Evaluation of the Impact of Cold Atmospheric Pressure Plasma on Soybean Seed Germination
Abstract
1. Introduction
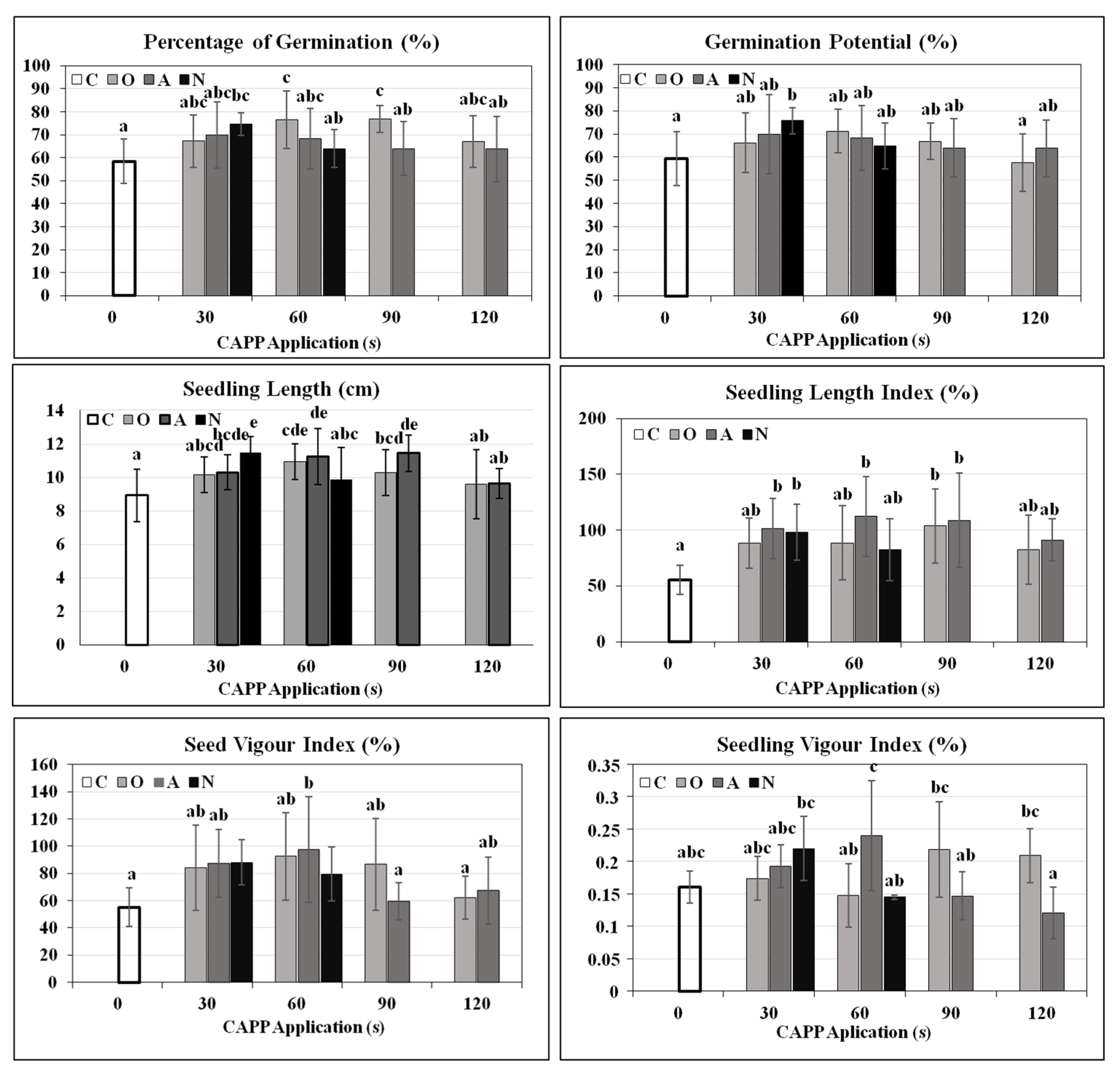
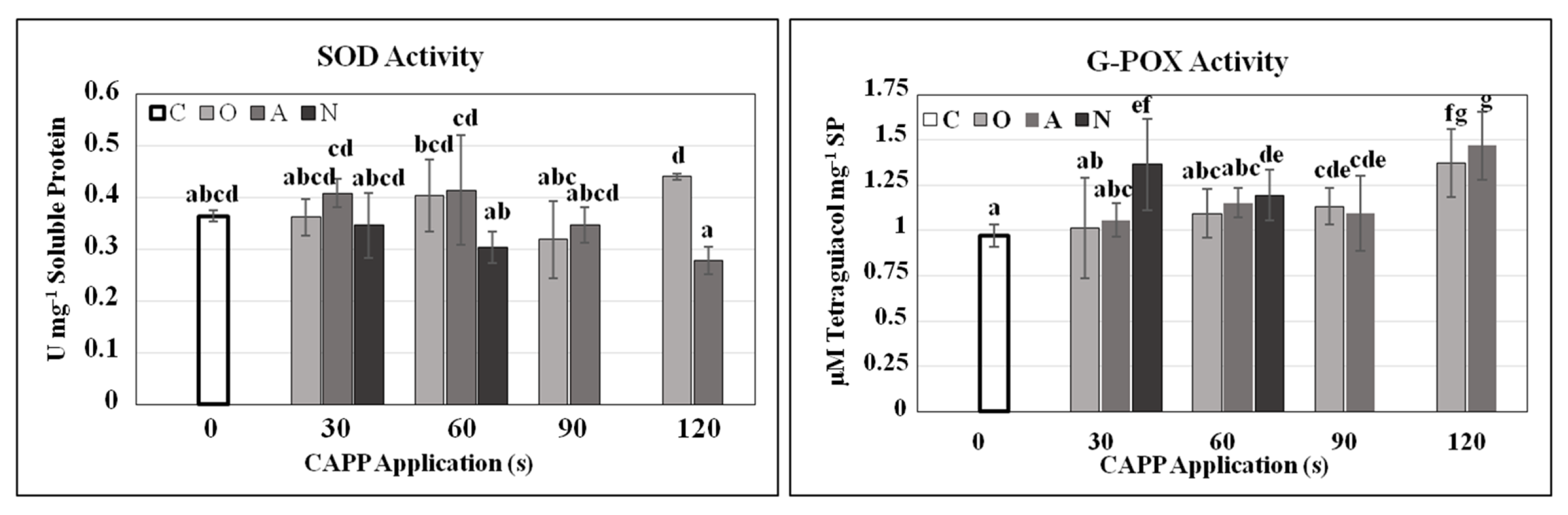
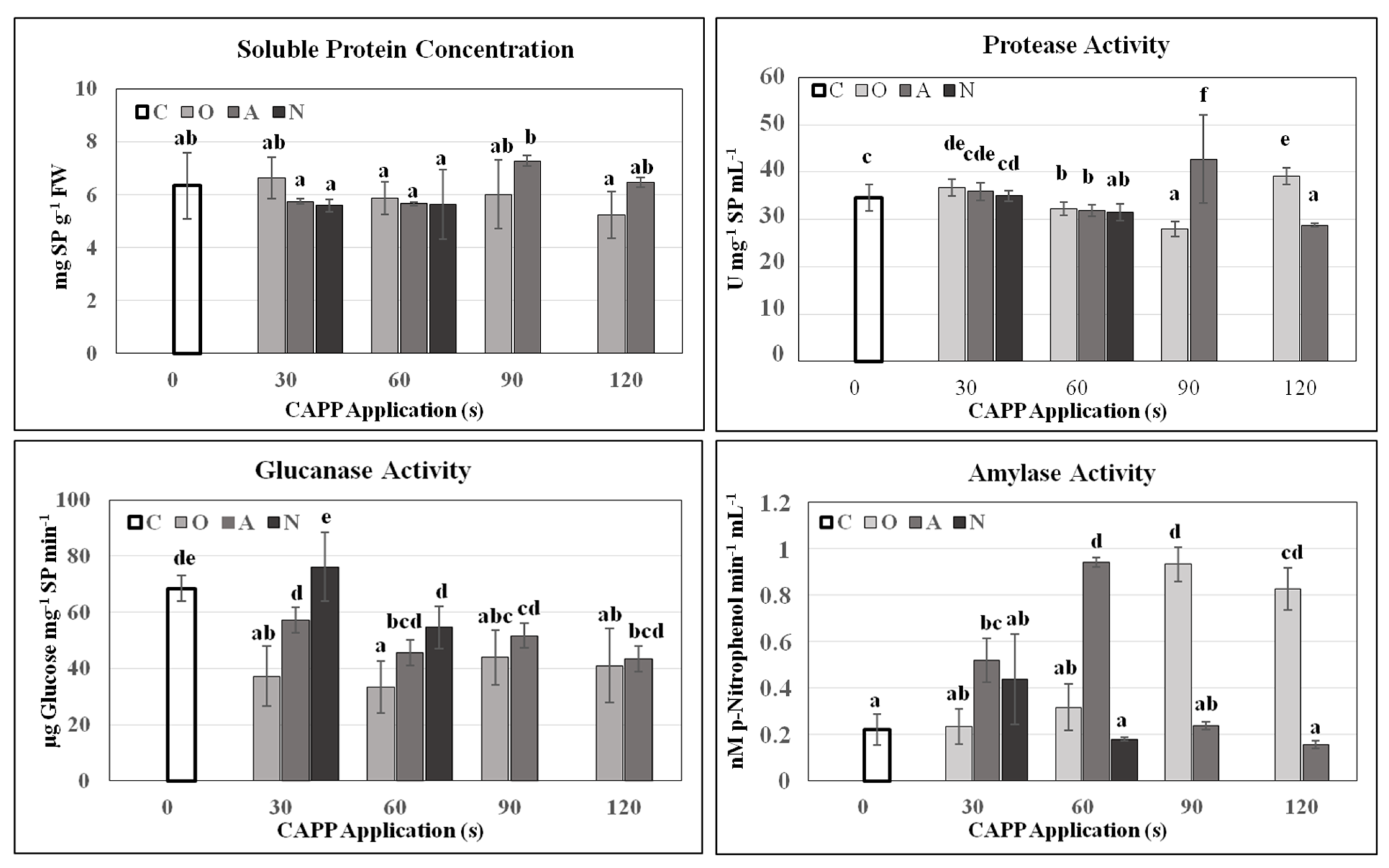
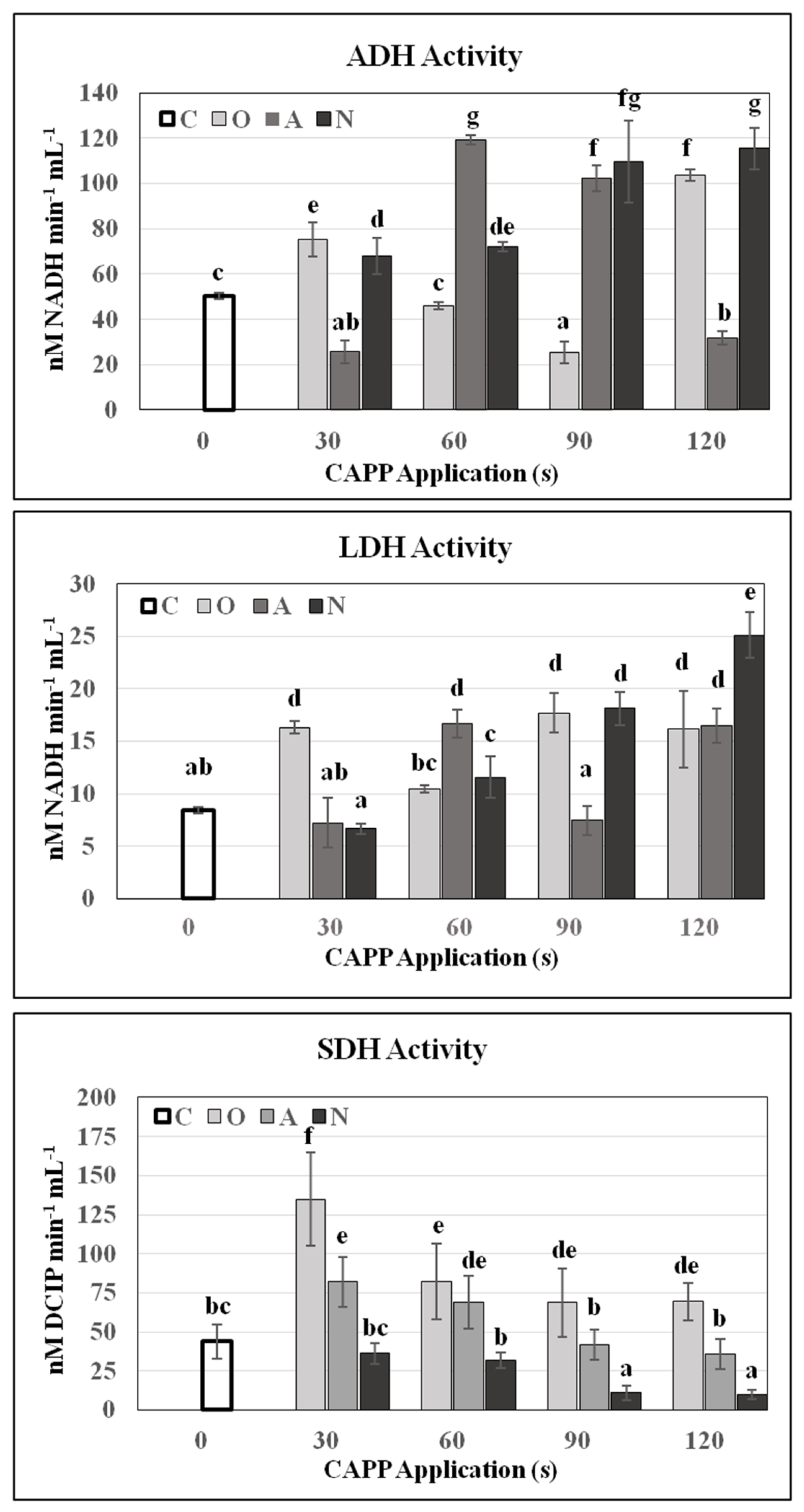
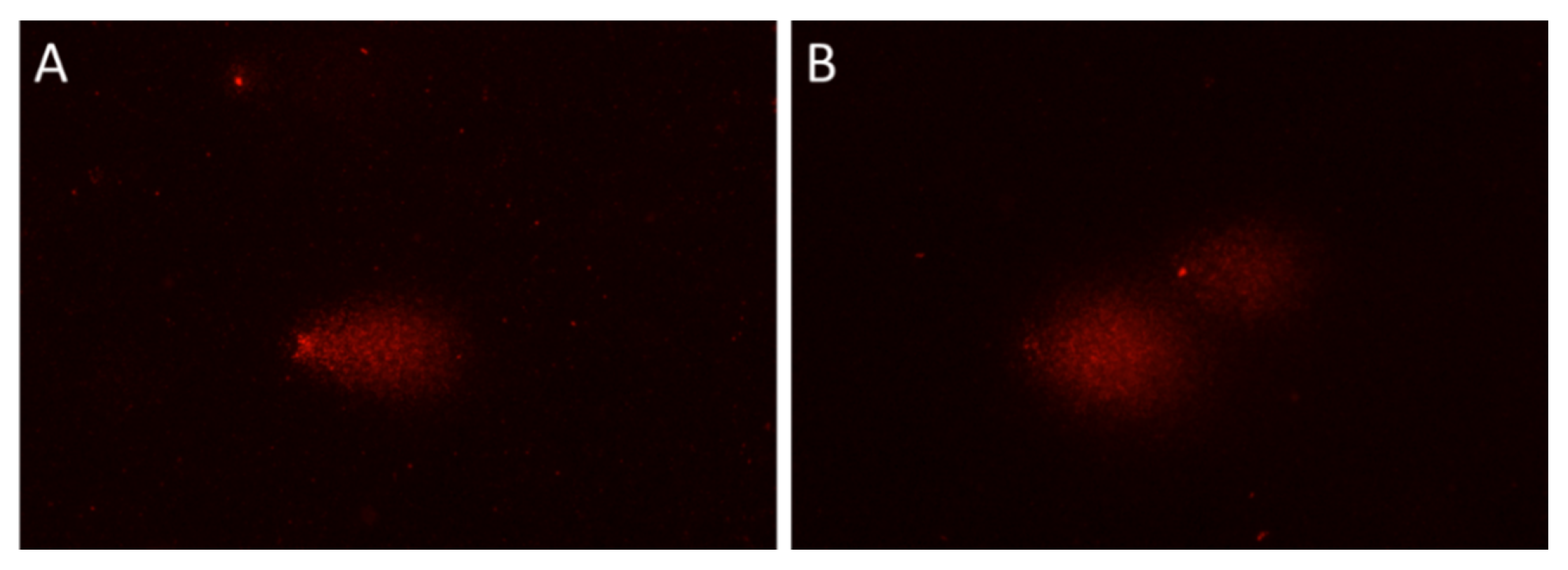
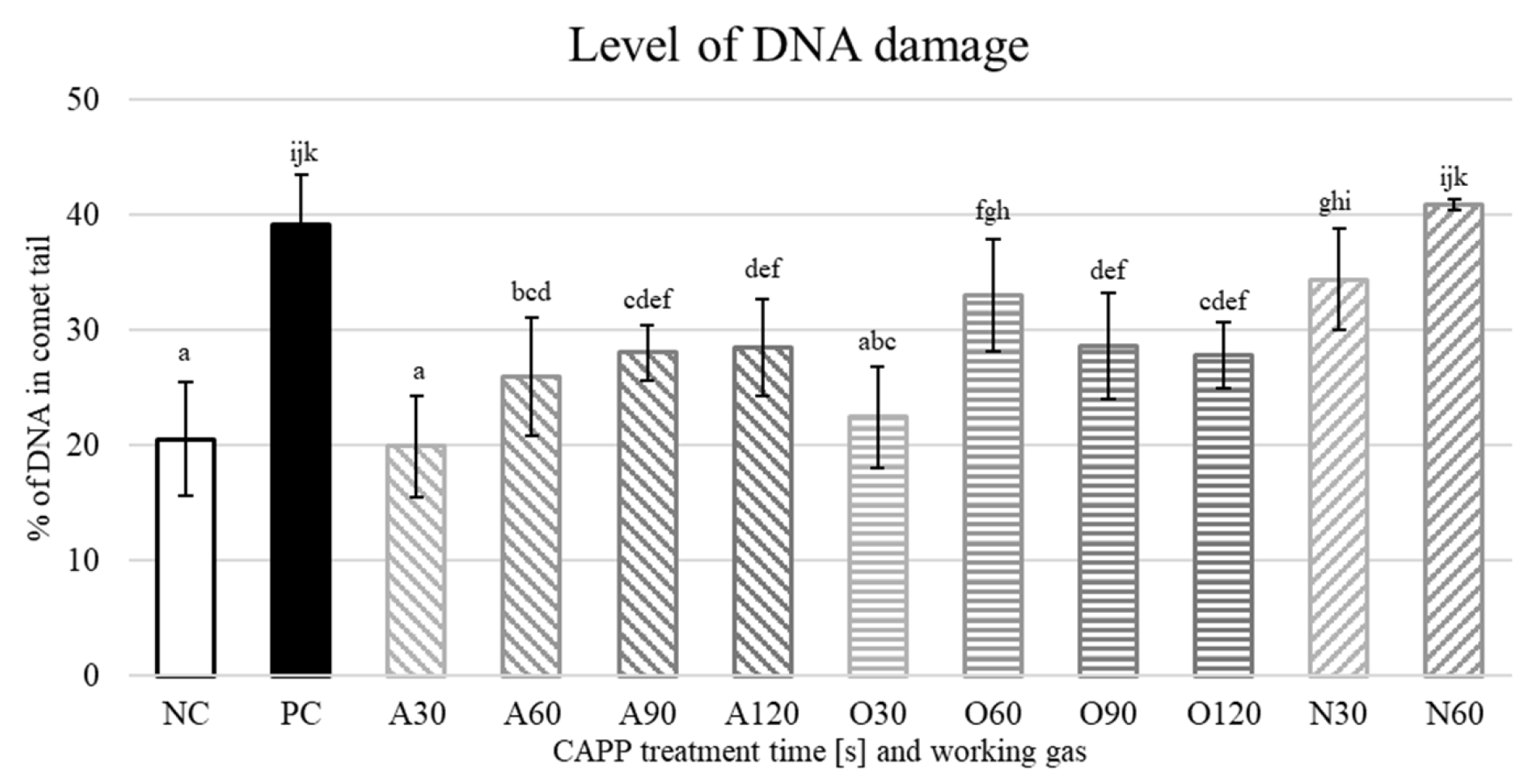
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
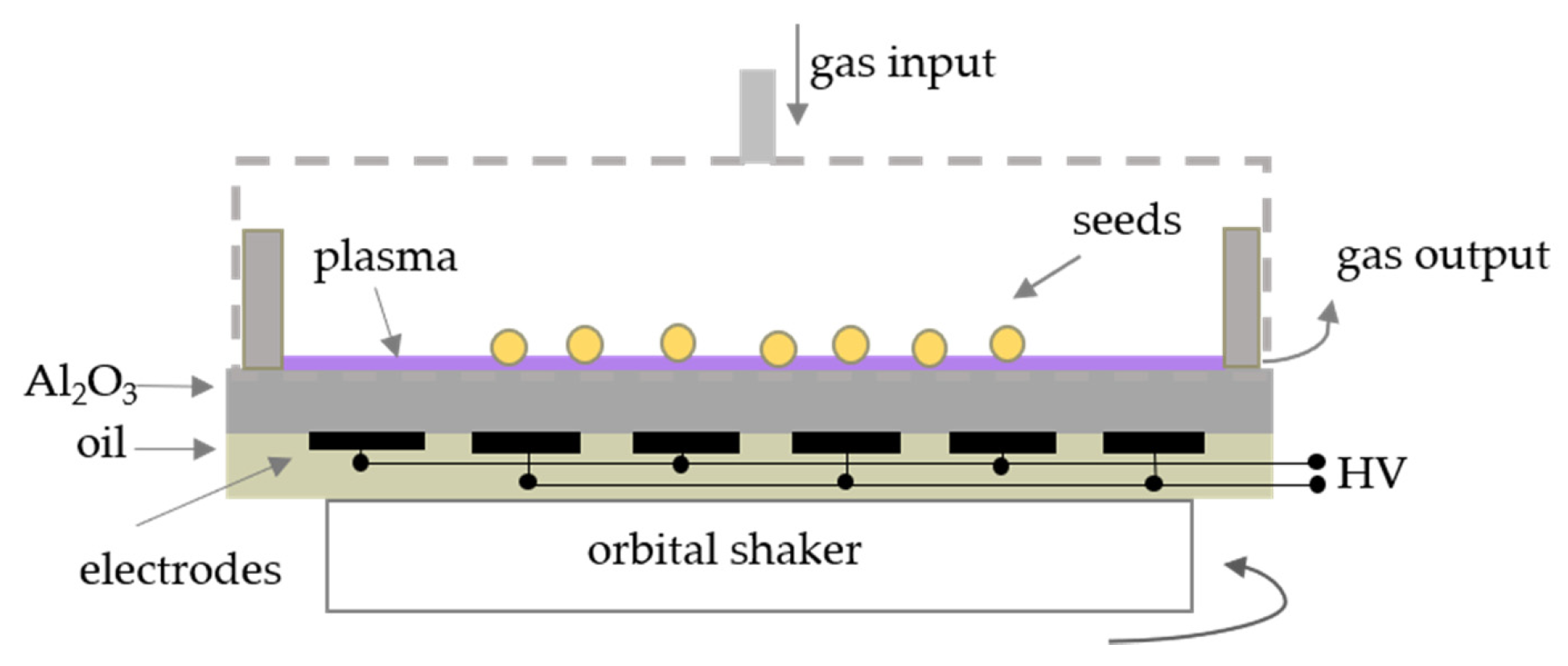
3.2. Plasma Source and Treatment of Soybean Seeds
3.3. Imbibition, Germination and Growth Conditions
3.4. Total Soluble Protein Content
3.5. Assay on Lytic Enzymes Assessment
3.6. Assay on Antioxidant Enzymes, Guaiacol Peroxidase (POX, E.C.1.11.1.7) and Superoxide Dismutase (SOD, E.C.1.15.1.1) Activities Assessment, and Visualization of ROS (H2O2 and ˙O2−)
3.7. Assay on Dehydrogenase Activity Evaluation
3.8. Comet Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Randeniya, L.; De Groot, G.J.J.B. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A Review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponse, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma agriculture: Review from the perspective of the plant and its ecosystem. Plasma Process. Polym. 2020, 18, e2000162. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Yousfi, M.; Merbahi, N.; Sarrette, P.; Eichwald, O.; Ricard, A.; Gardou, J.P.; Ducasse, O.; Benhenni, M. Non Thermal Plasma Sources of Production of Active Species for Biomedical Uses: Analyses, Optimization and Prospect. In Biomedical Engineering—Frontiers and Challenges; Intech Open: London, UK, 2011; pp. 99–124. [Google Scholar] [CrossRef]
