Abstract
Natural populations of Gentiana asclepiadea L., located at two mountainous sites, were HPLC-analyzed regarding the contents of six representative secondary metabolites. The contents of swertiamarin (SWM), gentiopicrin (GP), sweroside (SWZ), mangiferin (MGF), isoorientin (ISOOR), and isovitexin (ISOV) were determined in six populations (three per study site), and separately for aboveground and belowground plant parts. PCA showed a clear separation of four groups according to the contents of the analyzed secondary metabolites. Out of six analyzed compounds, five were present in all samples and only one (SWZ) was found in Golija populations (belowground parts) but not in Vlasina populations, and its presence can be indicative of the geolocation of populations. Clear separation of groups was mostly affected by the different contents of chemical compounds in plant parts (aboveground versus belowground) and by the differences related to population origin (higher content of SWM and GP in belowground parts of individuals from Vlasina populations and higher content of MGF and ISOOR of individuals from Golija populations). The results of this study contribute to the spatiochemical profiling of G. asclepiadea populations and a better understanding of inter- and intrapopulation variability of pharmacologically important compounds.
1. Introduction
The genus Gentiana comprises about 400 species primarily distributed throughout the Eurasian mountainous regions, while some species are found in lowlands and some are distributed in diverse habitats in the Andes, India, New Zealand, and Southern Australia [1]. Most gentian species are valued as medicinal and ornamental plants and are intensively researched due to the presence of pharmacologically important phytochemicals. Gentians are prescribed by many traditional pharmacopoeias and incorporated into more than a hundred official drugs, owing to their most important active constituents, secoiridoid glycosides and xanthones [2].
Gentiana asclepiadea L., Willow gentian, native to mountains of Central and Southern Europe, is 1 of 11 gentian species present in Serbian flora [3]. It mostly occurs at high altitudes approaching the subalpine belt, at mountain ores, wet meadows, peat bogs, light forests, and forest edges, and is most frequently found in spruce forests. Perennial species (15–60 cm high) blooming from July to September are characterized by the strong, thick rhizome that is used as a traditional remedy for hepatitis infections and digestive problems [4].
