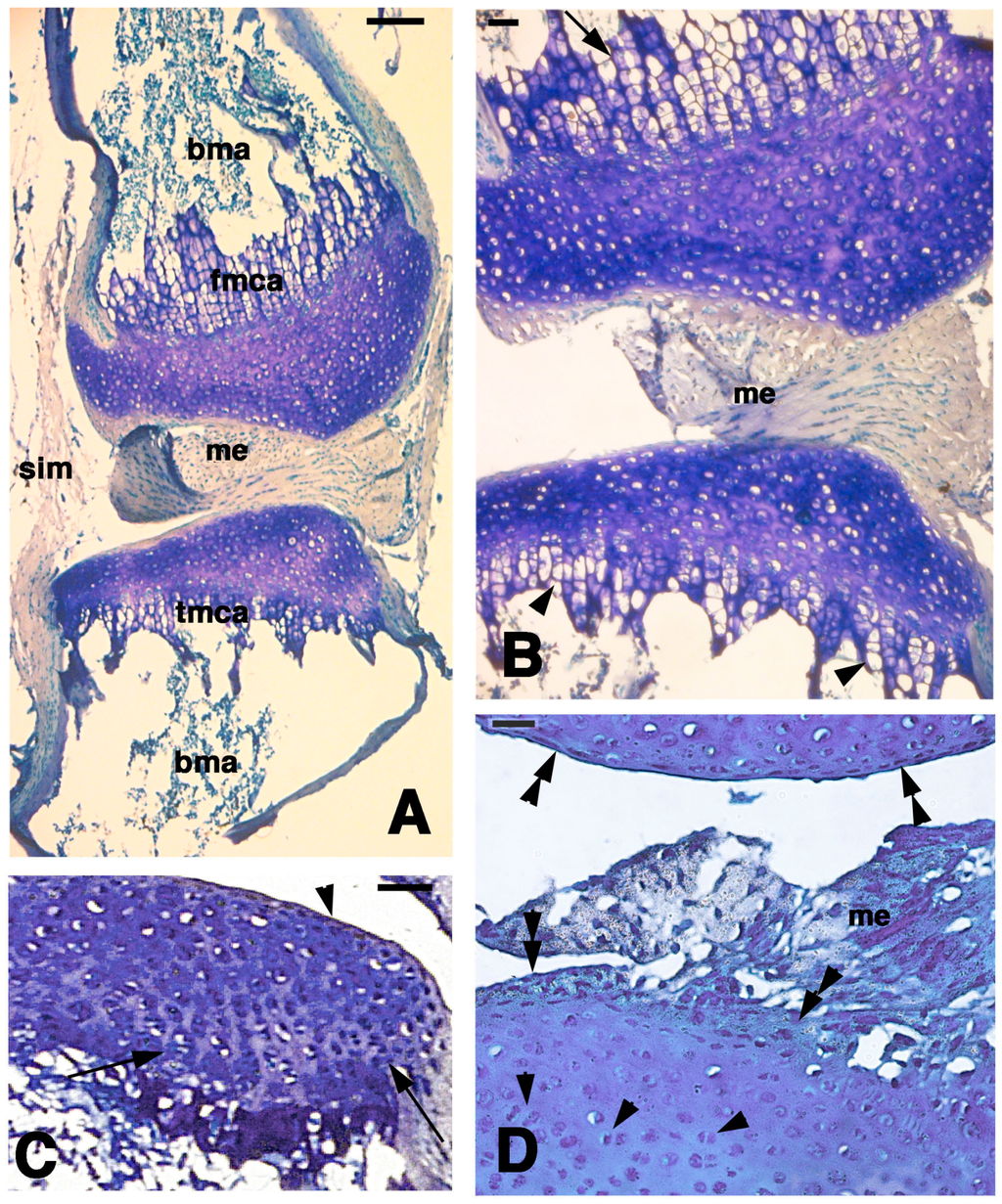
Figure 5.
Histological aspects of the knee 21 days after the lesion. (A) General view of a case showing the apposed cartilaginous epiphyses with the meniscus. Bar, 100 µm; (B) Detail on the highly cellularized, regenerated articular cartilages where a metaphyseal plate is formed in both femur (top, arrow) and tibia (bottom, arrowheads). Bar, 25 µm; (C) Other case with regenerated articular cartilage containing flat superficial chondrocytes (arrowhead) and the beginning of formation of piled chondrocytes (arrows) in the forming metaphyseal cartilage. Bar, 50 µm; (D) detail of highly cellularized cartilage with numerous isogenous groups (arrowheads). Bar, 25 µm. Legends: bma, bone marrow; fmca, femur metaphyseal cartilage; me, meniscus; tmca, tibia cartilaginous methaphysis.
3.4. Immunonocytochemical Observations
Four hours after injection of 5BrdU in the normal, uninjured knees, numerous labeled cells (nuclei) were seen on the surface layer of the articular cartilages of both femur and tibia. In addition, the meniscus contained labeled cells, especially along its surface (Figure 6A–C). Sparse labeled cells were, however, also present inside the cartilage and in the bone marrow, especially contacting the labeled piled cells present in the metaphyseal (growth) plate, and representing resting and proliferating chondrocytes (Figure 6D). Often the mineralizing matrix and the bone trabeculae located in the diaphysis non-specifically absorbed the fluorescent dye, and appeared more or less intensely fluorescent (asterisk in Figure 6B).
