Global Distribution of the Reniform Nematode Genus Rotylenchulus with the Synonymy of Rotylenchulus macrosoma with Rotylenchulus borealis
Abstract
:1. Introduction
2. Results
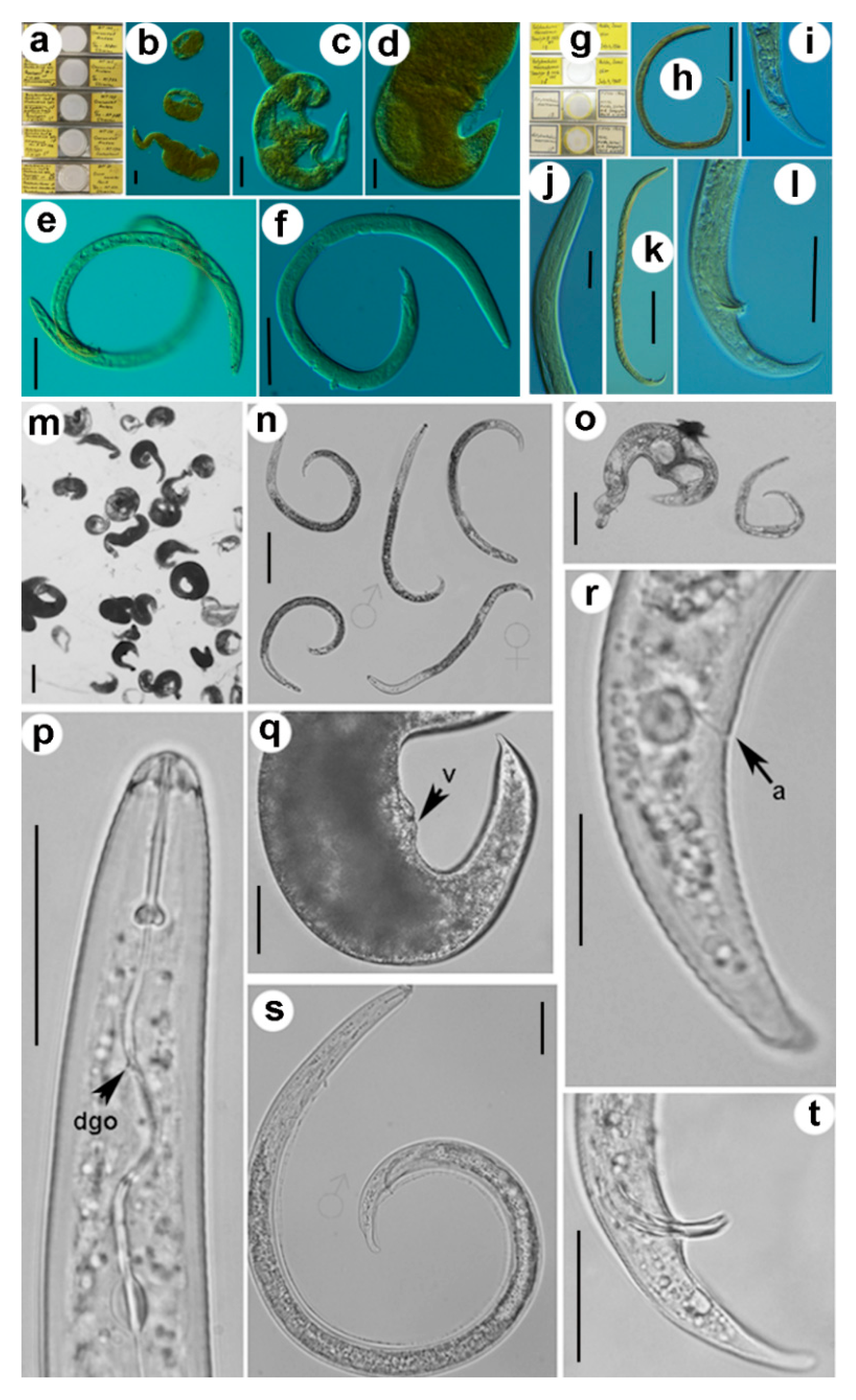
2.1. Morphometric Comparison of Paratypes and Several Populations of Rotylenchulus Borealis and Rotylenchulus Macrosoma
2.2. Molecular Characterisation and Phylogenetic Analysis of Rotylenchulus Borealis and Rotylenchulus Macrosoma Populations