- Tomeková, J.; Kyzek, S.; Medvecká, V.; Gálová, E.; Zahoranová, A. Influence of cold atmospheric pressure plasma on pea seeds: DNA damage of seedlings and optical diagnostics of plasma. Plasma Chem. Plasma Proces. 2020, 40, 1571–1584. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem. Plasma Proces. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Basaran, P.; Basaran-Akgulb, N.; Oksuz, L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol. 2008, 25, 626–632. [Google Scholar] [CrossRef]
- Mošovská, S.; Medvecká, V.; Gregová, M.; Tomeková, J.; Valík, Ľ.; Mikulajová, A.; Zahoranová, A. Plasma inactivation of Aspergillus flavus on hazelnut surface in a diffuse barrier discharge using different working gases. Food Control 2019, 104, 256–261. [Google Scholar] [CrossRef]
- Šerá, B.; Zahoranová, A.; Bujdáková, H.; Šerý, M. Disinfection from pine seeds contaminated with Fusarium circinatum Nirenberg & O’Donnell using non-thermal plasma treatment. Romanian Rep. Phys. 2019, 71, 701. [Google Scholar]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 4, 741–748. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Wannicke, N.; Horn, S.; Weltmann, K.-D.; Brust, H. A coaxial dielectric barrier discharge reactor for treatment of winter wheat seeds. Appl. Sci. 2020, 10, 7133. [Google Scholar] [CrossRef]
- Sadhu, S.; Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on the enzymatic activity in germinating mung beans (Vigna radiata). LWT-Food Sci. Technol. 2017, 78, 97–104. [Google Scholar] [CrossRef]
- Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, Ľ.; Gálová, E.; Zahoranová, A. Novel insight at the effect of cold atmospheric pressure plasma on the activity of enzymes essential for the germination of pea (Pisum sativum L. cv. Prophet) seeds. Plasma Chem. Plasma Proces. 2020, 40, 1221–1240. [Google Scholar] [CrossRef]
- Frączek, J.; Hebda, T.; Ślipek, Z.; Kurpaska, S. Effect of seed coat thickness on seed hardness. Can. Biosyst. Eng. 2005, 47, 4.1–4.5. [Google Scholar]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9738–9743. [Google Scholar] [CrossRef]
- Kyzek, S.; Holubová, Ľ.; Medvecká, V.; Zahoranová, A.; Ševčovičová, A.; Gálová, E. Genotoxic effect of low temperature plasma treatment on plant seeds. J. Toxicol. Lett. 2017, 280, S119. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signalling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Henselová, M.; Slováková, Ľ.; Martinka, M.; Zahoranová, A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 2012, 67, 490–497. [Google Scholar] [CrossRef]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L.). Plasma Chem. Plasma Proces. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Pauzaite, G.; Naucienė, Z.; Zukiene, R.; Degutyte-Fomins, L.; Pukalskas, A.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V. Seed treatment with cold plasma and electromagnetic field induces changes in red clover root growth dynamics, flavonoid exudation, and activates nodulation. Plasma Proces. Polym. 2020, 18, e2000160. [Google Scholar] [CrossRef]