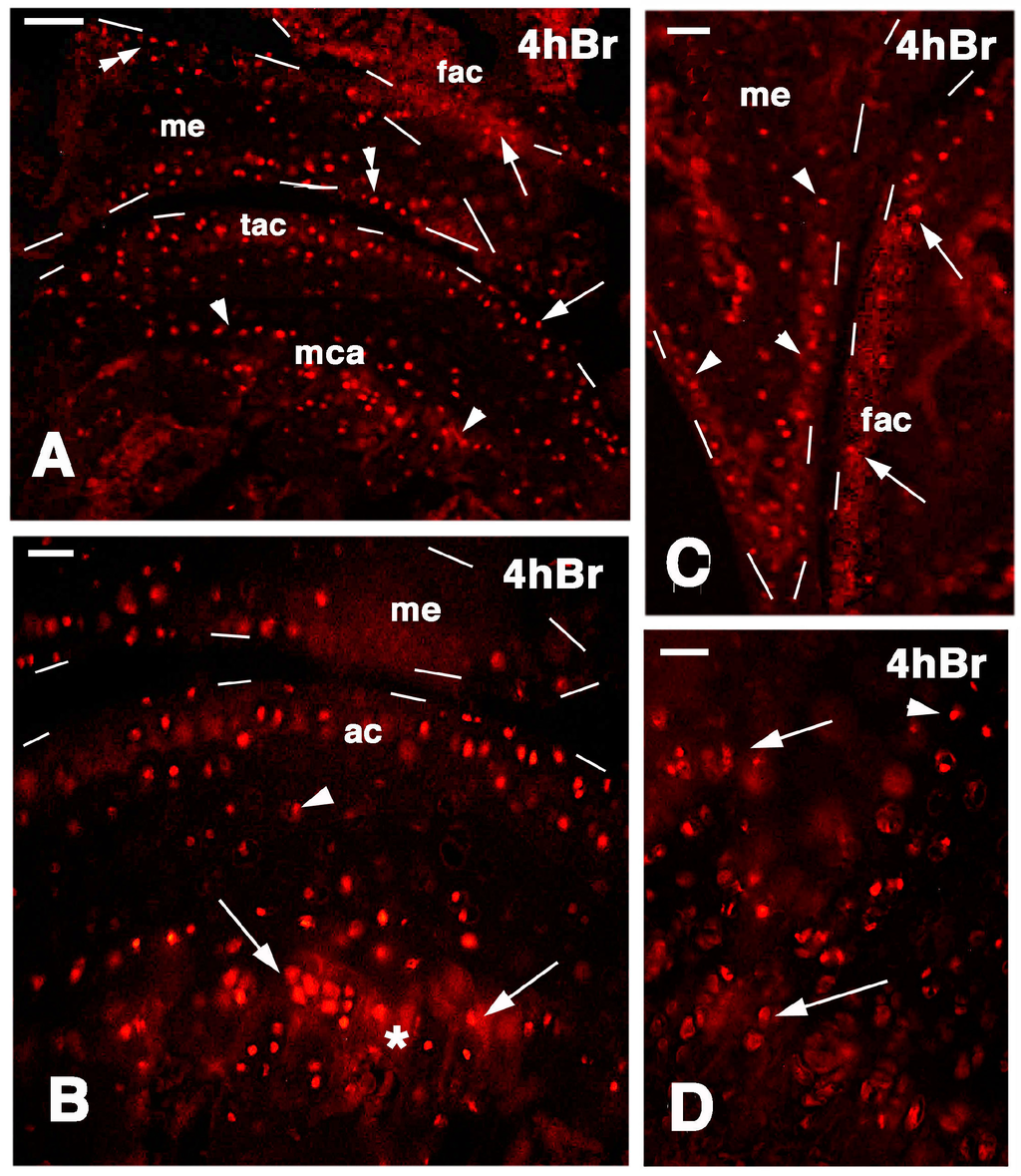
Figure 6.
Immunolocalization of labeled cells in normal knee epiphyses 4 h post-injection of 5BrdU. Dashes outline the perimetry of the epiphesis and meniscus. (A) The main distribution of labeled cells is observed along the surface of the articular cartilages (arrows), in the metaphyseal plate (arrowheads), and along the meniscus surface (double arrowheads). Bar, 50 µm; (B) Detail on labeled cells in the more superficial part of the articular cartilage, and in the serial cartilage of the metaphyseal plate (arrows, the asterisk indicates a non-specific fluorescence around cells due to fluorofore absorption in the mineralizing matrix). Sparse labeled cells are seen in the intermediate region (arrowhead). Bar, 25 µm; (C) Other detail showing labeled cells in the femur articular cartilage (arrows) and meniscus (arrowheads). Bar, 25 µm; (D) Detail on the labeled cells within the serial cartilage (proliferating region) of the metaphyseal plate and germinal area (arrowhead). Bar, 25 µm. Legends: fac, femur articular cartilage; me, meniscus; mca, metaphyseal cartilage; tac, tibia articular cartilage.
