Delta-Notch Signaling: The Long and the Short of a Neuron’s Influence on Progenitor Fates
Abstract
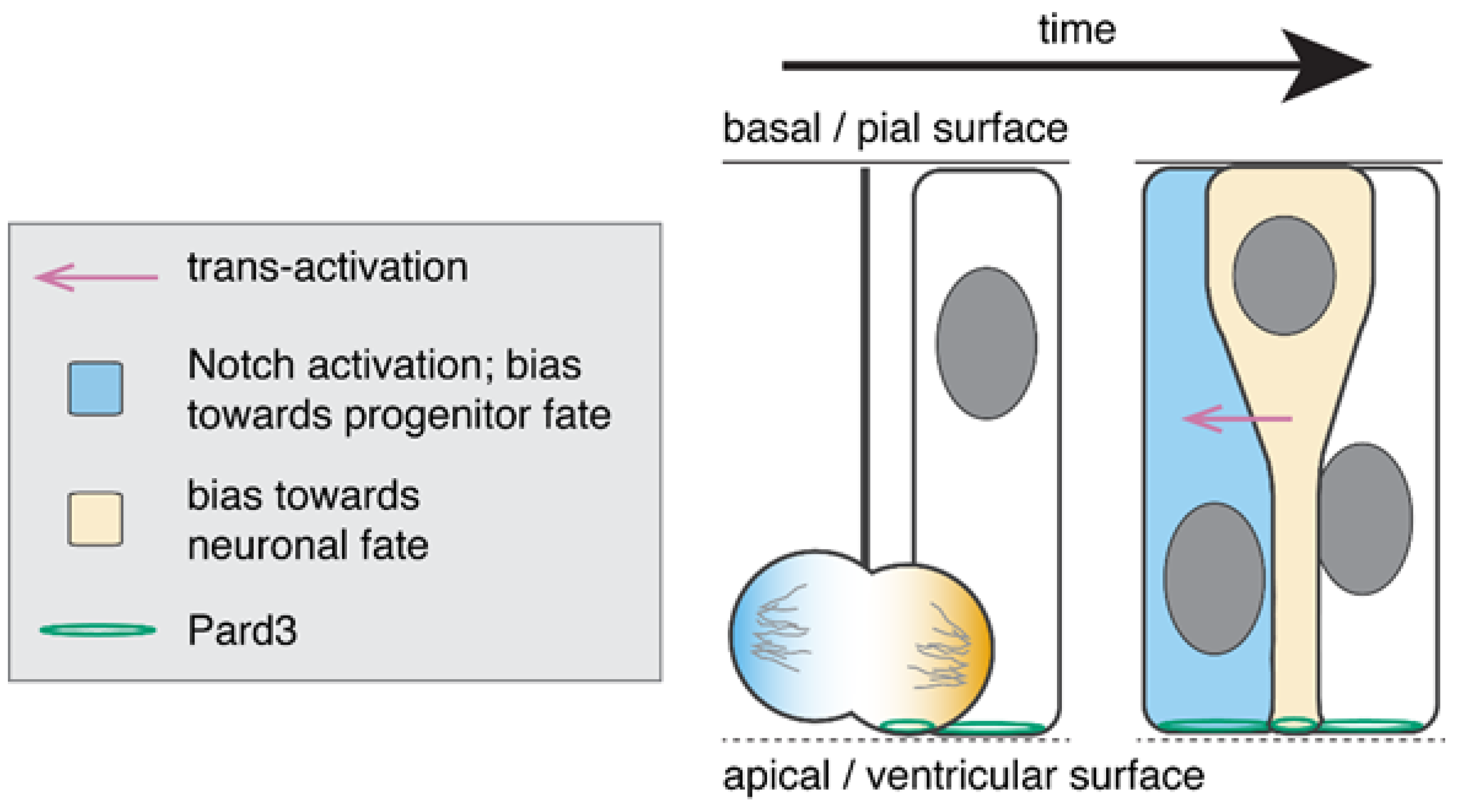
:1. Introduction
2. Neurons Derived from Asymmetric Divisions Can Influence Sister Cell Fate through Notch Signaling Pathway Activation
2.1. Neurons Inherit the Apical Attachment during Asymmetric Divisions
2.2. Recently Born Neurons Derived from Apical Progenitor Divisions May Activate the Notch Pathway in Sister Cells
2.3. Non-Apical Asymmetric Divisions—Do Newborn Neurons Influence Sister Cells Fates?
3. Differentiating Neurons can Influence the Fate of Surrounding Cells during Apical Detachment
3.1. During Apical Detachment, Differentiating Neurons Influence Surrounding Cells to Maintain Progenitor Fates and Tissue Integrity
3.2. Cellular Protrusions Developed by Differentiating Neurons Influence Neuronal Patterning in the Adjacent Tissue
3.3. Where Do Delta-Notch Interactions Occur?
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Chitnis, A.; Henrique, D.; Lewis, J.; Ish-Horowicz, D.; Kintner, C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature 1995, 375, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Henrique, D.; Hirsinger, E.; Adam, J.; Le Roux, I.; Pourquié, O.; Ish-Horowicz, D.; Lewis, J. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr. Biol. 1997, 7, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Appel, B.; Givan, L.A.; Eisen, J.S. Delta-Notch signaling and lateral inhibition in zebrafish spinal cord development. BMC Dev. Biol. 2001, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Okigawa, S.; Mizoguchi, T.; Okano, M.; Tanaka, H.; Isoda, M.; Jiang, Y.-J.; Suster, M.; Higashijima, S.-I.; Kawakami, K.; Itoh, M. Different combinations of Notch ligands and receptors regulate V2 interneuron progenitor proliferation and V2a/V2b cell fate determination. Dev. Biol. 2014, 391, 196–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, K.-J.; Koo, B.-K.; Im, S.-K.; Jeong, H.-W.; Ghim, J.; Kwon, M.-C.; Moon, J.-S.; Miyata, T.; Kong, Y.-Y. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron 2008, 58, 519–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, C.; Freem, L.; Goïame, R.; Sang, H.; Morin, X.; Tozer, S. Mib1 prevents Notch Cis-inhibition to defer differentiation and preserve neuroepithelial integrity during neural delamination. PLoS Biol. 2018, 16, e2004162. [Google Scholar] [CrossRef] [Green Version]
- Kressmann, S.; Campos, C.; Castanon, I.; Fürthauer, M.; González-Gaitán, M. Directional Notch trafficking in Sara endosomes during asymmetric cell division in the spinal cord. Nat. Cell Biol. 2015, 17, 333–339. [Google Scholar] [CrossRef]
- Coumailleau, F.; Fürthauer, M.; Knoblich, J.A.; González-Gaitán, M. Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature 2009, 458, 1051–1055. [Google Scholar] [CrossRef]
- Harris, W.A.; Holt, C.E.; Bonhoeffer, F. Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: A time-lapse video study of single fibres in vivo. Development 1987, 101, 123–133. [Google Scholar]