2.3. Global Distribution Rotylenchulus spp.
3. Discussion
4. Materials and Methods
4.1. Nematode Populations and Morphometric Studies
4.2. DNA Extraction, PCR, and Sequencing
4.3. Phylogenetic Analysis
4.4. Data Collection of Global Distribution of Rotylenchulus spp. and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, A.F.; Inserra, R.N.; Caswell-Chen, E.P.; Vovlas, N.; Troccoli, A. Rotylenchulus species: Identification distribution, host ranges, and crop plant resistance. Nematropica 1997, 27, 127–180. [Google Scholar]
- Linford, M.B.; Oliveira, J.M. Rotylenchulus reniformis nov. gen., n. sp., a nematode parasite of roots. Proc. Helm. Soc. Wash. 1940, 7, 35–42. [Google Scholar]
- Van den Berg, E.; Palomares-Rius, J.E.; Vovlas, N.; Tiedt, L.R.; Castillo, P.; Subbotin, S.A. Morphological and molecular characterisation of one new and several known species of the reniform nematode, Rotylenchulus Linford & Oliveira, 1940 (Hoplolaimidae: Rotylenchulinae), and a phylogeny of the genus. Nematology 2016, 18, 67–107. [Google Scholar]
- Palomares-Rius, J.E.; Archidona-Yuste, A.; Cantalapiedra-Navarrete, C.; Azpilicueta, A.; Saborido, A.; Tzortzakakis, E.A.; Cai, R.; Castillo, P. New distribution and molecular diversity of the reniform nematode Rotylenchulus macrosoma Dasgupta, Raski and Sher, 1968 (Nematoda: Rotylenchulinae) in Europe. Phytopathology 2020, 110. in press. [Google Scholar] [CrossRef]
- Colagiero, M.; Ciancio, A. Climate change and nematodes: Expected effects and perspectives for plant protection. Redia 2011, 94, 113–118. [Google Scholar]
- Jones, L.M.; Koehler, A.K.; Trnka, M.; Balek, J.; Challinor, A.J.; Atkinson, H.J.; Urwin, P.E. Climate change is predicted to alter the current pest status of Globodera pallida and G. rostochiensis in the United Kingdom. Glob. Chang. Biol. 2017, 23, 4497–4507. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Intergovernmental Panel on Climate Change. 2019. Available online: https://archive.ipcc.ch/index.htm (accessed on 17 September 2020).
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Palomares-Rius, J.E.; Cantalapiedra-Navarrete, C.; Archidona-Yuste, A.; Tzortzakakis, E.A.; Birmpilis, I.G.; Vovlas, N.; Subbotin, S.A.; Castillo, P. Prevalence and molecular diversity of reniform nematodes of the genus Rotylenchulus (Nematoda: Rotylenchulinae) in the Mediterranean Basin. Eur. J. Plant Pathol. 2018, 150, 439–455. [Google Scholar] [CrossRef]
- Dasgupta, D.R.; Raski, D.J.; Sher, S.A. A revision of the genus Rotylenchulus Linford and Oliveira, 1940 (Nematoda: Tylenchidae). Proc. Helminthol. Soc. Wash. 1968, 35, 169–192. [Google Scholar]
- Loof, P.A.A.; Oostenbrink, M. Rotylenchulus borealis n. sp. with a key to the species of Rotylenchulus. Nematologica 1962, 7, 83–90. [Google Scholar]
- Germani, G. Morphological and biometrical characters of three West-African species of Rotylenchulus Linford & Oliveira, 1940 (Nematoda: Tylenchida). Rev. Nématol. 1978, 1, 241–250. [Google Scholar]
- Castillo, P.; Vovlas, N.; Troccoli, A. The reniform nematode, Rotylenchulus macrosoma, infecting olive in southern Spain. Nematology 2003, 5, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Niragire, I. Morphological and Molecular Characterization of Plant-Parasitic Nematodes of Potato (Solanum tuberosum I.) in Rwanda. Master’s Thesis, University of Ghent, Ghent, Belgium, 2018; p. 44. [Google Scholar]
- Singh, P.R.; Kashando, B.E.; Couvreur, M.; Karssen, G.; Bert, W. Plant-parasitic nematodes associated with sugarcane in Kilimanjaro, Tanzania. J. Nematol. 2020, 52, e2020-59. [Google Scholar] [CrossRef]