High-altitude (over 1000 m) medicinal and aromatic flora deserve special attention due to their richness in valuable secondary metabolites, which become more abundant with increasing elevation [5]. The distribution of herbaceous mountainous flora is determined by specific topography, mountains’ directional extensions, slope, soil type, vegetation cover, and so forth. [6]. The high mountainous habitat is characterized by a set of environmental conditions, namely, temperature (including day–night temperature ranges), precipitation, insolation and UV radiation, duration of vegetation season, day length, and clear sky conditions, which together contribute to plants’ adaptive response regarding both primary and secondary metabolism [7]. Among the numerous environmental influences that have a synergistic impact on plant growth and reproduction, UV-B radiation is considered to be the most important factor for photosynthetic production and secondary metabolism. Under field conditions, plant biomass accumulation and phenophase shifting occur continually throughout the whole growing season, despite the negative effect of UV-B radiation on the photosynthetic apparatus [8]. Nevertheless, a substantial amount of energy produced in photosynthesis is required to acclimate and restore the damage caused by UV-B to photosynthetic pigments and cellular metabolism [9]. Light environment adaptation (UV-B radiation adaptation) at the secondary metabolism level is indicated by the enhancement of biosynthesis and aggregation of protecting UV-B compounds, namely, flavonoids (with strong free-radical scavenging potential) and sinapate esters (capable of absorption in the wavelength range from 280 to 340 nm) [10,11,12,13].
Other important external drivers for biosynthesis and accumulation of secondary metabolites are the attraction of pollinators, which decrease both in diversity and activity in high altitudes [14], and intensive herbivore pressure, considering that plant-eating insects have limited food choice to complete their life cycle [15]. High levels of biologically active secondary metabolites (potentially poisonous) in high-altitude habitats also contribute to shaping the community structure, as certain plant species are avoided by grazers. Low palatability (and in higher amounts toxicity) of gentians is an important grazing indicator and the cause for their increased abundance from the Neolithic period, as revealed by pollen records [16].
Eco-biochemical studies of natural populations are important for understanding species’ responses to specific site conditions in high-altitude environments, while plant-part-related differences provide further information on specific environmental drivers that are predicted to cause ecophysiological and metabolic adaptations. This research aimed to assess geographic variability, interpopulation variability, and plant-part-related differences in natural populations of Willow gentian based on the contents of six representative secondary metabolites.
2. Results
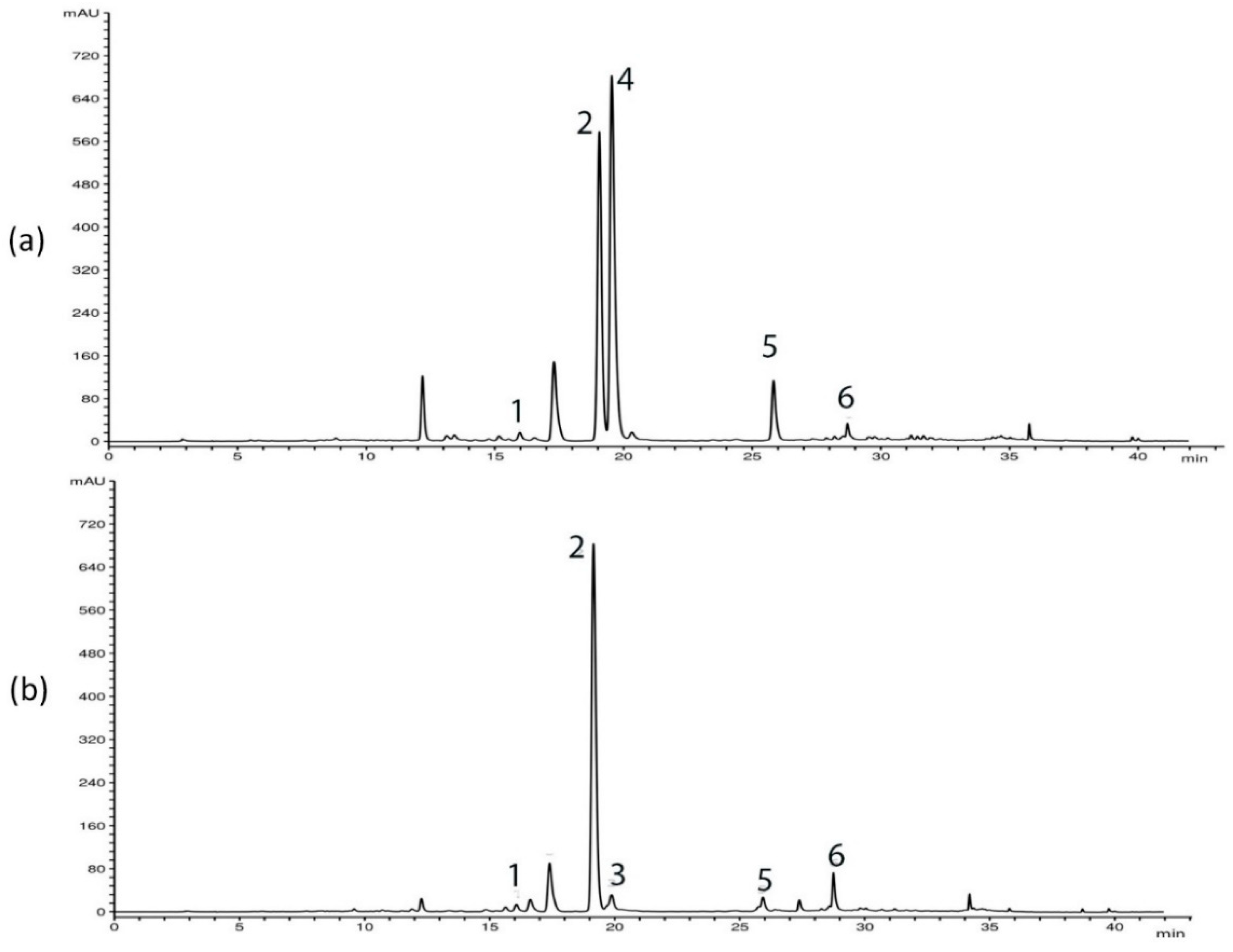