- Kyzek, S.; Holubová, Ľ.; Medvecká, V.; Tomeková, J.; Gálová, E.; Zahoranová, A. Cold atmospheric pressure plasma can induce adaptive response in pea seeds. J. Plasma Chem. Plasma Proces. 2019, 39, 475–486. [Google Scholar] [CrossRef]
- Šerá, B.; Gajdová, I.; Černák, M.; Gavril, B.; Hnatiuc, E.; Kováčik, D.; Kříha, V.; Sláma, J.; Šerý, M.; Špatenka, P. How various plasma sources may affect seed germination and growth. In Proceedings of the 13th International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Brasov, Romania, 24–26 May 2012. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Šerá, B.; Špatenka, P.; Šerý, M.; Vrchotová, N.; Hrušková, I. Influnce of plasma treatment on wheat and oat germination and early growth. IEE Transact. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Efects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci. Technol. 2014, 16, 260–266. [Google Scholar] [CrossRef]
- Ling, L.; Jiafeng, J.; Jiangang, L.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Jha, M.; Tuteja, N. DNA damage and repair in plants under ultraviolet and ionizing radiations. Sci. World J. 2015. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Huang, Y.F.; Yang, S.Z.; Chen, W. Introduction of a new atmospheric plasma device on tomato seeds. Agri. Sci. 2011, 2, 23–27. [Google Scholar]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci. Technol. 2014, 16, 54–58. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.D. The efect of non-thermal plasma treatment on wheat germination and early growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K. Effects of atmospheric-pressure N2, He, air, and O2 microplasmas on mung bean seed germination and seedling growth. Sci. Rep. 2016, 6, 32603. [Google Scholar] [CrossRef] [PubMed]
