A Quest for Mechanisms of Plant Root Exudation Brings New Results and Models, 300 Years after Hales
Abstract
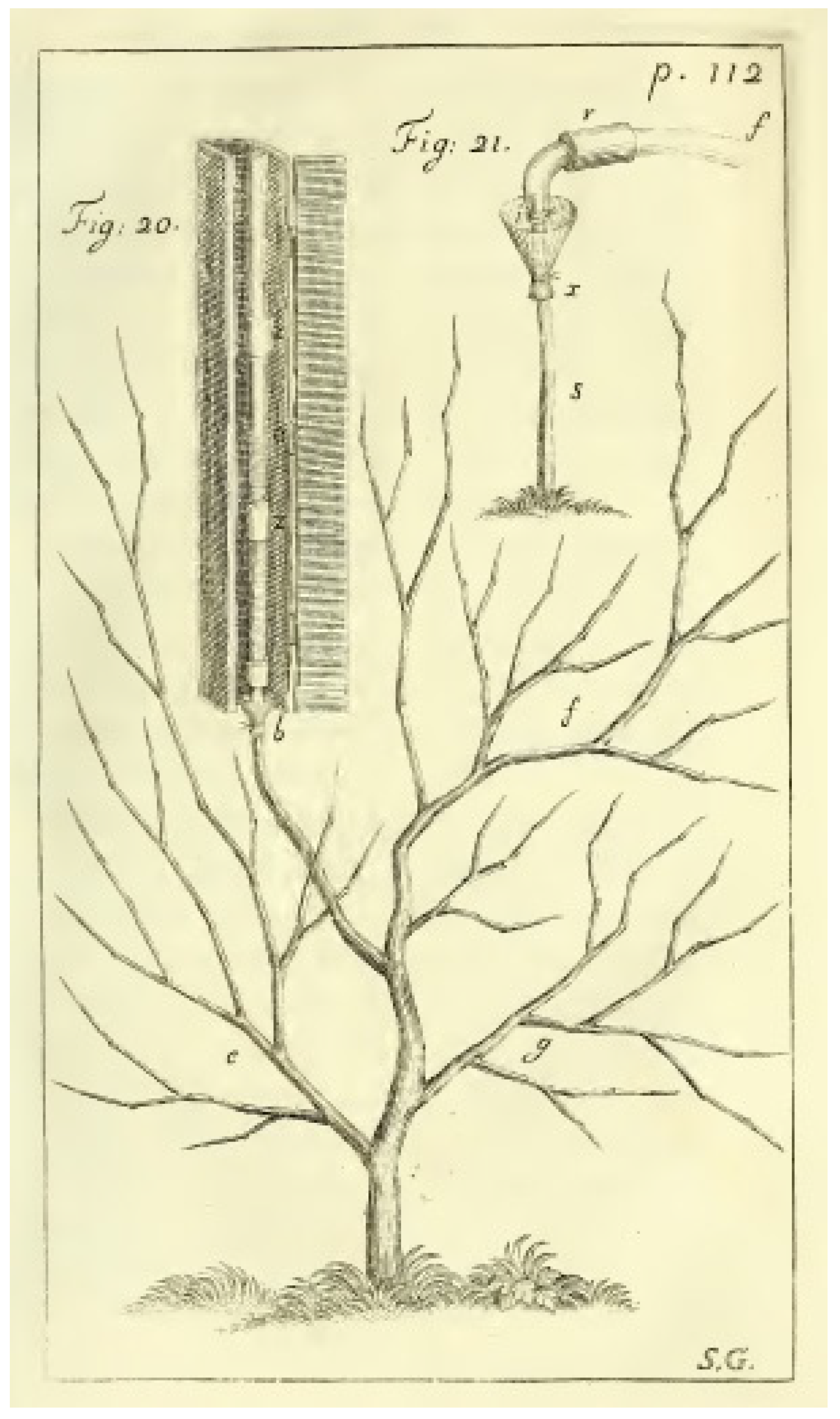
:1. Introduction
2. Root Pressure and the Ascent of Sap
3. Origin of Exudate
4. Initial Concepts and Further Models for Understanding Mechanisms of Root Pressure and Exudation
| Type of Model, Main Feature | Short Description | Principle | Proponents, References |
|---|---|---|---|
| Macroscopic | Simple osmotic idea that exudation is caused purely by difference of osmotic pressures between xylem sap and external medium; exudation rate is linearly proportional to this difference | osmotic | Atkins [92], Priestley [93], Sabinin [82], Eaton [94] and others |
| Macroscopic | Concentration gradient within the vessel lumens drives water flow and exudation | osmotic, standing gradient flow | Anderson, Aikman and Meiri [103] |
| Macroscopic | Iso-osmotic flow is realized due to different parameters and reflection coefficients for membranes located in a row | osmotic, iso-osmotic flow | Ginsburg [104] |
| Macroscopic | Concentration gradient exists within the cell walls inside the root stele; water follows osmotic forces which are caused by solute transport, potentially at the expenses of energy for ion fluxes | osmotic, standing gradient flow | Katou, Taura and Furumoto [105] |
| Macroscopic | High turgor pressure in the root symplast allows reverse osmosis and nonlinear dependence between exudation rate and difference in osmotic pressures between exudate and external medium | reverse osmosis at the level of whole root symplast | Lyalin [106] |
| Macroscopic | Detailed model with over tens of coefficients, microscopic coefficients are based on structure of plasmalemma and applied to averaged membrane interface between symplast and xylem; assumes active ion fluxes | models osmotic effects with reference to the microscopic level without irreversible thermodynamics | Pickard [36,107] |
| Subcellular | Reverse osmosis is realized in each plasmodesma of root endodermal cells; turgor oscillations within the cells at 1 Hz frequency result in pumping of water to the root xylem, produce uphill water flux and exudation; energy is pumped into the system via mechanic constrictions and cytoskeleton | reverse osmosis at cellular and subcellular levels | Kundt, Robnik [110,111] |
| Molecular | The first known model introducing specific ion channels to water transport; alterations in reflection coefficient due to mechanosensitive ion channels explain oscillations in exudation; combination with reverse osmosis may explain visible non-osmotic effects | alterations in reflection coefficient between root compartments with potential implications of reverse osmosis | Schwenke [21], Schwenke, Wagner [22] |
| Molecular | Model of active water transport based on water cotransport with cation-chloride cotransporters, transferred to plants from animal physiology; suggests the mechanism of water transport to xylem vessels; applicable to root exudation | energetically uphill water transport due to water molecules cotransport with cation-chloride cotransporters | Wegner [112,113,114]; Fricke [115] |
5. Rhizodermis Exudation
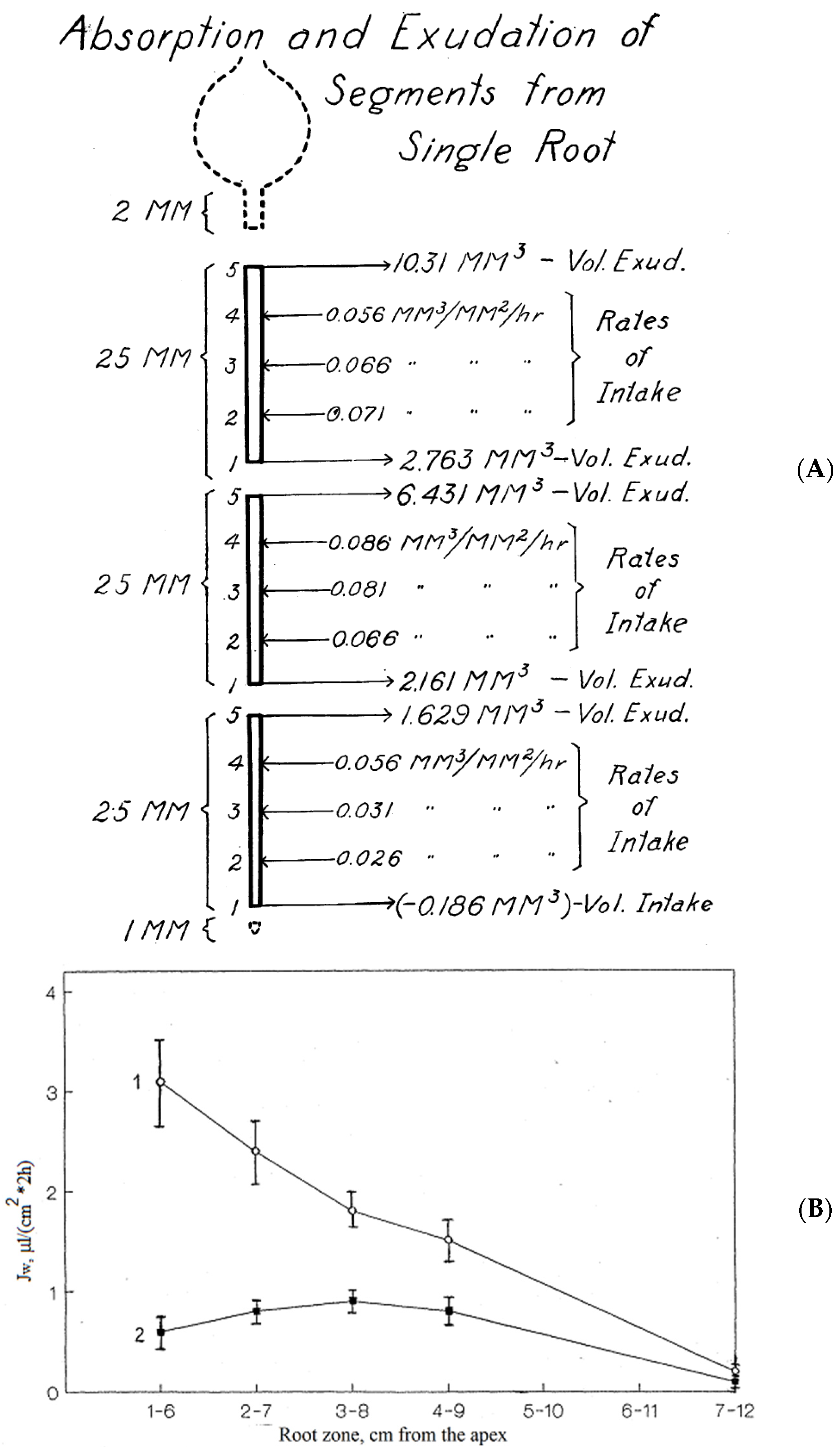
6. Exudation of Root Segments without Root Apex and Stem Exudation
7. Pulses and Oscillations in Exudation Rate, Coordinated Activity of Cells, and Estimates for Potential Changes of Cell Volume and Turgor Required
- (1)
- cytoskeleton-based turgor oscillations of root cells constituting the so called metabolic component of root pressure [19];
- (2)
8. Effects of Chemical Agents on Exudation and Root Pressure
8.1. Inhibitors of Metabolism and Substances Influencing the Integrity of Cell Membranes and Cytoskeleton
8.2. Plant Hormones, Biologically Active Compounds, and Modifiers of Signal Transduction Chains
8.3. Ion Channel Blockers
8.4. Summary on the Effects of Chemicals on Exudation
9. Energy Activation Barrier and Osmotic Compensation Pressure for Root Exudation
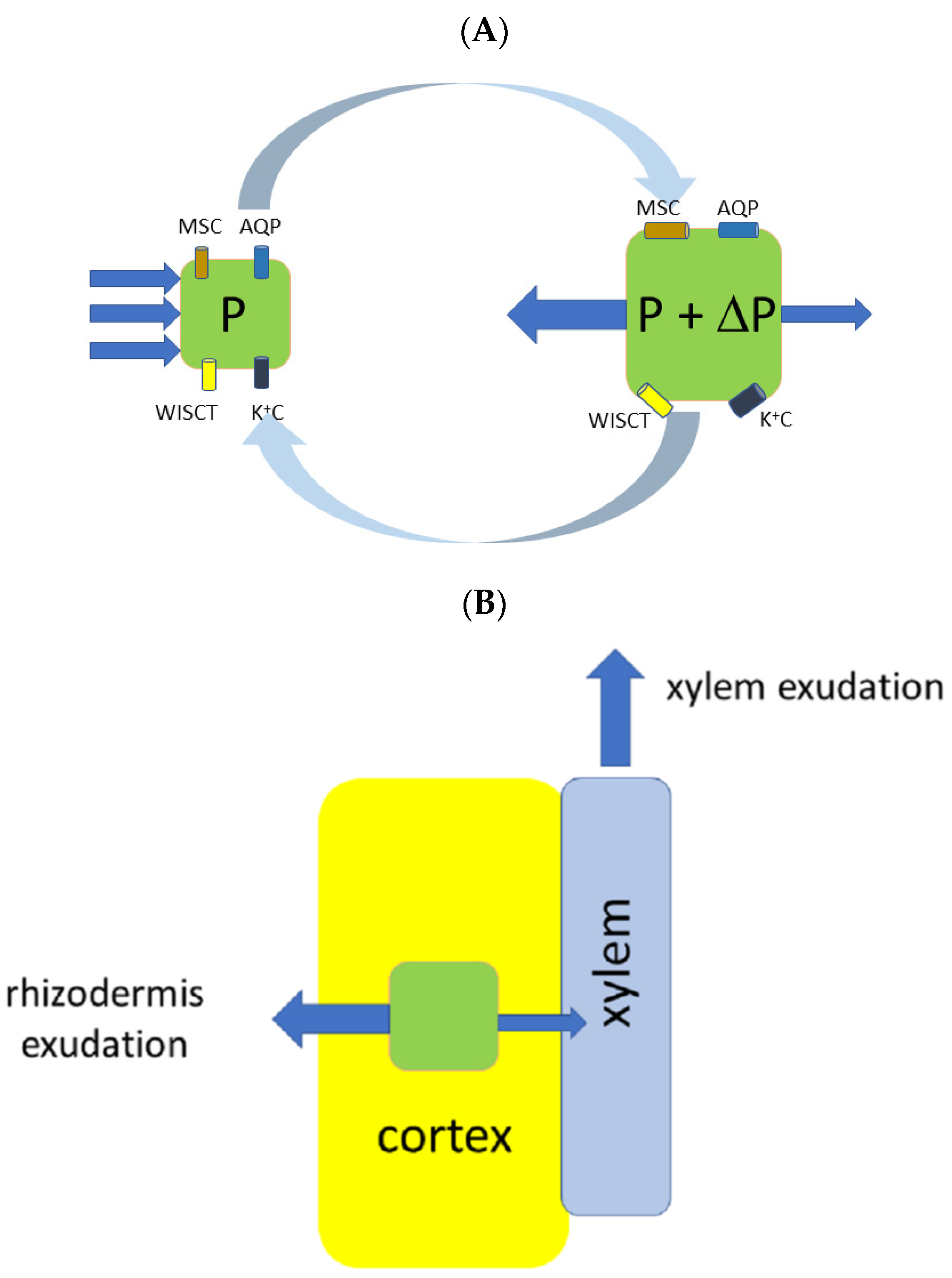
10. Proposed Sketch of the New Mechanism Which Explains Both Xylem and Rhizodermal Types of Exudation; Overall Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, S. Vegetable Staticks: Or, an Account of Some Statical Experiments on the Sap in Vegetables: Being an Essay towards a Natural History of Vegetation. Also a Specimen of an Attempt to Analyse the Air, by a Great Variety of Chymo-Statical Experiments, Which Were Read at Several Meetings before the Royal Society; W. and J. Innys: London, UK, 1727. [Google Scholar]
- Clark, W.S. The circulation of sap in plants. Mass. State Board Agric. Ann. Rep. 1874, 21, 159–204. [Google Scholar]
- Fisher, J.B.; Angeles, G.; Ewers, F.W.; Lòpez-Portillo, J. Survey of root pressure in tropical vines and woody species. Int. J. Plant. Sci. 1997, 158, 44–50. [Google Scholar] [CrossRef]