- Qing, X.; Bik, H.; Yergaliyev, T.M.; Gu, J.; Fonderie, P.; Brown-Miyara, S.; Szitenberg, A.; Bert, W. Widespread prevalence but contrasting patterns of intragenomic rRNA polymorphisms in nematodes: Implications for phylogeny, species delimitation and life history inference. Mol. Ecol. Resour. 2020, 20, 318–332. [Google Scholar] [CrossRef]
- Lišková, M.; Troccoli, A.; Vovlas, N.; Sasanelli, N. On the occurrence of Rotylenchulus borealis in the Slovak Republic. Helminthologia 2002, 39, 165–167. [Google Scholar]
- Cohn, E.; Mordechai, M.M. Morphology and parasitism of the mature female of Rotylenchulus macrosomus. Rev. Nématol. 1988, 11, 385–389. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolph, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Riascos-Ortiz, D.; Mosquera-Espinosa, A.T.; De Agudelo, F.V.; de Oliveira, C.M.G.; Muñoz-Flórez, J.E. Morpho-molecular characterization of Colombian and Brazilian populations of Rotylenchulus associated with Musa spp. J. Nematol. 2019, 51, e2019-47. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.P.; Tilman, D.; Purschke, O.; Ciobanu, M.; Cowles, J.; Isbell, F.; Wragg, P.D.; Eisenhauer, N. Climate warming promotes species diversity, but with greater taxonomic redundancy, in complex environments. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Neher, D.A. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Gaur, H.S. Dissemination and mode of survival of nematodes in dust storms. Ind. J. Nematol. 1988, 18, 84–98. [Google Scholar]
- Dasgupta, D.R.; Raski, D.J. The biology of Rotylenchulus parvus. Nematologica 1968, 14, 429–440. [Google Scholar]
- Siddiqi, M.R. Tylenchida Parasites of Plants and Insects, 2nd ed.; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Coolen, W.A. Methods for extraction of Meloidogyne spp. and other nematodes from roots and soil. In Root-Knot Nematodes (Meloidogyne species). Systematics, Biology, and Control; Lamberti, F.F., Taylor, C.E., Eds.; Academic Press: London, UK, 1979; pp. 317–329. [Google Scholar]
- Seinhorst, J.W. Killing nematodes for taxonomic study with hot f.a. 4:1. Nematologica 1966, 12, 178. [Google Scholar] [CrossRef]
- De Grisse, A.T. Redescription ou modification de quelques techniques utilisées dans l’étude des nematodes phytoparasitaires. Meded. Rijksfak. Landbouwet. 1969, 34, 351. [Google Scholar]
- De Ley, P.; Felix, M.A.; Frisse, L.A.; Nadler, S.; Sternberg, P.; Thomas, W. Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1999, 1, 591–612. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Vierstraete, A.; De Ley, P.; Rowe, J.; Waeyenberge, L.; Moens, M.; Vanfleteren, J.R. Phylogenetic relationships within the cyst-forming nematodes (Nematoda, Heteroderidae) based on analysis of sequences from the ITS regions of ribosomal DNA. Mol. Phylogenet. Evol. 2001, 21, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–174. [Google Scholar] [CrossRef]
- Altschul, S.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A. FigTreev1.4.2. A Graphical Viewer of Phylogenetic Trees. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 October 2020).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. Available online: http://qgis.osgeo.org (accessed on 12 November 2020).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- R_Core_Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Foundation: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 6 October 2020).