At four days post-lesion, numerous labeled cells were still seen among the piled chondrocytes of the metaphyseal plate after 4 h post-injection of 5BrdU (Figure 7A). Conversely, no labeled cells were localized in the degenerated areas of the articular cartilage or menisci (Figure 7B). However, sparse labeled nuclei of chondrocytes were still seen in undamaged areas of the superficial, articular cartilage, especially in areas not hit by the lesion that maintained chondrocytes like in the normal, uninjured knee surfaces (Figure 7C,D).
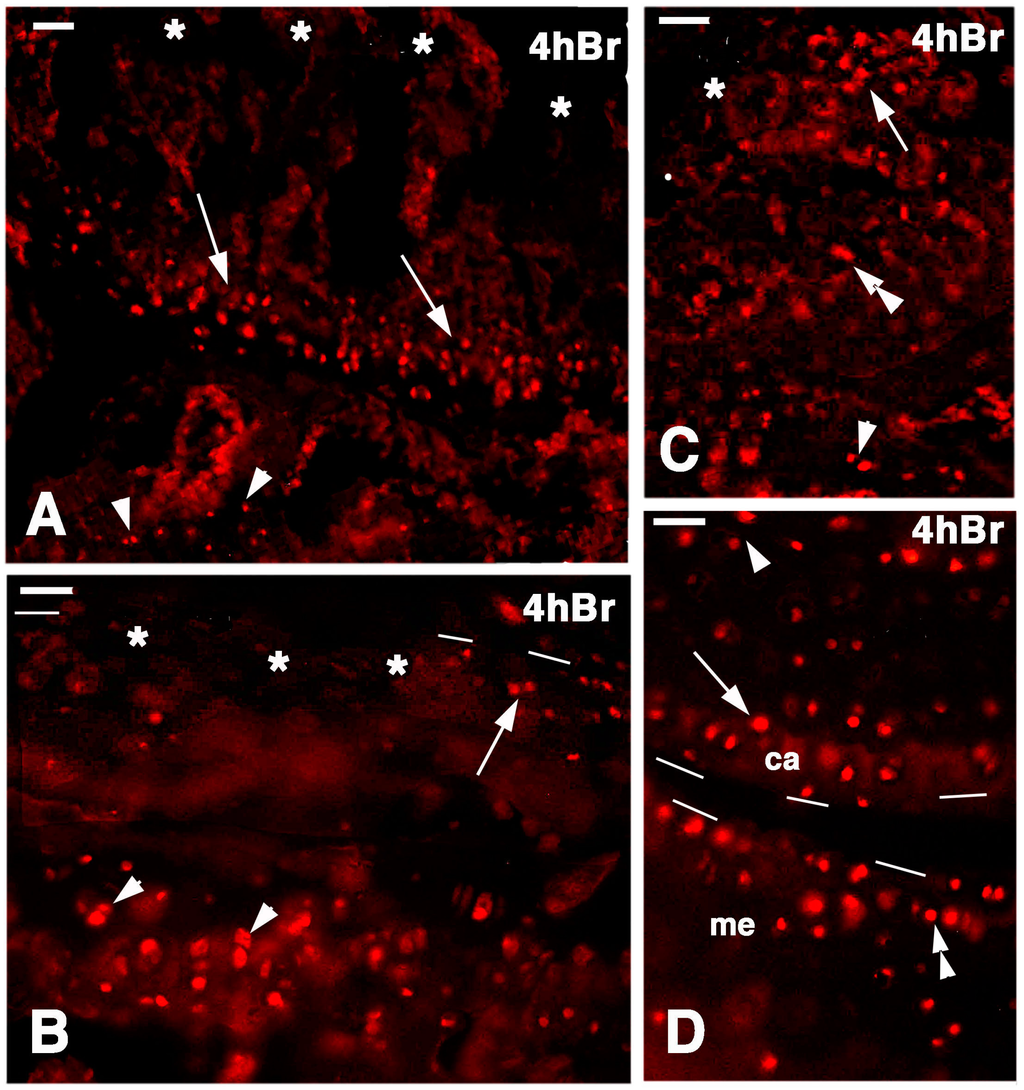
Figure 7.
Immunolocalization of labeled cells in 4-day lesioned knee epiphyses at 4 h post-injection of 5BrdU. Dashes outline the perimetry of the articular cartilage. (A) Numerous labeled cells (arrows) are still seen in the metaphyseal plate but not in the damaged superficial articular cartilage (asterisks). Arrowheads point to sparse labeled cells in the bone marrow of the diaphysis. Bar, 25 µm; (B) Detail of the labeled cells (arrowheads) present in the serial cartilage of the metaphysis that are absent in the superficial, damaged cartilage (asterisks) but are still seen in a confining region of relatively undamaged cartilage (arrow). Bar, 25 µm; (C) Area of relatively undamaged cartilage showing the presence of still labeled cells in the superficial part of the articular cartilage (arrow, the asterisk indicates the nearby damaged area). Sparse labeled cells are located in the intermediate region (double arrowhead) and by the metaphyseal plate (arrowhead). Bar, 25 µm; (D) Intact area of femur articular cartilage (ac) showing labeled cells (arrow), near the metaphyseal serial cartilage (arrowhead), and in the meniscus (me, double arrowhead). Bar, 25 µm.