- Kraus, C. Die Saftleistung der Wurzeln, besonders ihrer jüngsten Theile. I. Ueber Verbreitung und Nachweis des Blutungsdrucks der Wurzeln. Forschungen auf dem Gebiete der Agriculturphysik 1882, 5, 432–462. [Google Scholar]
- Wieler, A. Das Bluten der Pflanzen. Beitr. Biol. Pflanzen 1893, 6, 1–211. [Google Scholar]
- Lopushinsky, W. Occurrence of root pressure exudation in Pacific Northwest conifer seedlings. For. Sci. 1980, 26, 275–279. [Google Scholar]
- O’Leary, J.W.; Kramer, P.J. Root pressure in conifers. Science 1964, 145, 284–285. [Google Scholar] [CrossRef]
- White, P.R. “Root pressure”—An unappreciated force in sap movement. Am. J. Bot. 1938, 25, 223–227. [Google Scholar] [CrossRef]
- White, P. Root-Pressure as a Factor in the Rise of Sap. Nature 1938, 141, 581–583. [Google Scholar] [CrossRef]
- Rosene, H. Water balance in the onion roots: Relation of water intake to volume exudate of isolated roots and root segments. Plant Physiol. 1941, 16, 447–460. [Google Scholar] [CrossRef] [Green Version]
- Rosene, H. Control of water transport in local root regions of attached and isolated roots by means of the osmotic pressure of the external solution. Am. J. Bot. 1941, 28, 402–410. [Google Scholar] [CrossRef]
- O’Leary, J. Root pressure exudation from apical root segments. Nature 1966, 212, 96–97. [Google Scholar] [CrossRef]
- Mozhaeva, L.V.; Pil’shchikova, N.V. Effect of temperature on exudation rate and several aspects of energy exchange of sunflower roots. Izvestiya Timiryazevskoy Sel’skokhozyaistvennoi Akademii 1969, 4, 14–30. [Google Scholar]
- Mozhaeva, L.V.; Pil’shchikova, N.V. Nature of water pumping process by plant roots. Izvestiya Timiryazevskoy Sel’skokhozyaistvennoi Akademii 1972, 3, 3–15. [Google Scholar]
- Mozhaeva, L.V.; Pil’shchikova, N.V.; Zaitseva, N.V. The study of the contractile properties of root cells in relation to rhythmicity of plant exudation. Izvestiya Timiryazevskoy Sel’skokhozyaistvennoi Akademii 1975, 1, 3–12. [Google Scholar]
- Mozhaeva, L.V.; Pil’shchikova, N.V.; Kuzina, V.I. Study on the nature of the motive force of plant exudation with the use of chemical effects. Izvestiya Timiryazevskoy Sel’skokhozyaistvennoi Akademii 1979, 1, 3–9. [Google Scholar]
- Miller, D.M. Studies of root function in Zea mays: III. Xylem sap composition at maximum root pressure provides evidence of active transport into the xylem and a measurement of the reflection coefficient of the root. Plant Physiol. 1985, 77, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steudle, E.; Oren, R.; Schulze, E.D. (1987) Water transport in maize roots: Measurement of hydraulic conductivity, solute permeability, and of reflection coefficients of excised roots using the root pressure probe. Plant Physiol. 1987, 84, 1220–1232. [Google Scholar] [CrossRef] [Green Version]
- Zholkevich, V.N. Root pressure. In Plant Roots, the Hidden Half; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel-Dekker: New York, NY, USA, 1991; pp. 589–603. [Google Scholar]
- Zholkevich, V.N.; Chugunova, T.V.; Korolev, A.V. New data on the nature of root pressure. Stud. Biophys. 1990, 136, 209–216. [Google Scholar]
- Schwenke, H. Der Mechanismus der Wurzelexsudation; Hochschulverlag: Zurich, Switzerland, 1990; ISBN 3-8107-2233-2. [Google Scholar]
- Schwenke, H.; Wagner, E. A new concept of root exudation. Plant, Cell Environ. 1992, 15, 289–299. [Google Scholar] [CrossRef]
- Volkov, V.S.; Zholkevich, V.N. Exudation parameters depending on spatial orientation of root segments of Zea mays L. Dokl. Akad. Nauk 1993, 332, 526–528. [Google Scholar]
- Volkov, V.; Zholkevich, V. Oppositely-directed water flows in roots with respect to the plant integral hydrodynamic system. In Root Demographics and Their Efficiencies in Sustainable Agriculture, Grasslands and Forest Ecosystems. Developments in Plant and Soil Sciences; Box, J.E., Ed.; Springer: Dordrecht, The Netherlands, 1998; Volume 82, pp. 623–630. [Google Scholar] [CrossRef]
- Volkov, V.S. Investigation of oppositely directed water flows in roots as an approach to the identification of the plant’s integral hydrodynamic system. Acta Hortic. 1998, 458, 247–252. [Google Scholar] [CrossRef]
- Volkov, V.S. Peculiarities of Water Transport in Segments of Zea mays L. Roots. Ph.D. Thesis, K.A. Timiriazev Institute of Plant Physiology, Russian Academy of Sciences, Moscow, Russia, 1999. (In Russian). [Google Scholar]
- Zholkevich, V.N.; Korolev, A.V. Air-dried roots as a model for studying the nature of root pressure. Dokl. Biol. Sci. 1995, 344, 481–483. [Google Scholar]
- Zholkevich, V.N.; Chugunova, T.V. Effect of neurotransmitters on the pumping activity of plant roots. Dokl. Biochem. 1997, 356, 109–112. [Google Scholar]
- Zholkevich, V.N.; Zubkova, N.K.; Korolev, A.V. Effect of colchicine and noradrenaline on the exudate secretion by Helianthus annuus L. roots in the absence of water uptake from an environment. Dokl. Akad. Nauk. 1998, 359, 551–553. [Google Scholar]
