Red and Blue Netting Alters Leaf Morphological and Physiological Characteristics in Apple Trees
Abstract
:1. Introduction
2. Results
2.1. Light Relations
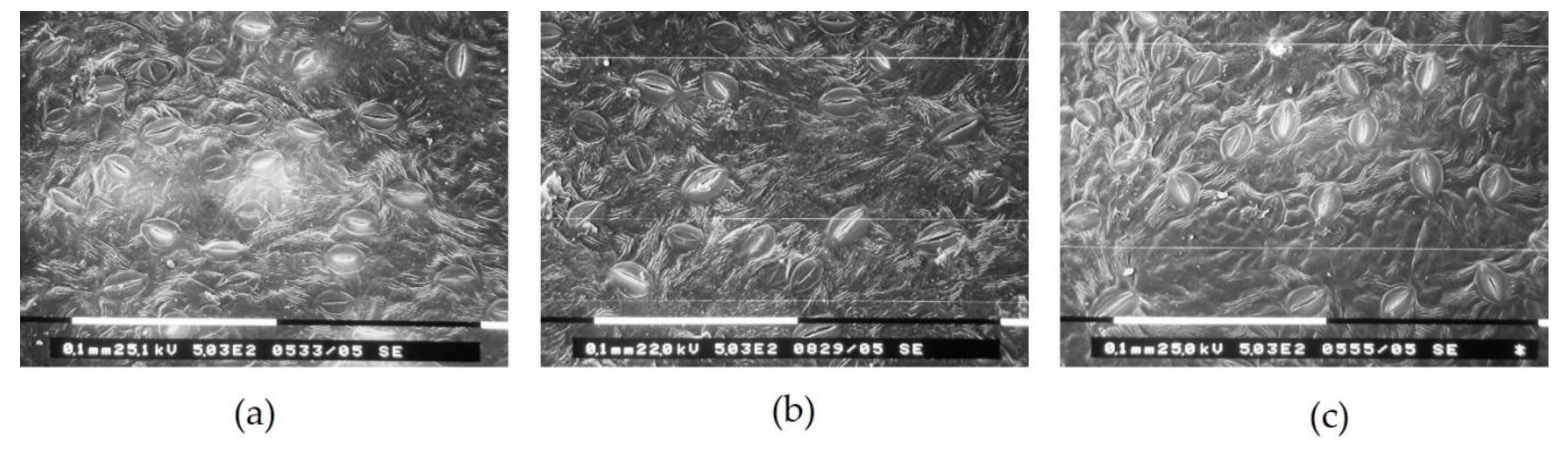
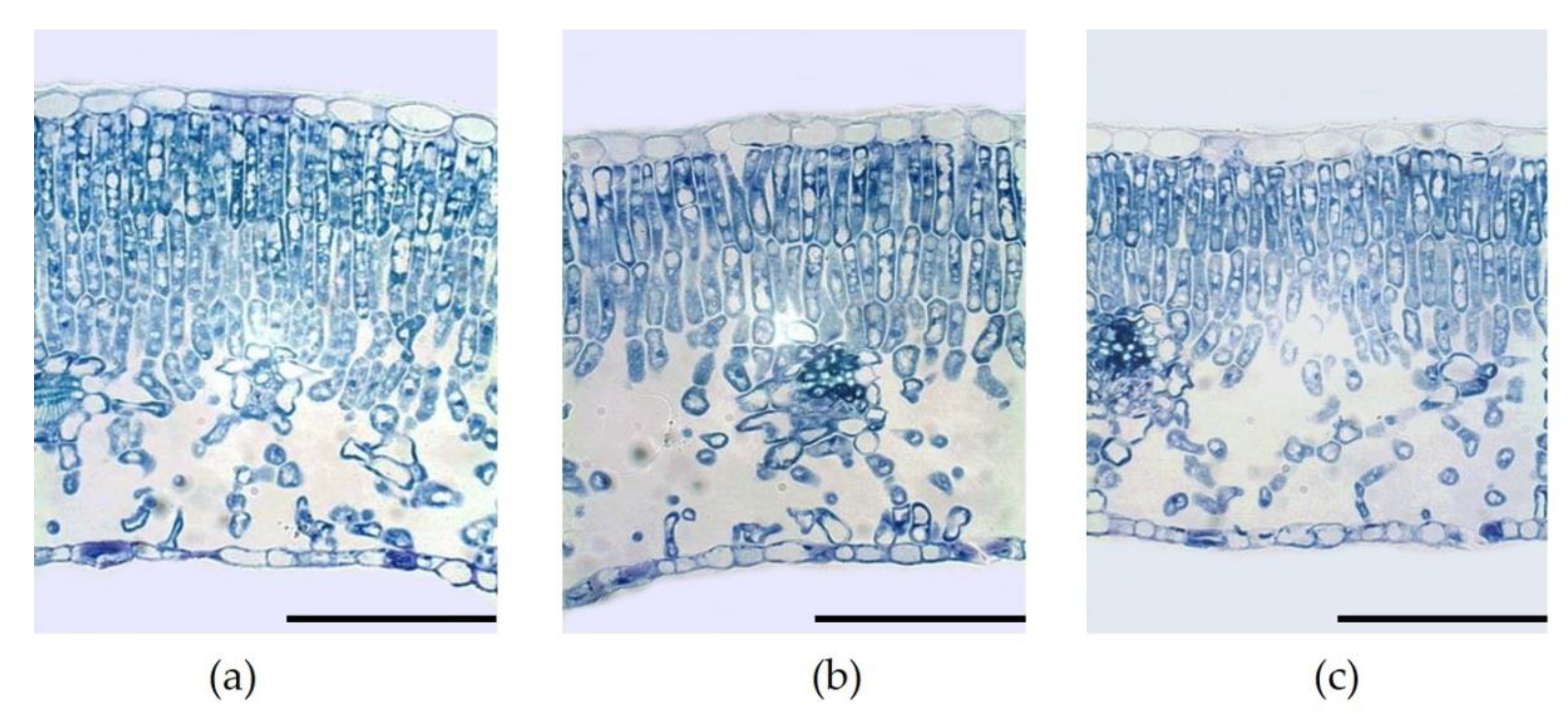
2.2. Trees and Leaf Characteristics
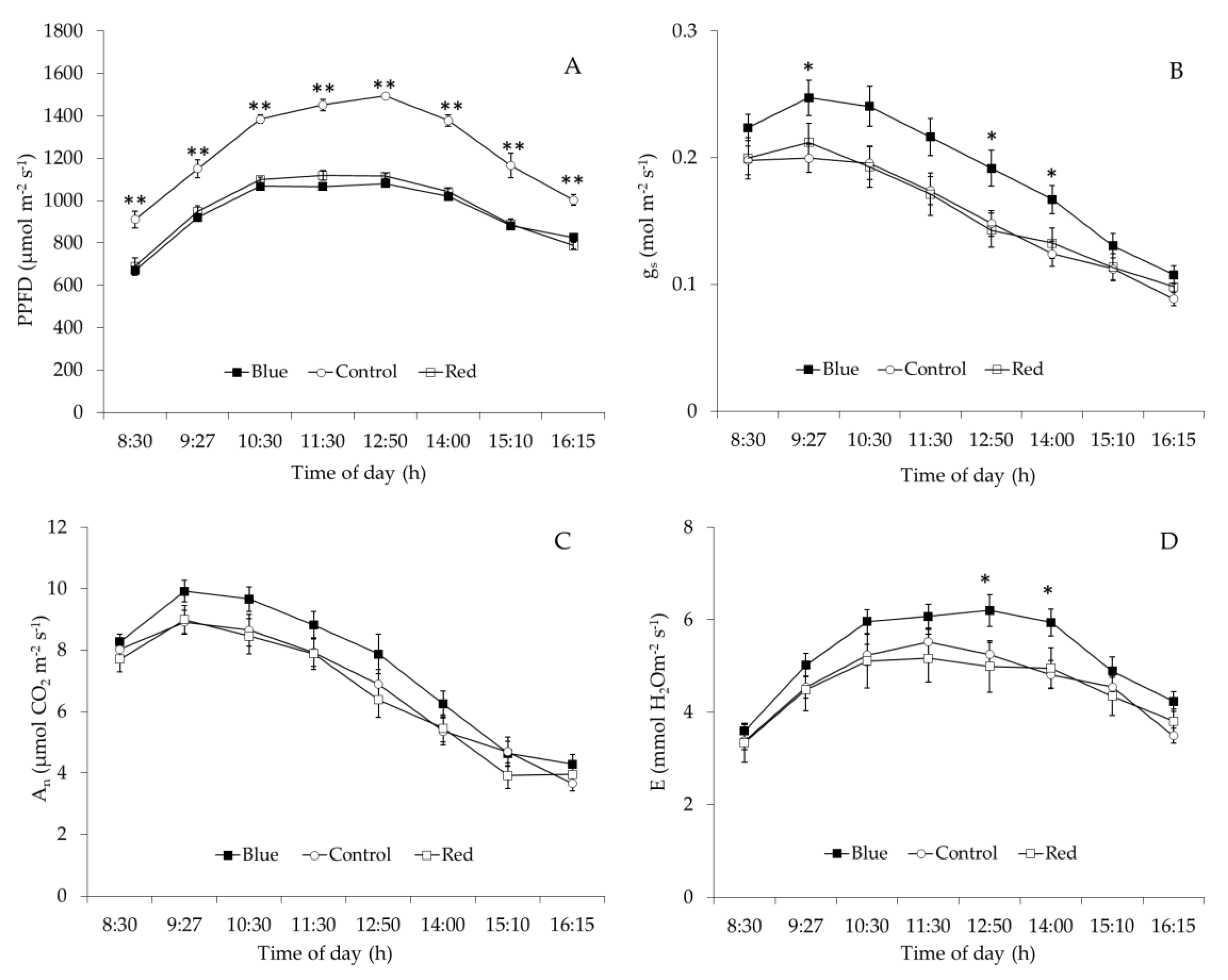
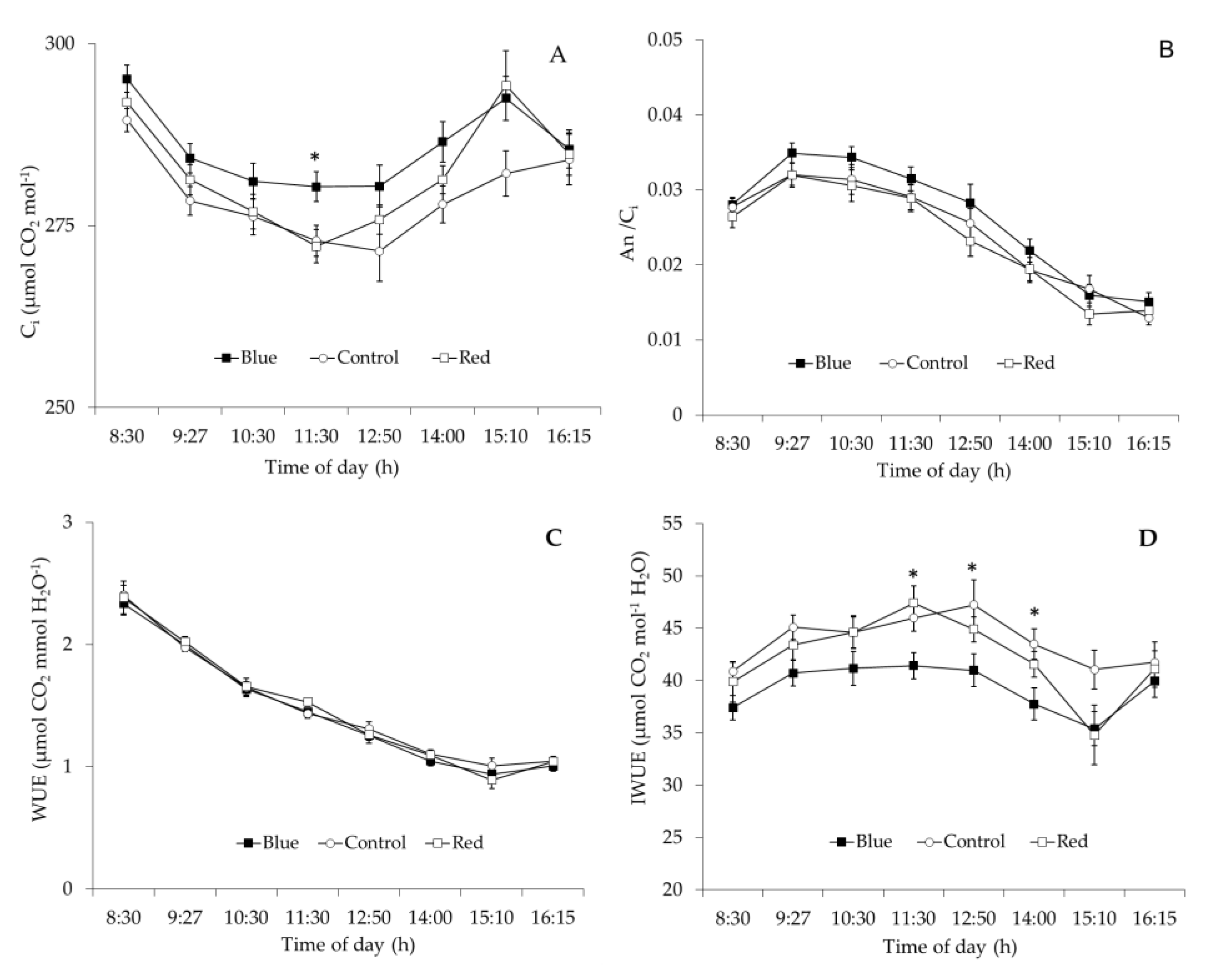
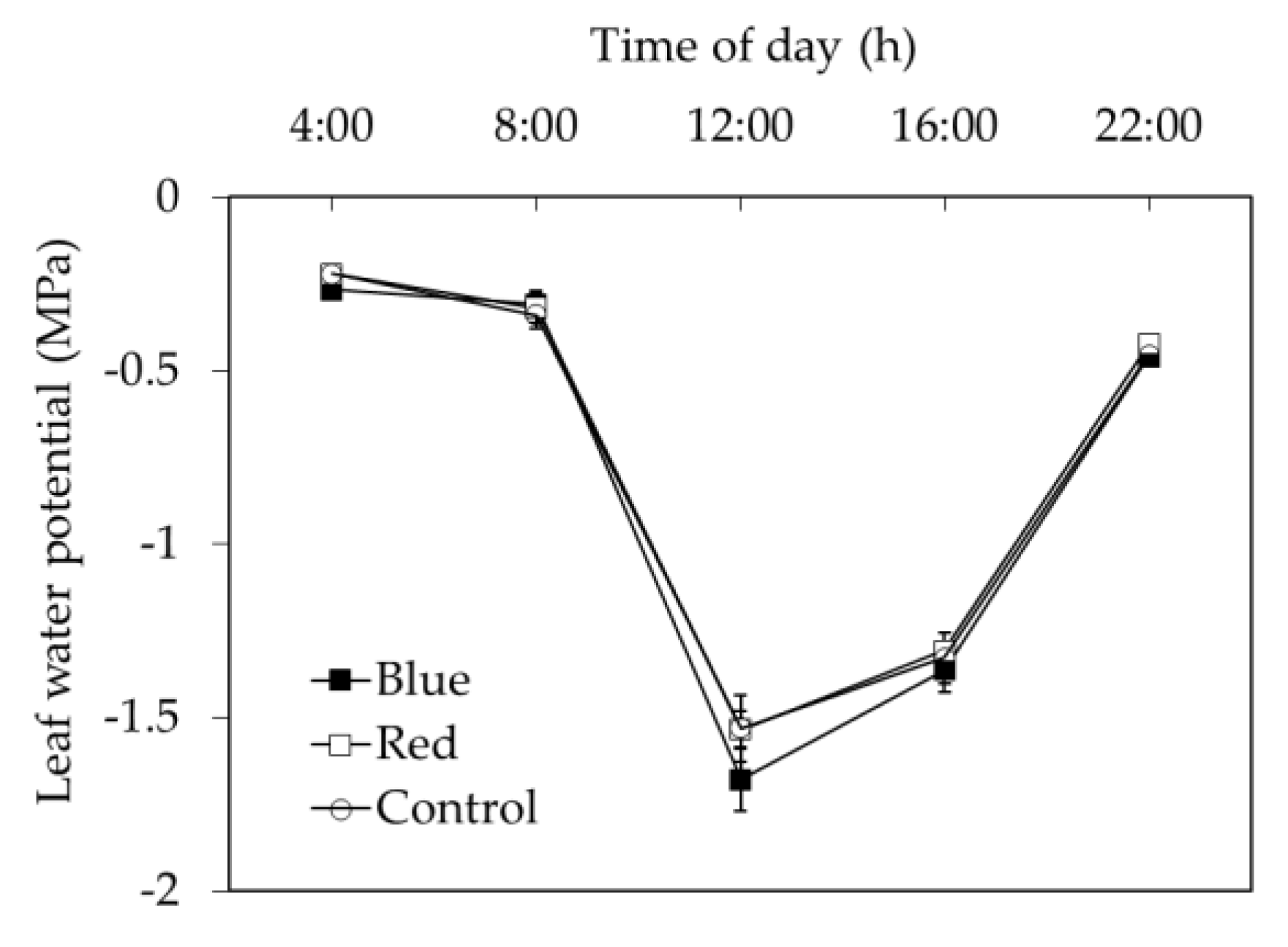
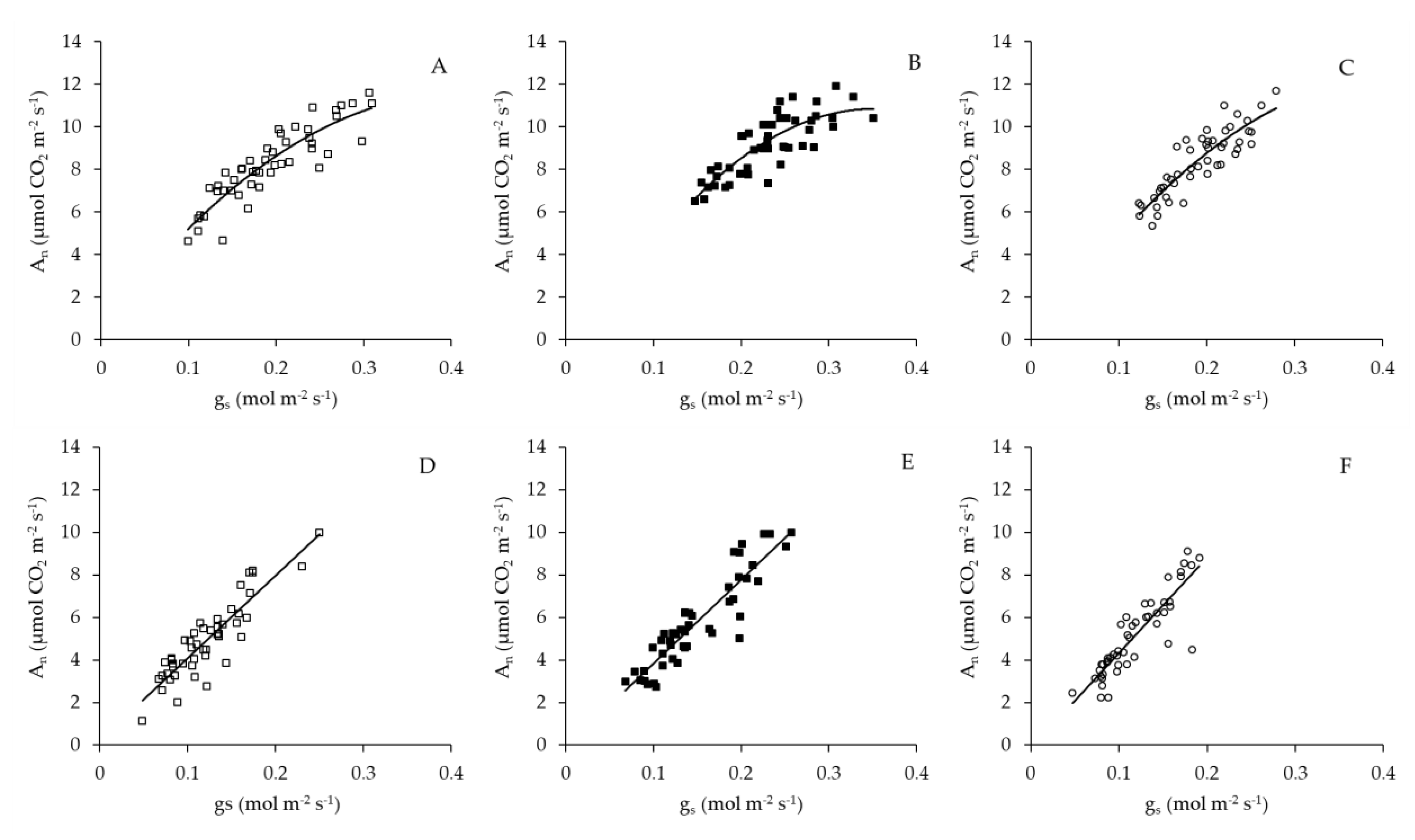
2.3. Leaf Gas Exchange
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Light Relations
4.3. Tree Growth
4.4. Leaf Morphology and Anatomy
4.5. Leaf Gas Exchange
4.6. Tree Water Status
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomakhin, A.; Blanke, M.M. Coloured hailnets alter light transmission, spectra and phytochrome, as well as vegetative growth, leaf chlorophyll and photosynthesis and reduce flower induction of apple. Plant. Growth Regul. 2008, 56, 211–218. [Google Scholar] [CrossRef]
- Gindaba, J.; Wand, S.J.E. Do fruit sunburn control measures affect leaf photosynthetic rate and stomatal conductance in ‘Royal Gala’ apple? Environ. Exp. Bot. 2007, 59, 160–165. [Google Scholar] [CrossRef]
- Mupambi, G.; Anthony, B.M.; Layne, D.R.; Musacchi, S.; Serra, S.; Schmidt, T.; Kalcsits, L.A. The influence of protective netting on tree physiology and fruit quality of apple: A review. Sci. Hortic. 2018, 236, 60–72. [Google Scholar] [CrossRef]
- Olivares-Soto, H.; Bastías, R.M. Photosynthetic efficiency of apples under protected shade nets. Chil. J. Agric. Res. 2018, 78, 126–138. [Google Scholar] [CrossRef]