At seven days post-lesion in the regenerating areas or in the large cartilage at 14 days post-lesion, the regenerated cartilage of the epiphyses contained sparse labeled chondrocytes at 4 h post-injection of 5BrdU. These cells were present in the superficial cartilaginous layers but also inside the new cartilaginous tissue (Figure 8A,B). In the latter, the formation of isogenous groups was noted within the cartilaginous mass, and some chondroblasts appeared labeled, indicating that an interstitial proliferation was taking place (Figure 8C). Sparse labeled cells were also seen in the piled chondroblasts present in the forming metaphyseal plate (Figure 8D). Control sections showed absence of labeled cells (nuclei) and a diffuse immunofluorescence in the mineralizing matrix around the chondrocytes of the metaphyseal plate (Figure 8E).
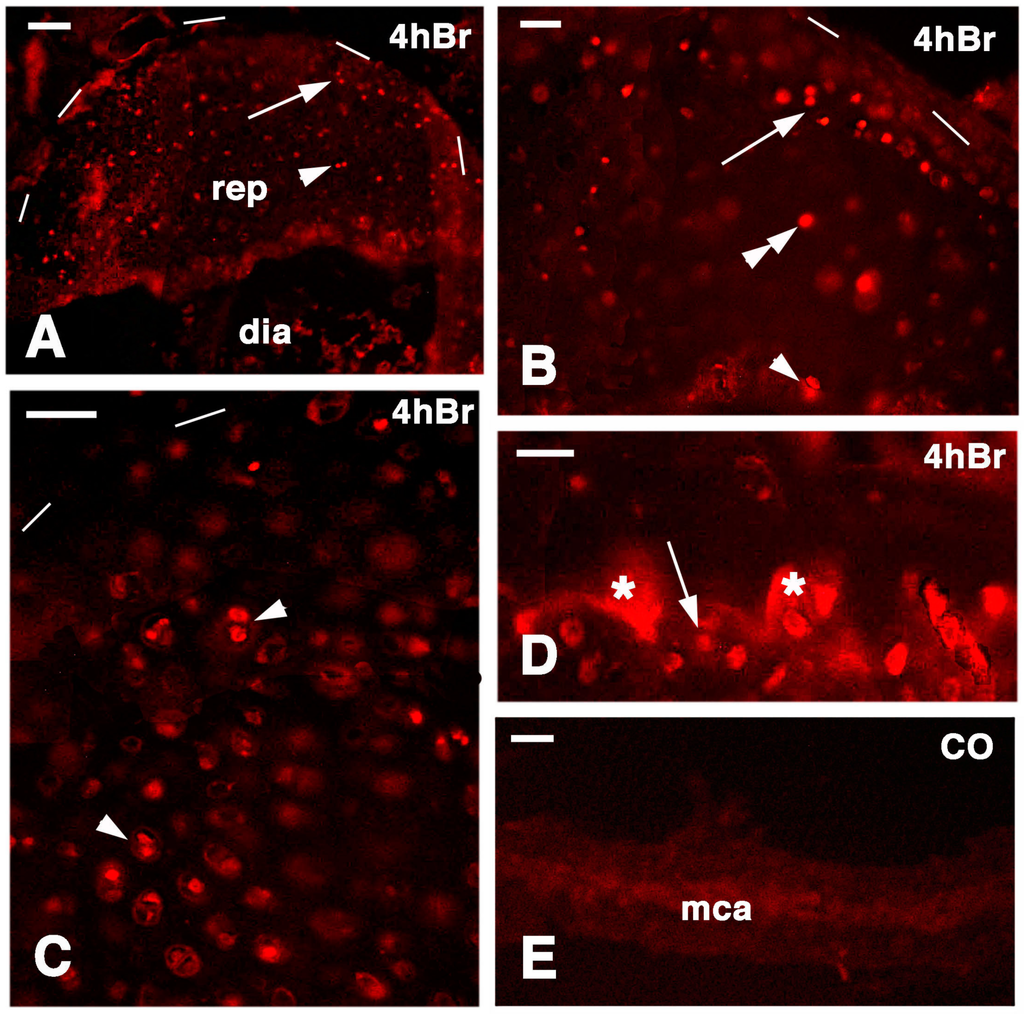
Figure 8.
Immunolocalization of labeled cells in 14-day lesion tibia epiphyses (A,C), and 7-day lesion tibia (B,D) at 4 hours post-injection of 5BrdU. Dashes outline the perimetry of the articular cartilage. (A) Low magnification view of regenerated epiphyses (rep; dia, diaphysis) with labeled cells in the superficial (arrow) and inner (arrowhead) mass of cartilaginous cells. Bar, 50 µm; (B) Labeled cells in the superficial (arrow), inner cartilaginous mass (double arrowhead), and in the serial metaphyseal cartilage (arrowhead). Bar, 25 µm; (C) Detail on the labeled chondroblasts of isogenous groups (arrowheads) of regenerated cartilage. Bar, 25 µm; (D) Detail on labeled chondrocytes (arrow) within metaphyseal cartilage (the asterisks indicate non-specific fluorescence of the mineralizing matrix). Bar, 25 µm; (E) Control section of the metaphyseal cartilaginous (growth) plate (mac). Bar, 25 µm.
