Elevated Carbon Dioxide Levels Decreases Cucumber Mosaic Virus Accumulation in Correlation with Greater Accumulation of rgs-CaM, an Inhibitor of a Viral Suppressor of RNAi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Atmospheric CO2 Treatments
2.2. Host Plants and Viruses
2.3. CMV Accumulation as Affected by CO2 Level in Wild-Type Plants (Group 1)
2.4. Suppression of RNA Silencing by 2b Protein under Elevated CO2 (Group 2)
2.5. The Gene Expression Involved in Plant RNA-Silencing Signaling Pathway
2.6. CMV Accumulation Determination
2.7. Ca2+ Fluorescence Microscopy
2.8. Statistical Analysis
3. Results
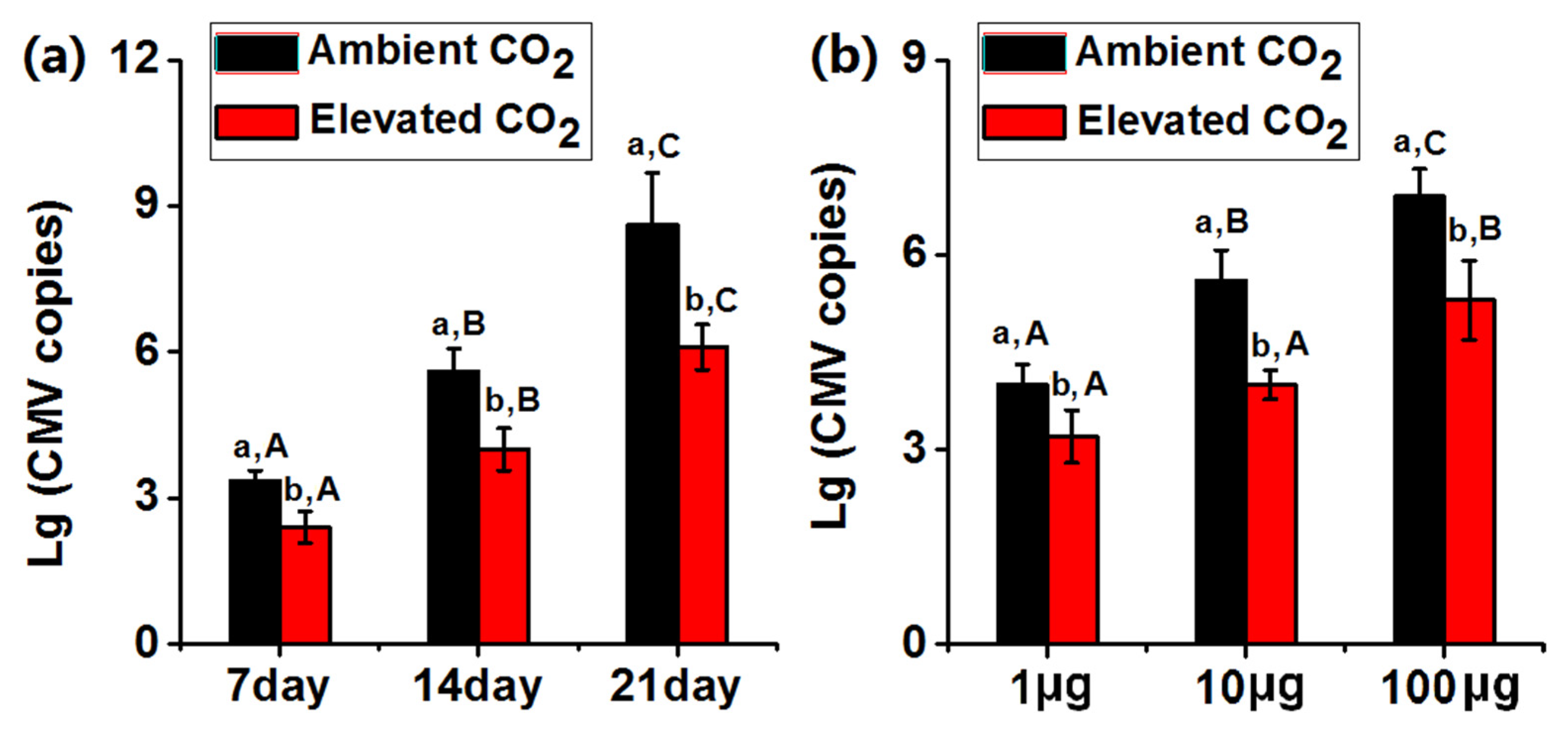
3.1. Elevated CO2 Decreased the Copy Numbers of CMV
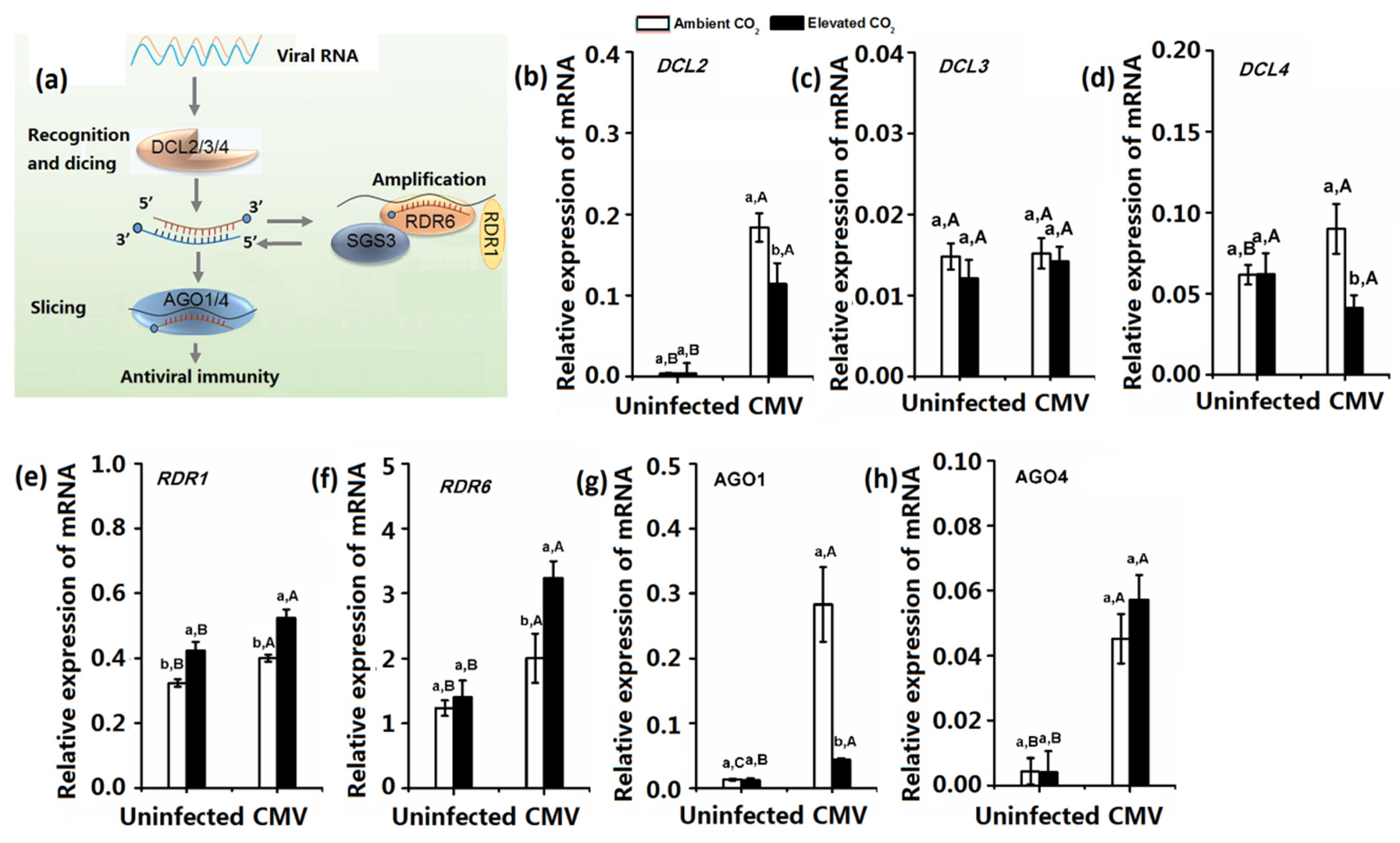
3.2. Elevated CO2 Affect Plant Key Genes Expression Associated with Plant Systemic RNA Silencing
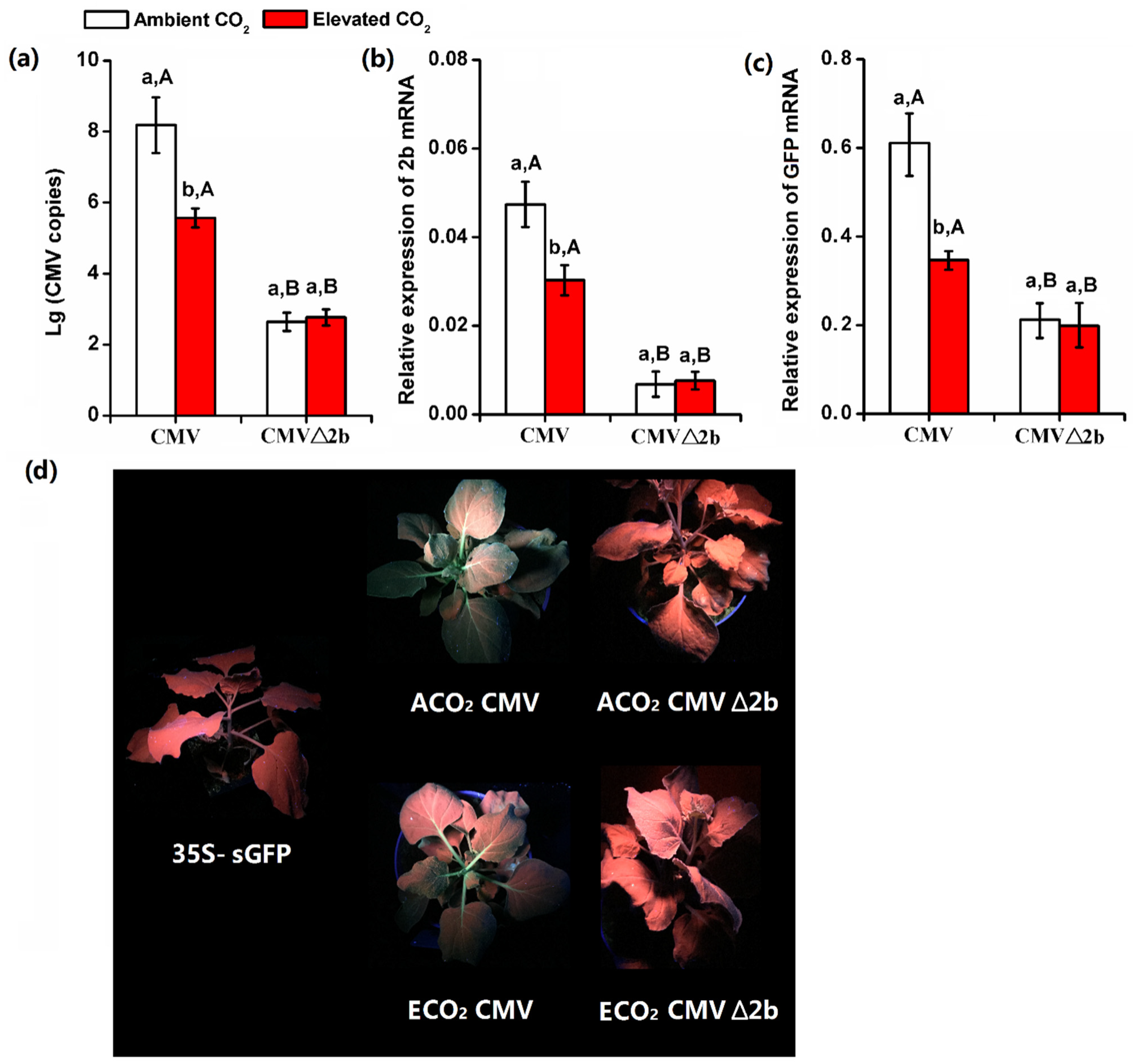
3.3. Elevated CO2 Suppressed the Expression of VSR 2b Protein of CMV
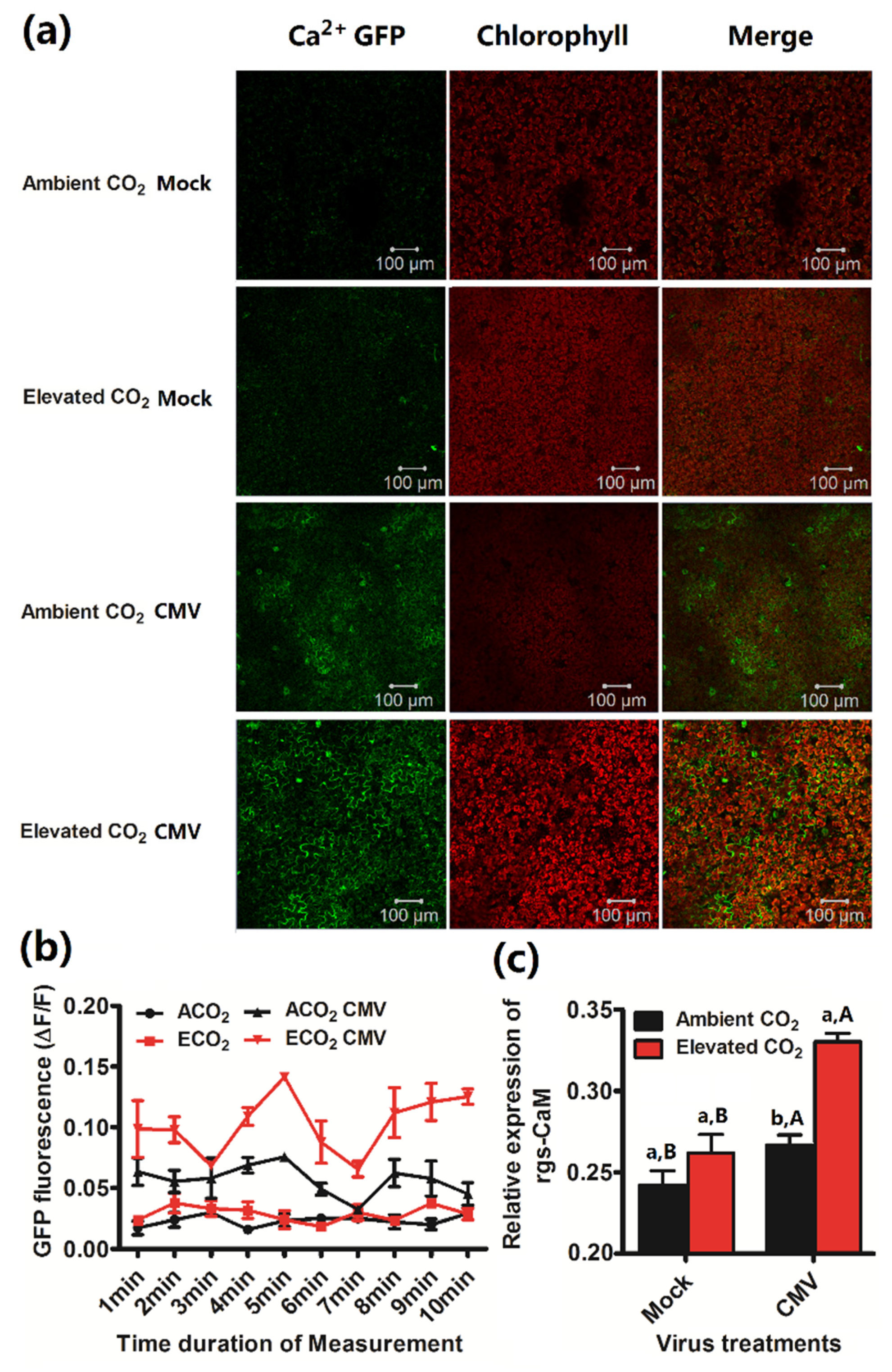
3.4. Calcium Concentration and Calmodulin Gene Expression of Plants Was Increased by Elevated CO2 When Infected by CMV
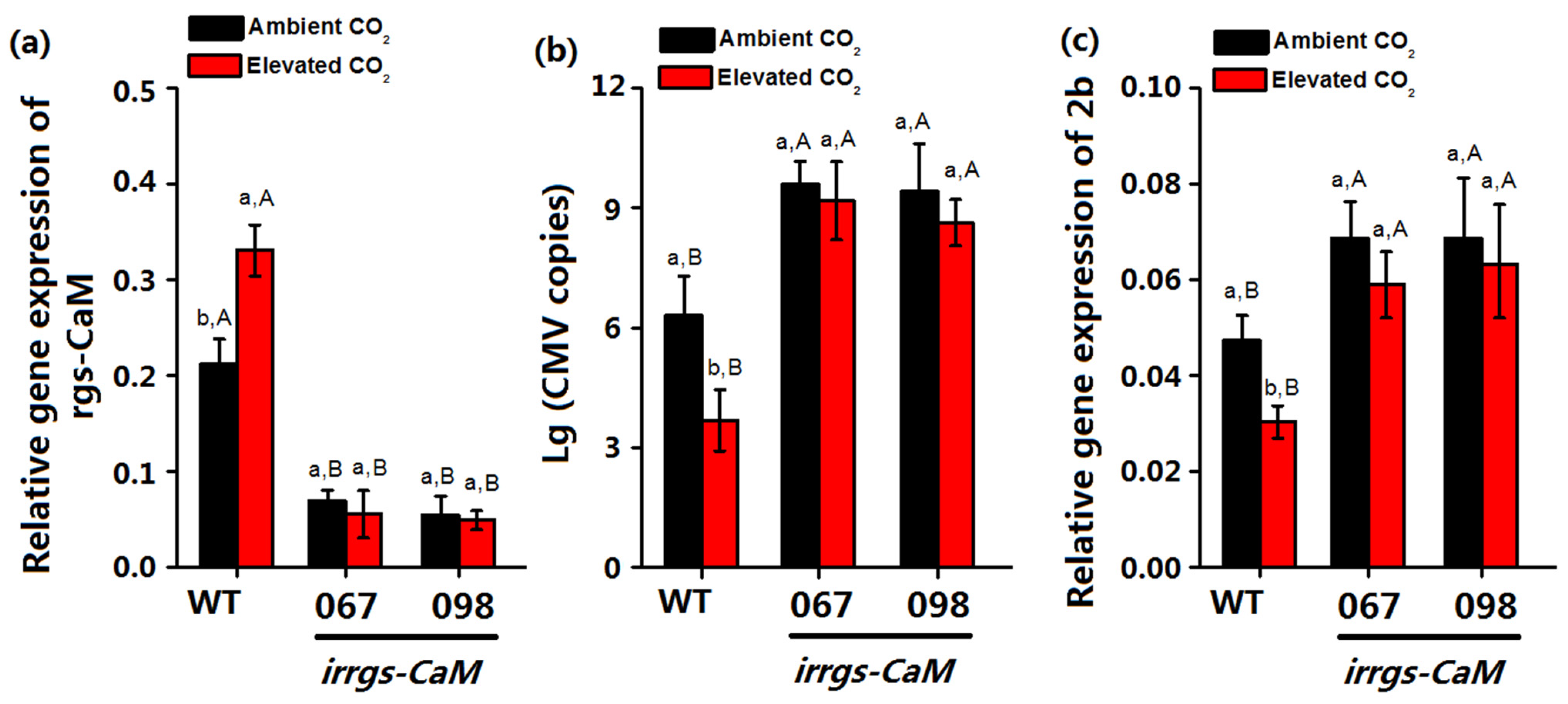
3.5. The CMV Copy Numbers and 2b Gene Expression on Irrgs-CaM Plants under Elevated CO2
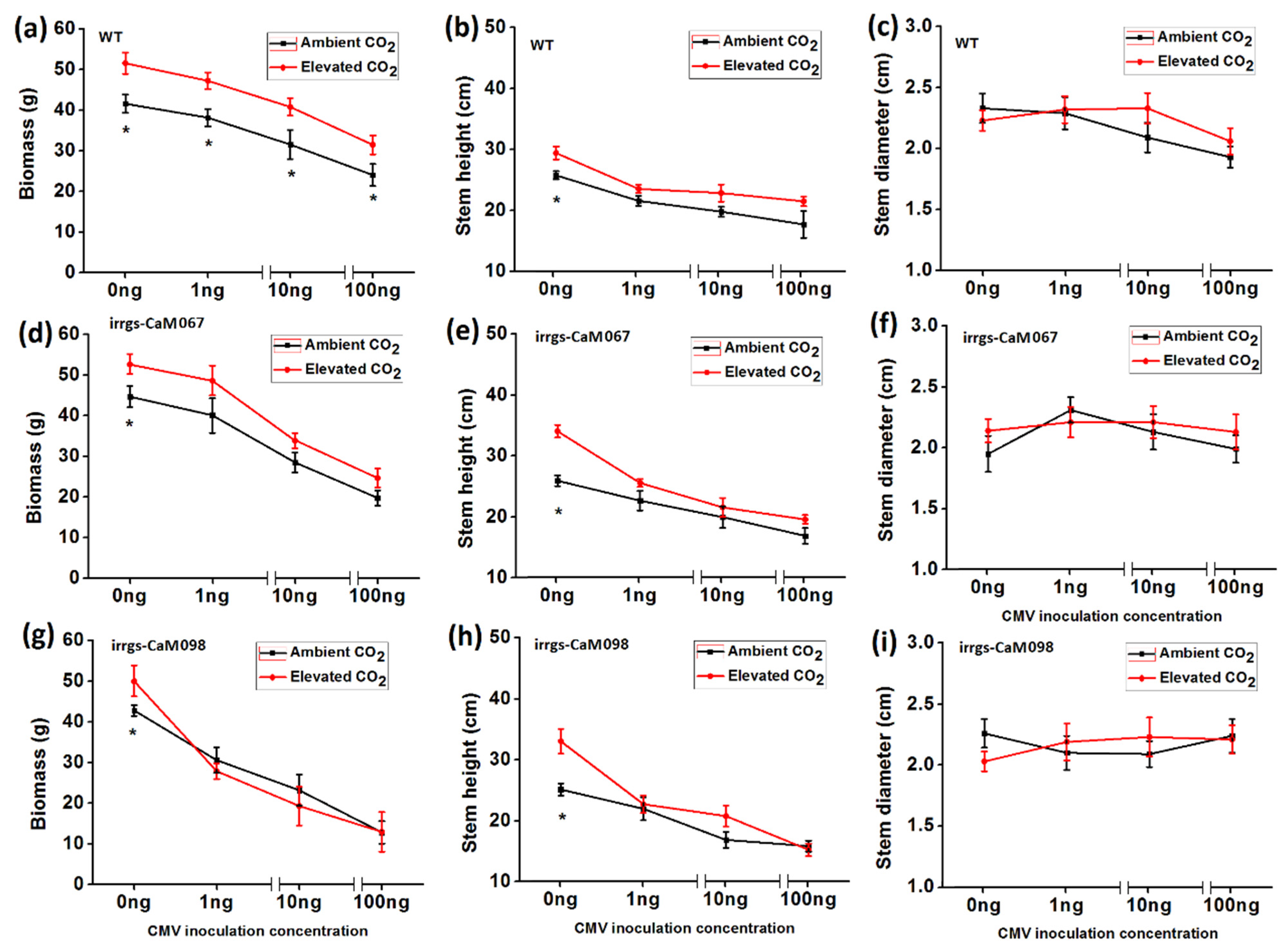