- Chouinard, G.; Veilleux, J.; Pelletier, F.; Larose, M.; Philion, V.; Joubert, V.; Cormier, D. Impact of Exclusion Netting Row Covers on ‘Honeycrisp’ Apple Trees Grown under Northeastern North American Conditions: Effects on Photosynthesis and Fruit Quality. Insects 2019, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Kalcsits, L.; Musacchi, S.; Layne, D.R.; Schmidt, T.; Mupambia, G.; Serra, S.; Mendozac, M.; Asteggiano, L.; Jarolmasjed, S.; Sankar, S.; et al. Above and below-ground environmental changes associated with the use of photoselective protective netting to reduce sunburn in apple. Agric. For. Meteorol. 2017, 237, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Shahak, Y.; Ratner, K.; Giller, Y.; Zur, N.; Or, E.; Gussakovsky, E.; Stern, R.; Sarig, P.; Raban, E.; Harcavi, E.; et al. Improving solar energy utilization, productivity and fruit quality in orchards and vineyeards by photoselective netting. Act. Hortic. 2008, 772, 65–72. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Romero-Bravo, S.; Del Pozo, A. Productivity and fruit quality of Vaccinium corymbosum cv. Elliott under photo-selective shading nets. Sci. Hortic. 2013, 153, 143–149. [Google Scholar] [CrossRef]
- Basile, B.; Giaccone, M.; Cirillo, C.; Ritieni, A.; Graziani, G.; Shahak, Y.; Forlania, M. Photo-selective hail nets affect fruit size and quality in Hayward kiwifruit. Sci. Hortic. 2012, 141, 91–97. [Google Scholar] [CrossRef]
- Zhou, K.; Jerszurki, D.; Avi Sadk, A.; Shlizerman, L.; Rachmilevitch, S.; Ephrath, J. Effects of photoselective netting on root growth and development of young grafted orange trees under semi-arid climate. Sci. Hortic. 2018, 238, 272–280. [Google Scholar] [CrossRef]
