Flaxseed and Camelina Meals as Potential Sources of Health-Beneficial Compounds
Abstract
:1. Introduction
2. Results
2.1. Seed Yield and Qualitative Characteristics
2.2. Phytochemical Screening and Anti-Radical Activity of Seed Meals
2.3. LC–PDA/UV–ESI–MS Profiles
2.3.1. Lignan Content of Flaxseed Meal
2.3.2. Glucosinolate and Phenol Contents of Camelina Meal
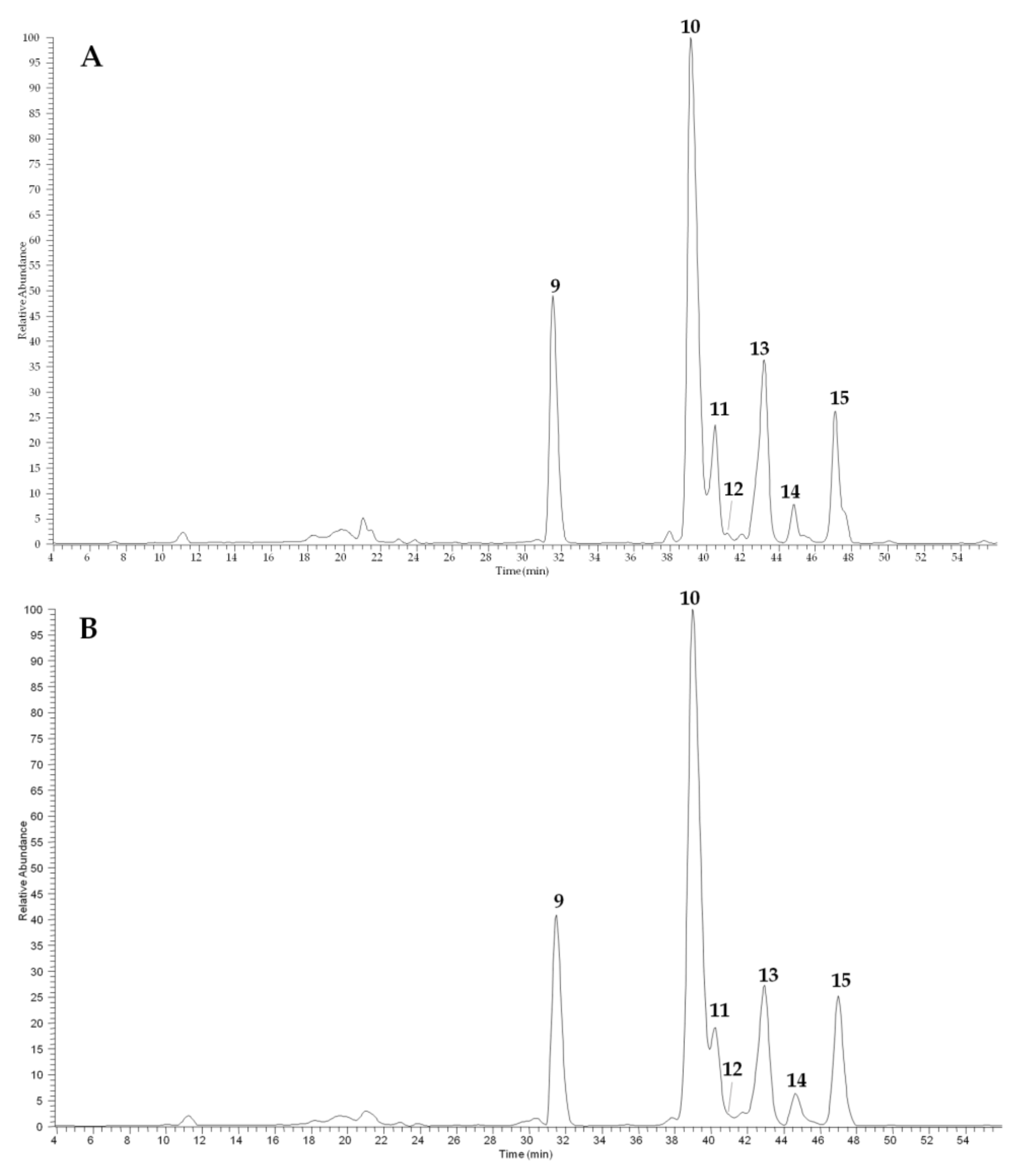
3. Discussion
4. Materials and Methods
4.1. Reagents and Standards
4.2. Experimental Conditions and Plant Material
4.3. Agronomic Evaluations
4.4. Seed Processing and Analysis
4.5. Extraction of Bioactive Compounds
4.6. Analysis of Total Phenols and Flavonoids
4.7. HPLC–PDA/UV–ESI–MS/MS Analyses of Camelina and Flaxseed Meal Extracts
4.7.1. Alkaline Hydrolysis of Flaxseed Oligomers
4.7.2. HPLC–UV–MS Analyses
4.8. Free Radical-Scavenging Assay
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ancuța, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes-A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of chemical composition of two linseed varieties as sources of health-beneficial substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crop. Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Tavarini, S.; Angelini, L.G.; Casadei, N.; Spugnoli, P.; Lazzeri, L. Agronomical evaluation and chemical characterization of Linum usitatissimum L. as oilseed crop for bio-based products in two environments of Central and Northern Italy. Ital. J. Agron. 2016, 11, 735. [Google Scholar] [CrossRef] [Green Version]
- Angelini, L.G.; Abou Chehade, L.; Foschi, L.; Tavarini, S. Performance and potentiality of camelina (Camelina sativa L. Crantz) genotypes in response to sowing date under Mediterranean environment. Agronomy 2020, 10, 1929. [Google Scholar] [CrossRef]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed cake as a tool for the improvement of nutraceutical and sensorial features of sourdough bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Adolphe, J.L.; Whiting, S.J.; Juurlink, B.H.J.; Thorpe, L.U.; Alcorn, J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br. J. Nutr. 2010, 103, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Zubr, J.; Matthäus, B. Effects of growth conditions on fatty acids and tocopherols in Camelina sativa oil. Ind. Crop. Prod. 2002, 15, 155–162. [Google Scholar] [CrossRef]
- Pagnotta, E.; Ugolini, L.; Matteo, R.; Lazzeri, L.; Foschi, L.; Angelini, L.G.; Tavarini, S. Exploring the Camelina sativa value chain: A new opportunity for bio-based products and overall crop sustainability. Riv. Ital. Sost. Grasse 2019, XCVI, 259–268. [Google Scholar]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B.; Angelini, L.G. Anti-nutritive constituents in oilseed crops from Italy. Ind. Crop. Prod. 2005, 21, 89–99. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Rubilar, M.; Jara, C.; Verdugo, M.; Sineiro, J.; Shene, C. Flaxseed and flaxseed cake as a source of compounds for food industry. J. Soil Sci. Plant Nutr. 2010, 10, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Strandås, C.; Kamal-Eldin, A.; Andersson, R.; Åman, P. Composition and properties of flaxseed phenolic oligomers. Food Chem. 2008, 110, 106–112. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.P.; Xu, S.P.; Wang, J.H.; Liu, X. Separation and determination of secoisolariciresinol diglucoside oligomers and their hydrolysates in the flaxseed extract by high-performance liquid chromatography. J. Chromatogr. A 2008, 1185, 223–232. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Barthet, V.J. Characterization of a partially purified extract from flax (Linum usitatissimum L.) seed. J. Am. Oil Chem. Soc. 2015, 92, 1183–1194. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.P.; Verhoef, R.; van Oostveen-van Casteren, W.H.; Voragen, A.G.; Gruppen, H. The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls. Phytochemistry 2007, 68, 1227–1235. [Google Scholar] [CrossRef]
- Das, N.; Berhow, M.A.; Angelino, D.; Jeffery, E.H. Camelina sativa defatted seed meal contains both alkyl sulfinyl glucosinolates and quercetin that synergize bioactivity. J. Agr. Food Chem. 2014, 62, 8385–8391. [Google Scholar] [CrossRef]
- Quéro, A.; Molinié, R.; Mathiron, D.; Thiombiano, B.; Fontaine, J.-X.; Brancourt, D.; Van Wuytswinkel, O.; Petit, E.; Demailly, H.; Mongelard, G.; et al. Metabolite profiling of developing Camelina sativa seeds. Metabolomics 2016, 12, 186. [Google Scholar] [CrossRef]
- Lafond, G.P.; Irvine, B.; Johnston, A.M.; May, W.E.; McAndrew, D.W.; Shirtlie, S.J.; Stevenson, F.C. Impact of agronomic factors on seed yield formation and quality in flax. Can. J. Plant Sci. 2008, 88, 485–500. [Google Scholar] [CrossRef]
- Berti, M.; Wilckens, R.; Fischer, S.; Solis, A.; Johnson, B. Seeding date influence on camelina seed yield, yield components, and oil content in Chile. Ind. Crops Prod. 2011, 34, 1358–1365. [Google Scholar] [CrossRef]
- Fila, G.; Bagatta, M.; Maestrini, C.; Potenza, E.; Matteo, R. Linseed as a dual-purpose crop: Evaluation of cultivar suitability and analysis of yield determinants. J. Agr. Sci. 2018, 156, 162–176. [Google Scholar] [CrossRef]
- Daun, J.; Barthet, V.; Chornick, T.; Duguid, S. Structure, composition, and variety development of flaxseed. In Flaxseed in Human Nutrition, 2nd ed.; Thompson, L., Cunanne, S., Eds.; AOCS Press: Champaign, IL, USA, 2003; pp. 1–40. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, M.; Singh Gill, B. Phenolic acid composition of flaxseed cultivars by ultra-performance liquid chromatography (UPLC) and their antioxidant activities: Effect of sand roasting and microwave heating. J. Food Process. Preserv. 2017, 41, e13181. [Google Scholar] [CrossRef]
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Klein, J.; Zikeli, S.; Claupein, W.; Gruber, S. Linseed (Linum usitatissimum) as an oil crop in organic farming: Abiotic impacts on seed ingredients and yield. Org. Agr. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Silska, G.; Walkowiak, M. Comparative analysis of fatty acid composition in 84 accessions of flax (Linum usitatissimum L.). J. Pre-Clin. Clin. Res. 2019, 13, 118–129. [Google Scholar] [CrossRef]