3.6. Growth Traits of Plants as Affected by Plant Genotype, CO2 Levels, and CMV Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2007: The physical science basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Leakey, A.D.; Ort, D.R.; Long, S.P. FACE-ing the facts: Inconsistencies and interdependence among field, chamber and modeling studies of elevated [CO2] impacts on crop yield and food supply. New Phytol. 2008, 179, 5–9. [Google Scholar] [CrossRef]
- Chakraborty, S.; Datta, S. How will plant pathogens adapt to host plant resistance at elevated CO2 under a changing climate? New Phytol. 2003, 159, 733–742. [Google Scholar] [CrossRef]
- Zavala, J.A.; Nabity, P.D.; DeLucia, E.H. An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 2013, 58, 79–97. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Guo, H.; Ge, F. Plant—Aphid interactions under elevated CO2: Some cues from aphid feeding behavior. Front. Plant Sci. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazan, K. Plant-biotic interactions under elevated CO2: A molecular perspective. Environ. Exp. Bot. 2018, 153, 249–261. [Google Scholar] [CrossRef]
- Melloy, P.; Hollaway, G.; Luck, J.O.; Norton, R.O.B.; Aitken, E.; Chakraborty, S. Production and fitness of Fusarium pseudograminearum inoculum at elevated carbon dioxide in FACE. Glob. Chang. Biol. 2010, 16, 3363–3373. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ishiguro, K.; Nakajima, T.; Kim, H.Y.; Okada, M.; Kobayashi, K. Effects of elevated atmospheric CO2 concentration on the infection of rice blast and sheath blight. Phytopathology 2006, 96, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Jwa, N.S.; Walling, L.L. Influence of elevated CO2 concentration on disease development in tomato. New Phytol. 2001, 149, 509–518. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Sun, Z.; Shao, S.; Hu, L.; Ye, M.; Shi, K. Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 2015, 66, 1951–1963. [Google Scholar] [CrossRef] [Green Version]
- Eastburn, D.M.; McElrone, A.J.; Bilgin, D.D. Influence of atmospheric and climatic change on plant–pathogen interactions. Plant Pathol. 2011, 60, 54–69. [Google Scholar] [CrossRef]
- Vaucheret, H. Post-transcriptional small RNA pathways in plants: Mechanisms and regulations. Genes Dev. 2006, 20, 759–771. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.W.; Voinnet, O. Antiviral immunity directed by small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef] [Green Version]
- Matros, A.; Amme, S.; Kettig, B.; Buck-sorlin, G.H.; Sonnewald, U.W.E.; Mock, H.P. Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant Cell Environ. 2006, 29, 126–137. [Google Scholar] [CrossRef]