- Pawłat, J.; Starek, A.; Sujak, A.; Terebun, P.; Kwiatkowski, M.; Budzeń, M.; Andrejko, D. Effects of atmospheric pressure plasma jet operating with DBD on Lavatera thuringiaca L. seeds germination. PLoS ONE 2018. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Baki, A.; Anderson, J. Vigour determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chach, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Felzer, B.S.; Cronin, T.; Reilly, J.M.; Melillo, J.M.; Wang, X. Impacts of ozone on trees and crops. Geoscience 2007, 339, 784–798. [Google Scholar] [CrossRef]
- Corpas, F.J. Reactive nitrogen species (RNS) in plants under physiological and adverse environmental conditions: Current view. Prog. Bot. 2016, 78, 97–119. [Google Scholar]
- Berwal, M.K.; Ram, C. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. In Abiotic and Biotic Stress in Plants; de Oliveira, A.B., Ed.; Intech Open: London, UK, 2018; Available online: https://www.intechopen.com/books/abiotic-and-biotic-stress-in-plants/superoxide-dismutase-a-stable-biochemical-marker-for-abiotic-stress-tolerance-in-higher-plants (accessed on 7 December 2018). [CrossRef]
- Krishnan, H.; Natarajan, S.S.; Mahmoud, A.A.; Nelson, R.L. Identification of glycinin and β-conglycinin subunits that contribute to the increased protein content of high-protein soybean lines. J. Agric. Food Chem. 2007, 55, 1839–1845. [Google Scholar] [CrossRef]