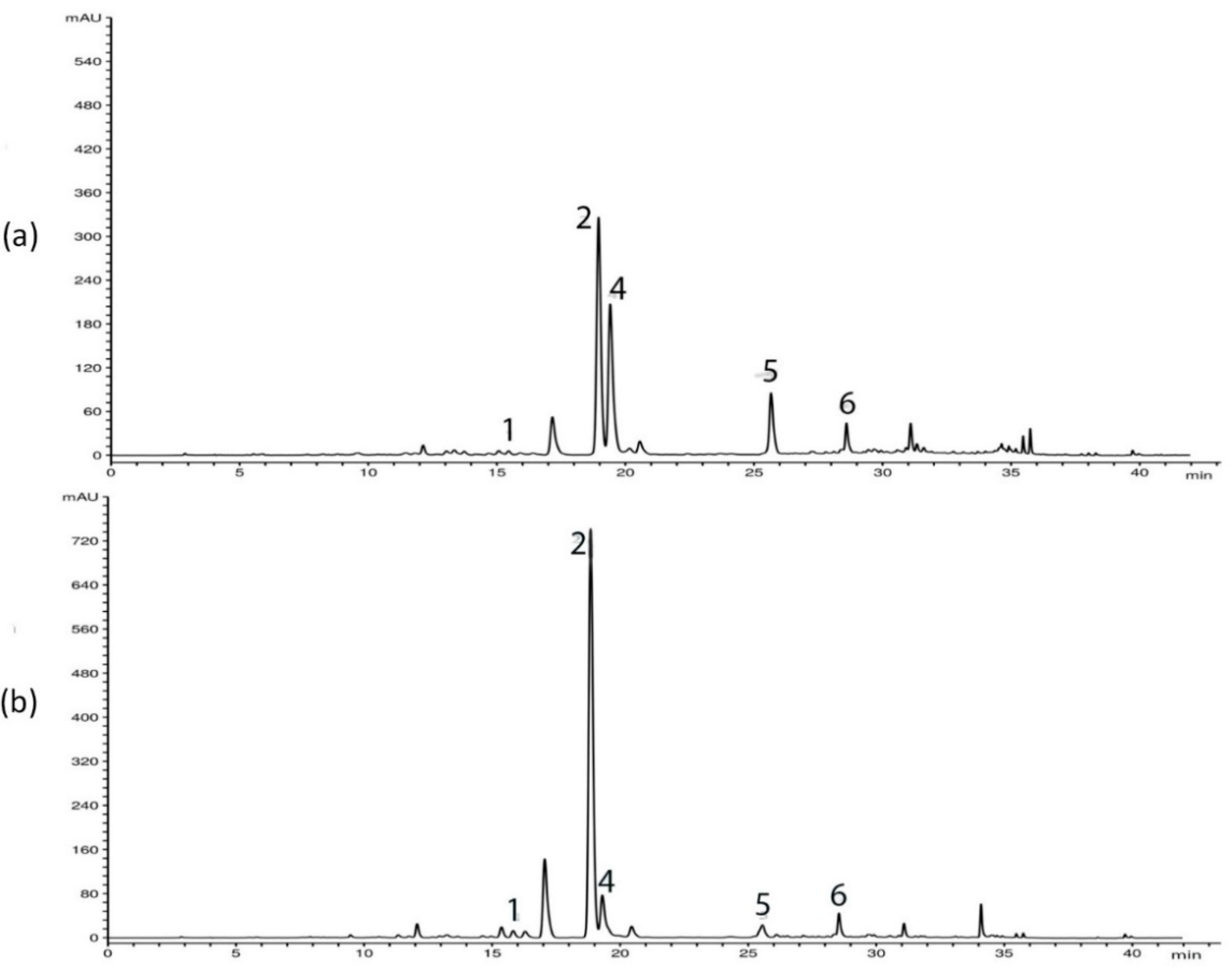
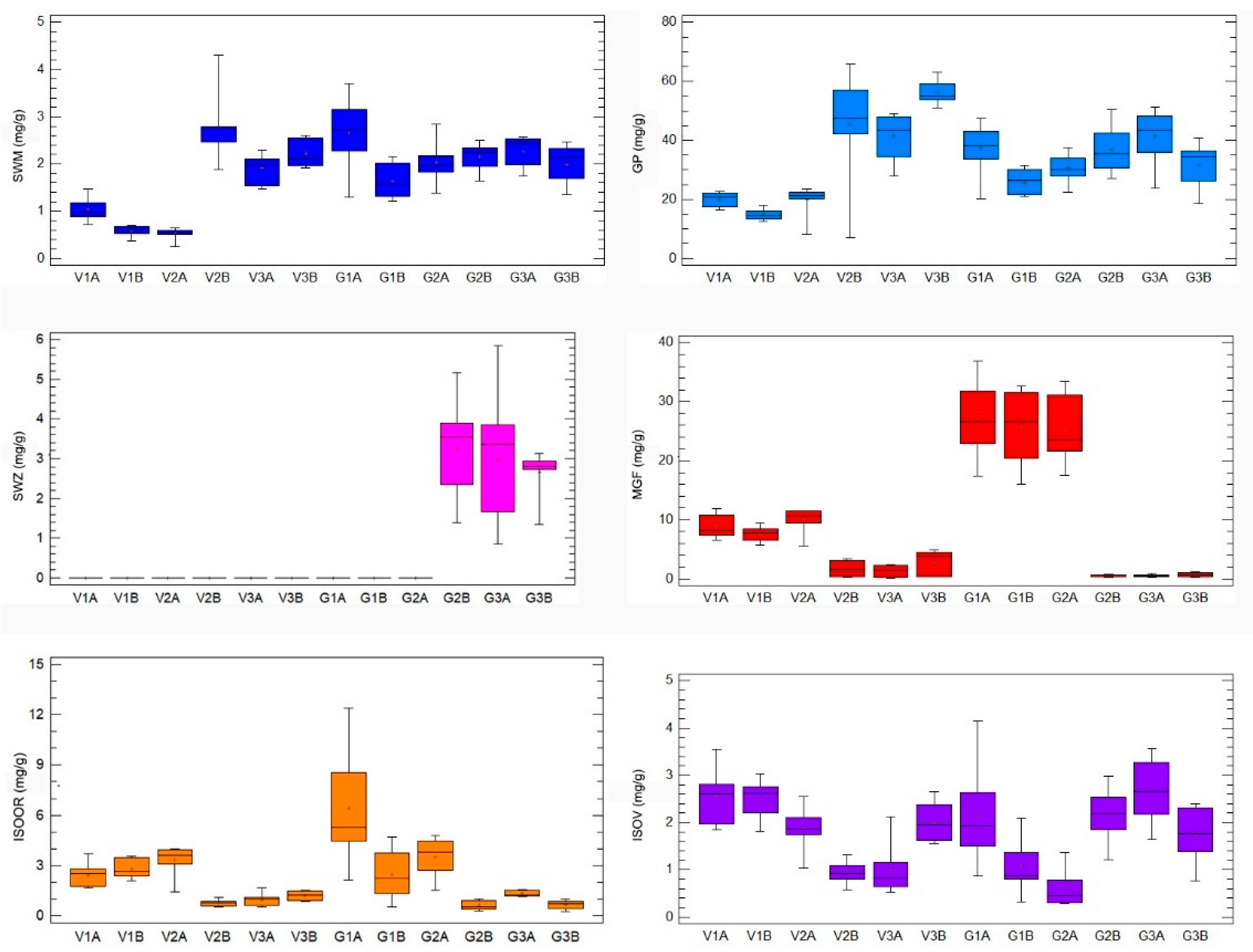
HPLC analysis of G. asclepiadea plants revealed the presence of six major compounds—secoiridoid glucosides swertiamarin (SWM), gentiopicrin (GP), and sweroside (SWZ); xanthone mangiferin (MGF); and two C-glucoflavones isoorientin (ISOOR) and isovitexin (ISOV). Aboveground and belowground plant parts contained varying contents of these compounds. Gentiopicrin was detected as the most abundant compound in all analyzed samples. Chromatograms of above- and belowground plant parts with identified peaks of secondary metabolites are presented in Figure 1 (Golija) and Figure 2 (Vlasina). The contents of six analyzed secondary metabolites (mg/g dry weight (dw)) in above- and belowground plant parts from Vlasina and Golija populations are shown in Figure 3.
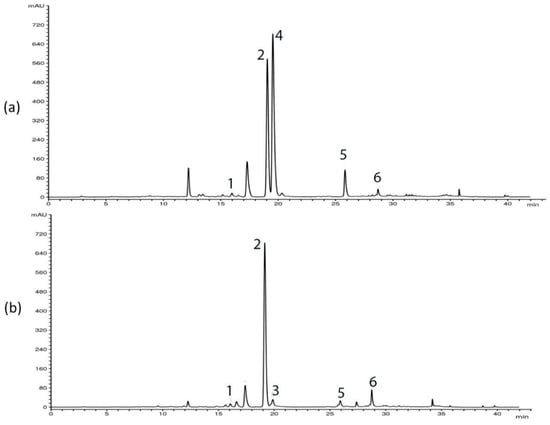
Figure 1.
HPLC profiles (λ = 260 nm) of Gentiana asclepiadea methanol extracts of plants collected from Golija: (a) aboveground parts; (b) belowground parts. Peaks: swertiamarin (1), gentiopicrin (2), sweroside (3), mangiferin (4), isoorientin (5), and isovitexin (6).
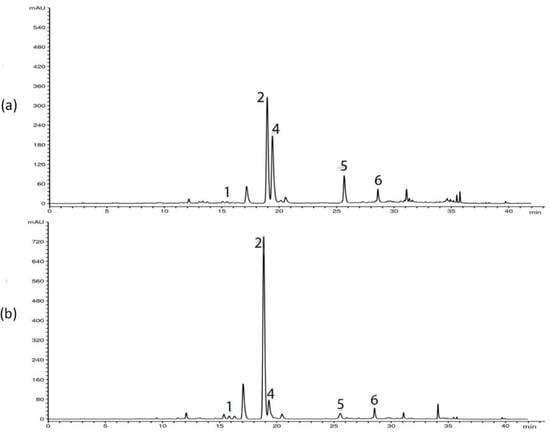
Figure 2.
HPLC profiles (λ = 260 nm) of Gentiana asclepiadea methanol extracts of plants collected from Vlasina: (a) aboveground parts; (b) belowground parts. Peaks: swertiamarin (1), gentiopicrin (2), mangiferin (4), isoorientin (5), and isovitexin (6).
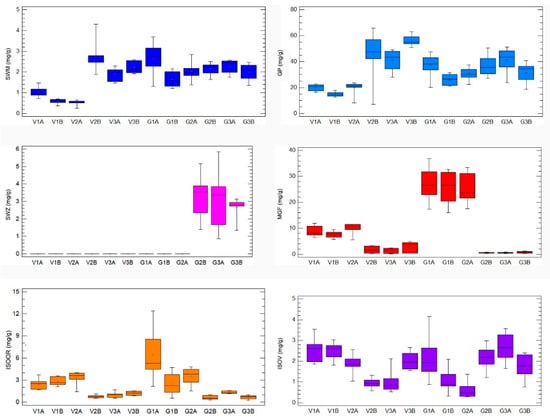
Figure 3.
The contents of six representative secondary metabolites (mg/g dry weight (dw)) in Gentiana asclepiadea by 12 groups of samples (original, nontransformed data): Vlasina aboveground plant parts (V1A, V2A, V3A), Vlasina belowground plant parts (V1B, V2B, V3B), Golija aboveground plant parts (G1A, G2A, G3A), Golija belowground plant parts (G1B, G2B, G3B). Boxplot major features (bottom to top): minimum value, box (interquartile range), and maximum value. Horizontal bar inside a box is median. Cross inside a box is mean value. Vertical bar is range.
The impacts of three factors (location, population, and plant part) on dependent variables (contents of secondary metabolites) are presented in Table 1. The applied model for nested ANOVA is highly significant for each analyzed property (p < 0.001; not shown). It is noticeable that for each property, there are statistically significant differences only between plant parts (above- and belowground). For half of the examined properties, there are differences between localities (Golija and Vlasina), while none of the properties show statistically significant differences between populations.

Table 1.
Results of nested ANOVA for the effects of locality (A), population (B), and plant part (C) on the content of six representative secondary metabolites in Gentiana asclepiadea.