- Klimek-Kopyra, A.; Zając, T.; Micek, P.; Borowiec, F. Effect of mineral fertilization and sowing rate on chemical composition of two linseed cultivars. J. Agric. Sci. 2013, 5, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Vollmann, J.; Moritz, T.; Kargl, C.; Baumgartner, S.; Wagentristl, H. Agronomic evaluation of camelina genotypes selected for seed quality characteristics. Ind. Crops Prod. 2007, 26, 270–277. [Google Scholar] [CrossRef]
- Righini, D.; Zanetti, F.; Monti, A. The bio-based economy can serve as the springboard for camelina and crambe to quit the limbo. OCL 2016, 23, D504. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, F.; Eynck, C.; Christou, M.; Krzyżaniak, M.; Righini, D.; Alexopoulou, E.; Stolarski, M.; Van Loo, E.; Puttick, D.; Monti, A. Agronomic performance and seed quality attributes of camelina (Camelina sativa L. Crantz) in multi-environment trials across Europe and Canada. Ind. Crops Prod. 2017, 107, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Teh, S.-S.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem. 2014, 21, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, J.R.; Côrrea, A.P.F.; Michel, I.; Brandeli, A.; Tessaro, I.C.; Marczak, L.D.F. Evaluation of the phenolic content and antioxidant activity of different seed and nut cakes from the edible oil industry. J. Am. Oil Chem. Soc. 2014, 91, 1773–1782. [Google Scholar] [CrossRef]
- Rahman, M.J.; Costa de Camargo, A.; Shahidi, F. Phenolic profiles and antioxidant activity of defatted camelina and Sophia seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Terpinc, P.; Polak, T.; Makuc, D.; Ulrih, N.P.; Abramovič, H. The occurrence and characterisation of phenolic compounds in Camelina sativa seed, cake and oil. Food Chem. 2012, 131, 580–589. [Google Scholar] [CrossRef]
- Tangney, C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Aziza, A.E.; Quezada, N.; Cherian, G. Antioxidative effect of dietary camelina meal in fresh, stored, or cooked broiler chicken meat. Poultry Sci. 2010, 89, 2711–2718. [Google Scholar] [CrossRef]
- Beltranena, E.; Oryschak, M. Camelina sativa Coproducts as Feedstuffs for Poultry. In Proceedings of the Western Poultry Conference, AB, Alberta, Canada, 29 February 2016. [Google Scholar]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: A review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Berhow, M.A.; Polat, U.; Glinski, J.A.; Glensk, M.; Vaughn, S.F.; Isbell, T.; Ayala-Diaz, I.; Marek, L.; Gardner, C. Optimized analysis and quantification of glucosinolates from Camelina sativa seeds by reverse-phase liquid chromatography. Ind. Crops Prod. 2013, 43, 119–125. [Google Scholar] [CrossRef]
