Transmission of Engineered Plastids in Sugarcane, a C4 Monocotyledonous Plant, Reveals that Sorting of Preprogrammed Progenitor Cells Produce Heteroplasmy
Abstract
:1. Introduction
2. Results
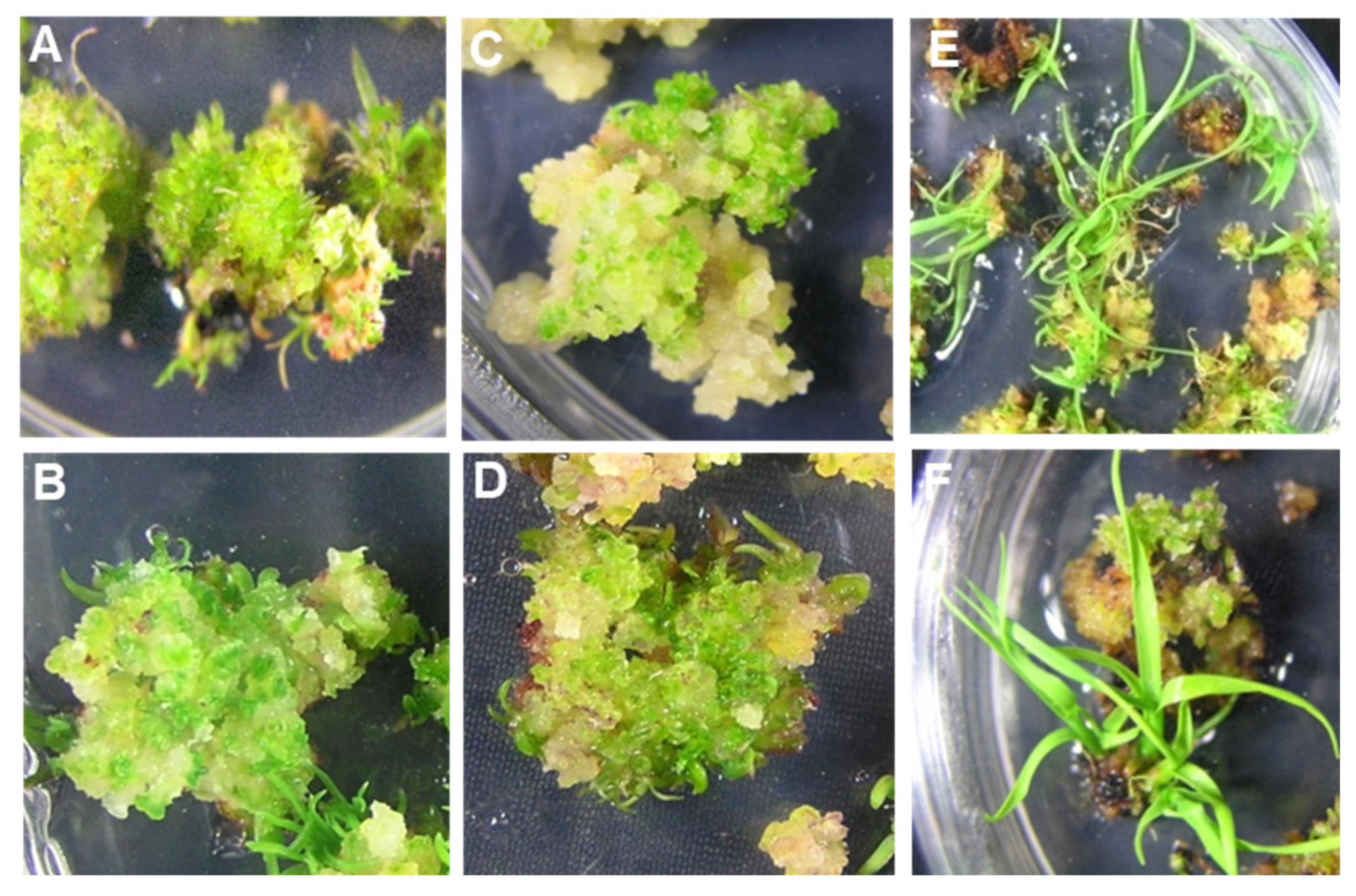
2.1. Development of a Proficient Regeneration Protocol
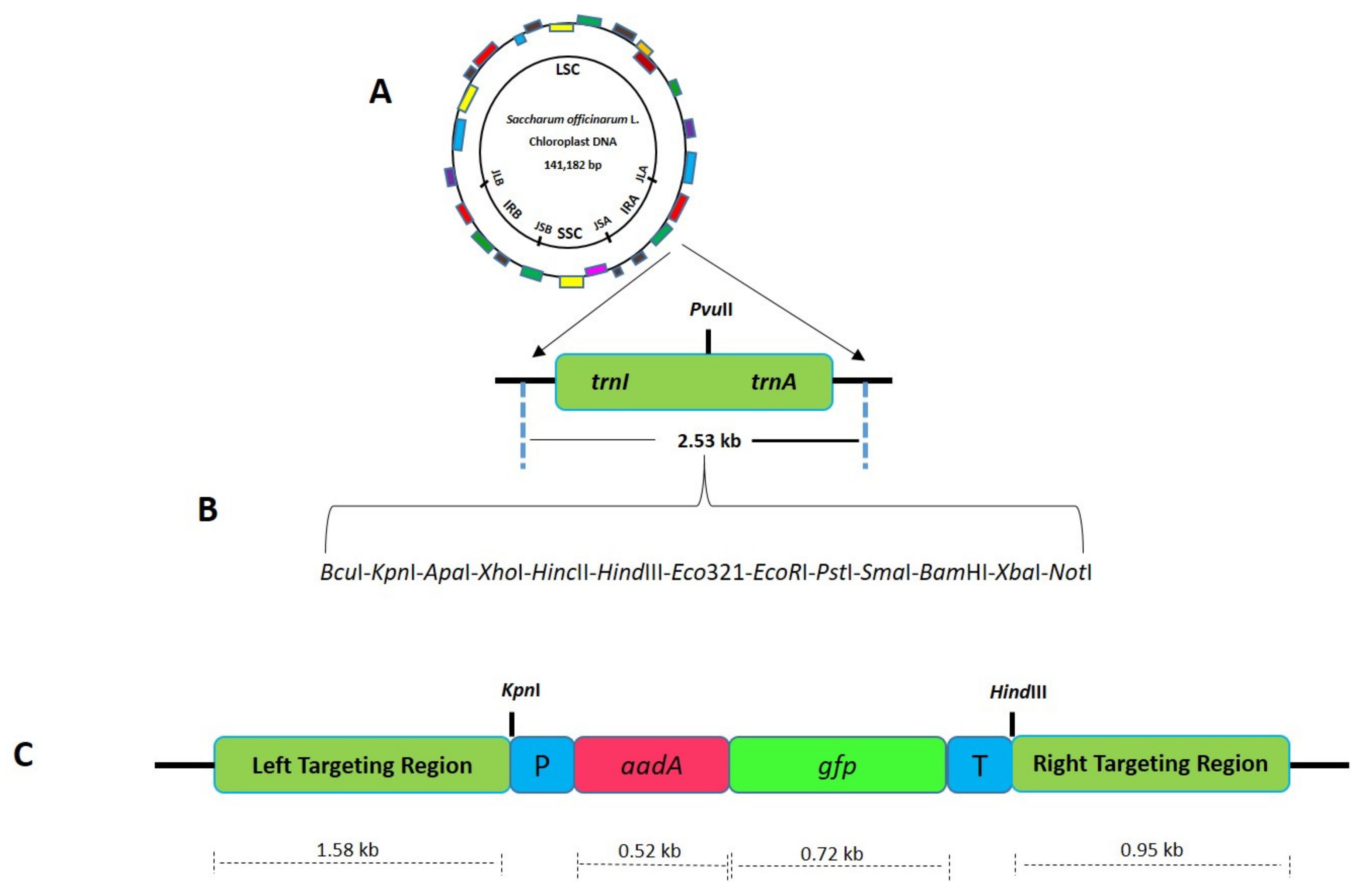
2.2. Development of Species-Specific Chloroplast Transformation Vectors
2.3. Plastid Transformation and Recovery of Transplastomic Plants
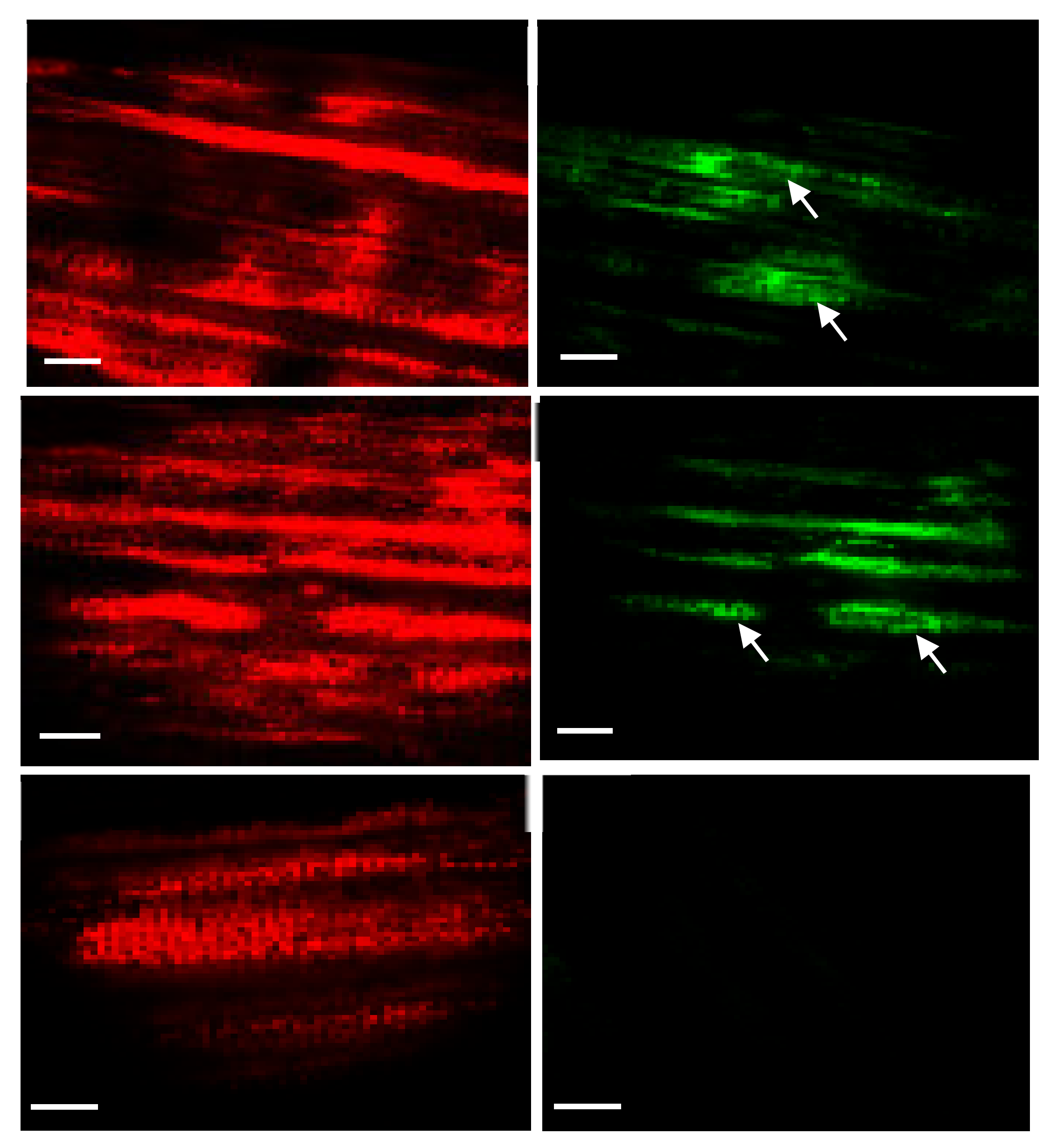
2.4. Tracking Transgenic Plastids Using Green Fluorescence Protein (GFP)
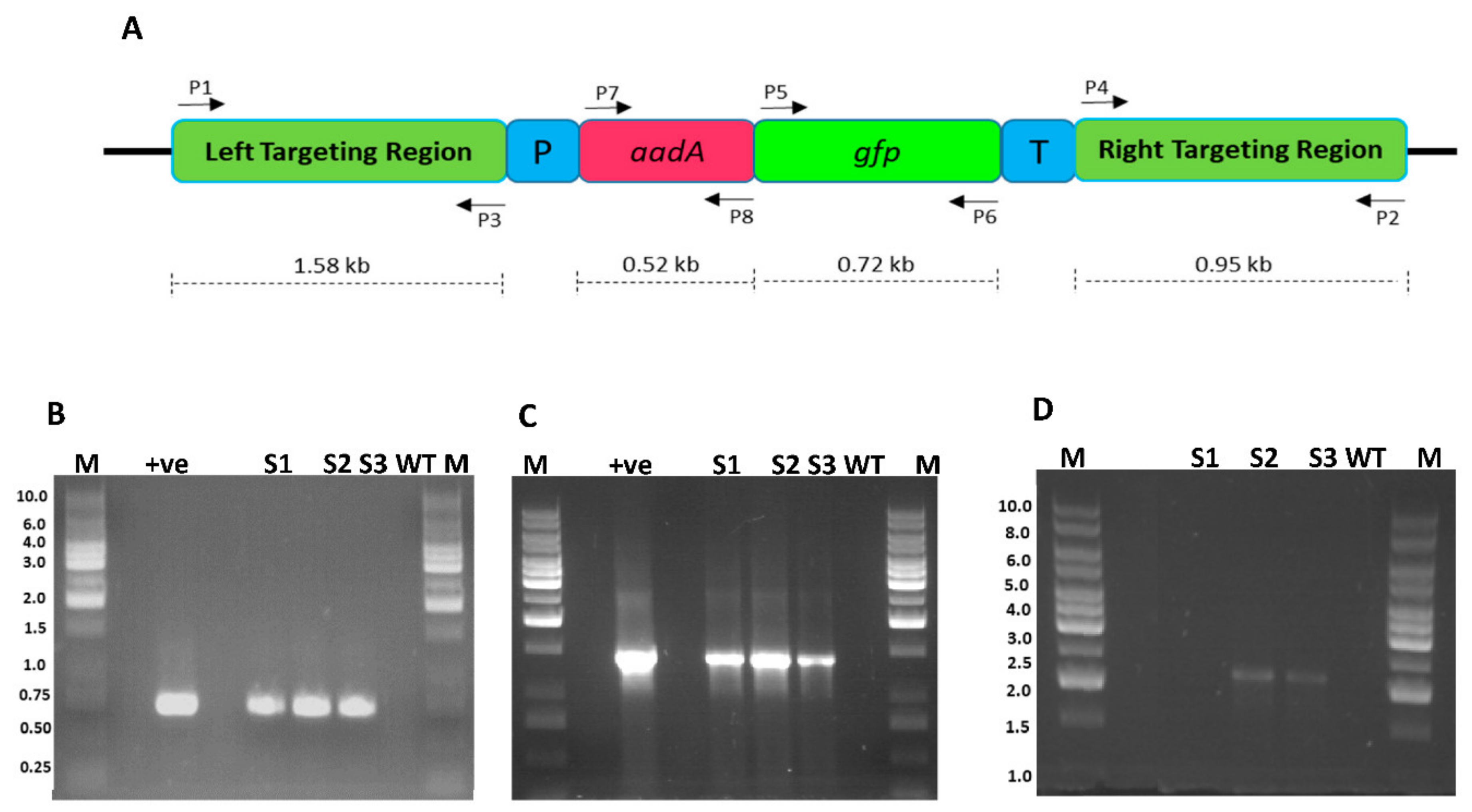
2.5. Tracking Transgene Integration through PCR Approach
3. Discussion
4. Materials and Methods
4.1. Choice of Explant Material and Callus Induction
4.2. Shoot Induction, Multiplication, and Rooting
4.3. Construction of Species-Specific Chloroplast Transformation Vectors
4.4. Sugarcane Plastome Transformation
4.5. Tracking Fluorescent Protein (gfp) in Plastids/Cells
4.6. Total Cellular DNA Extraction and PCR Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.S. Plastid genome engineering in plants: Present status and future Trends. Mol. Plant Breed. 2012, 8, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Kanwal, B.; Nazir, S. Metabolic engineering of the chloroplast genome reveals that the yeast ArDH gene confers enhanced tolerance to salinity and drought in plants. Front. Plant Sci. 2015, 6, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, J.L.; Daniell, H. Plastid