- Chenn, A.; McConnell, S.K. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell 1995, 82, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Miyata, T.; Kawaguchi, A.; Okano, H.; Ogawa, M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 2001, 31, 727–741. [Google Scholar] [CrossRef] [Green Version]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Dammerman, R.S.; Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001, 409, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Noctor, S.C.; Martínez-Cerdeño, V.; Ivic, L.; Kriegstein, A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004, 7, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Fietz, S.A.; Kelava, I.; Vogt, J.; Wilsch-Bräuninger, M.; Stenzel, D.; Fish, J.L.; Corbeil, D.; Riehn, A.; Distler, W.; Nitsch, R.; et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 2010, 13, 690–699. [Google Scholar] [CrossRef]
- Hansen, D.V.; Lui, J.H.; Parker, P.R.L.; Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010, 464, 554–561. [Google Scholar] [CrossRef]
- Haubensak, W.; Attardo, A.; Denk, W.; Huttner, W.B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: A major site of neurogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 3196–3201. [Google Scholar] [CrossRef] [Green Version]
- Miyata, T.; Kawaguchi, A.; Saito, K.; Kawano, M.; Muto, T.; Ogawa, M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 2004, 131, 3133–3145. [Google Scholar] [CrossRef] [Green Version]
- Reillo, I.; de Juan Romero, C.; García-Cabezas, M.Á.; Borrell, V. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb. Cortex 2011, 21, 1674–1694. [Google Scholar] [CrossRef] [Green Version]
- Shitamukai, A.; Konno, D.; Matsuzaki, F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J. Neurosci. 2011, 31, 3683–3695. [Google Scholar] [CrossRef]
- Wang, X.; Tsai, J.-W.; LaMonica, B.; Kriegstein, A.R. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat. Neurosci. 2011, 14, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Betizeau, M.; Cortay, V.; Patti, D.; Pfister, S.; Gautier, E.; Bellemin-Ménard, A.; Afanassieff, M.; Huissoud, C.; Douglas, R.J.; Kennedy, H.; et al. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 2013, 80, 442–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertz, C.C.; Lui, J.H.; LaMonica, B.E.; Wang, X.; Kriegstein, A.R. Diverse behaviors of outer radial glia in developing ferret and human cortex. J. Neurosci. 2014, 34, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.; Norris, J.; Clarke, J.D.; Alexandre, P. Spatial distribution and characterization of non-apical progenitors in the zebrafish embryo central nervous system. Open Biol 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Florio, M.; Huttner, W.B. Neural progenitors, neurogenesis and the evolution of the neocortex. Development 2014, 141, 2182–2194. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, P.; Reugels, A.M.; Barker, D.; Blanc, E.; Clarke, J.D.W. Neurons derive from the more apical daughter in asymmetric divisions in the zebrafish neural tube. Nat. Neurosci. 2010, 13, 673–679. [Google Scholar] [CrossRef]