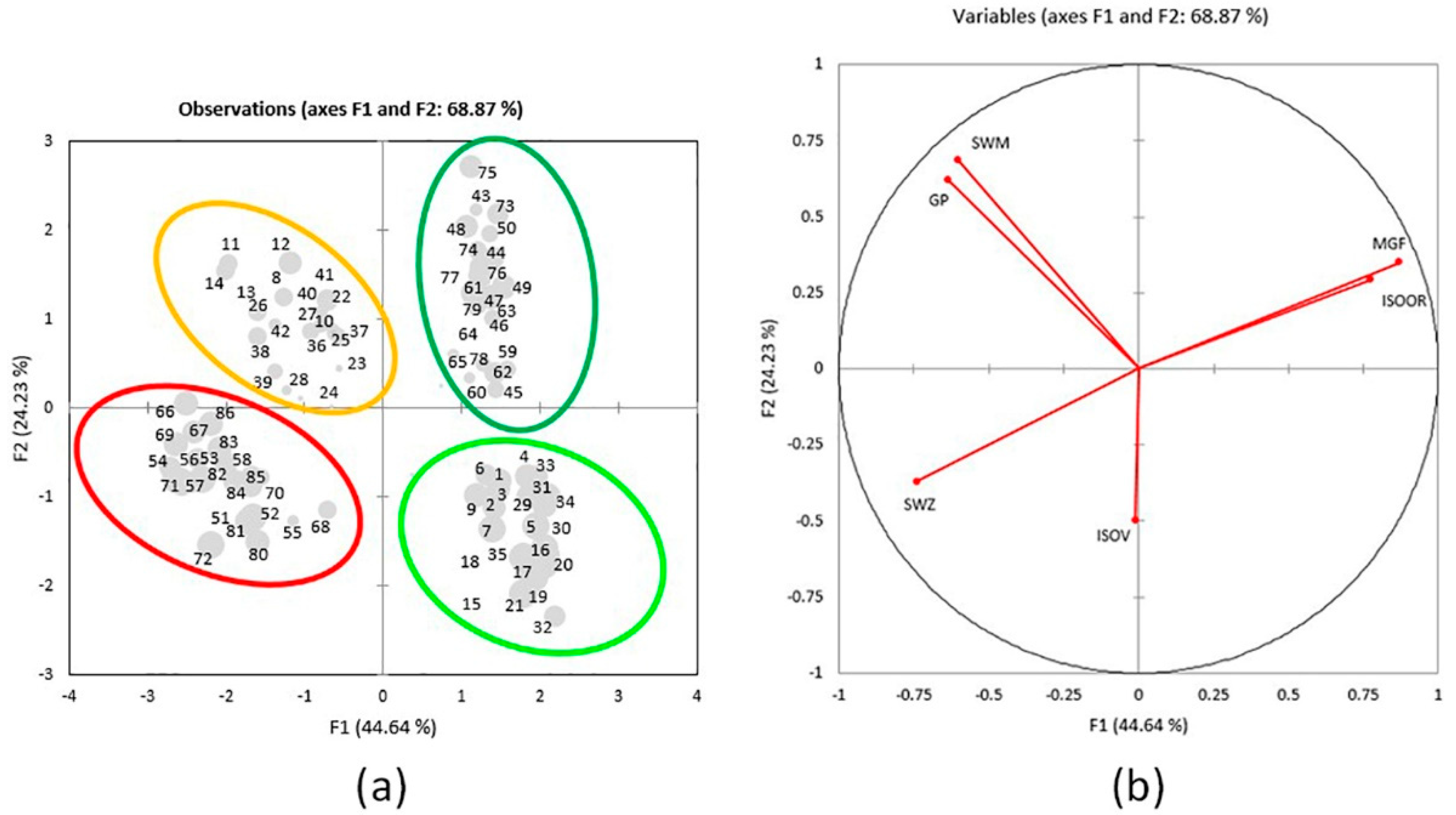
PCA separated 86 elements into 4 distinct assemblages (Figure 4a). The results show that the first two axes explain 68.87% of data variability (44.64% and 24.23%, respectively), and the information about well-compressed data was confirmed by their eigenvalues > 1. Based on the sum of squared correlations between the variables and factors in the PC 1–2 plane (sum of r2 > 0.87), it can be concluded that MGF, SWM, and GP are the best-represented properties and are likely to have the most important role in the total variability of the sample (Table 2). The formation of the first axis is mainly defined by the variability of MGF, ISOOR, SWZ, and GP, whereas the variability of SWM is the most important for the formation of the second axis. The structure of the properties and their relationships are presented in Figure 4b. The tendencies among the elements (i.e., their groupings (or separation)) are noticeable in the plane of the first two axes (Figure 4a). Well-represented elements, with the larger square cosine of the observations for the first two axes, are displayed by larger dots.
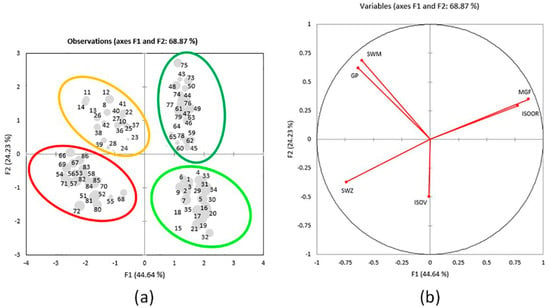
Figure 4.
(a) Score plot. Separation of 86 individuals in regard to interactions of parts of a plant and locality according to the contents of 6 variables in the plane of the first 2 axes. Larger-dot elements are better-represented elements, with the larger square cosine of the observations for the first two axes: 1–7 = V1A; 8–14 = V1B; 15–21 = V2A; 22–28 = V2B; 29–35 = V3A; 36–42 = V3B; 43–50 = G1A; 51–57 = G1B; 58–64 = G2A; 65–72 = G2B; 73–79 = G3A; 80–86 = G3B; (b) Loading plot. Structure of the six variables in the plane of the first 2 axes in 86 individuals.

Table 2.
Squared cosin es of the factor—correlations of variables.
The separation of 86 elements by different plant parts is clear, as the aboveground plant parts are grouped on the right part of the figure (Golija top right and Vlasina bottom right) and the belowground plant parts are grouped on the left part (Vlasina top left and Golija bottom left) (Figure 4a). The red ellipse encompasses elements that contain SWZ, and these are belowground parts from populations G1, G2, and G3 (G1B, G2B, and G3B). The yellow ellipse encompasses elements with the higher content of SWM and GP and these are belowground part populations V1, V2, and V3 (V1B, V2B, and V3B) (the only exception is element nine, which is poorly represented in the plane of the first two axes). A dark-green ellipse encompasses elements with the higher content of MGF and ISOOR and these are aboveground parts from populations G1, G2, and G3 (G1A, G2A, and G3A). A light-green ellipse encompasses elements with the lower content of SWM and GP and these are aboveground parts from populations V1, V2, and V3 (V1A, V2A, and V3A).
3. Discussion
Information on the chemical composition of essential oils and extracts obtained from G. asclepiadea has been provided in numerous publications, mostly aimed at evaluation of their biological activities [17]. The volatile profile of flowers and leaves was reported for the natural population of willow gentian [18], and Kozuharova et al. [19] suggested that specific secondary metabolites may have an important role against seed predators. However, information about locality-dependent differences and inter- and intrapopulation variability based on species’ phytochemistry has not been reported to date.