- Kim, T.H.; Choi, U.-K.; Ryu, H.S.; Lee, S.J.; Kwon, O.-S. Mobilization of storage proteins in soybean seed (Glycine max L.) during germination and seedling growth. Biochim. Biophys. Acta 2011, 1814, 1178–1187. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tadege, M.; Dupuis, I.; Kuhlemeier, C. Ethanolic fermentation: New functions for an old pathway. Trends Plant Sci. 1999, 4, 320–325. [Google Scholar] [CrossRef]
- Schnarrenberger, C.; Martin, W. Evolution of the enzymes of the citric acid cycle and the glyoxylate cycle of higher plants. Eur. J. Biochem. 2002, 269, 868–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Jin, J.O.; Do, L.H.; Raj, K.M.; Mrinmoy, G.; Amit, K.S.; Sang, B.L.; Young, S.M.; Park, H.; Dong, K.J. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef] [PubMed]
- Koppen, G.; Toncelli, L.M.; Triest, L.; Verschaeve, L. The comet assay: A tool to study alteration of DNA integrity in developing plant leaves. Mech. Ageing Dev. 1999, 110, 13–24. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.S.; Li, P.; Zhou, Q.; Sun, T. Root growth inhibition and induction of DNA damage in soybean (Glycine max) by chlorobenzenes in contaminated soil. Chemosphere 2004, 57, 101–106. [Google Scholar] [CrossRef]
- Abdelhaliem, E.; Al-Shalawi, J. Attenuation of lead genotoxicity in Glycine max by adsorbent nanosized titanium dioxide using phenotypic, cytogenetic and DNA status bioassays. Genet. Mol. Res. 2019, 8, GMR18350. [Google Scholar]
- Menke, M.; Chen, I.-P.; Angelis, K.J.; Schubert, I. DNA Damage and repair in Arabidopsis thaliana as measured by the comet assay after treatment with different classes of genotoxins. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2001, 493, 87–93. [Google Scholar] [CrossRef]