biotechnology for crop production: Present status and future perspectives. Plant Mol. Biol. 2011, 76, 207–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancin, M.; Sanz-Barrio, R.; Santamaria, E.; Millan, A.F.; Larraya, L.; Veramendi, J.; Farran, I. Functional improvement of human cardiotrophin 1 produced in tobacco chloroplasts by co-expression with plastid thioredoxin m. Plants 2020, 9, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, P.; Armarego-Marriott, T.; Bock, R. Plastid transformation and its application in metabolic engineering. Curr. Opin. Biotechnol. 2018, 49, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.; Warzecha, H. Solar-powered factories for new vaccines and antibiotics. Trends Biotechnol. 2010, 28, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Bock, R. Engineering plastid genomes: Methods, tools, and applications in basic research and biotechnology. Annu. Rev. Plant Biol. 2015, 66, 211–241. [Google Scholar] [CrossRef] [Green Version]
- Gan, Q.; Jiang, J.; Han, X.; Wang, S.; Lu, Y. Engineering the chloroplast genome of oleaginous marine microalga Nannochloropsis oceanica. Front. Plant Sci. 2018, 9, 439. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.; Daniell, H. Chloroplast vector systems for biotechnology applications. Plant Physiol. 2007, 145, 1129–1143. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Mustafa, G.; Joyia, F.A. Technical Advances in Chloroplast Biotechnology. In Transgenic Crops; Khan, M.S., Ed.; InTech-Open Science: London, UK, 2019; pp. 1–13. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Maliga, P. Fluorescent antibiotic resistance marker for tracking plastid transformation in higher plants. Nat. Biotechnol. 1999, 17, 910–915. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, K.; Chung, H.; Yoo, S.H.; Xu, X.M.; Lee, S.B.; Cheong, J.J.; Daniell, H.; Kim, M. Plastid transformation in the monocotyledonous cereal crop, rice (Oryza sativa) and transmission of transgenes to their progeny. Mol. Cells 2006, 21, 401–410. [Google Scholar] [PubMed]
- Daniell, H.; Khan, M.S.; Allison, L. Milestones in chloroplast genetic engineering: An environmentally friendly era in biotechnology. Trends Plant Sci. 2002, 7, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Biradar, S.; Biradar, D.P.; Patil, V.C.; Patil, S.S.; Kambar, N.S. In vitro plant regeneration using shoot tip culture in commercial cultivar of sugarcane. Karnat. J. Agri. Sci. 2009, 22, 21–24. [Google Scholar]
- Chen, W.H.; Davey, M.R.; Power, J.B.; Cocking, E.C. Control and maintenance of plant regeneration in sugarcane callus cultures. J. Exp. Bot. 1988, 39, 251–261. [Google Scholar] [CrossRef]
- Ather, A.; Khan, S.; Rehman, A.; Nazir, M. Optimization of the protocols for callus induction, regeneration and acclimatization of sugarcane cv. Thatta-10. Pak. J. Bot. 2009, 41, 815–820. [Google Scholar]
- Mustafa, G.; Khan, M.S. Reproducible in vitro regeneration system for purifying sugarcane clones. Afr. J. Biotechnol. 2012, 11, 9961–9969. [Google Scholar] [CrossRef]