Five of six analyzed secondary metabolites were detected in all samples of G. asclepiadea, and only one (SWZ) which was found in Golija populations but not in Vlasina populations can be considered as a distinctive compound between the two geographic locations. However, this finding should be interpreted with a note that it relates to only one sampling period, and the occurrence of SWZ in earlier developmental stages of the Vlasina population should not be excluded. Although high ontogenetic variability in the content of secondary metabolites has been documented, the same gender-specific compounds were present in the preflowering, flowering, and postflowering stages in Gentiana pneumonanthe [20]. Considering that the full maturity phase at the time of flowering is the period of collection and harvesting of medicinal plants [21], the information regarding the Vlasina populations is important and deserves further research. Gentiopicrin was the most abundant compound in all samples (36.36 ± 7.53 mg/g and 26.98 ± 5.04 mg/g in aboveground parts of Golija and Vlasina populations, respectively; 31.28 ± 6.56 mg/g and 38.96 ± 8.22 mg/g in belowground parts of Golija and Vlasina populations, respectively). It was also a dominant component in belowground parts of the natural populations of G. asclepiadea from Hungary (varying from 45 to 68 mg/g depending on the sampling site), and SWM and SWZ were also found in all studied populations [22]. Gentiopicroside was the dominant iridoid in Caucasian populations of G. asclepiadea (about 90 mg/g in herbs and 65 mg/g in roots, respectively), SWM was detected at a lower level (about 1.5 mg/g in herbs and 6 mg/g in roots, respectively), and SWZ was only found in trace amounts in all plant parts [23]. The content of MGF in the aboveground plant parts found in the populations from Golija (17.62 ± 4.03 mg/g) was very similar to that previously reported for Caucasian populations (17.48 ± 0.33 mg/g), although it was lower in the populations from Vlasina (6.49 ± 1.04 mg/g). However, this xanthone compound was detected in belowground parts of the populations from Vlasina and Golija but not in the populations from Azerbaijan [23]. Flavonoids ISOOR and ISOV, which are predominantly abundant in aboveground plant parts of gentians, were detected in all plant parts of G. asclepiadea, comparable to a previous report [23].
Differences between individuals from different locations are also confirmed by the higher content of SWM and GP in belowground parts obtained from Vlasina individuals and the higher content of MGF and ISOOR in aboveground parts of Golija individuals. Besides specific local climatic and topoedaphic conditions at two localities, sampled populations were members of different forest stands (beech-dominated in Vlasina and spruce-dominated in Golija), and differences in local flora and the composition of plant communities may also affect the production of secondary metabolites. Comparative analyses related to phytochemical profiling of geographically distinct populations of numerous species revealed the need for more research regarding the impact of both genetic origin and a specific set of environmental conditions on the composition and content of secondary metabolites [24]. Several studies documented the chemical variability among natural populations in Gentiana species [20,25,26,27,28,29,30]. The study of high-altitude G. straminea showed significant interpopulation variability for several secondary metabolites, providing the basis for a geo-authentic production area for this traditional medicinal plant [28], and our results show that particular metabolites can even be absent in the chemical profile of some populations. Sweroside, a secoiridoid with anti-inflammatory and analgesic properties, has been used in the treatment against osteoporosis in traditional Chinese medicine and can be a promising therapeutic product for this medical issue [31]. Information regarding its abundance in certain geolocations can be valuable for further investigation regarding the plant–environment biochemical response and indicative of exploitation and production.
The most prominent differences in the presence and content of the six secondary metabolites were recorded between distinct plant parts, which is in accordance with the rich literature data on the distribution of main chemical compounds in gentians [2]. Secoiridoids are predominantly present in the belowground plant parts but can also be found in aboveground parts at a prominent level [32]. Isoorientin and isovitexin are the most frequently found C-glucoflavones in Gentiana sp., confirmed in all representatives of a genus [33,34]. Flavonoids are a large group of phenolic compounds with pronounced antioxidant and chelating effects, and their ultraviolet-absorbing properties are an important adaptive feature at higher altitudes [35]. The accumulation of flavonoids occurs in the preflowering period and then reaches the maximum value during flowering [36]. Iridoids and secoiridoid glycosides have a primarily defensive function against herbivores and pathogens and can be detected in different plant organs [32]. The high amount of these compounds in belowground organs, which is found in all ontogenetic stages for gentians [20,37], may imply a response to specific biotic pressures. However, more information regarding species autecology (i.e., photosynthesis, morphology, biotic interactions) would provide a better understanding of plant–environment relations among gentians [38,39].
For a large number of phytochemically investigated plants, specific secondary metabolites have been determined exclusively or predominantly in a particular organ, and most of the differences in the quantitative and/or qualitative composition of secondary metabolites are reported between the below- and aboveground plant parts [40]. Different chemical compositions of below- and aboveground plant parts arise from the different environmental pressures to which they are exposed. The secondary metabolites predominantly present in aboveground plant organs have multiple functions, as pollinator attractants (volatile attractants in flower petals) [41], for seed dispersal (esters in fruits) [42], toxic and repellent functions against herbivores and pathogens (phenolic glucosides, furanocoumarins, tannins in aerial parts, alkaloids in nectar) [43], and in protection against abiotic factors (flavonoids in epidermal tissues for UV protection) [35]. In belowground parts of the plant, the most important function of secondary metabolites (e.g., emodin) is a defense against herbivores that can seriously damage or destroy the root and consequently the whole plant [44], but their allelopathic and self-regulating functions are achieved as well (phenolic acids, flavanols, flavones, flavanones, anthocyanins) [45]. Regarding the pharmacological activities of a plant species, the “medicinal part” of a plant is usually indicated either in pharmacognostic literature or ethnopharmacobotanical sources [21].
Roots are recognized to be able to synthesize and accumulate a diversity of secondary metabolites in response to environmental stressors [46], and significant variability between the root and shoot defensive chemicals may be caused by habitat conditions [47,48]. In reviewing the root/shoot differences regarding the secondary metabolites, Rasmann and Agrawal [49] pointed that (1) compounds present in aboveground parts are most often present in roots; (2) considering their surface area and exposition to belowground herbivores, roots are pronouncedly chemically defended; and (3) even though differential allocation to aboveground and belowground parts is not completely clarified, it depends on the plant family, species, genotype, and ontogenetic stage of tissues.
4. Materials and Methods
4.1. Study Sites
The Vlasina Plateau is located in southeastern Serbia (42°43′55″ N, 22°19′18″ E) at an altitude of over 1200 m, surrounded by the mountains of Rhodope massif: Bukova glava, Čemernik, Plana, and Vardenik. Mountains around the plateau are very rich in springs that form streams and smaller rivers, and along with watercourses from the plateau, they flow into the Vlasina Lake located in the center of the plateau. Edaphic factors are determined by topography (plateau and surrounding mountains) and soils (peat and district brown forest soils). The climate is humid and submountainous, with cool summers and very cold winters. Lake Vlasina and the surrounding area are classified in the first protection category—Outstanding Natural Landscape “Vlasina”—due to the exceptional richness of flora and fauna, both freshwater and terrestrial (under state protection since 2006). The vegetation of the area is a unique mosaic of meadows, pastures, and high-altitude forests (birch, beech, pine, and juniper).