- Černák, M.; Černáková, L.; Hudec, I.; Kovacik, D. Difuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials. Eur. Phys. J. Appl. Phys. 2009, 47, 22806. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Nelson, N.A. Photometric adaptation of the Somogyi method for determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Matušíková, I.; Salaj, J.; Moravčíková, J.; Mlynárová, L.; Nap, J.-P.; Libantová, J. Tentacles of in vitro-grown round-leaf sundew (Drosera rotundifolia L.) show induction of chitinase activity upon mimicking the presence of prey. Planta 2005, 222, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Frič, F.; Fuchs, W. Veränderungen der Aktivität einiger Enzyme im Weizenblatt in Abhängigkeit von der temperaturlabilen Verträglichkeit für Puccinia graminis tritici. J. Phytopathol. 1970, 67, 161–174. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio Protoc. 2014, 4, 3–6. [Google Scholar] [CrossRef]
- Collins, A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Boiteux, S.; Gajewski, E.; Laval, J.; Dizdaroglu, M. Substrate specificity of the Escherichia coli Fpg protein formamidopyrimidine-DNA glycosylase: Excision of purine lesions in DNA produced by ionizing radiation or photosensitization. Biochemistry 1992, 31, 106–110. [Google Scholar] [CrossRef]
- Dušinská, M.; Collins, A. Detection of oxidised purines and UV-induced photoproducts in DNA of single cells, by inclusion of lesion-specific enzymes in the comet assay. Altern. Lab. Anim. 1996, 24, 405–411. [Google Scholar] [CrossRef]