- Zholkevich, V.N.; Puzakov, M.M.; Monakhova, O.F. The involvement of actin in the formation of root pressure. Dokl. Biochem. Biophys. 2001, 380, 332–335. [Google Scholar] [CrossRef]
- Zholkevich, V.N.; Sushchenko, S.V.; Emel’yanova, I.B.; Monakhova, O.F. Peculiarities of root exudation in the absence of water uptake. Dokl. Biol. Sci. 2005, 400, 42–44. [Google Scholar] [CrossRef]
- Zholkevich, V.N.; Emel’yanova, I.B.; Sushchenko, S.V. Self-oscillations of water transport in the plant root. Dokl. Biol. Sci. 2005, 403, 269–271. [Google Scholar] [CrossRef]
- Zholkevich, V.N.; Popova, M.S.; Zhukovskaya, N.V. Stimulatory effects of adrenalin and noradrenalin on root water-pumping activity and the involvement of G-proteins. Russ. J. Plant Physiol. 2007, 54, 790–796. [Google Scholar] [CrossRef]
- Steudle, E. Water uptake by plant roots: An integration of views. Plant Soil 2000, 226, 45–56. [Google Scholar] [CrossRef]
- Rowan, A.; McCully, M.E.; Canny, M.J. The origin of the exudate from cut maize roots. Plant Physiol. Biochem. 2000, 38, 957–967. [Google Scholar] [CrossRef]
- Pickard, W.F. The riddle of root pressure: II. Root exudation at extreme osmolalities. Funct. Plant Biol. 2003, 30, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Knipfer, T.; Fricke, W. Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare). New Phytol. 2010, 187, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E.; Meshcheryakov, A.B. Hydraulic and osmotic properties of oak roots. J. Exp. Bot. 1996, 47, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, O. Long-Distance water transport in aquatic plants. Plant Physiol. 1993, 103, 1369–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschner, S. Die Theorie vom Saftkreislauf der Pflanzen. Ein Wenig Bekanntes Kapitel in der Geschichte der Pflanzenphysiologie. Habilitation Thesis, Ludwig Maximilian University of Munich, Munich, Germany, 2002. [Google Scholar]
- Biddulph, S.; Biddulph, O. The circulatory system of plants. Sci. Am. 1959, 200, 44–49. [Google Scholar]
- Zyalalov, A.A. Water flows in higher plants: Physiology, evolution, and system analysis. Russ. J. Plant Physiol. 2004, 51, 547–556. [Google Scholar] [CrossRef]
- Scholander, P.F.; Love, W.E.; Kanwisher, J.W. The rise of sap in tall grapevines. Plant Physiol. 1955, 30, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 19–38. [Google Scholar] [CrossRef]
- Sperry, J.S.; Holbrook, N.M.; Zimmerman, M.H.; Tyree, M.T. Spring filling of xylem vessels in wild grapevine. Plant Physiol. 1987, 83, 414–417. [Google Scholar] [CrossRef] [Green Version]
- Jansen, S.; Choat, B.; Pletsers, A. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am. J. Bot. 2009, 96, 409–419. [Google Scholar] [CrossRef]
- Choat, B.; Cobb, A.R.; Jansen, S. Tansley Review: Structure and function of bordered pits: New discoveries and impacts on whole plant hydraulic function. New Phytol. 2008, 177, 608–625. [Google Scholar] [CrossRef] [PubMed]
- Pagay, V.; Santiago, M.; Sessoms, D.A.; Huber, E.J.; Vincent, O.; Pharkya, A.; Corso, T.N.; Lakso, A.N.; Stroock, A.D. A microtensiometer capable of measuring water potentials below -10 MPa. Lab Chip 2014, 14, 2806–2817. [Google Scholar] [CrossRef] [PubMed]
- Lakso, A.N.; Zhu, S.; Santiago, M.; Shackel, K.; Volkov, V.; Stroock, A.D. A microtensiometer sensor to continuously monitor stem water status in woody plants—Design and field testing. Acta Hortic. (ISHS) 2021, in press. [Google Scholar]
- Tyree, M.T.; Fiscus, S.D.; Wullschleger, M.A.; Dixon, M.A. Detection of xylem cavitation in corn under field conditions. Plant Physiol. 1986, 82, 597–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Tian, X.; Ding, Y.; Wan, X.; Tyree, M.T. A survey of root pressure in 53 Asian species of bamboo. Ann. For. Sci. 2011, 68, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Tyree, M.T.; Salleo, S.; Nardini, A.; Lo Gullo, M.A.; Mosca, R. Refilling of embolized vessels in young stems of Laurel. Do we need a new paradigm? Plant Physiol. 1999, 120, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Zwieniecki, M.A.; Holbrook, N.M. Bordered pit structure and vessel wall surface properties: Implications for embolism repair. Plant Physiol. 2000, 123, 1015–1020. [Google Scholar] [CrossRef] [Green Version]
- De Boer, A.H.; Volkov, V. Logistics of water and salt transport through the plant: Structure and functioning of the xylem. Plant Cell Environ. 2003, 26, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Zwieniecki, M.A.; Holbrook, N.M. Confronting Maxwell’s demon: Biophysics of xylem embolism repair. Trends in Plant Science 2009, 14, 530–534. [Google Scholar] [CrossRef]
- Williams, C.B.; Næsborg, R.R.; Dawson, T.E. Coping with gravity: The foliar water relations of giant sequoia. Tree Physiol. 2017, 37, 1312–1326. [Google Scholar] [CrossRef] [Green Version]
- Singh, S. Guttation: Path, principles and functions. Aust. J. Bot. 2013, 61, 497–515. [Google Scholar] [CrossRef]