Golija Mountain is part of the Dinaric mountain range, situated in southwestern Serbia (43°20′16″ N, 20°16′36″ E), with the highest peak Jankov kamen at 1833 m. Climatic patterns in this area are moderate continental; in higher zones (over 1300 m), the climate is mountainous with severe winters with high snow cover and short summers. Overall edaphic, hydrological, and climatic conditions and the refugial character of many habitats lead to a great diversity of flora and fauna, which is the reason for a high degree of protection within the Golija–Studenica Biosphere Reserve, the first UNESCO-MAB registered biosphere reserve in Serbia (2001). Golija is the most forested Serbian mountain, with the largest, best-preserved, and highest-quality forest complexes, with primeval forest patches. Deciduous and deciduous–coniferous old-grown forests (beech, oak, beech–fir, and beech–spruce) predominate along the elevational gradient, and above 1700 m, only spruce is represented.
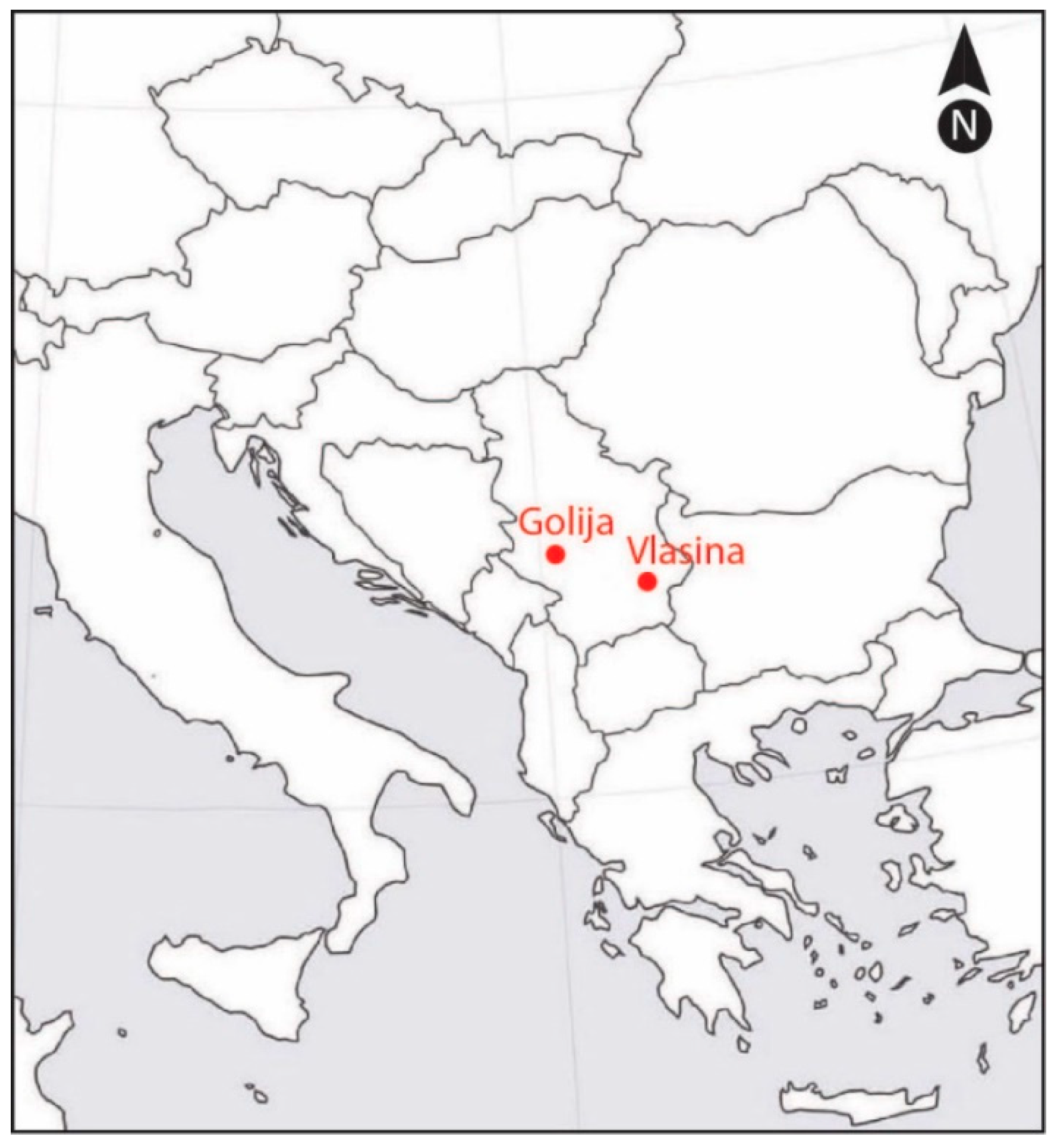
The positions of the Vlasina and Golija study sites are shown in Figure 5. Geographical coordinates of the studied populations and climate characteristics of locations are given in Table 3.
Figure 5.
The positions of the Vlasina and Golija study sites.

Table 3.
Geographic locations and climate characteristics of studied populations.
4.2. Plant Material and Sample Preparation
At both study sites—Vlasina (V) and Golija (G)—individuals from three distinct populations of G. asclepiadea were sampled during the flowering period (August 2019). All specimens were healthy individuals without any visible damage caused by herbivores or pathogens. Populations were positioned at the edges of forest communities dominated by beech, beech–birch (Vlasina), or spruce and beech–spruce (Golija), with the distance between them of about several kilometers. From one population, seven to eight individuals were sampled; from each individual, a mixed sample of three stems was considered as the aboveground part (A), and the common rhizome as the belowground part (B). Above- and belowground parts were divided after air-drying of whole plants and analyzed separately. The total number of samples was 2 (locations) × 3 (populations) × 7–8 (individuals) × 2 (plant parts) = 86. Representative samples are in the herbarium at the Institute for Biological Research, Belgrade (voucher codes V1, V2, V3, G1, G2, G3).
4.3. Chemical Analysis
4.3.1. Reagents and Chemicals
Standard compounds mangiferin, isoorientin, and isovitexin were purchased from Sigma-Aldrich (Steinheim, Germany). Swertiamarin, gentiopicrin, and sweroside were purchased from Cfm Oscar Tropitzsch (Marktredwitz, Germany).
4.3.2. Extraction
Each sample of G. asclepiadea was divided into aboveground and belowground plant parts (A—stems, leaves, and reproductive parts, B—roots and rhizomes) and analyzed separately. Air-dried samples were ground to a fine powder (250 mg) and extracted with 5 mL of methanol in an ultrasonic bath for 20 min. After sonication, extraction was continued by maceration for 48 h in the dark at room temperature. The extracts were filtered into 5 mL volumetric flasks, adjusted to the volume with methanol, and stored at 4 °C until use for HPLC analysis.