| Locality, Country | Nematode Code | D2-D3 | ITS1 | coxI |
|---|---|---|---|---|
| Rotylenchulus borealis | ||||
| Huissen, Betuwe region (the Netherlands) | AV23 | MW173970 | MW1742399 | MW182432 |
| Huissen, Betuwe region (the Netherlands) | AV25 | MW173971 | MW174240 | - |
| Huissen, Betuwe region (the Netherlands) | AV26 | MW173972 | - | MW182433 |
| Huissen, Betuwe region (the Netherlands) | AV27 | MW173973 | MW174241 | MW182434 |
| Huissen, Betuwe region (the Netherlands) | AV28 | MW173974 | MW174242 | MW182435 |
| Huissen, Betuwe region (the Netherlands) | AV29 | MW173975 | - | |
| Huissen, Betuwe region (the Netherlands) | AV30 | MW173976 | - | |
| Rotylenchulus macrosoma | ||||
| Beit She’an (Israel) | C26 | MW173977 | - | - |
| Beit She’an (Israel) | C27 | MW173978 | - | - |
| Beit She’an (Israel) | C29 | MW173979 | - | - |
| Beit She’an (Israel) | C30 | MW173980 | - | - |
| Beit She’an (Israel) | C31 | MW173981 | MW174243 | - |
| Beit She’an (Israel) | C32 | MW173982 | MW174244 | - |
| Beit She’an (Israel) | C45 | MW173983 | MW174245 | - |
| Beit She’an (Israel) | C46 | MW173984 | MW174246 | - |
| Beit She’an (Israel) | C47 | MW173985 | - | - |
| Grasses, Arnhem, the Netherlands, Paratypes | Paratypes from WANECO Nematode Collection, Re-Measured in This Study | (R. variabilis = R. borealis) Rumex sp., Inyanga Orchard Area, Southern Rhodesia (Dasgupta et al. [10] | Upland Rice, Ferkessedougou, Ivory Coast [12] | Sweet Potato, Ferkessedougou, Ivory Coast [12] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Character/Ratio | Immature Females | Males | Immature Females | Males | Immature Females | Males | Immature Females | Males | Immature Females | Males |
| n | 20 | 40 | 3 | 5 | 22 | 21 | 18 | 17 | 5 | 8 |
| L a | (370–460) | (400–490) | 408 ± 24 (381–424) | 418 ± 12 (404–435) | (300–370) | (340–410) | 420 (360–550) | 480 (400–540) | 470 (430–500) | 520 (480–570) |
| a | (22.5–32.5) | (30.3–40.2) | 27.9 ± 2.3 (25.4–29.9) | 24.1 ± 2.8 (21.6–28.9) | (22–26) | (22–33) | 27 (23–31) | 29.2 (25–42) | 29 (26–31) | 28 (24–36) |
| b | (2.5–3.4) | (3.2–4.0) | 4.1 ± 0.2 (3.9–4.2) | 3.8 ± 0.2 (3.4–3.9) | (3.3–3.9) | (3.1–4.1) | 2.9 (2.0–3.3) | 4.4 (3.8–5.2) | 3.0 (2.8–3.6) | 4.5 (4.5–5.0) |
| c | (11.3–14.8) | (12.1–15.8) | 14.9 ± 0.5 (14.6–15.5) | 14.7 ± 1.0 (13.6–16.1) | (13.0–16.0) | (14–20) | 14.7 (12.5–17.3) | 16 (14–18) | 14.3 (12.0–16.0) | 15 (11–18) |
| c’ | - | - | 2.8 ± 0.2 (2.6–2.9) | 2.8 ± 0.1 (2.6–2.9) | (2.6–3.2) | - | 3.4 (3.0–4.0) | - | 3.5 (3.1–4.4) | - |
| V or T | (57.6–64.8) | (25.0–54.0) | 62.2 ± 1.0 (61.0–63.0) | 39.7 ± 13.5 (23.4–56.4) | (59–66) | (29–51) | 61 (57–67) | - | 62 (59–66) | - |
| o | - | - | 124.4 ± 3.7 (121.4–128.6) | 136.7 ± 8.1 (130.8–150.0) | (120–138) | - | 131 (113–160) | - | 124 (113–147) | - |
| DGO | - | - | 17.0 ± 1.0 (16.0–18.0) | 17.2 ± 0.8 (16.0–18.0) | - | - | - | - | - | - |