- Singh, S. Root Pressure: Getting to the Root of Pressure. In Progress in Botany; Lüttge, U., Cánovas, F., Matyssek, R., Eds.; Springer: Cham, Germany, 2016; Volume 77, pp. 105–150. [Google Scholar] [CrossRef]
- Singh, S. Guttation: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Dutrochet, H. L’agent Immédiat du Mouvement Vital Dévoilé dans sa Nature et dans son Mode D’action, Chez les Végétaux et Chez les Animaux; Dentu: Paris, France, 1826. [Google Scholar]
- Dutrochet, H. Mémoires Pour Servir à L’histoire Anatomique et Physiologique des Vegetaux et des Animaux; Baillière: Paris, France, 1837. [Google Scholar]
- Rominger, C.L. Versuche über die Saftführung der Gefässe. Bot. Ztg. 1843, 1, 177–185. [Google Scholar]
- Brücke, E. Ueber das Bluten des Rebstockes. Annalen der Physik und Chemie 1844, 63, 177–214. [Google Scholar] [CrossRef] [Green Version]
- Kraus, C. Untersuchungen über den Säftedruck der Pflanzen; Flora Oder Allgemeine Botanische Zeitung; Regensburgische Botanische Gesellschaft: Regensburg, Germany, 1881–1883. [Google Scholar]
- James, W.O.; Baker, H. Sap pressure and the movements of sap. New Phytol. 1933, 32, 317–343. [Google Scholar] [CrossRef]
- Köckenberger, W.; Pope, J.M.; Xia, Y.; Jeffrey, K.R.; Komor, E.; Callaghan, P.T. A non-invasive measurement of phloem and xylem water flow in castor bean seedlings by nuclear magnetic resonance microimaging. Planta 1997, 201, 53–63. [Google Scholar] [CrossRef]
- Schobert, C.; Komor, E. Transport of nitrate and ammonium into the phloem and the xylem of Ricinus communis seedlings. J. Plant Physiol. 1992, 140, 306–309. [Google Scholar] [CrossRef]
- Scheenen, T.W.; Vergeldt, F.J.; Heemskerk, A.M.; Van As, H. Intact plant magnetic resonance imaging to study dynamics in long-distance sap flow and flow-conducting surface area. Plant Physiol. 2007, 144, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Köckenberger, W. Functional imaging of plants by magnetic resonance experiments. Trends Plant Sci. 2001, 6, 286–292. [Google Scholar] [CrossRef]
- Van As, H. Intact plant MRI for the study of cell water relations, membrane permeability, cell-to-cell and long distance transport. J. Exp. Bot. 2007, 58, 743–756. [Google Scholar] [CrossRef] [Green Version]
- Hofmeister, W. Tabellen zu W. Hofmeisters Aufsatz über Spannung, Ausflussmenge und Ausflussgeschwindigkeit von Säften lebender Pflanzen. Flora Oder Allgemeine Botanische Zeitung 1862, 45, I–XXXIX. [Google Scholar]
- Hocking, P.J. The composition of phloem exudate and xylem sap from tree tobacco (Nicotiana glauca Grah.). Ann. Bot. 1980, 45, 633–643. [Google Scholar] [CrossRef]
- Richardson, P.T.; Baker, D.A.; Ho, L.C. The chemical composition of Cucurbit vascular exudates. J. Exp. Bot. 1982, 33, 1239–1247. [Google Scholar] [CrossRef]
- Shelp, B.J. The composition of phloem exudate and xylem sap from broccoli (Brassica oleracea var. italica) supplied with NH4+, NO3- or NH4NO3. J Exp. Bot. 1987, 38, 1619–1636. [Google Scholar] [CrossRef]
- Schurr, U.; Schulze, E.D. The concentration of xylem sap constituents in root exudate, and in sap from intact, transpiring castor bean plants (Ricinus communis L.). Plant Cell Environ. 1995, 18, 409–420. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Sanchez, A.; Lorente, F.A.; Guzman, M. A daily rhythmic model for pH and volume from xylem sap of tomato plants. Communications in Soil Science and Plant Analysis 1995, 27, 1859–1874. [Google Scholar] [CrossRef]
- Wallace, A.; Abou-Zamzam, A.M.; Motoyama, E. Cation and anion balance in the xylem exudate of tobacco roots. Plant Soil 1971, 35, 433–438. [Google Scholar] [CrossRef]
- Siebrecht, S.; Tischner, R. Changes in the xylem exudate composition of poplar (Populus tremula × P. alba)—dependent on the nitrogen and potassium supply. J. Exp. Bot. 1999, 50, 1797–1806. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.J.; Holland, P.T.; Smith, G.S. Chemical composition of bleeding xylem sap from kiwifruit vines. Ann. Bot. 1986, 58, 353–362. [Google Scholar] [CrossRef]
- Andersen, P.C.; Brodbeck, B.V. Diurnal and temporal changes in the chemical profile of xylem exudate from Vitis rotundifolia. Physiol. Plant. 1989, 75, 63–70. [Google Scholar] [CrossRef]
- Glad, C.; Regnard, J.L.; Querou, Y.; Brun, O.; Morot-Gaudry, J.F. Flux and chemical composition of xylem exudates from chardonnay grapevines: Temporal evolution and effect of recut. Am. J. Enol. Vitic. 1992, 43, 275–282. [Google Scholar]
- Sabinin, D.A. On the root system as an osmotic apparatus. Perm Univ. Bull. 1925, 4, 1–128. [Google Scholar]
- Ketchie, D.O.; Lopushinsky, W. Composition of Root Pressure Exudate from Conifers; Research Note PNW-395; USDA, Forest Service: Washington, DC, USA, 1981; pp. 1–6.
- Nollet, J.-A. Recherches sur les causes du bouillonnement des liquides. In Mémoires de Mathématique et de Physique, tirés des registres de l’Académie Royale des Sciences de l’année; Imprimerie Nationale: Tournai, Belgium, 1748; pp. 57–104. [Google Scholar]