- Bastías, R.M.; Manfrini, L.; Corelli-Grappadelli, L. Exploring the potential use of photoselective nets for fruit growth regulation in apple. Chil. J. Agric. Res. 2012, 72, 224–231. [Google Scholar] [CrossRef]
- Corollaro, M.L.; Manfrini, L.; Endrizzi, I.; Aprea, E.; Demattè, M.L.; Charles, M.; Bergamaschi, M.; Biasioli, F.; Zibordi, M.; Corelli Grappadelli, L.; et al. The effect of two orchard light management practices on the sensory quality of apple: Fruit thinning by shading or photo-selective nets. J. Hort. Sci. Biotech. 2015, 90, 99–107. [Google Scholar] [CrossRef]
- Olivares-Soto, H.; Bastías, R.M.; Calderón-Orellana, A.; López, M.D. Sunburn control by nets differentially affects the antioxidant properties of fruit peel in ‘Gala’ and ‘Fuji’ apples. Hortic. Environ. Biotechnol. 2020, 61, 241–254. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Cobo, N.; Del Pozo, A. Spectral irradiance, gas exchange characteristics and leaf traits of Vaccinium corymbosum L. ‘Elliott’ grown under photo-selective nets. Environ. Exp. Bot. 2012, 75, 142–149. [Google Scholar] [CrossRef]
- Medina, C.L.; Souza, R.P.; Machado, E.C.; Ribeiro, R.V.; Silva, J.A.B. Photosynthetic response of citrus grown under reflective aluminized polypropylene shading nets. Sci. Hortic. 2002, 1821, 1–11. [Google Scholar] [CrossRef]
- Mupambi, G.; Musacchi, S.; Serra, S.; Kalcsits, L.A.; Layne, D.R.; Schmidt, T. Protective netting improves leaf-level photosynthetic light use efficiency in ‘Honeycrisp’ apple under heat stress. HortScience 2018, 53, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Bastías, R.M.; Corelli-Grappadelli, L. Light quality management in fruit orchards: Physiological and technological aspects. Chil. J. Agric. Res. 2012, 72, 574–582. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.C. Long-Term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant. Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Baraldi, R.; Rapparini, F.; Rotondi, A.; Bertazza, G. Effects of simulated light environments on growth and leaf morphology of peach plants. J. Hort. Sci. Biotech. 1998, 73, 251–258. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical features of pepper plants (Capsicum annuum L.) Grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. 2009, 96, 30–37. [Google Scholar] [CrossRef]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant. Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [Green Version]
- Corelli-Grappadelli, L. Light Relations. In Apples: Botany, Production and Uses; Ferree, D.C., Warrington, I.J., Eds.; CAB International: Wallingford, UK, 2003; pp. 195–216. [Google Scholar]
- Gregoriou, K.; Pontikis, K.; Vemmos, S. Effects of reduced irradiance on leaf morphology, photosynthetic capacity, and fruit yield in olive (Olea europea L.). Photosynthetica 2007, 45, 172–181. [Google Scholar] [CrossRef]
- Smith, K.W.; Vogelmann, T.C.; De Lucia, E.; Bell, D.T.; Shepherd, K.A. Leaf form and photosynthesis. Do leaf structure and orientation interact to regulate light and carbon dioxide? Bioscience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Solomakhin, A.; Blanke, M. The microclimate under coloured hailnets affects leaf and fruit temperature, leaf anatomy, vegetative and reproductive growth as well as fruit colouration in apple. Ann. App. Biol. 2010, 156, 121–136. [Google Scholar] [CrossRef]