- Das, R.M.; Storey, K.G. Mitotic spindle orientation can direct cell fate and bias Notch activity in chick neural tube. EMBO Rep. 2012, 13, 448–454. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, N.; Yeo, S.-Y.; Chitnis, A.; Guo, S. Intralineage directional Notch signaling regulates self-renewal and differentiation of asymmetrically dividing radial glia. Neuron 2012, 74, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Saade, M.; Gutiérrez-Vallejo, I.; Le Dréau, G.; Rabadán, M.A.; Miguez, D.G.; Buceta, J.; Martí, E. Sonic hedgehog signaling switches the mode of division in the developing nervous system. Cell Rep. 2013, 4, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Paolini, A.; Duchemin, A.-L.; Albadri, S.; Patzel, E.; Bornhorst, D.; González Avalos, P.; Lemke, S.; Machate, A.; Brand, M.; Sel, S.; et al. Asymmetric inheritance of the apical domain and self-renewal of retinal ganglion cell progenitors depend on Anillin function. Development 2015, 142, 832–839. [Google Scholar] [CrossRef] [Green Version]
- Tozer, S.; Baek, C.; Fischer, E.; Goiame, R.; Morin, X. Differential routing of mindbomb1 via centriolar satellites regulates asymmetric divisions of neural progenitors. Neuron 2017, 93, 542–551.e4. [Google Scholar] [CrossRef] [Green Version]
- Fujita, I.; Shitamukai, A.; Kusumoto, F.; Mase, S.; Suetsugu, T.; Omori, A.; Kato, K.; Abe, T.; Shioi, G.; Konno, D.; et al. Endfoot regeneration restricts radial glial state and prevents translocation into the outer subventricular zone in early mammalian brain development. Nat. Cell Biol. 2020, 22, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, J.; Wakamatsu, Y.; Nagafuchi, A.; Kageyama, R.; Shigemoto, R.; Shimamura, K. Cadherin-based adhesions in the apical endfoot are required for active Notch signaling to control neurogenesis in vertebrates. Development 2014, 141, 1671–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.R.; Wen, G.; Lepier, A.; Schroeder, T.; Götz, M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development 2008, 135, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Bingham, S.; Chaudhari, S.; Vanderlaan, G.; Itoh, M.; Chitnis, A.; Chandrasekhar, A. Neurogenic phenotype of mind bomb mutants leads to severe patterning defects in the zebrafish hindbrain. Dev. Dyn. 2003, 228, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, M.; Kim, C.-H.; Palardy, G.; Oda, T.; Jiang, Y.-J.; Maust, D.; Yeo, S.-Y.; Lorick, K.; Wright, G.J.; Ariza-McNaughton, L.; et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 2003, 4, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cerdeño, V.; Noctor, S.C.; Kriegstein, A.R. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb. Cortex 2006, 16 (Suppl. 1), i152-61. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Satou, C.; Higashijima, S.-I. V2a and V2b neurons are generated by the final divisions of pair-producing progenitors in the zebrafish spinal cord. Development 2008, 135, 3001–3005. [Google Scholar] [CrossRef] [Green Version]