- Hofmeister, W. Ueber Spannung, Ausflussmenge und Ausflussgeschwindigkeit von Säften lebender Pflanzen. Flora Oder Allgemeine Botanische Zeitung 1862, 45, 97–108, 113–120, 138–144, 145–152, 170–175. [Google Scholar]
- Sachs, J. Handbuch der Experimental-Physiologie der Pflanzen; Engelmann: Leipzig, Germany, 1865. [Google Scholar]
- Pfeffer, W. Osmotische Untersuchungen. Studien zur Zellmechanik; Engelmann: Leipzig, Germany, 1877. [Google Scholar]
- Van’t Hoff, J.M. Die Gesetze des chemischen Gleichgewichts; Engelmann: Leipzig, Germany, 1900. [Google Scholar]
- Pfeffer, W. Zur Kenntniss der Plasmahaut und der Vacuolen nebst Bemerkungen über den Aggregatzustand des Protoplasmas und über osmotische Vorgänge; Hirzel: Leipzig, Germany, 1890. [Google Scholar]
- Pfeffer, W. Studien zur Energetik der Pflanze; Hirzel: Leipzig, Germany, 1892. [Google Scholar]
- Pfeffer, W. Pflanzenphysiologie. Ein Handbuch der Lehre vom Stoffwechsel und Kraftwechsel in der Pflanze, 2nd ed.; Engelmann: Leipzig, Germany, 1897; Volume 1. [Google Scholar]
- Atkins, W.R.G. Some Recent Researches in Plant Physiology; Whittaker: London, UK, 1916. [Google Scholar]
- Priestley, J.H. Further observations upon the mechanism of root pressure. New Phytol. 1922, 21, 41–47. [Google Scholar] [CrossRef]
- Eaton, F. The osmotic and vitalistic interpretations of exudation. Am. J. Bot. 1943, 30, 663–674. [Google Scholar] [CrossRef]
- Kramer, P.J. Physical and physiological aspects of water absorption. In Encyclopedia of Plant Physiology, 3 (Water Relations of Plants); Ruhland, W., Ed.; Springer: Berlin/Göttingen/Heidelberg, Germany, 1956; pp. 124–159. [Google Scholar]
- Marbach, S.; Bocquet, L. Osmosis, from molecular insights to large-scale applications. Chem. Soc. Rev. 2019, 48, 3102–3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staverman, A.J. Non-equilibrium thermodynamics of membrane processes. Trans. Faraday Soc. 1952, 48, 176–185. [Google Scholar] [CrossRef]
- Kedem, O.; Katchalsky, A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim. Biophys. Acta 1958, 27, 229–246. [Google Scholar] [CrossRef]
- Kedem, O.; Katchalsky, A. A physical interpretation of the phenomenological coefficients of membrane permeability. J. Gen. Physiol. 1961, 45, 143–179. [Google Scholar] [CrossRef] [Green Version]
- Kedem, O.; Katchalsky, A. Permeability of composite membranes. Part 1—Electric current, volume flow and flow of solute through membranes. Trans. Faraday Soc. 1963, 59, 1918–1930. [Google Scholar] [CrossRef]
- Dainty, J. Water relations of plant cells. Adv. Bot. Res. 1963, 1, 279–326. [Google Scholar]
- Van Overbeek, J. Water uptake by excised root systems of the tomato due to non-osmotic forces. Am. J. Bot. 1942, 29, 677–683. [Google Scholar] [CrossRef]
- Anderson, W.P.; Aikman, D.P.; Meiri, A. Excised root exudation—A standing-gradient osmotic flow. Proc. R. Soc. Lond. B Biol. Sci. 1970, 174, 445–458. [Google Scholar]
- Ginsburg, H. Model for iso-osmotic water flow in plant roots. J. Theor. Biol. 1971, 32, 147–158. [Google Scholar] [CrossRef]
- Katou, K.; Taura, T.; Furumoto, M. A model for water transport in the stele of plant roots. Protoplasma 1987, 140, 123–132. [Google Scholar] [CrossRef]
- Lyalin, O.O. Theory of transcellular osmosis: Reverse osmosis model of root exudation. Fiziol. Rast. (Sov. Plant Physiol. Engl. Transl.) 1989, 38, 421–434. [Google Scholar]
- Pickard, W.F. The riddle of root pressure: I. Putting Maxwell’s demon to rest. Funct. Plant Biol. 2003, 30, 121–134. [Google Scholar]
- Enns, L.C.; McCully, M.E.; Canny, M.J. Solute concentrations in xylem sap along vessels of maize primary roots at high root pressure. J. Exp. Bot. 1998, 49, 1539–1544. [Google Scholar] [CrossRef]
- Enns, L.C.; Canny, M.J.; McCully, M.E. An investigation of the role of solutes in the xylem sap and in the xylem parenchyma as the source of root pressure. Protoplasma 2000, 211, 183–197. [Google Scholar] [CrossRef]
- Kundt, W.; Robnik, M. Water pumps in plant roots. Russ. J. Plant Physiol. 1998, 45, 262–269. [Google Scholar]
- Kundt, W. The hearts of the plants. Curr. Sci. 1998, 75, 98–102. [Google Scholar]
- Wegner, L.H. Root pressure and beyond: Energetically uphill water transport into xylem vessels? J. Exp. Bot. 2014, 65, 381–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegner, L.H. A thermodynamic analysis of the feasibility of water secretion into xylem vessels against a water potential gradient. Funct. Plant Biol. 2015, 42, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.H. Cotransport of water and solutes in plant membranes: The molecular basis, and physiological functions. AIMS Biophys. 2017, 4, 192–209. [Google Scholar] [CrossRef]