- Fernandez-San, M.A.; Mingo-Castel, A.; Miller, M.; Daniell, H. A chloroplast transgenic approach to hyper express and purify human serum albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol. 2003, 1, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, T.A.; Thanh, N.D.; Lao, N.T.; Grath, N.M.; Peter, S.O.; Horvath, E.M.; Dix, P.J.; Medgyesy, P. Homologous plastid DNA transformation in tobacco is mediated by multiple recombination events. Genetics 1999, 152, 1111–1122. [Google Scholar] [PubMed]
- Khan, M.S.; Khalid, A.M.; Malik, K.A. Intein-mediated protein trans-splicing and transgene containment in plastids. Trends Biotechnol. 2005, 23, 217–220. [Google Scholar] [CrossRef]
- Dhingra, A.; Portis, A., Jr.; Daniell, H. Enhanced translation of a chloroplast-expressed RbcS gene restores small subunit levels and photosynthesis in nuclear RbcS antisense plants. Proc. Natl. Acad. Sci. USA 2004, 101, 6315–6320. [Google Scholar] [CrossRef] [Green Version]
- Day, A.; Goldschmidt-Clermont, M. The chloroplast transformation toolbox: Selectable markers and marker removal. Plant Biotechnol. J. 2011, 9, 540–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, G.; Khan, M.S. Prospecting the utility of antibiotics as lethal selection agents for chloroplast transformation of sugarcane. Int. J. Agri. Biol. 2012, 14, 307–310. [Google Scholar]