- Boije, H.; Edqvist, P.-H.D.; Hallböök, F. Horizontal cell progenitors arrest in G2-phase and undergo terminal mitosis on the vitreal side of the chick retina. Dev. Biol. 2009, 330, 105–113. [Google Scholar] [CrossRef]
- Nomura, T.; Ohtaka-Maruyama, C.; Yamashita, W.; Wakamatsu, Y.; Murakami, Y.; Calegari, F.; Suzuki, K.; Gotoh, H.; Ono, K. The evolution of basal progenitors in the developing non-mammalian brain. Development 2016, 143, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Haldipur, P.; Aldinger, K.A.; Bernardo, S.; Deng, M.; Timms, A.E.; Overman, L.M.; Winter, C.; Lisgo, S.N.; Razavi, F.; Silvestri, E.; et al. Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science 2019, 366, 454–460. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Ishibashi, M.; Gradwohl, G.; Nakanishi, S.; Guillemot, F.; Kageyama, R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999, 18, 2196–2207. [Google Scholar] [CrossRef] [Green Version]
- Minaki, Y.; Mizuhara, E.; Morimoto, K.; Nakatani, T.; Sakamoto, Y.; Inoue, Y.; Satoh, K.; Imai, T.; Takai, Y.; Ono, Y. Migrating postmitotic neural precursor cells in the ventricular zone extend apical processes and form adherens junctions near the ventricle in the developing spinal cord. Neurosci. Res. 2005, 52, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Zolessi, F.R.; Poggi, L.; Wilkinson, C.J.; Chien, C.-B.; Harris, W.A. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 2006, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, R.M.; Storey, K.G. Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 2014, 343, 200–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto-Torii, K.; Torii, M.; Sarkisian, M.R.; Bartley, C.M.; Shen, J.; Radtke, F.; Gridley, T.; Sestan, N.; Rakic, P. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 2008, 60, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Sibbe, M.; Förster, E.; Basak, O.; Taylor, V.; Frotscher, M. Reelin and Notch1 cooperate in the development of the dentate gyrus. J. Neurosci. 2009, 29, 8578–8585. [Google Scholar] [CrossRef]
- Ohata, S.; Aoki, R.; Kinoshita, S.; Yamaguchi, M.; Tsuruoka-Kinoshita, S.; Tanaka, H.; Wada, H.; Watabe, S.; Tsuboi, T.; Masai, I.; et al. Dual roles of Notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron 2011, 69, 215–230. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Saraswathy, V.M.; Xiang, L.; Fürthauer, M. Notch-mediated inhibition of neurogenesis is required for zebrafish spinal cord morphogenesis. Sci. Rep. 2019, 9, 9958. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Sakane, F.; Hashimoto, K. N-cadherin-based adherens junction regulates the maintenance, proliferation, and differentiation of neural progenitor cells during development. Cell Adhes. Migr. 2015, 9, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Sheen, V.L. Filamin A mediated Big2 dependent endocytosis: From apical abscission to periventricular heterotopia. Tissue Barriers 2014, 2, e29431. [Google Scholar] [CrossRef] [Green Version]