A similar general labeling pattern was observed in the regenerated cartilaginous epiphyses of the femur and tibia at 21 days post-injury. In fact, 4 h after injection of 5 BrdU numerous labeled cells were irregularly distributed inside the cartilaginous mass and less frequently on the articular surface or in the metaphyseal plate (Figure 9A). This suggested that most of the cartilage regeneration was interstitial more than appositional. Furthermore, at 48 hours post-injection of 5BrdU the number of intensely or less intensely labeled cells increased in the cartilage and metaphyseal plate, further suggesting the prevalence of an interstitial proliferation in addition to the less intense proliferation of the piled chondrocytes of the metaphyseal (growth) plate (Figure 9B–D). Relatively few flat and labeled chondroblasts were seen on the synovial surface of the regenerated articular cartilage.
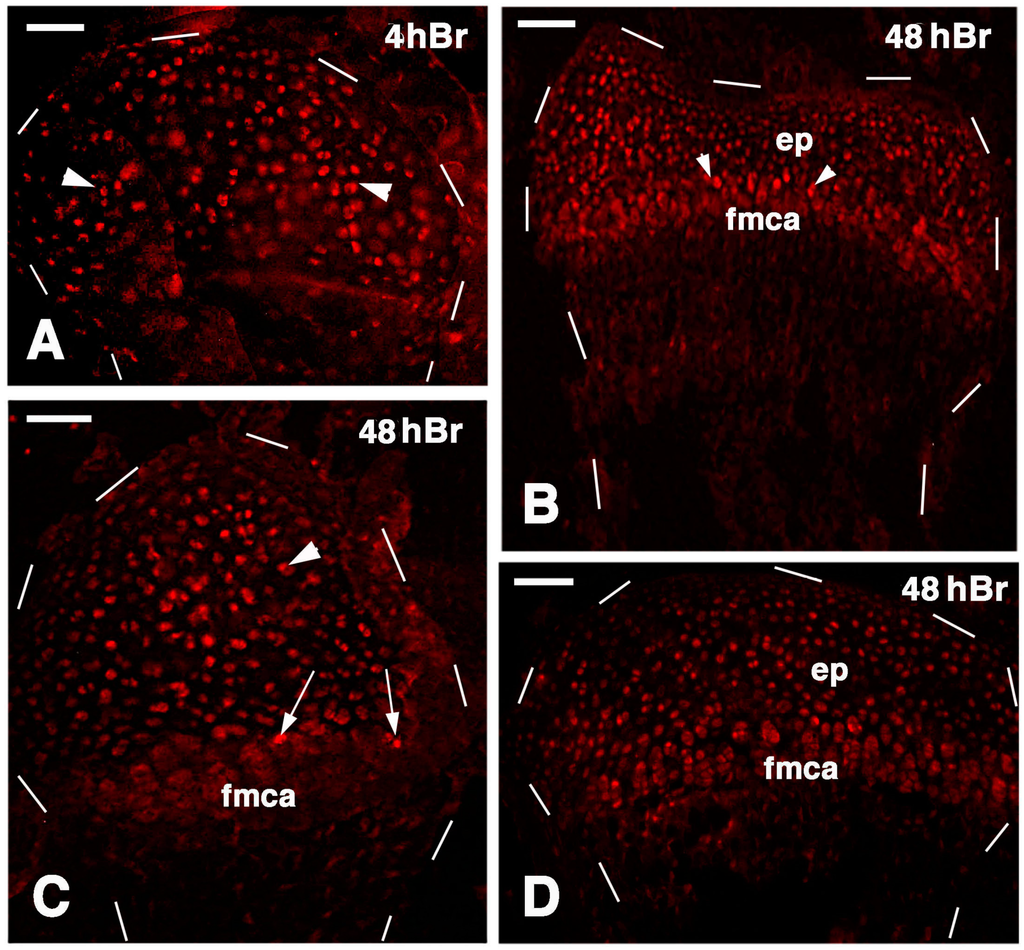
Figure 9.
Immunolocalization of labeled cells in 21 lesioned knee epiphyses 4 h post-injection of 5Brdu (A) and 48 h post-injection (B–D). Dashes outline the perimetry of the articular cartilage down to the diaphysis. (A) Tibia epiphyses formed by numerous labeled inner chondrocytes (arrowheads). Bar, 50 µm; (B) Sparse labeled cells in the femur epiphysis (ep) where the forming metaphyseal plate (fmca) also contains few clearly labeled cells (arrowheads). Bar, 50 µm; (C) Distributed labeled cells in a cartilaginous epiphyses (arrowhead) and in the forming metaphyseal plate (fmca, arrows) of a fibula. Bar, 50 µm; (D) Distributed labeled cells in the epiphysis (ep) and sparse labeled cells in the serial cartilage of the forming metaphyseal cartilage (fmca) of a tibia. 50 µm.