- Fricke, W. The significance of water co-transport for sustaining transpirational water flow in plants: A quantitative approach. J. Exp. Bot. 2015, 66, 731–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeuthen, T. Molecular water pumps. Rev.Physiol. Biochem. Pharmacol. 2000, 141, 97–151. [Google Scholar] [PubMed]
- Zeuthen, T. Water-transporting proteins. J. Membr. Biol. 2010, 234, 57–73. [Google Scholar] [CrossRef]
- Zeuthen, T.; McAulay, N. Cotransport of water by Na+–K+–2Cl– cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J. Physiol. 2012, 590, 1139–1154. [Google Scholar] [CrossRef]
- Zeuthen, T.; Gorraitz, E.; Her, K.; Wright, E.M.; Loo, D.D.F. Water permeation through transporters. Proc. Nat. Acad. Sci. USA 2016, 113, E6887–E6894. [Google Scholar] [CrossRef] [Green Version]
- Steudle, E.; Henzler, T. Water channels in plants: Do basic concepts of water transport change? J. Exp. Bot. 1995, 46, 1067–1076. [Google Scholar] [CrossRef]
- Javot, H.; Maurel, C. The role of aquaporins in root water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Gambetta, G.A.; Knipfer, T.; Fricke, W.; McElrone, A.J. Aquaporins and root water uptake. In Plant Aquaporins. Signalling and Communication in Plants; Chaumont, F., Tyerman, S., Eds.; Springer: Cham, Germany, 2017; pp. 133–153. [Google Scholar] [CrossRef]
- Steudle, E.; Peterson, C.A. How does water get through roots? J. Exp. Bot. 1998, 49, 775–788. [Google Scholar] [CrossRef]
- Blasel, D. Untersuchungen zur Wurzelexsudation an Mais, Rebe und verschiedenen Laubbaumarten. Diploma Thesis, Albert Ludwig University of Freiburg, Freiburg im Breisgau, Germany, 1991. [Google Scholar]
- McCully, M.E. Water efflux from the surface of field-grown grass roots: Observations by cryo-scanning electron microscopy. Physiol. Plant. 1995, 95, 217–224. [Google Scholar] [CrossRef]
- De Candolle, A.-P. Physiologie Végétale; Béchet Jeune: Paris, France, 1832; Volume 1. [Google Scholar]
- Molisch, H. Über Wurzelausscheidungen und deren Einwirkung auf organische Substanzen. Sitzungsberichte der Wiener Akademie der Wissenschaften Mathematisch-Naturwissenschaftliche Classe 1888, 96, 84–109. [Google Scholar]
- Stahl, E. Pflanzen und Schnecken: Eine Biologische Studie über die Schutzmittel der Pflanze Gegen Schneckenfrass; Gustav Fischer: Jena, Germany, 1888. [Google Scholar]
- Czapek, F. Zur Lehre von den Wurzelausscheidungen. Jahrbücher für Wissenschaftliche Botanik 1896, 29, 321–390. [Google Scholar]
- Rogers, W.S. Root Studies VIII. Apple root growth in relation to rootstock, soil, seasonal and climatic factors. J. Pomol. Horticult. Sci. 1940, 17, 99–130. [Google Scholar] [CrossRef]
- Richardson, S.D. Root growth of Acer pseudoplatanus L. in relation to grass cover and nitrogen deficiency. Mededelingen van de Landbouwhogeschool te Wageningen/Nederland 1953, 54, 75–97. [Google Scholar]
- Cailloux, M. Observations sur l’exsudation par les poils radiculaires. Ann. ACFAS (Assoc. Can.-Fr. Av. Sci.) 1950, 16, 158–162. [Google Scholar]
- Cailloux, M. Metabolism and the absorption of water by root hairs. Can. J. Bot. 1972, 50, 557–573. [Google Scholar] [CrossRef]
- Ahmad, M.; Cailloux, M. The effects of malonate on absorption of water by root hairs of Avena sativa. Can. J. Bot. 1971, 49, 521–528. [Google Scholar] [CrossRef]
- Ahmad, M.; Cailloux, M. Effects of some respiratory inhibitors on water flux in root hairs of Avena sativa. Can. J. Bot. 1972, 50, 575–579. [Google Scholar] [CrossRef]
- Head, G.C. A study of ‘Exudation’ from the root hairs of apple roots by time-lapse cine-photomicrography. Ann. Bot. 1964, 28, 495–498. [Google Scholar] [CrossRef]
- Moradi, A.B.; Carminati, A.; Vetterlein, D.; Vontobel, P.; Lehmann, E.; Weller, U.; Hopmans, J.W.; Hans-Jörg Vogel, H.-J.; Oswald, S.E. Three-dimensional visualization and quantification of water content in the rhizosphere. New Phytol. 2011, 192, 653–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carminati, A.; Zarebanadkouki, M.; Kroener, E.; Ahmed, M.A.; Holz, M. Biophysical rhizosphere processes affecting root water uptake. Ann. Bot. 2016, 118, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, I.; Armas, C.; Pugnaire, F.I. Water release through plant roots: New insights into its consequences at the plant and ecosystem level. New Phytol. 2012, 193, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.B.; Cardon, Z.G. The magnitude of hydraulic redistribution by plant roots: A review and synthesis of empirical and modeling studies. New Phytol. 2012, 194, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M.; Rothfuss, Y.; Raynaud, T.B.; Biron, P.; Richard, P.; Durand, J.-L.; Couvreur, V.; Vanderborght, J.; Javaux, M. Measuring and modeling hydraulic lift of Lolium multiflorum using stable water isotopes. Vadose Zone J. 2017, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.H.; Caldwell, M.M. Hydraulic lift: Substantial nocturnal water transport between soil layers by Artemisia tridentate roots. Oecologia 1987, 73, 486–489. [Google Scholar] [CrossRef]