4.3.3. HPLC Analysis
Chromatographic analysis of the secondary metabolites was carried out on an Agilent series 1100 HPLC instrument (Agilent Technologies, Waldronn, Germany) with a DAD, on a reverse-phase Zorbax SB C-18 (Agilent, Newport, Delaware, USA) analytical column (250 × 4.6 mm, 5 µm particle size) thermostatted at 25 °C. Prior to HPLC analysis, the extracts were filtered through nylon syringe filters (Captiva syringe filters, 0.45 µm, 13 mm, Agilent Technologies). The mobile phase consisted of solvent A (1%, v/v solution of orthophosphoric acid in water) and solvent B (acetonitrile, J.T. Baker, Deventer, the Netherlands), using gradient elution previously published by Popović et al. [20]. Briefly, samples of belowground and aboveground plant parts were separated as follows: 98–90% A 0–5 min, 90–85% A 5–17 min, 85% A 17–20 min, 85–70% A 20–30 min, 70–0% A 30–39 min, and 0% A 39–42 min. The flow rate was 1 mL/min. The injection volume of samples was 5 µL; the detection wavelengths were set at 260 and 320 nm. The quantification of secondary metabolites was done using the external standard method by preparing calibration standards ranging from 0.01 to 0.5 mg/mL and recording the calibration curves at 260 nm for secoiridoids and flavonoids, and at 320 nm for xanthone mangiferin. The results are presented as milligrams per gram of dry weight (dw).
4.4. Statistical Analysis
A total of 516 numerical data points related to G. asclepiadea were analyzed: two mountainous sites (V—Vlasina and G—Golija) × three populations (V1, V2, V3; G1, G2, G3) × two plant parts (A—aboveground and B—belowground) × seven (eight) elements (from seven to eight plants per population) × six secondary metabolites (SWM, GP, MGF, SWZ, ISOOR, and ISOV). The choice of statistical tests was made based on preliminary verification of conditions for the application of the majority of the parametric tests: normal distribution (chi-square test, p ≥ 0.05) and the equality of variances (Levene’s and Batlett’s tests, p > 0.05). After the transformation (y’ = log10 (y + 1), y = original data value), the data fulfilled the abovementioned conditions. To determine differences between the mean values of localities (fixed factor), populations, and plant parts (random factors), we used nested ANOVA, a very common analysis in ecological studies (levels of random factors are similar but not identical to each other). Principal component analysis (PCA) was carried out with log-transformed data in order to determine the trends, structure, and relationships of properties and grouping of individuals. Visualizing the groups of samples with boxplots enhanced our understanding of the original (nontransformed) data and helped us to make comparisons across groups. Statgraphics Plus (version 5.0; Statistical Graphics Corporation, The Plains, VA, USA), Statistica (version 10, Stat. Soft. Inc., Tulsa, OK, USA, 2011), and Addinsoft XLSTAT software (free trial version) were used.
5. Conclusions
The phytochemical analysis of G. asclepiadea based on six representative secondary metabolites showed differences in chemical profiles among populations from different mountainous sites. Due to its absence in Vlasina populations, SWZ was the most prominently indicative of the chemical fingerprint of the plants related to the geolocation. Further research is warranted to clarify the occurrence of this compound in earlier developmental stages of Vlasina populations. The contents of other studied compounds (SWM, GP, MF, ISOOR, and ISOV) significantly differed concerning the study site. Different accumulations of analyzed secondary metabolites between above- and belowground plant parts mostly contributed to the separation of groups by PCA. This study confirms the organ-specific distribution of specific secondary metabolites, which is probably directed by different environmental pressures to above- and belowground plant parts. Moreover, it points to the indicative value of the studied compounds for the phytochemical profiling of populations from specific locations.
Author Contributions
Conceptualization, Z.P. and S.B.; methodology, Z.P., S.B., and D.K.-M.; validation, Z.P. and S.B.; formal analysis, S.B.; investigation, D.K.-M., M.M., and V.V.; data curation, S.B.; writing—original draft preparation, Z.P.; visualization, V.V.; supervision, Z.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia under contract 451-03-68/2020-14/200007.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the authors.
Acknowledgments
Authors thank the management of Landscape of Outstanding Features “Vlasina” for fieldwork support. We also thank the three anonymous reviewers for their suggestions and comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zając, A.; Pindel, A. Review of the Willow Gentian, Gentiana asclepiadea L. Biodiversity 2011, 12, 181–185. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.-L.; Zhang, J.; Li, W.-Y.; Wang, Y.-Z. Phytochemistry and Pharmacological Activities of the Genus Gentiana (Gentianaceae). Chem. Biodivers. 2016, 13, 107–150. [Google Scholar] [CrossRef]
- Josifović, M. Flora SR Srbije; Srpska Akademija Nauka i Umetnosti: Belgrade, Serbia, 1973; Volume 5. [Google Scholar]
- Sarić, M.R. Lekovite Biljke SR Srbije; Srpska Akademija Nauka i Umetnosti: Belgrade, Serbia, 1989. [Google Scholar]
- Gairola, S.; Shariff, N.M.; Bhatt, A.; Chandra Prakash, K. Influence of climate change on production of secondary chemicals in high altitude medicinal plants: Issues needs immediate attention. J. Med. Plants Res. 2010, 4, 1825–1829. [Google Scholar] [CrossRef]
- Körner, C.; Jetz, W.; Paulsen, J.; Payne, D.; Rudmann-Maurer, K.; Spehn, E.M. A global inventory of mountains for bio-geographical applications. Alp. Bot. 2017, 127, 1–15. [Google Scholar] [CrossRef]
- Nagy, L.; Grabherr, G.; Körner, C.; Thompson, D.B.A. Alpine Biodiversity in Europe; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2003; Volume 167. [Google Scholar] [CrossRef]
- Teramura, A.H.; Sullivan, J.H. Effects of UV-B radiation on photosynthesis and growth of terrestrial plants. Photosynth. Res. 1994, 39, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant. Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Sheahan, J.J. Sinapate esters provide greater UV-B attenuation than flavonoids in Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 1996, 83, 679–686. [Google Scholar] [CrossRef]
- Paul, N. Plant responses to UV-B: Time to look beyond stratospheric ozone depletion? New Phytol. 2001, 150, 5–8. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant. Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Dean, J.C.; Kusaka, R.; Walsh, P.S.; Allais, F.; Zwier, T.S. Plant Sunscreens in the UV-B: Ultraviolet Spectroscopy of Jet-Cooled Sinapoyl Malate, Sinapic Acid, and Sinapate Ester Derivatives. J. Am. Chem. Soc. 2014, 136, 14780–14795. [Google Scholar] [CrossRef]
- Duan, Y.-W.; Zhang, T.-F.; Liu, J.-Q. Interannual fluctuations in floral longevity, pollinator visitation and pollination limitation of an alpine plant (Gentiana straminea Maxim., Gentianaceae) at two altitudes in the Qinghai-Tibetan Plateau. Plant. Syst. Evol. 2007, 267, 255–265. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. 2005, 80, 489–513. [Google Scholar] [CrossRef] [PubMed]