| Stylet length | (13.0–16.0) | (12.0–14.0) | 13.7 ± 0.6 (13.0–14.0) | 12.6 ± 0.5 (12.0–13.0) | (13.0–15.0) | (10.0–12.0) | 14.0 (12.0–15.0) | 13.0 (10.0–14.0) | 14.5 (13.0–15.0) | 14.0 (13.0–14.0) |
| Lip region width | - | - | 6.3 ± 0.6 (6.0–7.0) | 6.5 ± 0.5 (6.0–7.0) | - | - | - | - | - | - |
| Tail length | - | - | 27.3 ± 1.5 (26.0–29.0) | 28.6 ± 2.3 (26.0–32.0) | - | - | - | - | - | - |
| h | - | - | 10.0 ± 1.0 (9.0–11.0) | 9.9 ± 0.7 (9.0–11.0) | (3.0–6.0) | (3.0–7.0) | 9.0 (7.0–12.0) | 8.0 (6.0–10.0) | 8.2 (6.0–11.0) | 9.0 (6.0–11.0) |
| Spicule length | - | (20.0–21.0) | - | 21.2 ± 0.8 (20.0–22.0) | - | (19.0–23.0) | - | 22.7 (18.0–24.0) | - | 23.0 (20.0–26.0) |
| Gubernaculum length | - | (7.0–8.0) | - | 7.0 ± 0.7 (6.0–8.0) | - | (7.0–9.0) | - | 9.0 (6.0–10.0) | - | 10.0 (8.0–12.0) |
| Sweet Potato, Bouaké, Ivory Coast [12] | Upland Rice, Bambari, Central African Republic [12] | Cotton, North Republic of Benin [12] | ||||
|---|---|---|---|---|---|---|
| Character/Ratio | Immature Females | Males | Immature Females | Males | Immature Females | Males |
| n | 6 | 2 | 9 | 16 | 6 | 9 |
| L a | 420 (370–500) | 500, 520 | 390 (370–420) | 380 (360–410) | 330 (300–360) | 360 (320–370) |
| a | 25 (24–26) | 26, 27 | 25.2 (23–27) | 27 (21–30) | 23 (21–26) | 24 (21–28) |
| b | 2.9 (2.6–3.4) | 5.0, 5.7 | 2.8 (2.6–3.2) | 3.4 (2.7–3.9) | 2.7 (2.6–2.9) | 3.3 (2.6–3.7) |
| c | 16.7 (13.4–25.5) | 14.6, 17.2 | 15.5 (14.0–17.2) | 17 (14–21) | 14.2 (12.7–15.4) | 15.0 (8.7–16.7) |
| c’ | 3.2 (2.9–3.3) | - | 2.7 (2.3–3.1) | - | 2.7 | - |
| V or T | 62 (60–64) | - | 63.7 (61–65) | - | 63.6 (64–65) | - |
| o | 119 (100–127) | - | 112 (100–128) | - | 134 (130–143) | - |
| DGO | 6.8 (5.0–8.0) | 6.0 (3.0–10.0) | 6.0 (5.0–8.0) | - | ||
| Stylet length | 14.0 (13.0–15.0) | - | 15.0 (14.0–15.0) | 12.0 (11.0–13.0) | 13.0 (13.0–14.0) | 6.3 (4.0–8.0) |
| Lip region width | - | - | - | 22.0 (17.0–25.0) | - | 19.0 (17.0–22.0) |
| Tail length | - | - | - | 8.0 (7.0–11.0) | - | 8.0 (7.0–10.0) |
| h | 7.5 (5.0–11.0) | 8.0, 9.0 | (3.0–6.0) | (3.0–7.0) | 9.0 (7.0–12.0) | 8.0 (6.0–10.0) |
| Spicule length | - | 22.0, 24.0 | - | (19.0–23.0) | - | 22.7 (18.0–24.0) |
| Gubernaculum length | - | - | - | (7.0–9.0) | - | 9.0 (6.0–10.0) |
| Cotton, North Cameroon [12] | Peanut, Burkina Faso [12] | Corn, Somotor, Slovak Republic [17] | Grasses, the Netherlands, This Study | |||||
|---|---|---|---|---|---|---|---|---|
| Character/Ratio | Immature Females | Males | Immature Females | Males | Immature Females | Males | Immature Females | Males |
| n | 10 | 10 | 15 | 2 | 8 | 5 | 5 | 10 |
| L a | 370 (320–400) | 390 (360–420) | 400 (360–450) | 480, 490 | 428 ± 18 (410–457) | 445 ± 17 (416–459) | 427 ± 361 (381–465) | 432 ± 28 (400–490) |
| a | 22.6 (18–29) | 25.6 (23–28) | 24.5 (21–31) | 21, 31 | 29 ± 2.3 (27.0–34.5) | 31 ± 1.5 (28.7–32.1) | 27.5 ± 3.9 (22.5–32.5) | 29.2 ± 4.3 (25.1–40.2) |
