Spartina alterniflora Leaf and Soil Eco-Stoichiometry in the Yancheng Coastal Wetland
Abstract
:1. Introduction
2. Materials and Methods
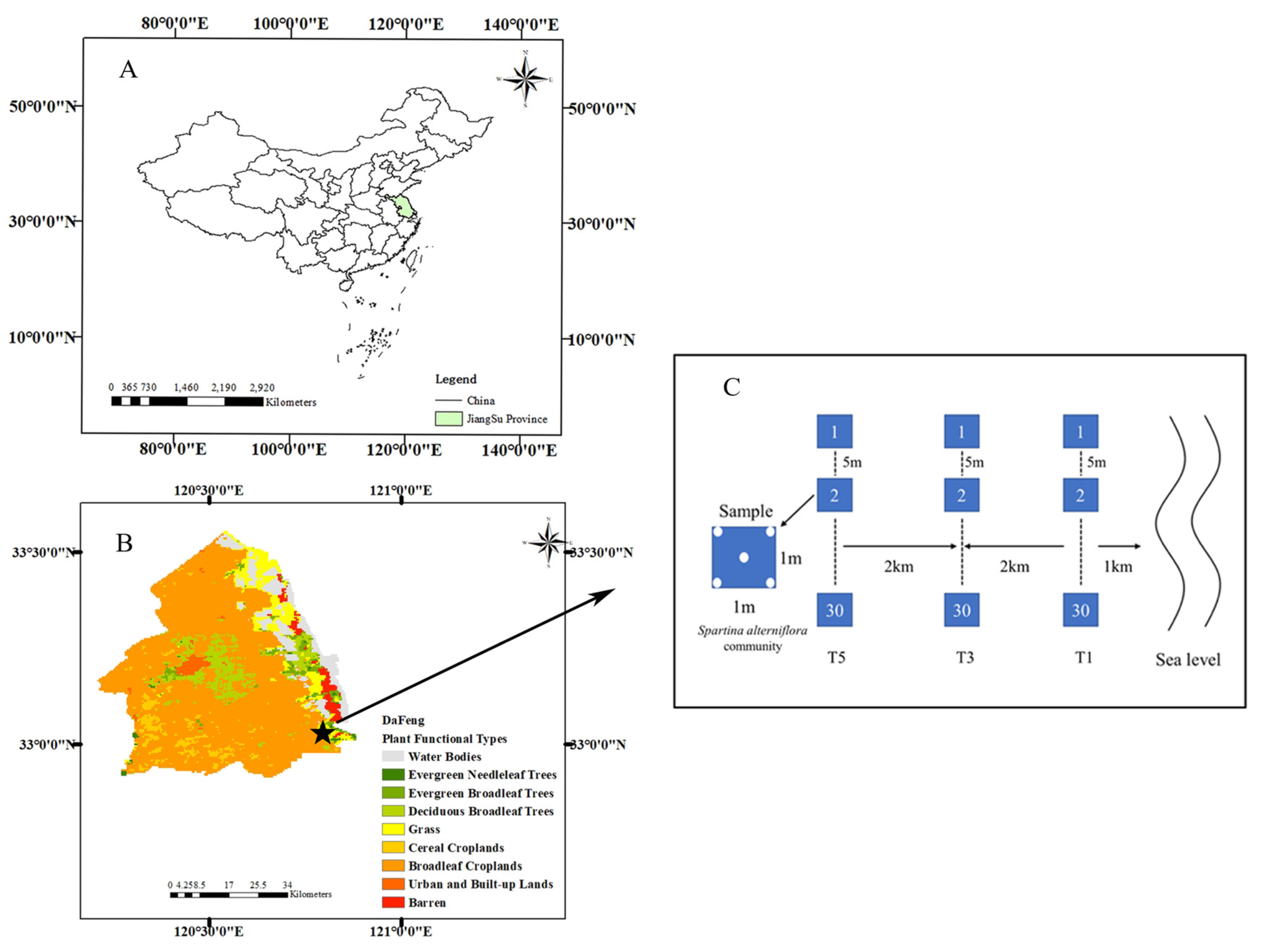
2.1. Study Site
2.2. Sample Collection and Measurement
2.3. Data Analysis
3. Results
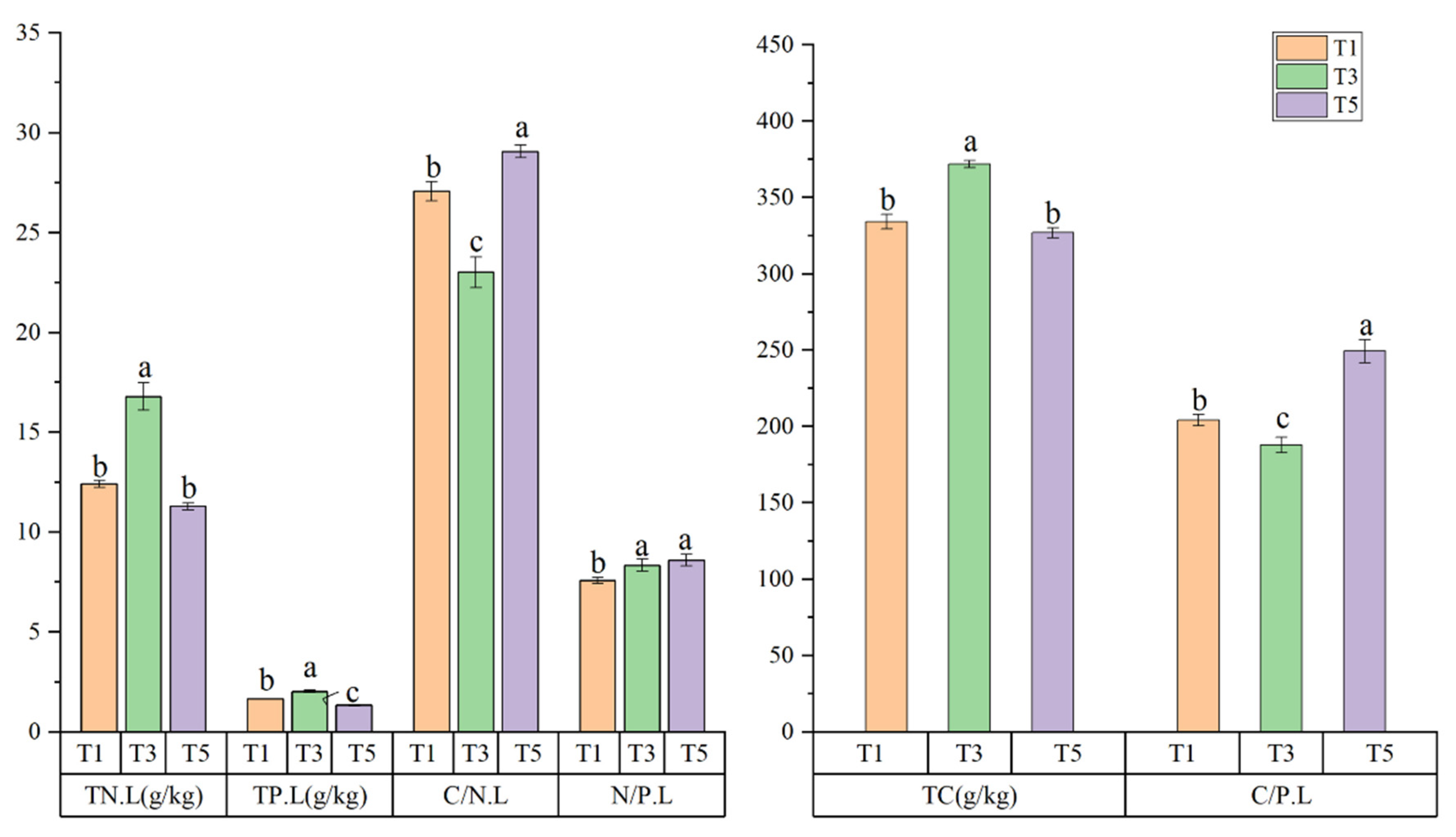
3.1. Stoichiometric Characteristics of C, N, and P in Leaves of S. alterniflora Leaves
3.2. Stoichiometric and Other Characteristics of Soil C, N, and P
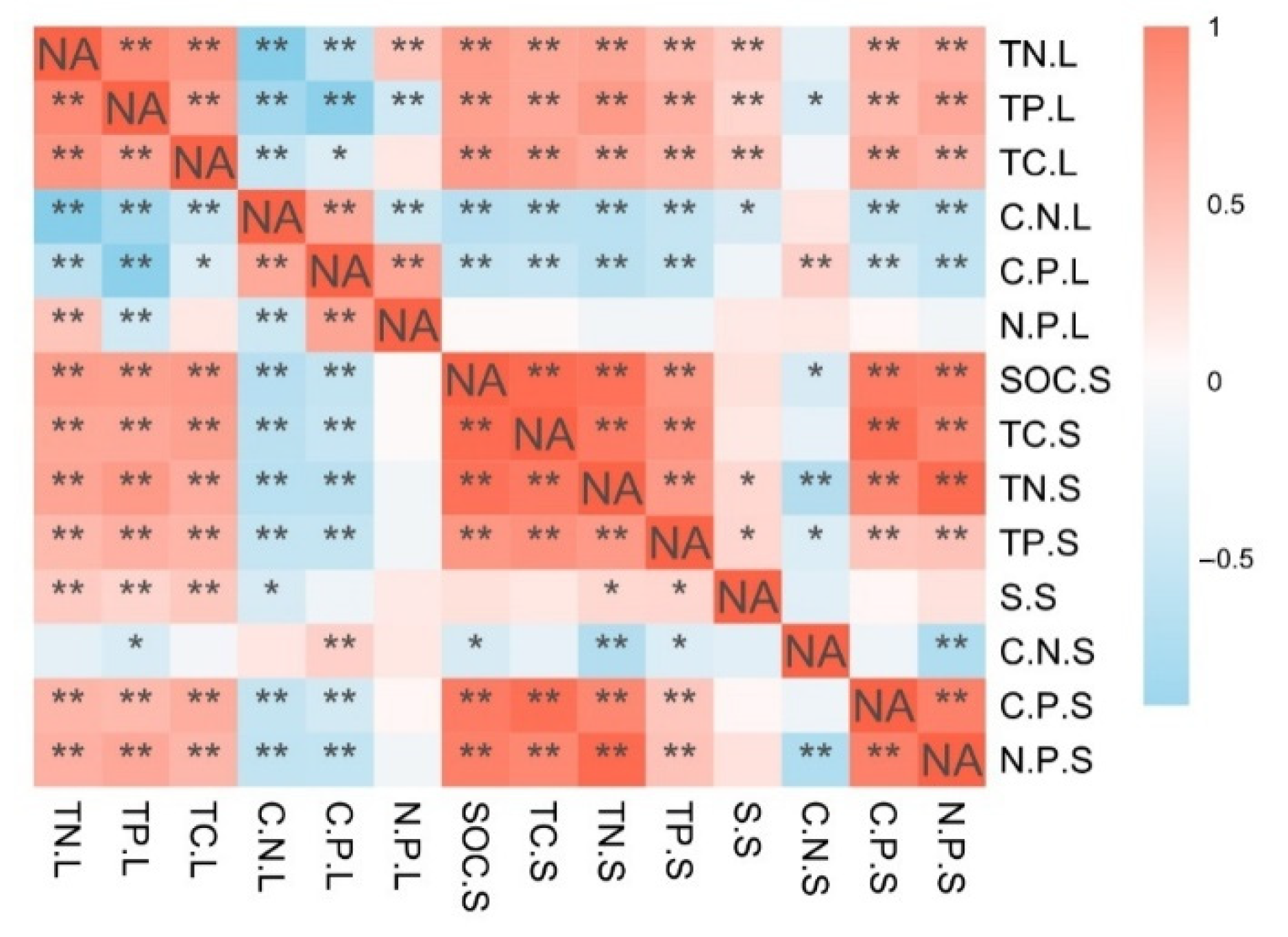
3.3. Relationship between the Leaf and Soil C, N, P Stoichiometric Characteristics and Other Physical and Chemical Properties
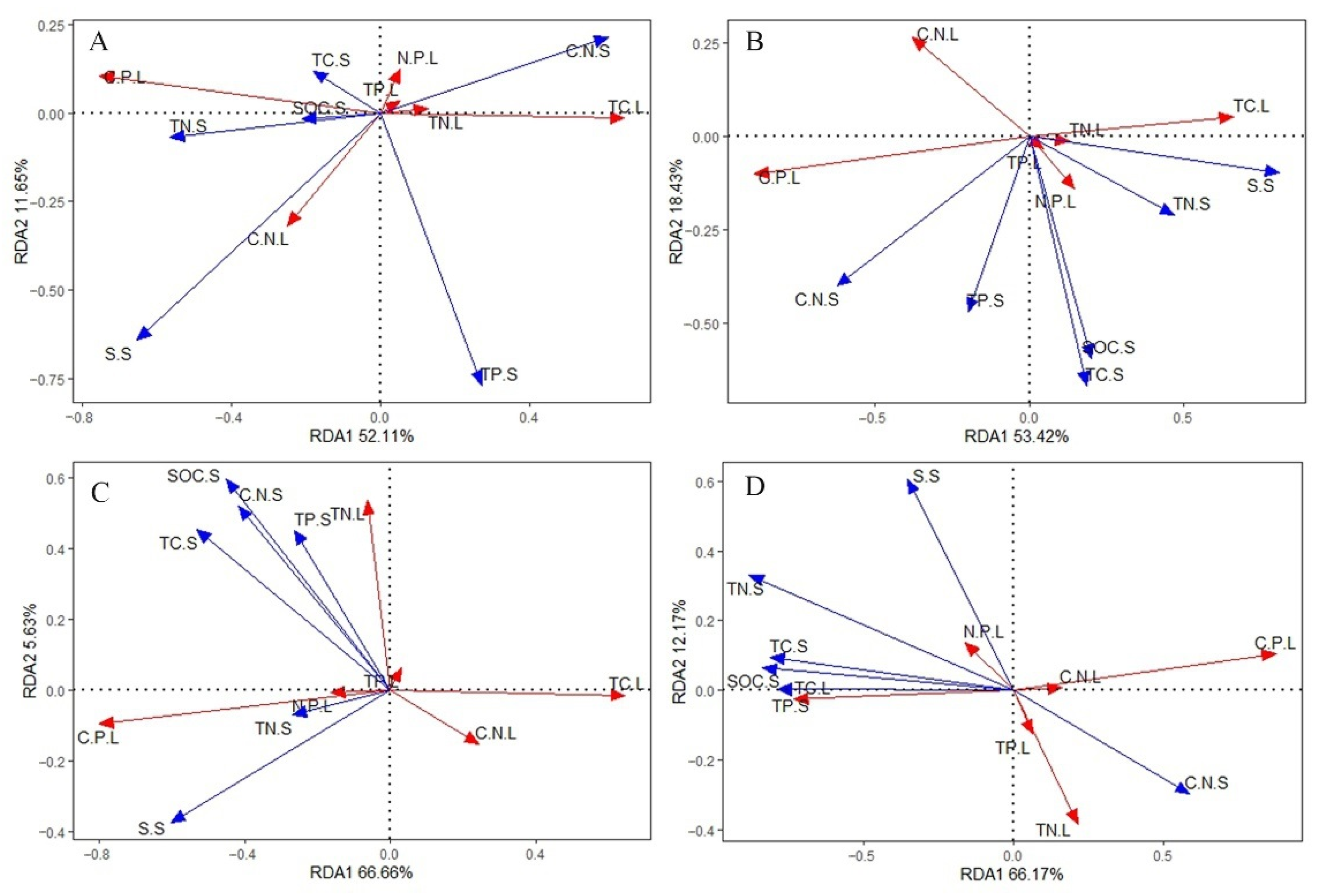
3.4. Effects of Soil Eco-Stoichiometry on Leaf Eco-Stoichiometry
4. Discussion
4.1. Relationships between the Eco-Stoichiometry of S. alterniflora Leaves and Soil Physical and Chemical Properties
4.2. Key Soil Factors That Affect Leaf Eco-Stoichiometry
4.3. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Wei, Y. Revised normalized difference nitrogen index (NDNI) for estimating canopy nitrogen concentration in wetlands. Optik 2016, 127, 7676–7688. [Google Scholar] [CrossRef]
- Sheaves, M.; Brookes, J.; Coles, R.; Freckelton, M.; Groves, P.; Johnston, R.; Wingberg, P. Repair and revitalisation of Australia’s tropical estuaries and coastal wetlands: Opportunities and constraints for the reinstatement of lost function and productivity. Mar. Policy 2014, 47, 23–38. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Cai, F.; Su, X.Z.; Liu, J.H.; Li, B.; Lei, G. Coastal erosion in China under the condition of global climate change and measures for its prevention. Prog. Nat. Sci. 2009, 19, 415–426. [Google Scholar] [CrossRef]