- Bortenschlager, S. The Iceman’s environment. In The Iceman and His Natural Environment: Palaeobotanical Results; Bortenschlager, S., Oeggl, K., Eds.; The Man in the Ice; Springer: Vienna, Austria, 2000; pp. 11–24. [Google Scholar] [CrossRef]
- Yang, J.-L.; Liu, L.-L.; Shi, Y.-P. Phytochemicals and biological activities of Gentiana species. Nat. Prod. Commun. 2010, 5, 1934578X1000500432. [Google Scholar] [CrossRef]
- Georgieva, E.; Handjieva, N.; Popov, S.; Evstatieva, L. Comparative analysis of the volatiles from flowers and leaves of three Gentiana species. Biochem. Syst. Ecol. 2005, 33, 938–947. [Google Scholar] [CrossRef]
- Kozuharova, E.; Lapeva-Gjonova, A.; Shishiniova, M. Plant–insect interactions: Gentians, seed predators and parasitoid wasps. Arthropod-Plant. Interact. 2018, 12, 453–463. [Google Scholar] [CrossRef]
- Popović, Z.; Milošević, D.K.; Stefanović, M.; Vidaković, V.; Matić, R.; Janković, J.; Bojović, S. Variability of six secondary metabolites in plant parts and developmental stages in natural populations of rare Gentiana pneumonanthe. Plant. Biosyst. Int. J. Deal. Asp. Plant. Biol. 2020. [Google Scholar] [CrossRef]
- Popović, Z.; Matić, R.; Bojović, S.; Stefanović, M.; Vidaković, V. Ethnobotany and herbal medicine in modern complementary and alternative medicine: An overview of publications in the field of I&C medicine 2001–2013. J. Ethnopharmacol. 2016, 181, 182–192. [Google Scholar] [CrossRef]
- Szucs, Z.; Dános, B.; Nyiredy, S. Comparative analysis of the underground parts of Gentiana species by HPLC with diode-array and mass spectrometric detection. Chromatographia 2002, 56, S19–S23. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Gadimli, A.I.; Isaev, J.I.; Kashchenko, N.I.; Prokopyev, A.S.; Kataeva, T.N.; Chirikova, N.K.; Vennos, C. Caucasian Gentiana Species: Untargeted LC-MS Metabolic Profiling, Antioxidant and Digestive Enzyme Inhibiting Activity of Six Plants. Metabolites 2019, 9, 271. [Google Scholar] [CrossRef]
- Popović, Z.; Matić, R.; Stefanović, M.; Vidaković, V.; Bojović, S. Chemodiversity in natural plant populations as a base for biodiversity conservation. In Biodiversity and Biomedicine; Ozturk, M., Egamberdieva, D., Pešić, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 11–41. [Google Scholar] [CrossRef]
- Zhou, D.; Hou, Q.; Si, Q.; Liu, J.; Yang, H. Concentrations of the Active Constituents of the Tibetan Folk Medicine Qinjiao (Gentiana sect. Cruciata) within and between Taxonomic Species across the Qinghai-Tibetan Plateau. Chem. Biodivers. 2010, 7, 2088–2094. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Wang, Z.-J.; Wang, Z.-Z. Comparative analysis of contents of four iridoid glucosides in different organs of four species of Gentiana L. J. Plant. Resour. Environ. 2012, 21, 58–63. [Google Scholar]
- Aiello, N.; Bontempo, R.; Vender, C. Use of morphological features and amarogentin content for characterization of wild yellow gentian (Gentiana lutea L.) populations in north-east Italy. Acta Bot. Gallica 2013, 160, 33–41. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Chen, S.; Hu, F.; Zhou, D. Spatial variation profiling of four phytochemical constituents in Gentiana straminea (Gentianaceae). J. Nat. Med. 2014, 68, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Popović, Z.; Krstić-Milošević, D.; Stefanović, M.; Matić, R.; Vidaković, V.; Bojović, S. Chemical and Morphological Inter- and Intrapopulation Variability in Natural Populations of Gentiana pneumonanthe L. Chem. Biodivers. 2019, 16, e1800509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, T.; Wu, L.; Zhang, J.; Zuo, Z.; Wang, Y. Identification of Gentiana rigescens from different geographical origins based on HPLC and FTIR fingerprints. Anal. Methods 2020, 12, 2260–2271. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, J.; Wang, Y.; Zhang, N.; Gober, H.-J.; Qiu, X.; Li, D.; Wang, L. Chinese single herbs and active ingredients for postmenopausal osteoporosis: From preclinical evidence to action mechanism. Biosci. Trends 2017, 11, 496–506. [Google Scholar] [CrossRef]
- Bowers, M.D. Iridoid Glycosides. In Herbivores: Their Interactions with Secondary Plant Metabolites, 2nd ed.; Rosenthal, G.A., Berenbaum, M.R., Eds.; Academic Press: San Diego, CA, USA, 1991; Volume 1, pp. 297–325. [Google Scholar] [CrossRef]
- Hostettmann, K.; Jacot-Guillarmod, A. Xanthones et C-glucosides flavoniques du genre Gentiana (section Cyclostigma). Phytochemistry 1977, 16, 481–482. [Google Scholar] [CrossRef]
- Rybczyński, J.J.; Davey, M.R.; Mikula, A. The Gentianaceae—Volume 2: Biotechnology and Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Schmitz-Hoerner, R.; Weissenböck, G. Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry 2003, 64, 243–255. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Maffucci, K.G.; Huang, L.; Zeng, R. Analytical Methods of Phytochemicals from the Genus Gentiana. Molecules 2017, 22, 2080. [Google Scholar] [CrossRef]
- Menković, N.; Šavikin-Fodulović, K.; Momcilović, I.; Grubišić, D. Quantitative Determination of Secoiridoid and γ-Pyrone Compounds in Gentiana lutea Cultured in vitro. Planta Med. 2000, 66, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T. Diversity and variability of plant secondary metabolism: A mechanistic view. Entomol. Exp. Appl. 1996, 80, 177–188. [Google Scholar] [CrossRef]
- Kolosova, N.; Sherman, D.; Karlson, D.; Dudareva, N. Cellular and Subcellular Localization of S-Adenosyl-L-Methionine:Benzoic Acid Carboxyl Methyltransferase, the Enzyme Responsible for Biosynthesis of the Volatile Ester Methylbenzoate in Snapdragon Flowers. Plant. Physiol. 2001, 126, 956–964. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant. Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Ibanez, S.; Gallet, C.; Després, L. Plant Insecticidal Toxins in Ecological Networks. Toxins 2012, 4, 228–243. [Google Scholar] [CrossRef]
- Izhaki, I. Emodin—A secondary metabolite with multiple ecological functions in higher plants. New Phytol. 2002, 155, 205–217. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant. Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Flores, H.E.; Vivanco, J.M.; Loyola-Vargas, V.M. ‘Radicle’ biochemistry: The biology of root-specific metabolism. Trends Plant. Sci. 1999, 4, 220–226. [Google Scholar] [CrossRef]
- Wainhouse, D.; Ashburner, R.; Ward, E.; Rose, J. The effect of variation in light and nitrogen on growth and defence in young Sitka Spruce. Funct. Ecol. 1998, 12, 561–572. [Google Scholar] [CrossRef]
- Mattson, W.J.; Julkunen-Tiitto, R.; Herms, D.A. CO2 enrichment and carbon partitioning to phenolics: Do plant responses accord better with the protein competition or the growth differentiation balance models? Oikos 2005, 111, 337–347. [Google Scholar] [CrossRef]
- Rasmann, S.; Agrawal, A.A. In Defense of Roots: A Research Agenda for Studying Plant Resistance to Belowground Herbivory. Plant Physiol. 2008, 146, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).