- Kim, G.-T.; Yano, S.; Kozuka, T.; Tsukaya, H. Photomorphogenesis of leaves: Shade avoidance and differentiation of sun and shade leaves. Photochem. Photobiol. Sci. 2005, 4, 770–774. [Google Scholar] [CrossRef]
- Stamps, R.H.; Chandler, A.L. Differential effect of colored shade nets on three cut foliage crops. Act. Hortic. 2008, 770, 169–176. [Google Scholar] [CrossRef]
- Takemiya, A.; Inue, S.I.; Doi, M.; Kinoshita, T.; Shimazaki, K.I. Phototropins promotes plant growth in Responses to Blue Light in Low Light Environments. Plant. Cell. 2005, 17, 110–1127. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, R.; Nerozzi, F.; Magnanini, E.; Corelli-Grappadelli, L. Influence of environmental and plant factors on canopy photosynthesis and transpiration in apple trees. Tree Physiol. 1997, 17, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Lakso, A.N. 2003. Water Relations of Apples. In Apples: Botany, Production and Uses; Ferree, D.C., Warrington, I.J., Eds.; CAB International: Wallingford, UK, 2003; pp. 167–194. [Google Scholar]
- Atkinson, C.J.; Policarpo, M.; Webster, A.D.; Kingswell, G. Drought tolerance of clonal Malus determined from measurements of stomatal conductance and leaf water potential. Tree Physiol. 2000, 20, 557–563. [Google Scholar] [CrossRef]
- Smith, H. Phytochromes and light signal perception by plants–an emerging synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Franks, P.; Drake, P.L.; Berling, D.J. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: An analysis using Eucalyptus globulus. Plant. Cell Environ. 2009, 32, 1737–1748. [Google Scholar] [CrossRef]