- Caldwell, M.; Dawson, T.; Richards, J. Hydraulic lift: Consequences of water efflux from the roots of plants. Oecologia 1998, 113, 151–161. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Caldwell, M.M.; Canadell, J.; Mooney, H.A.; Jackson, R.B.; Parson, D.; Scholes, R.; Sala, O.E.; Trimborn, P. Downward flux of water through roots (i.e., Inverse hydraulic lift) in dry Kalahari sands. Oecologia 1998, 115, 460–462. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Ong, C.K. 1998. The redistribution of soil water by tree root systems. Oecologia 1998, 115, 306–311. [Google Scholar] [CrossRef]
- Breazeale, J.F.; Crider, F.J. Plant association and survival, and the build-up of moisture in semi-arid soils. Tech. Bull. Univ. Ariz. 1934, 53, 95–123. [Google Scholar]
- Gessner, F. Die Wasseraufnahme durch Blätter und Samen. In Encyclopedia of Plant Physiology, 3 (Water Relations of Plants); Ruhland, W., Ed.; Springer: Berlin/Göttingen/Heidelberg, Germany, 1956; pp. 215–246. [Google Scholar]
- Smart, D.R.; Carlisle, E.; Goebel, M.; Núñez, B.A. Transverse hydraulic redistribution by a grapevine. Plant Cell Environ. 2005, 28, 157–166. [Google Scholar] [CrossRef]
- Emerman, S.H. Towards a theory of hydraulic lift in trees and shrubs. In Sixteenth American Geophysical Union Hydrology Days; Morel-Seytoux, H.J., Ed.; Hydrology Days Publication: Atherton, CA, USA, 1996; pp. 147–157. [Google Scholar]
- Cardon, Z.G.; Stark, J.M.; Herron, P.M.; Rasmussen, J.A. Sagebrush carrying out hydraulic lift enhances surface soil nitrogen cycling and nitrogen uptake into inflorescences. Proc. Natl. Acad. Sci. USA 2013, 110, 18988–18993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.C.; Liste, H.H. Plant hydraulic lift of soil water—Implications for crop production and land restoration. Plant Soil 2008, 313, 1–17. [Google Scholar]
- Izumi, Y.; Okaichi, S.; Awala, S.K.; Kawato, Y.; Watanabe, Y.; Yamane, K.; Iijima, M. Water supply from pearl millet by hydraulic lift can mitigate drought stress and improve productivity of rice by the close mixed planting. Plant Prod. Sci. 2018, 21, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wang, T.T.; Zhao, X.L.; Qi, Y.S.; Xu, X. Study on the hydraulic lift of Medicago sativa and Astragalus laxmannii and its effect on their neighborhood plants. Chin. J. Plant Ecol. 2020, 44, 752–762. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Han, Q.Q.; Lü, X.P.; Bai, J.P.; Qiao, Y.; Paré, P.W.; Wang, S.M.; Zhang, J.L.; Wu, Y.N.; Pang, X.P.; Xu, W.B.; et al. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014, 5, 525:1–525:8. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157:1–157:19, Correction in Front Plant Sci. 2019, 10, 420. [Google Scholar] [CrossRef] [Green Version]
- Dietz, S.; Herz, K.; Gorzolka, K.; Jandt, U.; Bruelheide, H.; Scheel, D. Root exudate composition of grass and forb species in natural grasslands. Sci. Rep. 2020, 10, 10691:1–10691:15. [Google Scholar] [CrossRef] [PubMed]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3874–3883. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ataka, M.; Han, M.; Han, Y.; Gan, D.; Xu, T.; Guo, Y.; Zhu, B. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol. 2021, 229, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; de Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef] [Green Version]
- Solov’ev, V.A.; Verenchikov, S.P. Characterization of xylem exudate obtained without shoot detachment. Fiziol. Rast. (Sov. Plant Physiol. Engl. Transl.) 1987, 34, 554–559. [Google Scholar]
- Ionenko, I.F.; Zyalalov, A.A. Effect of potassium and abscisic and indole-3-acetic acids on maize root xylem exudation and potassium efflux. Biol. Plant. 1999, 42, 137–141. [Google Scholar] [CrossRef]
- Stevens, C.L.; Eggert, R.L. Observations on the causes of flow of sap in red maple. Plant Physiol. 1945, 20, 636–648. [Google Scholar] [CrossRef] [Green Version]
- Marvin, J.W.; Greene, M.T. Temperature induced sap flow in excised stems of Acer. Plant Physiol. 1951, 26, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Ewers, F.W.; Améglio, T.; Cochard, H.; Martignac, M.; Vandame, M.; Bodet, C.; Cruiziat, P. Seasonal variation of xylem pressure in walnut trees: Root and stem pressure. Tree Physiol 2001, 21, 1123–1132. [Google Scholar] [CrossRef]
- Hamilton, E.S.; Schlegel, A.M.; Haswell, E.S. United in diversity: Mechanosensitive ion channels in plants. Ann. Rev. Plant. Biol. 2015, 66, 113–137. [Google Scholar] [CrossRef] [Green Version]
- Basu, D.; Haswell, E.S. Plant mechanosensitive ion channels: An ocean of possibilities. Curr. Opin. Plant Biol. 2017, 40, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.; Wege, S.; Gilliham, M. Plant cation-chloride cotransporters (CCC): Evolutionary origins and functional insights. Int. J. Mol. Sc. 2018, 19, 492. [Google Scholar] [CrossRef] [Green Version]