- Khan, M.S.; Ali, S.; Iqbal, J. Developmental and photosynthetic regulation of δ-endotoxin reveals that engineered sugarcane conferring resistance to ‘dead heart’ contains no toxins in cane juice. Mol. Biol. Rep. 2011, 38, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, S.R.; Serino, G.; Chaudhuri, S.; Maliga, P. Plastid transformation in Arabidopsis thaliana. Plant Cell Rep. 1998, 18, 20–24. [Google Scholar] [CrossRef]
- Yu, Q.; Lutz, K.A.; Maliga, P. Efficient plastid transformation in Arabidopsis. Plant Physiol. 2017, 175, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Sidorov, V.A.; Kasten, D.; Pang, S.Z.; Hajdukiewicz, P.T.J.; Staub, J.M.; Nehra, N.S. Stable chloroplast transformation in potato: Use of green fluorescent protein as a plastid marker. Plant J. 1999, 19, 209–216. [Google Scholar] [CrossRef]
- Valkov, V.T.; Gargano, D.; Manna, C.; Formisano, G.; Dix, P.J.; Gray, J.C.; Scotti, N.; Cardi, T. High efficiency plastid transformation in potato and regulation of transgene expression in leaves and tubers by alternative 5′ and 3′ regulatory sequences. Transgenic Res. 2011, 20, 137–151. [Google Scholar] [CrossRef] [Green Version]
- Valkov, V.T.; Gargano, D.; Scotti, N.; Cardi, T. Plastid transformation in potato: Solanum tuberosum. Methods Mol. Biol. 2014, 1132, 295–303. [Google Scholar] [CrossRef]
- Ruf, S.; Hermann, M.; Berger, I.J.; Carrer, H.; Bock, R. Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat. Biotechnol. 2001, 19, 870–875. [Google Scholar] [CrossRef]