- Franks, P.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant. Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Massonnet, C.; Costes, E.; Rambal, S.; Dreyer, E.; Regnard, J.L. Stomatal regulation of photosynthesis in apple leaves: Evidence for different water-use strategies between two cultivars. Ann. Bot. 2007, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Ann. Rev. Plant. Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Wilton, J. Apple production trends in Europe. Compact Fruit Tree. 2001, 34, 29–31. [Google Scholar]
- Kittas, C.; Baille, A.; Giaglaras, P. Influence of covering material and shading on the spectral distribution of light in greenhouses. J. Agric. Eng. Res. 1999, 73, 341–351. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequlibria determination using spectral data. Trans. Am. Soc. Agric. Eng. 1988, 31, 1883–1889. [Google Scholar]
- Gitz, D.; Baker, J. Methods for creating stomatal impressions directly onto archivable slides. Agron. J. 2009, 101, 232–236. [Google Scholar] [CrossRef]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An ethanol-based fixation method for anatomical and micro-morphological characterization of leaves of various tree species. Biotech. Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef]
- Baldini, E.; Facini, O.; Nerozzi, F.; Rossi, F.; Rotondi, A. Leaf characteristics and optical properties of different woody species. Trees Struct. Fun 1997, 12, 73–81. [Google Scholar] [CrossRef]
- Balzarini, M.G.; González, L.; Tablada, M.; Casanoves, F.; Di Rienzo, J.A.; Robledo, C.W. InfoStat: Statistical Software; Grupo InfoStat—Universidad Nacional de Córdoba: Córdoba, Argentina, 2008; p. 329. [Google Scholar]