- Meng, W.; Feagin, R.A.; Innocenti, R.A.; Hu, B.; He, M.; Li, H. Invasion and ecological effects of exotic smooth cordgrass Spartina alterniflora in China. Ecol. Eng. 2020, 143, 105670. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, Y.; Liu, M.; Chang, Y.; Yan, X.; Bu, R.; Zhao, D.; Li, Z. Introduction and Spread of an Exotic Plant, Spartina alterniflora, Along Coastal Marshes of China. Wetlands 2017, 37, 1181–1193. [Google Scholar] [CrossRef]
- An, S.Q.; Gu, B.H.; Zhou, C.F.; Wang, Z.S.; Deng, Z.F.; Zhi, Y.B.; Li, H.L.; Chen, L.; Yu, D.H.; Liu, Y.H. Spartina invasion in China: Implications for invasive species management and future research. Weed Res. 2007, 47, 183–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.; Wang, W.; Chen, L.; Lin, G. Interactions between mangroves and exotic Spartina in an anthropogenically disturbed estuary in southern China. Ecology 2012, 93, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, B.; Zhong, Y.; Chen, J. Local competitive effects of introduced Spartina alterniflora on Scirpus mariqueter at Dongtan of Chongming Island, the Yangtze River estuary and their potential ecological consequences. Hydrobiologia 2004, 528, 99–106. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.M.; Zheng, R.S.; Li, X.; Elmer, W.H.; Wolfe, L.M.; Li, B. Indirect effects of non-native and its fungal pathogen (fusarium palustre) on native saltmarsh plants in China. J. Ecol. 2014, 102, 1112–1119. [Google Scholar] [CrossRef]
- Levin, L.A.; Neira, C.; Grosholz, E.D. Invasive Cordgrass Modifies Wetland Trophic Function. Ecology 2006, 87, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Z.; Luo, Y.Q.; Fang, C.M.; Chen, J.K.; Li, B. Litter pool sizes, decomposition, and nitrogen dynamics in Spartina alterniflora-invaded and native coastal marshlands of the Yangtze Estuary. Oecologia 2008, 156, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Zhang, L.H.; Wang, W.Q.; Gauci, V.; Marrs, R.; Liu, B.G.; Jia, R.X.; Zeng, C.S. Contrasting