- Russo, R.; Reggiani, R. Glucosinolates and sinapine in camelina meal. Food Nutr. Sci. 2017, 8, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Piragine, E.; Flori, L.; Di Cesare Mannelli, L.; Ghelardini, C.; Pagnotta, E.; Matteo, R.; Lazzeri, L.; Martelli, A.; Miragliotta, V.; Pirone, A.; et al. Eruca sativa Mill. seed extract promotes anti-obesity and hypoglycemic effects in mice fed with a high-fat diet. Phytother. Res. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Pagnotta, E.; Micheli, L.; Parisio, C.; Testai, L.; Martelli, A.; Calderone, V.; Matteo, R.; Lazzeri, L.; Di Cesare, M.L.; et al. Eruca sativa meal against diabetic neuropathic pain: An H2S-mediated effect of glucoerucin. Molecules 2019, 24, 3006. [Google Scholar] [CrossRef] [Green Version]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Lazzeri, L.; Malaguti, L.; Cinti, S.; Ugolini, L.; De Nicola, G.R.; Bagatta, M.; Casadei, N.; D’Avino, L.; Matteo, R.; Patalano, G. The Brassicaceae biofumigation system for plant cultivation and defence. An Italian twenty-year experience of study and application. Acta Hortic. 2013, 1005, 375–382. [Google Scholar] [CrossRef]
- Conte, L.S.; Leoni, O.; Palmieri, S.; Capella, P.; Lercker, G. Half-seed analysis: Rapid chromatographic determination of the main fatty acids of sunflower seed. Plant Breed. 1998, 102, 158–165. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Jia, Z.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-Świgło, A.; Barthet, V.; Skręty, J. Effect of extraction method on the phenolic and cyanogenic glucoside profile of flaxseed extracts and their antioxidant capacity. J. Am. Oil Chem. Soc. 2015, 92, 1609–1619. [Google Scholar] [CrossRef]
- Felice, F.; Fabiano, A.; De Leo, M.; Piras, A.M.; Beconcini, D.; Cesare, M.M.; Braca, A.; Zambito, Y.; Di Stefano, R. Antioxidant effect of cocoa by-product and cherry polyphenol extracts: A comparative study. Antioxidants 2020, 9, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]




| Crop/Variety | Total Phenolic Content (mg GAE/gDW) | Total Flavonoids (mg CAE/gDW) | ||||
|---|---|---|---|---|---|---|
| Pisa | Bologna | Mean Variety | Pisa | Bologna | Mean Variety | |
| Flaxseed Sideral | 2.57 ± 0.28 | 2.74 ± 0.39 | 2.66 | 1.27 ± 0.05 | 1.36 ± 0.19 | 1.32 |
| Flaxseed B.Aires | 2.86 ± 0.15 | 2.74 ± 0.17 | 2.80 | 1.16 ± 0.23 | 1.23 ± 0.16 | 1.20 |
| Mean Site | 2.71 | 2.74 | 1.22 | 1.30 | ||
| Significance | Variety (V) = n.s. | Variety (V) = n.s. | ||||
| Site (S) = n.s. | Site (S) = n.s. | |||||
| VxS = n.s. | VxS = n.s. | |||||
| Camelina Italia | 7.00 ± 0.18 a | 6.26 ± 0.28 b | 6.63 | 6.15 ± 0.79 | 5.48 ± 0.45 | 5.82 |
| Significance | Site (S) = ** | Site (S) = n.s. | ||||
| Crop/Variety | EC50 DPPH (mg mL−1) | EC50 ABTS (mg mL−1) | ||||
|---|---|---|---|---|---|---|
| Pisa | Bologna | Mean Variety | Pisa | Bologna | Mean Variety | |
| Flaxseed Sideral | 3.60 ± 0.20 | 4.30 ± 0.30 | 3.95 A | 3.10 ± 0.30 | 2.80 ± 0.20 | 2.95 |
| Flaxseed B.Aires | 3.10 ± 0.20 | 3.80 ± 0.20 | 3.45 B | 3.00 ± 0.30 | 3.20 ± 0.30 | 3.10 |
| Mean Site | 3.35 B | 4.05 A | 3.05 | 3.00 | ||
| Significance | Variety (V) = ** | Variety (V) = n.s. | ||||
| Site (S) = *** | Site (S) = n.s. | |||||
| VxS = n.s. | VxS = n.s. | |||||
| Camelina Italia | 1.50 ± 0.10 b | 1.90 ± 0.10 a | 1.70 | 2.10 ± 0.30 b | 2.90 ± 0.30 a | 2.50 |
| Significance | Site (S) = ** | Site (S) = ** | ||||