- Ruf, S.; Bock, R. Plastid transformation in tomato. Methods Mol. Biol. 2014, 1132, 265–276. [Google Scholar] [CrossRef]
- Langbecker, C.L.; Ye, G.N.; Broyles, D.L.; Duggan, L.L.; Xu, C.W.; Hajdukiewicz, P.T.J.; Armstrong, C.L.; Staub, J.M. High-frequency transformation of undeveloped plastids in tobacco suspension cells. Plant Physiol. 2004, 135, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silhavy, D.; Maliga, P. Mapping of promoters for the nucleus-encoded plastid RNA polymerase (NEP) in the iojap maize mutant. Curr. Genet. 1998, 33, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Maliga, P. Progress towards commercialization of plastid transformation technology. Trends Biotechnol. 2003, 21, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Gill, N.K.; Gill, R.; Gosal, S.S. Factors enhancing somatic embryogenesis and plant regeneration in sugarcane (Saccharum officinarum L.). Ind. J. Biotechnol. 2004, 3, 119–123. [Google Scholar]
- Gallo-Meagher, M.; English, R.G.; Abouzid, A. Thidiazuron stimulates shoot regeneration of sugarcane embryogenic callus. Vitro Cell. Dev. Biol. Plant 2000, 36, 37–40. [Google Scholar] [CrossRef]
- Ali, S.; Iqbal, J.; Khan, M.S. Genotype independent in vitro regeneration system in elite varieties of sugarcane. Pak. J. Bot. 2010, 42, 3783–3790. [Google Scholar]
- Sud, R.M.; Dengler, N.G. Cell lineage of vein formation in variegated leaves of the C4 grass Stenotaphrum secundatum. Ann. Bot. 2000, 86, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.E. Making leaves. Curr. Opin. Plant Biol. 2012, 15, 24–30. [Google Scholar] [CrossRef]