4. Discussion
4.1. Articular Cartilage Regeneration in Lizards
The present study shows that cartilage regeneration is not only restricted to tail vertebrae of lizards [5] but that also long bones in adult lizards maintain a high regenerative capability in their cartilaginous tissue. In vertebrae, from the inter-vertebral and intra-vertebral cartilage but also likely from the periosteum, a noteworthy mass of new cartilaginous cells is produced, repairing the neural arch and the vertebral body at high temperature conditions (summer daily exposition). Previous studies on long bone fractures in lizards also showed that a large mass of chondrocytes derived from the reactive periosteum [24] and also somewhat from the bone marrow (medullary regeneration, see [24]).
The present study shows that, like in lesion bones and inter-vertebral cartilage of the vertebra, a massive regeneration takes place in the knee after lesions, at least at the high temperature conditions utilized in our experiments. The new chondroblasts rapidly form a large mass of cartilaginous cells that appears to derive not only from the flat chondroblasts of the articular cartilage but likely also from some chondroblasts present in the germinal (resting and proliferating) region of the piled flat chondrocytes of the nearby metaphyseal plate.
The study indicated that after three weeks from the lesion at the high temperatures present in our conditions the entire epiphyses or large part of them become cartilaginous and largely or totally replace the secondary ossification centers, reassuming the aspect of the epiphyses during embryonic stages (Figure 10). From the present observation, it appears that the injured chondrocytes rapidly die and form pyknotic cells during the first days post-trauma, while the basophilic ground extracellular matrix loses most of its glycosominoglycans. The degenerated cells are, however, rapidly replaced within 7–14 days with the differentiation of new chondrocytes in our experimental conditions. A large mass of cartilaginous cells also appeared from 7 days in fractured femurs in lizards kept at 32–37 °C [24], confirming that a rapid regeneration of cartilaginous cells can occur in lizards at higher temperatures.
The present study unfortunately cannot specify which proliferating region contributes more to the new chondrocytes, or whether also chondrogenic cells derive from the injured bone marrow of the injured secondary ossification center of the epiphyses (medullary regeneration, see [24]). The presence of proliferating cells in both articular and metaphyseal regions of the long bones, as indicated by the 5BrdU pattern at 4 h and 48 h post-injection, suggests that in these lizards the new cartilaginous epiphyses are derived from the expansion of cells from both the articular cartilage down and from the metaphyseal cartilage up so that the secondary bone center is temporary replaced by newly formed chondrocytes. Initially cell proliferation is mainly appositional but later most interstitial cell division appears to contribute to the rapid regeneration of a large mass of cartilage. The latter gives rise to new cartilaginous epiphyses, possibly resembling those present during embryonic development. Interstitial chondroblasts therefore represent the transient amplifying cells, likely derived from the flat stem chondroblasts of the articular cartilage and, possibly, from the germinal (resting and proliferating) chondroblasts of the metaphyseal plate. It is likely that at later stages of recovery, not studied in the present account, secondary centers of ossification might be re-formed like in adult bones [18,23]. As indicated from the 5BrdU labeling in normal knees, it appears that in lizards the growing centers very likely maintain a population of resident stem cells, at least with a chondrogenic-osteogenic potential, in both articular and metaphyseal cartilages of long bones.
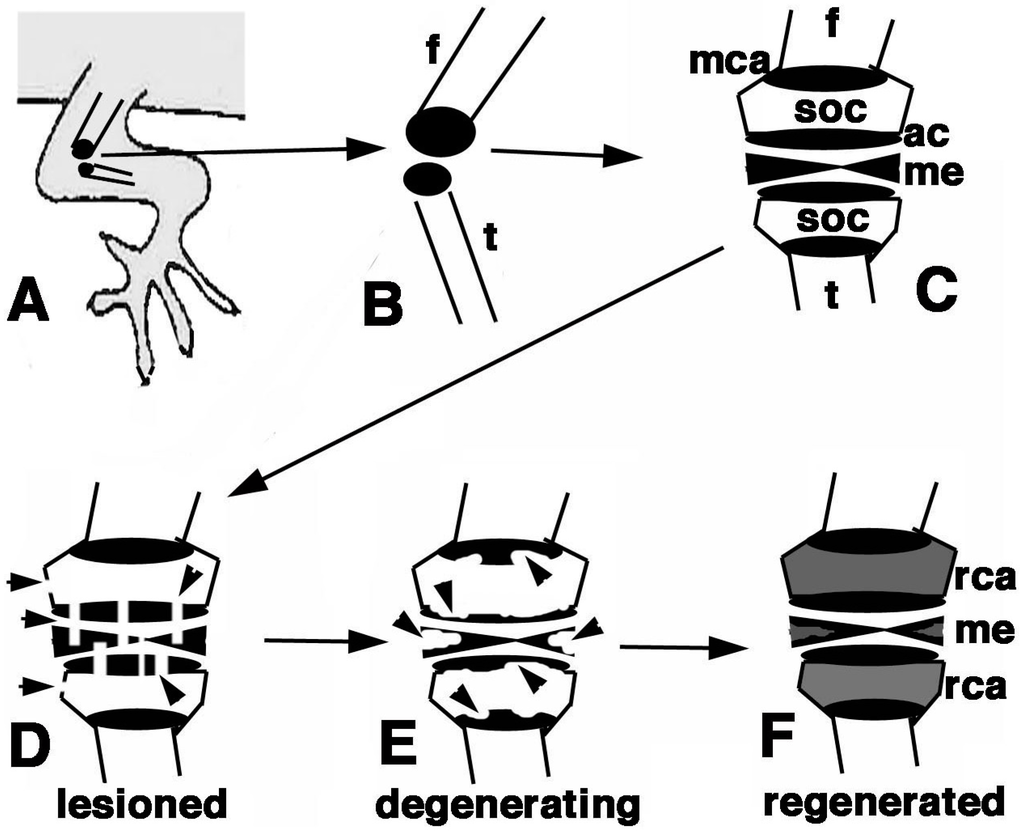
Figure 10.