- Huang, L.; Ren, Q.; Sun, Y.; Ye, L.; Cao, H.; Ge, F. Lower incidence and severity of tomato virus in elevated CO2 is accompanied by modulated plant induced defence in tomato. Plant Biol. 2012, 14, 905–913. [Google Scholar] [CrossRef]
- Voinnet, O. Use, tolerance and avoidance of amplified RNA silencing by plants. Trends Plant Sci. 2008, 13, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Vaucheret, H. Plant argonautes. Trends Plant Sci. 2008, 13, 350–358. [Google Scholar] [CrossRef]
- Bai, M.; Yang, G.-S.; Chen, W.-T.; Mao, Z.; Kang, H.-X.; Chen, G.-H.; Yang, Y.; Xie, B. Genome-wide identification of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families and their expression analyses in response to viral infection and abiotic stresses in Solanum lycopersicum. Gene 2012, 501, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.G.; Fang, Y.Y.; Zhou, B.J.; Zhao, J.H.; Hou, W.N.; Zhu, H.; Ding, S.; Guo, H.S. Suppression of Arabidopsis ARGONAUTE1-mediated slicing, transgene-induced RNA silencing, and DNA methylation by distinct domains of the Cucumber mosaic virus 2b protein. Plant Cell 2012, 24, 259–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, K.S.; Masuta, C.; Yamada, S.; Shimura, H.; Kashihara, Y.; Wada, T.S.; Sekiguchi, T. Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus RNA silencing suppressors. Proc. Natl. Acad. Sci. USA 2012, 109, 10113–10118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Duplessis, S.; White, H.; Karnosky, D.F.; Martin, F.; Podila, G.K. Gene expression patterns of trembling aspen trees following long-term exposure to interacting elevated CO2 and tropospheric O3. New Phytol. 2005, 167, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, H.; Zhu-Salzman, K.; Ge, F. Elevated CO2 increases the abundance of the peach aphid on Arabidopsis by reducing jasmonic acid defenses. Plant Sci. 2013, 210, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liao, L.; Zhang, Y.; Xu, K.; Zhang, J.; Liu, K.; Li, X.; Tan, G.; Zheng, J.; He, Y.; et al. Development of a Gateway-compatible pCAMBIA binary vector for RNAi-mediated gene knockdown in plants. Plasmid 2018, 98, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Krügel, T.; Lim, M.; Gase, K.; Halitschke, R.; Baldwin, I.T. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 2002, 12, 177–183. [Google Scholar] [CrossRef]
- Guo, H.; Gu, L.; Liu, F.; Chen, F.; Ge, F.; Sun, Y. Phid-borne viral spread is enhanced by virus-induced accumulation of plant reactive oxygen species. Plant Physiol. 2019, 179, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.Y.; Zhao, J.H.; Liu, S.W.; Wang, S.; Duan, C.G.; Guo, H.S. CMV2b-AGO interaction is required for the suppression of RDR-dependent antiviral silencing in Arabidopsis. Front. Microbiol. 2016, 7, 1329. [Google Scholar] [CrossRef] [Green Version]
- Schaad, M.C.; Jensen, P.; Carrington, J.C. Formation of plant RNA virus replication complexes on membranes: Role of an endoplasmic reticulum-targeted viral protein. EMBO J. 1997, 16, 4049–4059. [Google Scholar] [CrossRef]
- Brigneti, G.; Voinnet, O.; Wan-Xiang, L.; Ding, S.W.; Baulcombe, D.C. Viral pathogenicity determinants are suppressors of transgene silencing. EMBO J. 1998, 17, 6739–6746. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.P.; Liu, D.S.; Yan, T.; Fang, X.D.; Dong, K.; Xu, J.; Wang, Y.; Yu, J.; Wang, X.B.L. Cucumber mosaic virus coat protein modulates the accumulation of 2b protein and antiviral silencing that causes symptom recovery in planta. PLoS Pathog. 2017, 13, e1006522. [Google Scholar] [CrossRef] [Green Version]
- Candresse, T.; Hammond, R.W.; Hadidi, A. Detection and identification of plant viruses and viroids using polymerase chain reaction (PCR). In Plant Virus Disease Control; Hadidi, A., Khetarpal, R.K., Koganezawa, H., Eds.; APS Press: St. Paul, MN, USA, 1998; pp. 399–416. [Google Scholar]