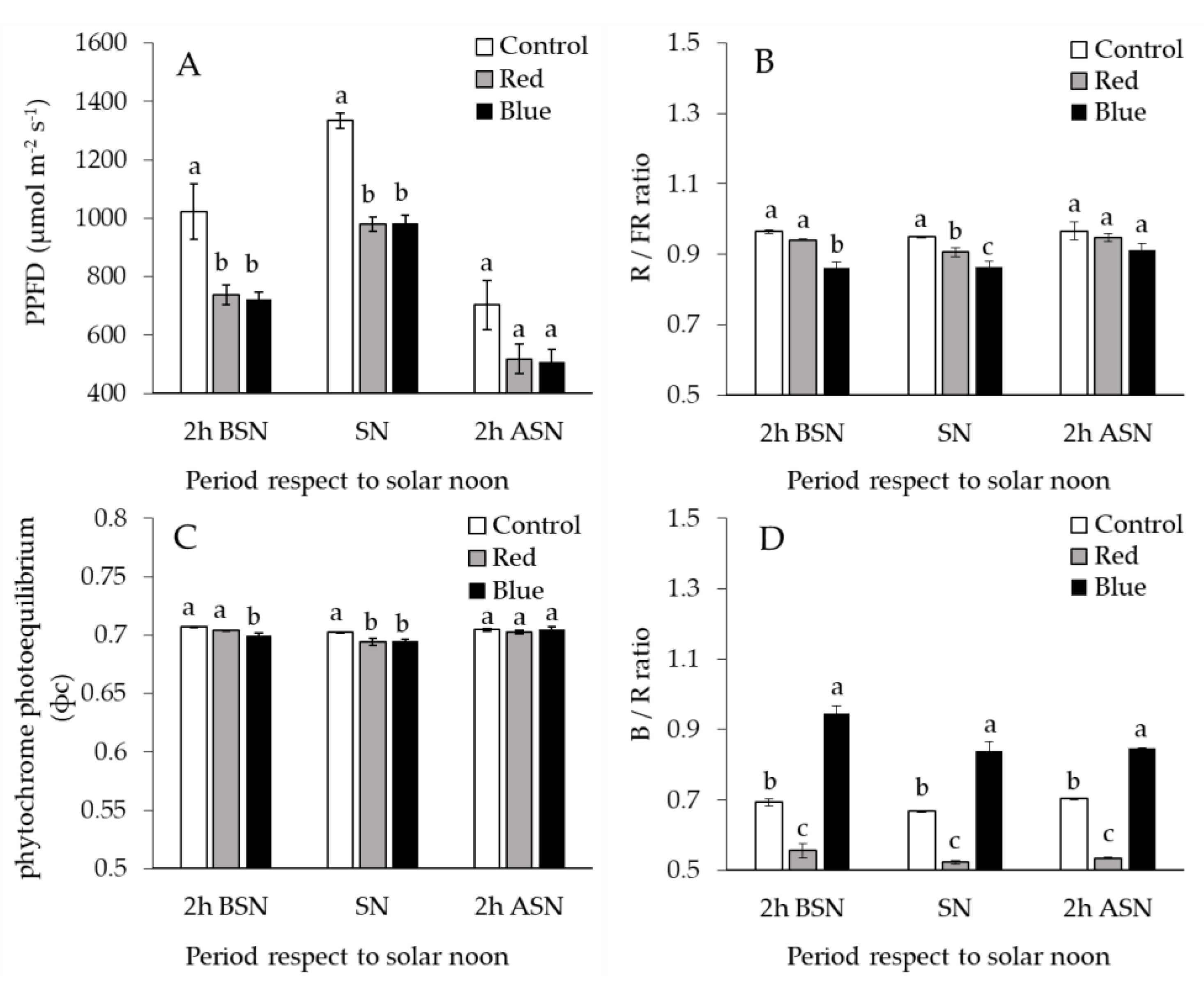


| Factors | LA (cm2) | LMA (mg cm−2) | SD (n° mm−2) | ChC (SPAD Unit) | TCSA (cm−2) | TSL (m) |
|---|---|---|---|---|---|---|
| Net system (NS) | ||||||
| Control | 16.4 | 12.8 a 1 | 531.8 a | 48.5 b | 4.8 | 3.3 b |
| Red | 20.6 | 12.3 ab | 442.2 b | 47.9 b | 4.6 | 4.2 b |
| Blue | 24.9 | 11.8 b | 438.7 b | 50.9 a | 4.6 | 5.6 a |
| p-value | 0.0640 ns | 0.0286 * | 0.0343 * | <0.0001 ** | 0.8164 ns | 0.0129 * |
| Tree vigor (TV) | ||||||
| High | 21.9 | 12.3 | 478.4 | 49.4 | 4.8 | 4.2 |
| Low | 21.5 | 12.3 | 463.3 | 48.8 | 4.5 | 4.4 |
| p-value | 0.8161 ns | 0.8798 ns | 0.6331 ns | 0.0945 ns | 0.3361 ns | 0.7030 ns |
| p-value NS × TV | 0.7462 ns | 0.6693 ns | 0.5154 ns | 0.3903 ns | 0.4059 ns | 0.4886 ns |
| Net Systems | SL (µm) | SW (µm) | L/W | Stomata Frequency by Length (%) | |||
|---|---|---|---|---|---|---|---|
| <15 µm | 15–20 µm | 20–25 µm | >25 µm | ||||
| Control | 20.2 b | 14.5 | 1.4 b | 5.0 | 45.0 | 40.0 b | 10.0 |
| Red | 22.5 a | 14.6 | 1.5 a | 1.0 | 26.0 | 51.0 a | 22.0 |
| Blue | 21.3 ab | 13.8 | 1.5 a | 1.0 | 32.0 | 56.0 a | 11.0 |
| p-value | 0.0262 * | 0.3531 ns | 0.0029 ** | 0.0537 ns | 0.0667 ns | 0.0193 * | 0.1104 ns |
| Net System | Leaf Tissues Thickness (µm) | Palisade/Spongy Mesophyll Ratio | |||
|---|---|---|---|---|---|
| Total | Upper Epidermis | Lower Epidermis | Palisade | ||
| Control | 228.4 | 13.7 | 9.7 | 116.2 a 1 | 1.4 a |
| Red | 218.9 | 13.4 | 9.7 | 96.2 b | 1.0 b |
| Blue | 215.2 | 13.6 | 9.8 | 99.0 b | 1.1 b |
| p-value | 0.4980 ns | 0.9123 ns | 0.9616 ns | 0.0404 * | 0.0015 ** |
| Factors | An (µmol CO2 m−2 s−1) | gs (mol m−2 s−1) | E (mmol H2O m−2 s−1) | Ci (µmol mol−1) |