Schematic summary of the present results. (A) Hind limb with indicated operated area of the knee; (B) Detail of the epiphysis (in black) of the femur and tibia; (C) Detailed view of the regions of the femur and tibia epiphyses present in a normal knee; (D) The damaged areas are schematically indicated by the arrowheads on the epiphyses and meniscus/ligaments; (E) The following degeneration of the cartilaginous tissue is indicated by arrowheads; (F) The regenerated cartilage in (in grey) after 3 weeks in our experimental conditions fills up most of the space initially occupied by the secondary ossification centers. Also the cartilaginous part of the meniscus is likely regenerated. Legends: ac, articular cartilage; f, femur; me, meniscus; mca, metaphyseal cartilaginous (growth) plate;rca, regenerated cartilage; soc, secondary ossification center; t, tibia.
The possible contribution of connective cells located in the injured bone marrow (secondary center of ossification) has not been specifically determined in the present study, and this point requires a kinetic study using bone marrow cell markers. Among other tissues the skeletal system grows continuously in lizards so that we are expecting cell proliferation and the presence of cell niches in the growing centers of different bones. Also the growth of vertebrae in lizards during lifetime likely requires the presence of stem cells in their inter-vertebral cartilages and in their epiphysis [5]. It is also likely that the permanence of these regions storing chondrogenic stem/progenitor cells allows repairing or even regeneration of vertebrae and of long bones after injuries.
4.2. Articular Cartilage Regeneration in Terrestrial Vertebrates
A large regenerative ability of the articular cartilage is present in amphibian knees and elbows, considering that the epiphyses of their long bones are still largely made of cartilaginous cells [18,19,20] that likely include numerous chondrogenic stem cells. Also amphibians generally grow for most of their lifetime, a condition different from that of mammals, so that amphibians are pre-adapted to cartilage regeneration after injury [21,22].
The present study shows that a broad regenerative power of epiphyseal cartilages is present in lizards, including in the articular cartilage. The study however indicates that this outstanding cartilaginous regeneration appears related to the permanence of proliferating cells in the growing centers of the articular cartilage and of the metaphyseal cartilage (growth plate) during most of the lifetime in these reptiles that present a more or less continuous growth [3]. The present study made use of adult lizards, estimated by the size in this species above 12 cm for both sexes although it was unknown whether they were young or old adults. However the case, the fact that in adult epiphyses a cartilaginous and proliferating metaphyseal plate are still present, as shown by the uptake of 5BrdU, indicates that bone elongation is still active in these adult lizards, and this represents a pre-adaptation to regenerative potential. Lizards, as amniotes closer to the mammalian organization and physiology than amphibians, may give clues to analyze the mechanism that allows the persistence of chondrogenic cells in adult vertebrae and long bones throughout most of their lives. The knowledge of the stimulating molecular clues that maintain these regions likely populated by stem cells in lizards may be useful for the study of cartilage regeneration in mammals [15].
In mammals, characterized by a determinate growth, the epiphyses terminate cartilage production with maturity and the articular cartilage is very reduced: this condition indicates that no proliferating stem cells remain in mammalian epiphyses after cessation of growth [2,16,17]. In adult mammalian epiphyses, the accidental injury or the progressive chronic degeneration of the articular cartilage does not generally elicit the neo-formation of new cartilage [16,17]. The different outcome between the repair ability between reptilian-amphibian versus mammalian-avian articular cartilage is therefore related to the different biological conditions that characterize the epiphyses of many reptiles and amphibians versus those of birds and mammals [18,25]; the former is conducive to cartilage regeneration after injury. Therefore repopulating human articular cartilage with chondrogenic stem/progenitor cells or feeding the cartilage with these cells appears a valuable attempt to be pursued in clinical trials.
5. Conclusions
In conclusion, after knee injury both the femur and tibia epiphysis in lizard regenerate a large mass of cartilaginous tissue which cells mainly derive by an intense proliferation from the articular cartilage and growth plate that represent dormient but reactivating growth centers.
Acknowledgments
Comparative Histolab supported most of the study.
Conflicts of Interest
The author declare no conflict of interest.