- Tran, D.; Galletti, R.; Neumann, E.D.; Dubois, A.; Sharif-Naeini, R.; Geitmann, A.; Frachisse, J.-M.; Hamant, O.; Ingram, G.C. A mechanosensitive Ca2+ channel activity is dependent on the developmental regulator DEK1. Nat. Commun. 2017, 8, 1009. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Alfonso, Y.; Faulkner, C.; Pendle, A.; Miyashima, S.; Helariutta, Y.; Maule, A. Symplastic intercellular connectivity regulates lateral root patterning. Dev. Cell. 2013, 26, 136–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.R.; Schuler, E.; Kern, J.R.; Fuller, F.H. “Root pressure” in gymnosperms. Science 1958, 128, 308–309. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Wagner, E. Probing short-term root exudation in Zea mays. Environ. Exp. Bot. 1998, 40, 229–235. [Google Scholar] [CrossRef]
- Bose, J.C. The Motor Mechanism of Plants; Longmans, Green: London, UK, 1928. [Google Scholar]
- Martinac, B.; Cox, C.D. Mechanosensory transduction: Focus on ion channels. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; 46p, ISBN 978-012-809-633-8. [Google Scholar] [CrossRef]
- Han, X.; Zhang, C.; Wang, C.; Huang, Y.; Liu, Z. Gadolinium inhibits cadmium transport by blocking non-selective cation channels in rice seedlings. Ecotoxicol Environ Saf. 2019, 179, 160–166. [Google Scholar] [CrossRef]
- Quiquampoix, H.; Ratcliffe, R.G.; Ratkovic, S.; Vucinic, Z. Proton and phosphorus-31 NMR investigation of gadolinium uptake in maize roots. J. Inorganic Biochem. 1990, 38, 265–276. [Google Scholar] [CrossRef]
- Millet, B.; Pickard, B.G. Gadolinium ion is an inhibitor suitable for testing the putative role of stretch-activated ion channels in geotropism and thigmotropism. Biophys. J. 1998, 53, 155a. [Google Scholar]
- Lee, E.; Santana, B.V.N.; Samuels, E.; Benitez-Fuente, F.; Corsi, E.; Botella, M.A.; Perez-Sancho, J.; Vanneste, S.; Friml, J.; Macho, A.; et al. Rare earth elements induce cytoskeleton-dependent and PI4P-associated rearrangement of SYT1/SYT5 endoplasmic reticulum–plasma membrane contact site complexes in Arabidopsis. J. Exp. Bot. 2020, 71, 3986–3998. [Google Scholar] [CrossRef] [Green Version]
- Lane, J.W.; McBride, D.W., Jr.; Hamill, O.P. Amiloride block of the mechanosensitive cation channel in Xenopus oocytes. J. Physiol. 1991, 441, 347–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, N.; Fox, D.; Satter, R.L. Interaction of depolarization-activated K+ channel of Samanea saman with inorganic ion: A patch-clamp study. Plant Physiol. 1990, 94, 424–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, I.W.M. The temperature-dependence of elementary reaction rates: Beyond Arrhenius. Chem. Soc. Rev. 2008, 37, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Volkov, V. Quantitative description of ion transport via plasma membrane of yeast and small cells. Front. Plant Sci. 2015, 6, 425. [Google Scholar] [CrossRef] [Green Version]
- Loo, D.D.; Wright, E.M.; Zeuthen, T. Water pumps. J. Physiol. 2002, 542 Pt 1, 53–60. [Google Scholar] [CrossRef]
- Ramos, R.S.; Caldeira, M.T.; Arruda, P.; De Meis, L. The two Km’s for ATP of corn-root H+-ATPase and the use of Glucose-6-Phosphate and Hexokinase as an ATP-Regenerating System. Plant Physiol. 1994, 105, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Steffensen, A.B.; Oernbo, E.K.; Stoica, A.; Gerkau, N.J.; Barbuskaite, D.; Tritsaris, K.; Rose, C.R.; MacAulay, N. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat. Commun. 2018, 9, 2167:1–2167:13. [Google Scholar] [CrossRef]






| IIi, MPa | K+ | Ca2+ | Cl- | Amino Acids | Sucrose | Fructose | pH | |
|---|---|---|---|---|---|---|---|---|
| Exudation from detached roots | 0.13 ± 0.03 | 17.2 ± 2.9 | 2.4 ± 0.8 | 18.7 ± 3.2 | 7.8 ± 3.5 | 6 ± 1 | 29 ± 11 | 5.2 ± 0.2 |
| Treatment | Jw, μL × cm−2 × h−1 | ||
|---|---|---|---|
| Detached Roots | Segments | ||
| Basal End | Apical End | ||
| No treatment (water) | 1.9 ± 1.0 (100) | 1.6 ± 1.2 (100) | 0.7 ± 0.5 (100) |
| 2, 4-Dinitrophenol, 2.5 × 10−4 M | 0.5 ± 0.4 (26) | 0.4 ± 0.4 (25) | 0.2 ± 0.3 (29) |
| Cytochalasin B, 2 × l0−5 M | 1.3 ± 1.0 (68) | 1.2 ± 1.2 (75) | 0.5 ± 0.3 (71) |
| d-Tubocurarine, 6.7 × 10−5 M | 1.1 ± 0.5 (58) | 0.9 ± 0.8 (56) | 0.5 ± 0.3 (71) |
| Acetylcholine, 10−4 M | 2.2 ± 0.9 (116) | 1.7 ± 0.7 (106) | 1.1 ± 0.8 (157) |
| Noradrenaline, 10−5 M | 2.3 ± 1.1 (121) | 2.1 ± 1.2 (131) | 1.2 ± 1.0 (171) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkov, V.; Schwenke, H. A Quest for Mechanisms of Plant Root Exudation Brings New Results and Models, 300 Years after Hales. Plants 2021, 10, 38. https://doi.org/10.3390/plants10010038
Volkov V, Schwenke H. A Quest for Mechanisms of Plant Root Exudation Brings New Results and Models, 300 Years after Hales. Plants. 2021; 10(1):38. https://doi.org/10.3390/plants10010038
Chicago/Turabian StyleVolkov, Vadim, and Heiner Schwenke. 2021. "A Quest for Mechanisms of Plant Root Exudation Brings New Results and Models, 300 Years after Hales" Plants 10, no. 1: 38. https://doi.org/10.3390/plants10010038