nutrient stocks and litter decomposition in stands of native and invasive species in a sub-tropical estuarine marsh. Environ. Res. 2011, 111, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Van Wilgen, B.W.; Raghu, S.; Sheppard, A.W.; Schaffner, U. Quantifying the social and economic benefits of the biological control of invasive alien plants in natural ecosystems. Curr. Opin. Insect Sci. 2020, 38, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Lei, Y.B.; Tan, Y.H.; Sun, X.C.; Xu, H.; Liu, C.Q.; Liu, X.Y. Plant nitrogen and phosphorus utilization under invasive pressure in a montane ecosystem of tropical China. J. Ecol. 2019, 107, 372–386. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Traits Associated with Invasiveness in Alien Plants: Where Do we Stand? In Biological Invasions; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 97–125. [Google Scholar]
- Bachmann, D.; Both, S.; Bruelheide, H.; Ding, B.-Y.; Gao, M.; Härdtle, W.; Scherer-Lorenzen, M.; Erfmeier, A. Functional trait similarity of native and invasive herb species in subtropical China—Environment-specific differences are the key. Environ. Exp. Bot. 2012, 83, 82–92. [Google Scholar] [CrossRef]
- Funk, J.L.; Glenwinkel, L.A.; Sack, L. Differential Allocation to Photosynthetic and Non-Photosynthetic Nitrogen Fractions among Native and Invasive Species. PLoS ONE 2013, 8, e64502. [Google Scholar] [CrossRef] [Green Version]
- Penuelas, J.; Sardans, J.; Llusià, J.; Owen, S.M.; Carnicer, J.; Giambelluca, T.W.; Rezende, E.L.; Waite, W.; Niinemets, Ü. Faster returns on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Glob. Chang. Biol. 2009, 16, 2171–2185. [Google Scholar] [CrossRef] [Green Version]