- Laetsch, W.M.; Price, I. Development of the dimorphic chloroplasts of sugarcane source. Am. J. Bot. 1969, 56, 77–87. [Google Scholar] [CrossRef]
- Poethig, R.S. Cellular parameters of leaf morphogenesis in maize and tobacco. In Contemporary Problems in Plant Anatomy; White, R.A., Dickison, W.C., Eds.; Academic Press: New York, NY, USA, 1984; pp. 235–259. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, G.; Khan, M.S. Transmission of Engineered Plastids in Sugarcane, a C4 Monocotyledonous Plant, Reveals that Sorting of Preprogrammed Progenitor Cells Produce Heteroplasmy. Plants 2021, 10, 26. https://doi.org/10.3390/plants10010026
Mustafa G, Khan MS. Transmission of Engineered Plastids in Sugarcane, a C4 Monocotyledonous Plant, Reveals that Sorting of Preprogrammed Progenitor Cells Produce Heteroplasmy. Plants. 2021; 10(1):26. https://doi.org/10.3390/plants10010026
Chicago/Turabian StyleMustafa, Ghulam, and Muhammad Sarwar Khan. 2021. "Transmission of Engineered Plastids in Sugarcane, a C4 Monocotyledonous Plant, Reveals that Sorting of Preprogrammed Progenitor Cells Produce Heteroplasmy" Plants 10, no. 1: 26. https://doi.org/10.3390/plants10010026
APA StyleMustafa, G., & Khan, M. S. (2021). Transmission of Engineered Plastids in Sugarcane, a C4 Monocotyledonous Plant, Reveals that Sorting of Preprogrammed Progenitor Cells Produce Heteroplasmy. Plants, 10(1), 26. https://doi.org/10.3390/plants10010026