- De Joussineau, C.; Soulé, J.; Martin, M.; Anguille, C.; Montcourrier, P.; Alexandre, D. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature 2003, 426, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Georgiou, M.; Stevenson, N.L.; Miodownik, M.; Baum, B. Dynamic filopodia transmit intermittent Delta-Notch signaling to drive pattern refinement during lateral inhibition. Dev. Cell 2010, 19, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjivasiliou, Z.; Hunter, G.L.; Baum, B. A new mechanism for spatial pattern formation via lateral and protrusion-mediated lateral signalling. J. R. Soc. Interface 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Hadjivasiliou, Z.; Moore, R.E.; McIntosh, R.; Galea, G.L.; Clarke, J.D.W.; Alexandre, P. Basal protrusions mediate spatiotemporal patterns of spinal neuron differentiation. Dev. Cell 2019, 49, 907–919.e10. [Google Scholar] [CrossRef] [Green Version]
- Hunter, G.L.; Hadjivasiliou, Z.; Bonin, H.; He, L.; Perrimon, N.; Charras, G.; Baum, B. Coordinated control of Notch/Delta signalling and cell cycle progression drives lateral inhibition-mediated tissue patterning. Development 2016, 143, 2305–2310. [Google Scholar] [CrossRef] [Green Version]
- Hunter, G.L.; He, L.; Perrimon, N.; Charras, G.; Giniger, E.; Baum, B. A role for actomyosin contractility in Notch signaling. BMC Biol. 2019, 17, 12. [Google Scholar] [CrossRef] [Green Version]
- Dale, N.; Roberts, A.; Ottersen, O.P.; Storm-Mathisen, J. The development of a population of spinal cord neurons and their axonal projections revealed by GABA immunocytochemistry in frog embryos. Proc. R Soc. Lond. Series B Biol. Sci. 1987, 232, 205–215. [Google Scholar]
- Roberts, A.; Dale, N.; Ottersen, O.P.; Storm-Mathisen, J. The early development of neurons with GABA immunoreactivity in the CNS of Xenopus laevis embryos. J. Comp. Neurol. 1987, 261, 435–449. [Google Scholar] [CrossRef]
- Higashijima, S.; Masino, M.A.; Mandel, G.; Fetcho, J.R. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J. Neurosci. 2004, 24, 5827–5839. [Google Scholar] [CrossRef]
- Batista, M.F.; Jacobstein, J.; Lewis, K.E. Zebrafish V2 cells develop into excitatory CiD and Notch signalling dependent inhibitory VeLD interneurons. Dev. Biol. 2008, 322, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Gajendra, S.; Falenta, K.; Oudin, M.J.; Peschard, P.; Feng, S.; Wu, B.; Marshall, C.J.; Doherty, P.; Guo, W.; et al. RalA promotes a direct exocyst-Par6 interaction to regulate polarity in neuronal development. J. Cell Sci. 2014, 127, 686–699. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.R.; Hodge, R.D.; Bedogni, F.; Hevner, R.F. Dynamic interactions between intermediate neurogenic progenitors and radial glia in embryonic mouse neocortex: Potential role in Dll1-Notch signaling. J. Neurosci. 2013, 33, 9122–9139. [Google Scholar] [CrossRef] [PubMed]