- Neves, J.P.; Simões, M.P.; Ferreira, L.F.; Madeira, M.; Gazarini, L.C. Comparison of Biomass and Nutrient Dynamics Between an Invasive and a Native Species in a Mediterranean Saltmarsh. Wetlands 2010, 30, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.L.; Kominoski, J.S.; Danger, M.; Ishida, S.; Iwai, N.; Rubach, A. Can ecological stoichiometry help explain patterns of biological invasions? Oikos 2010, 119, 779–790. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: Biology of Elements from Molecules to the Biosphere. J. Plankton Res. 2002, 25, 1183. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the Plant-Soil System. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar] [CrossRef] [Green Version]
- Zeng, D.H.; Chen, G.S. Ecological stoichiometry: A science to explore the complexity of living systems. Acta Phytoecol. Sin. 2005, 29, 1007–1019. [Google Scholar]
- Xie, R.; Zhu, Y.; Li, J.; Liang, Q. Changes in sediment nutrients following Spartina alterniflora invasion in a subtropical estuarine wetland, China. Catena 2019, 180, 16–23. [Google Scholar] [CrossRef]
- Liao, C.; Luo, Y.; Jiang, L.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Invasion of Spartina alterniflora Enhanced Ecosystem Carbon and Nitrogen Stocks in the Yangtze Estuary, China. Ecosystems 2007, 10, 1351–1361. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Liu, L.; Tian, Y.; Yu, Z. Effects of Spartina alterniflora invasion on biogenic elements in a subtropical coastal mangrove wetland. Environ. Sci. Pollut. Res. 2015, 22, 3107–3115. [Google Scholar] [CrossRef]
- Wang, W.; Sardans, J.; Wang, C.; Zeng, C.; Tong, C.; Chen, G.; Huang, J.; Pan, H.; Peguero, G.; Vallicrosa, H.; et al. The response of stocks of C, N, and P to plant invasion in the coastal wetlands of China. Glob. Chang. Biol. 2019, 25, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Dostálek, T.; Münzbergová, Z.; Kladivová, A.; Macel, M. Plant–soil feedback in native vs. invasive populations of a range expanding plant. Plant Soil 2015, 399, 209–220. [Google Scholar] [CrossRef]