References
- Scadden, D.T. The stem-cell niche as an entity of action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Stocum, D.L. Regenerative Biology and Medicine; Academic Press-Elsevier: San Diego, CA, USA, 2006. [Google Scholar]
- Avery, R.H. Growth in reptiles. Gerontology 1994, 40, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Bellairs, A. d’A.; Bryant, S.V. Autotomy and regeneration in reptiles. In Biology of the Reptilia; Gans, C., Billet, F., Maderson, P.F.A., Eds.; John Wiley& Sons: New York, NY, USA, 1985; Volume 15B, pp. 302–410. [Google Scholar]
- Alibardi, L. Morphological and cellular aspects of tail and limb regeneration in lizard: A model system with implications for tissue regeneration in mammals. Adv. Anat. Embryol. Cell Biol. 2010, 207, 1–112. [Google Scholar]
- Alibardi, L. Histochemical, Biochemical and Cell Biological aspects of tail regeneration in lizard, an amniote model for studies on tissue regeneration. Progr. Histoch. Cytoch. 2014, 48, 143–244. [Google Scholar] [CrossRef]
- Quattrini, D. Ricerche anatomiche e sperimentali sull’autotomia della coda delle lucertole. II. Muscolatura, adipe sottomuscolare e scheletro. (Osservazioni nella Lacerta sicula sicula Raf. e nella L. sicula campestris De Betta). Arch. Zool. Ital. 1952, 37, 465–515. [Google Scholar]
- Quattrini, D. Piano di autotomia e rigenerazione della coda nei Sauri. Arch. Ital. Anat. Embriol. 1954, 59, 225–282. [Google Scholar]
- Werner, Y.L. Regeneration of the caudal axial skeleton in a gekkonid lizard (Hemidactylus) with particular reference to the “latent” period. Acta Zool. 1967, 48, 103–125. [Google Scholar] [CrossRef]
- Alibardi, L.; Sala, M. Indagini istochimiche sulla struttura della cartilagine rigenerata nella coda di Lacerta sicula. Arch. Ital. Anat. Embr. 1981, 88, 163–182. [Google Scholar]
- Alibardi, L.; Meyer-Rochow, V.B. Comparative fine structure of the axial skeleton inside the regenerated tail of lizards and the tuatara (Sphenodon punctatus). Gegemb. Morphol. Jahrb 1989, 135, 705–716. [Google Scholar]
- Alibardi, L. Development of the axial cartilaginous skeleton in the regenerating tail of lizards. Bull. Associat. Anatom. 1995, 79, 3–9. [Google Scholar]
- McLean, C.E.; Vickaryous, M.K. A novel amniote model of epimorphic regeneration: The leopard gecko, Eublepharis macularius. BMC Dev. Biol. 2011, 11, 50–74. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.E.; Geiger, L.A.; Stroik, L.K.; Hutchins, E.D.; George, R.M.; Denardo, D.F.; Kosumi, K.; Rawls, J.A.; Wilson-Rawls, J. A histological comparison of the original and regenerated tail in the green anole, Anolis carolinensis. Anat. Rec. 2012, 295, 1609–1619. [Google Scholar] [CrossRef]
- Lozito, T.P.; Tuan, R.S. Lizard tail regeneration: Regulation of two distinct cartilage regions by Indian hedgehog. Dev. Biol. 2015. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Kapfinger, E.; Geiss, J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr. Cartil. 2007, 15, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, C.E.; de la Corte-Garcia, H. Regeneration of articular cartilage of the knee: basic concept. In Articular Cartilage Defects of the Knee; Rodriguez-Merchan, E.C., Ed.; Sprinter-Verlag: Berlin, Germany, 2012; pp. 1–16. [Google Scholar]
- Haines, R.W. The evolution of epiphyses and of endochondral bone. Biol. Rev. 1942, 17, 267–292. [Google Scholar] [CrossRef]
- Hanken, J. Appendicular skeletal morphology in minute salamanders, Genus Thorius (Amphibia: Plethodontidae): Growth regulation, adult size determination, and natural variation. J. Morphol. 1982, 174, 57–77. [Google Scholar] [CrossRef]
- Dell’Orbo, C.; Gioglio, L.; Quacci, D. Morphology of epiphyseal apparatus of a ranid frog (Rana esculenta). Histol. Histopath. 1992, 7, 267–273. [Google Scholar]
- Cosden, R.S.; Lattermann, C.; Romine, S.; Gao, J.; Voss, S.R.; MacLeod, J.N. Intrinsic repair of full-thickness articular cartilage defects in the axolotl salamander. Osteoarthr. Cartil. 2011, 19, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Gardiner, D.M. Regeneration of limb joints in the axolotl (Ambystoma mexicanum). PloS ONE 2006, 7, e50615. [Google Scholar] [CrossRef]
- Haines, R.W. Epiphyses and sesamoids. In Biology of the Reptilia; Gans, C., Ed.; Academic Press: London, UK, 1969; Volume 1, pp. 81–115. [Google Scholar]
- Pritchard, J.J.; Ruzicka, A.J. Comparison of fracture repair in the frog, lizard and rat. J. Anat. 1950, 84, 236–262. [Google Scholar] [PubMed]
- Holliday, C.M.; Ridgely, R.C.; Sedlmayer, J.C.; Witmer, L.M. Cartilaginous epiphyses in extant archosaurs and their implications for reconstructuring limb function in dinosaurs. PloS ONE 2010, 5, e13120. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).