- Banda, E.; McKinsey, A.; Germain, N.; Carter, J.; Anderson, N.C.; Grabel, L. Cell polarity and neurogenesis in embryonic stem cell-derived neural rosettes. Stem Cells Dev. 2015, 24, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, F.; Wehman, A.M.; Link, B.A.; Baier, H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell 2008, 134, 1055–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murciano, A.; Zamora, J.; López-Sánchez, J.; Frade, J.M. Interkinetic nuclear movement may provide spatial clues to the regulation of neurogenesis. Mol. Cell. Neurosci. 2002, 21, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.S.; Cui, S.; Miesfeld, J.B.; Klezovitch, O.; Vasioukhin, V.; Link, B.A. Loss of Llgl1 in retinal neuroepithelia reveals links between apical domain size, Notch activity and neurogenesis. Development 2012, 139, 1599–1610. [Google Scholar] [CrossRef] [Green Version]
- Mizuhara, E.; Nakatani, T.; Minaki, Y.; Sakamoto, Y.; Ono, Y.; Takai, Y. MAGI1 recruits Dll1 to cadherin-based adherens junctions and stabilizes it on the cell surface. J. Biol. Chem. 2005, 280, 26499–26507. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, N.; Sasamura, T.; Ishikawa, H.O.; Kanai, M.; Ueda, R.; Saigo, K.; Matsuno, K. Polarized exocytosis and transcytosis of Notch during its apical localization in Drosophila epithelial cells. Genes Cells 2007, 12, 89–103. [Google Scholar] [CrossRef]
- Sanders, P.G.T.; Muñoz-Descalzo, S.; Balayo, T.; Wirtz-Peitz, F.; Hayward, P.; Arias, A.M. Ligand-independent traffic of Notch buffers activated Armadillo in Drosophila. PLoS Biol. 2009, 7, e1000169. [Google Scholar] [CrossRef] [Green Version]
- Benhra, N.; Lallet, S.; Cotton, M.; Le Bras, S.; Dussert, A.; Le Borgne, R. AP-1 controls the trafficking of Notch and Sanpodo toward E-cadherin junctions in sensory organ precursors. Curr. Biol. 2011, 21, 87–95. [Google Scholar] [CrossRef]
- Couturier, L.; Vodovar, N.; Schweisguth, F. Endocytosis by Numb breaks Notch symmetry at cytokinesis. Nat. Cell Biol. 2012, 14, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Trylinski, M.; Mazouni, K.; Schweisguth, F. Intra-lineage Fate Decisions Involve Activation of Notch Receptors Basal to the Midbody in Drosophila Sensory Organ Precursor Cells. Curr. Biol. 2017, 27, 2239–2247.e3. [Google Scholar] [CrossRef] [PubMed]
- Breunig, J.J.; Silbereis, J.; Vaccarino, F.M.; Sestan, N.; Rakic, P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 20558–20563. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, R.; Alexandre, P. Delta-Notch Signaling: The Long and the Short of a Neuron’s Influence on Progenitor Fates. J. Dev. Biol. 2020, 8, 8. https://doi.org/10.3390/jdb8020008
Moore R, Alexandre P. Delta-Notch Signaling: The Long and the Short of a Neuron’s Influence on Progenitor Fates. Journal of Developmental Biology. 2020; 8(2):8. https://doi.org/10.3390/jdb8020008
Chicago/Turabian StyleMoore, Rachel, and Paula Alexandre. 2020. "Delta-Notch Signaling: The Long and the Short of a Neuron’s Influence on Progenitor Fates" Journal of Developmental Biology 8, no. 2: 8. https://doi.org/10.3390/jdb8020008
APA StyleMoore, R., & Alexandre, P. (2020). Delta-Notch Signaling: The Long and the Short of a Neuron’s Influence on Progenitor Fates. Journal of Developmental Biology, 8(2), 8. https://doi.org/10.3390/jdb8020008