- Niu, J.; Lu, T.; Lin, Y.; Zhang, W. Effects of Nitrogen Addition on the Characteristics of Foliar and Soil Ecological Stoichiometry in Xishuangbanna Tropical Rainforest, Southwest China. J. Trop. For. Sci. 2020, 32, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Zhu, H. Ecological stoichiometry and invasive strategies of two alien species (Bidens pilosa and Mikania micrantha) in subtropical China. Ecol. Res. 2019, 34, 612–623. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, S.; Tian, Y.; Li, Y.; Wen, R.; Tsou, J.Y.; Zhang, Y. Monitoring Suaeda salsa Spectral Response to Salt Conditions in Coastal Wetlands: A Case Study in Dafeng Elk National Nature Reserve, China. Remote Sens. 2020, 12, 2700. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Qin, P.; Zhou, J.; Wang, G. Comparisons of ecosystem services among three conversion systems in Yancheng National Nature Reserve. Ecol. Eng. 2009, 35, 609–629. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhu, X.D.; Zou, X.Q.; Gao, J.H. Study on landscape ecosystem of coastal wetlands in Yancheng, Jiangsu Province. Bull. Mar. Sci. Miami 2005, 24, 46–51. [Google Scholar]
- Smith, K.A.; Tabatabai, M.A. Automated instruments for the determination of total Carbon, Hydrogen, Nitrogen, Sulfur, and Oxygen. Soil Environ. Anal. Mod. Instrum. Tech. 2003, 6, 202–246. [Google Scholar]
- Wardle, D.A.; Walker, L.R.; Bardgett, R.D. Ecosystem Properties and Forest Decline in Contrasting Long-Term Chronosequences. Science 2004, 305, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Tessier, J.T.; Raynal, D.J. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Wang, S.Q.; Yu, G.R. Ecological stoichiometry characteristics of ecosystem carbon, nitrogen and phosphorus elements. Acta Ecol. Sin. 2008, 40, 3937–3947. [Google Scholar]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.F.; Interlandi, S.J.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nat. Cell Biol. 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Tree growth changes with climate and forest type are associated with relative allocation of nutrients, especially phosphorus, to leaves and wood. Glob. Ecol. Biogeogr. 2012, 22, 494–507. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Wang, C.; Sardans, J.; Zeng, C.-S.; Tong, C.; Peñuelas, J. Plant invasive success associated with higher N-use efficiency and stoichiometric shifts in the soil–plant system in the Minjiang River tidal estuarine wetlands of China. Wetl. Ecol. Manag. 2015, 23, 865–880. [Google Scholar] [CrossRef] [Green Version]
- Hobbie, S.E.; Vitousek, P.M. Nutrient limitation of decomposition in hawaiian forests. Ecology 2000, 81, 1869–1877. [Google Scholar] [CrossRef]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon Sequestration in Ecosystems: The Role of Stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P Stoichiometry in Forests worldwide: Implications of Terrestrial Redfield-Type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Xie, L.; Wang, B.; Xin, M.; Wang, M.; He, X.; Wei, Q.; Shi, X.; Sun, X. Characteristics of Vegetation Carbon, Nitrogen, and C/N Ratio in a Tamarix chinensis Coastal Wetland of China. Clean Soil Air Water 2019, 47, 1800452. [Google Scholar] [CrossRef]
- Chen, Y.; Han, W.; Tang, L.; Tang, Z.; Fang, J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 2013, 36, 178–184. [Google Scholar] [CrossRef]
- Li, L.; Zerbe, S.; Han, W.; Thevs, N.; Li, W.; He, P.; Schmitt, A.O.; Liu, Y.; Ji, C. Nitrogen and phosphorus stoichiometry of common reed (Phragmites australis) and its relationship to nutrient availability in northern China. Aquat. Bot. 2014, 112, 84–90. [Google Scholar] [CrossRef]