| Trolox | 0.052 ± 0.001 | 0.046 ± 0.001 | ||||
| BHA | 0.031 ± 0.001 | 0.029 ± 0.001 | ||||
| Peak a | Compound | tR (min) | [M − H]− | [M + CH3COO]− | [M + HCOO]− | MS/MS Ions (m/z) b | λmax (nm) |
|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||
| 1/2 | p-Coumaric acid glucoside | 10.3, 11.2 | 325 | 385 | 371 | 325, 163 | 228, 295 |
| 3/4 | Ferulic acid glucoside | 12.1, 13.0 | 355 | 415 | 401 | 355, 193 | 235, 290 |
| 8 | Ferulic acid | 21.2 | 193 | 253 | 239 | 178, 149, 134 | 237, 323 |
| Flavonoids | |||||||
| 5 | Herbacetin diglucoside | 16.2 | 625 | ─ | ─ | 463, 301 | 235, 284, 323 |
| Lignans | |||||||
| 6–7 | Secoisolariciresinol diglucoside (SDG) | 17.1, 17.6 | 685 | 745 | 731 | 685, 583 | 232, 280 |
| Peak a | Compound | tR (min) | [M − H]− | MS/MS ions (m/z) | λmax (nm) |
|---|---|---|---|---|---|
| Glucosinolates | |||||
| 9 | Glucoarabin (9-(methylsulfinyl)nonylglucosinolate) | 31.4 | 506 | 491, 442, 248 | 240 |
| 10 | Glucocamelinin (10-(methylsulfinyl)decylglucosinolate) | 38.9 | 520 | 505, 456, 262 | 239 |
| 15 | 11-(methylsulfinyl)undecylglucosinolate | 47.1 | 534 | 519, 470 | 256 |
| Flavonol glycosides | |||||
| 11 | Quercetin 2”-O-apiosyl-3-O-rutinoside | 40.2 | 741 | 609, 300, 301 | 256, 354 |
| 12 | Quercetin O-apiosyl-glucoside | 40.9 | 595 | 463, 300, 301 | 258, 350 |
| 13 | Quercetin 3-O-rutinoside (rutin) | 42.9 | 609 | 463, 301 | 257, 356 |
| Other compound | |||||
| 14 | Synapoil derivative | 44.6 | 623 | 417, 399, 209 | 249, 328 |
| Peak n. (in Figure 4) | Pisa | Bologna | |
|---|---|---|---|
| Glucosinolates | |||
| Glucoarabin | 9 | 3.4 ± 0.04 | 3.9 ± 0.08 |
| Glucocamelinin | 10 | 9.3 ± 0.4 | 12.3 ± 0.2 |
| 11-(methylsulfinyl)undecylglucosinolate | 15 | 1.8 ± 0.06 | 2.4 ± 0.04 |
| Total | 14.5 ± 0.5 | 18.6 ± 0.3 | |
| Flavonol glycosides | |||
| Quercetin 2”-O-apiosyl-3-O-rutinoside | 11 | 1.8 ± 0.03 | 1.7 ± 0.02 |
| Quercetin apiosyl-glucoside | 12 | 0.14 ± 0.004 | 0.21 ± 0.005 |
| Quercetin 3-O-rutinoside | 13 | 2.9 ± 0.05 | 2.6 ± 0.04 |
| Total | 4.8 ± 0.08 | 4.5 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavarini, S.; De Leo, M.; Matteo, R.; Lazzeri, L.; Braca, A.; Angelini, L.G. Flaxseed and Camelina Meals as Potential Sources of Health-Beneficial Compounds. Plants 2021, 10, 156. https://doi.org/10.3390/plants10010156
Tavarini S, De Leo M, Matteo R, Lazzeri L, Braca A, Angelini LG. Flaxseed and Camelina Meals as Potential Sources of Health-Beneficial Compounds. Plants. 2021; 10(1):156. https://doi.org/10.3390/plants10010156
Chicago/Turabian StyleTavarini, Silvia, Marinella De Leo, Roberto Matteo, Luca Lazzeri, Alessandra Braca, and Luciana G. Angelini. 2021. "Flaxseed and Camelina Meals as Potential Sources of Health-Beneficial Compounds" Plants 10, no. 1: 156. https://doi.org/10.3390/plants10010156
APA StyleTavarini, S., De Leo, M., Matteo, R., Lazzeri, L., Braca, A., & Angelini, L. G. (2021). Flaxseed and Camelina Meals as Potential Sources of Health-Beneficial Compounds. Plants, 10(1), 156. https://doi.org/10.3390/plants10010156