- Trębicki, P.; Nancarrow, N.; Cole, E.; Bosque-Pérez, N.A.; Constable, F.E.; Freeman, A.J.; Fitzgerald, G.J. Virus disease in wheat predicted to increase with a changing climate. Glob. Chang. Biol. 2015, 21, 3511–3519. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Yang, Y.; Hong, N.; Wang, G.; Wang, A.; Wang, L. Characterization of virus-derived small interfering RNAs in Apple stem grooving virus-infected in vitro-cultured Pyrus pyrifolia shoot tips in response to high temperature treatment. Virol. J. 2016, 13, 166. [Google Scholar] [CrossRef] [Green Version]
- Paudel, D.B.; Sanfaçon, H. Exploring the Diversity of Mechanisms Associated with Plant Tolerance to Virus Infection. Front. Plant Sci. 2018, 9, 01575. [Google Scholar] [CrossRef] [PubMed]
- Haxim, Y.; Ismayil, A.; Jia, Q.; Wang, Y.; Zheng, X.; Chen, T.; Cheng, J. Autophagy functions as an antiviral mechanism against geminiviruses in plants. eLife 2017, 6, e23897. [Google Scholar] [CrossRef]
- Assmann, S.M. The cellular basis of guard cell sensing of rising CO2. Plant Cell Environ. 1999, 22, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.A.; McAinsh, M.R.; Mansfield, T.A.; Hetherington, A.M. Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J. 1996, 9, 297–304. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Ge, P.; Tong, J.; Zhang, Y.; Peng, X.; Zhao, Z.; Ge, F.; Sun, Y. Elevated Carbon Dioxide Levels Decreases Cucumber Mosaic Virus Accumulation in Correlation with Greater Accumulation of rgs-CaM, an Inhibitor of a Viral Suppressor of RNAi. Plants 2021, 10, 59. https://doi.org/10.3390/plants10010059
Guo H, Ge P, Tong J, Zhang Y, Peng X, Zhao Z, Ge F, Sun Y. Elevated Carbon Dioxide Levels Decreases Cucumber Mosaic Virus Accumulation in Correlation with Greater Accumulation of rgs-CaM, an Inhibitor of a Viral Suppressor of RNAi. Plants. 2021; 10(1):59. https://doi.org/10.3390/plants10010059
Chicago/Turabian StyleGuo, Huijuan, Panpan Ge, Jiahui Tong, Yanjing Zhang, Xinhong Peng, Zihua Zhao, Feng Ge, and Yucheng Sun. 2021. "Elevated Carbon Dioxide Levels Decreases Cucumber Mosaic Virus Accumulation in Correlation with Greater Accumulation of rgs-CaM, an Inhibitor of a Viral Suppressor of RNAi" Plants 10, no. 1: 59. https://doi.org/10.3390/plants10010059
APA StyleGuo, H., Ge, P., Tong, J., Zhang, Y., Peng, X., Zhao, Z., Ge, F., & Sun, Y. (2021). Elevated Carbon Dioxide Levels Decreases Cucumber Mosaic Virus Accumulation in Correlation with Greater Accumulation of rgs-CaM, an Inhibitor of a Viral Suppressor of RNAi. Plants, 10(1), 59. https://doi.org/10.3390/plants10010059