- Springob, G.; Kirchmann, H. Bulk soil C to N ratio as a simple measure of net N mineralization from stabilized soil organic matter in sandy arable soils. Soil Biol. Biochem. 2003, 35, 629–632. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, J.; Zhang, G.; Jia, J.; Wang, W.; Wang, X. Effects of water and salinity regulation measures on soil carbon sequestration in coastal wetlands of the Yellow River Delta. Geoderma 2018, 319, 219–229. [Google Scholar] [CrossRef]
- Jiang, L.L.; Zeng, C.S.; Shao, J.J.; Zhou, X.H. Plant nutrient dynamics and stoichiometric homeostasis of invasive species Spartina alterniflora and native Cyperus malaccensis var. brevifolius in the Minjiang River estuarine wetlands. Chin. J. Plant Ecol. 2017, 41, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Wilcox, K.R.; Pierre, K.L.; Knapp, A.K.; Han, X.; Smith, M.D. Stoichiometric homeostasis predicts plant species dominance, temporal stability, and responses to global change. Ecology 2015, 96, 2328–2335. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Cui, L.; Li, W.; Lei, Y.; Dou, Z.; Liu, Z.; Cai, Y.; Zhai, X. Spartina alterniflora Leaf and Soil Eco-Stoichiometry in the Yancheng Coastal Wetland. Plants 2021, 10, 13. https://doi.org/10.3390/plants10010013
Zuo X, Cui L, Li W, Lei Y, Dou Z, Liu Z, Cai Y, Zhai X. Spartina alterniflora Leaf and Soil Eco-Stoichiometry in the Yancheng Coastal Wetland. Plants. 2021; 10(1):13. https://doi.org/10.3390/plants10010013
Chicago/Turabian StyleZuo, Xueyan, Lijuan Cui, Wei Li, Yinru Lei, Zhiguo Dou, Zhijun Liu, Yang Cai, and Xiajie Zhai. 2021. "Spartina alterniflora Leaf and Soil Eco-Stoichiometry in the Yancheng Coastal Wetland" Plants 10, no. 1: 13. https://doi.org/10.3390/plants10010013