- Horvathova, E.; Kozics, K.; Melusova, M.; Galova, E.; Sevcovicova, A.; Kusznierewicz, B.; Chmiel, T.; Slamenova, D. Chromatographic analyses of Lavandula angustifolia and Rosmarinus officinalis extracts and their biological effects in mammalian cells and cell-free systems. Neoplasma 2017, 64, 856–868. [Google Scholar] [CrossRef]
- Chankova, S.G.; Dimova, E.; Dimitrova, M.; Bryant, P.E. Induction of DNA double-strand breaks by zeocin in Chlamydomonas reinhardtii and the role of increased DNA double-strand breaks rejoining in the formation of an adaptive response. Radiat. Environ. Biophys. 2007, 46, 409–416. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Švubová, R.; Slováková, Ľ.; Holubová, Ľ.; Rovňanová, D.; Gálová, E.; Tomeková, J. Evaluation of the Impact of Cold Atmospheric Pressure Plasma on Soybean Seed Germination. Plants 2021, 10, 177. https://doi.org/10.3390/plants10010177
Švubová R, Slováková Ľ, Holubová Ľ, Rovňanová D, Gálová E, Tomeková J. Evaluation of the Impact of Cold Atmospheric Pressure Plasma on Soybean Seed Germination. Plants. 2021; 10(1):177. https://doi.org/10.3390/plants10010177
Chicago/Turabian StyleŠvubová, Renáta, Ľudmila Slováková, Ľudmila Holubová, Dominika Rovňanová, Eliška Gálová, and Juliána Tomeková. 2021. "Evaluation of the Impact of Cold Atmospheric Pressure Plasma on Soybean Seed Germination" Plants 10, no. 1: 177. https://doi.org/10.3390/plants10010177
APA StyleŠvubová, R., Slováková, Ľ., Holubová, Ľ., Rovňanová, D., Gálová, E., & Tomeková, J. (2021). Evaluation of the Impact of Cold Atmospheric Pressure Plasma on Soybean Seed Germination. Plants, 10(1), 177. https://doi.org/10.3390/plants10010177