|---|---|---|---|---|
| Net system (NS) | ||||
| Control | 11.2 c 1 | 0.16 b | 4.0 b | 248.0 |
| Red | 12.9 b | 0.23 a | 5.2 a | 265.4 |
| Blue | 14.7 a | 0.23 a | 5.2 a | 253.7 |
| p-value | 0.0037 ** | 0.0090 ** | 0.0199 * | 0.1158 ns |
| Tree Vigor (TV) | ||||
| High | 13.2 | 0.19 | 4.8 | 246.3 |
| Low | 12.7 | 0.22 | 4.8 | 265.1 |
| p-value | 0.7369 ns | 0.2670 ns | 0.9112 ns | 0.0565 ns |
| p-value NS × TV | 0.6004 ns | 0.6720 ns | 0.4490 ns | 0.6075 ns |
| Regression Coefficients | Net Systems | ||
|---|---|---|---|
| Control | Red | Blue | |
| Before Solar Noon | |||
| β0 | −0.5 ns | 0.49 ns | −1.6 ns |
| β1 | 60.4 ** | 53.6 ** | 71.3 ** |
| β2 | −70.4 ns | −64.8 ns | −101.5 * |
| R2 | 0.77 ** | 0.80 ** | 0.67 ** |
| After Solar Noon | |||
| β0 | −0.15 ns | 0.19 ns | −0.13 ns |
| β1 | 44.8 ** | 38.8 ** | 39.4 ** |
| R2 | 0.77 ** | 0.80 ** | 0.83 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastías, R.M.; Losciale, P.; Chieco, C.; Corelli-Grappadelli, L. Red and Blue Netting Alters Leaf Morphological and Physiological Characteristics in Apple Trees. Plants 2021, 10, 127. https://doi.org/10.3390/plants10010127
Bastías RM, Losciale P, Chieco C, Corelli-Grappadelli L. Red and Blue Netting Alters Leaf Morphological and Physiological Characteristics in Apple Trees. Plants. 2021; 10(1):127. https://doi.org/10.3390/plants10010127
Chicago/Turabian StyleBastías, Richard M., Pasquale Losciale, Camilla Chieco, and Luca Corelli-Grappadelli. 2021. "Red and Blue Netting Alters Leaf Morphological and Physiological Characteristics in Apple Trees" Plants 10, no. 1: 127. https://doi.org/10.3390/plants10010127