| b | 2.6 (2.3–3.0) | 3.1 (2.4–3.6) | 2.7 (2.4–3.2) | 2.4, 3.2 | 4.2 ± 0.3 (3.9–4.6) | 4.0 ± 0.1 (3.6–3.9) | 3.2 ± 0.5 (2.5–3.8) | 3.6 ± 0.2 (3.2–4.0) |
| c | 15.5 (12–21) | 14.9 (13.5–16.0) | 15.8 (13.8–18.1) | 17.4, 21.3 | 13.9 ± 1.4 (12.7–16.7) | 14.0 ± 0.1 (12.8–15.3) | 13.8 ± 1.6 (11.3–15.0) | 14.6 ± 0.9 (13.3–16.0) |
| c’ | 3.2 (2.0–4.3) | - | 2.6 (2.2–3.2) | - | 3.4 ± 0.3 (2.9–3.8) | 3.0 ± 0.3 (2.6–3.5) | 3.0 ± 0.1 (2.9–3.1) | 2.5 ± 0.2 (2.3–2.9) |
| V or T | 62 (59–64) | - | 62 (56–65) | - | 63 ± 1.3 (62–65) | - | 61.9 ± 3.2 (57.0–65.0) | 39.2 ± 10.0 (25.0–54.0) |
| o | 139 (121–167) | - | 131 (108–171) | - | 145 ± 12.8 (122–163) | 145 (130–160) | 119.3 ± 11.9 (106.7–133.3) | 120.7 ± 5.0 (114.3–129.0) |
| DGO | - | - | - | - | - | - | 17.6 ± 1.5 (16.0–20.0) | 16.8 ± 0.6 (16.0–18.0) |
| Stylet length | 13.6 (12.0–14.0) | 12.5 (11.0–14.0) | 14.4 (13.0–15.0) | 13.0, 14.0 | 15.5 ± (15.0–16.5) | 13.0 ± 0.8 (11.5–13.5) | 14.8 ± 1.1 (13.0–16.0) | 13.7 ± 0.6 (12.0–14.0) |
| Lip region width | - | - | - | - | - | - | 6.7 ± 0.6 (6.0–7.0) | 6.4 ± 0.4 (6.0–7.0) |
| Tail length | - | - | - | - | - | - | 30.7 ± 1.2 (30.0–33.0) | 30.5 ± 1.5 (28.5–33.0) |
| h | 4.9 (3.0–7.0) | 7.0 (4.0–9.0) | 6.5 (5.0–8.0) | 7.0, 10.0 | 10.0 ± 2.3 (7.0–14.5) | 10.5 ± 1.0 (9.5–11.5) | 10.0 ± 0.5 (9.5–10.5) | 9.8 ± 0.8 (9.0–10.5) |
| Spicule length | - | 21.0 (18.0–25.0) | - | 19.0, 21.0 | - | 19.0 ± 0.7 (18.5–20.0) | - | 20.4 ± 0.5 (20.0–21.0) |
| Gubernaculum length | - | 7.0 (4.0–9.0) | - | 8.0, 9.0 | - | 7.0 ± 0.4 (6.5–7.5) | - | 7.4 ± 0.5 (7.0–8.0) |
| Olive, Hulda, Israel, Type Population [10] | Paratypes from WANECO and USDA NEMATODE Collections, Re-Measured in This Study | Olive, Growth Chamber Built Population [18] | Wild Olive, Vejer, Cádiz, Spain [13] | Cultivated Olive, Jerez de la Frontera, Cádiz, Spain [3] | |||||
|---|---|---|---|---|---|---|---|---|---|
| Character/Ratio | Immature Females | Males | Immature females | Males | Immature Females | Immature Females | Males | Immature Females | Male |
| n | 21 | 21 | 2 | 2 | 11 | 12 | 11 | 6 | 3 |
| L a | 520–640 | 500–568 | 612, 634 | 493, 517 | 490 (470–510) | 453 ± 28 (408–510) | 467 ± 13 (449–495) | 476 ± 26 (438–502) | 475.0 ± 28 (446–501) |
| a | 30–38 | 30–41 | 27.6, 37.1 | 32.9, 39.5 | 26.8 (24.5–29.5) | 29.8 ± 2.1 (26.3–34.2) | 31.5 ± 2.1 (27.5–34.0) | 30.1 ± 1.2 (28.7–31.4) | 31.7 ± 0.3 (31.3–31.9) |
| b | 3.8–5.7 | 3.7–5.7 | 3.6, 4.1 | 3.8, 5.2 | 3.5 (3.0–3.8) | 3.9 ± 0.3 (3.5–4.4) | 4.7 ± 0.7 (3.5–5.2) | 3.7 ± 0.3 (3.3–4.3) | 3.4 ± 0.3 (3.1–3.6) |
| c | 12–16 | 12–16 | 14.6, 15.3 | 12.0, 12.6 | 12.7 (11.8–14.7) | 13.5 ± 1.3 (11.7–16.8) | 13.9 ± 0.6 (13.1–15.0) | 15.6 ± 1.3 (13.8–17.6) | 15.2 ± 0.3 (14.9–15.4) |
| c’ | 3.7–5.0 | - | 3.5, 4.2 | 3.5, 3.9 | - | 3.7 ± 0.5 (2.8–4.4) | 3.2 ± 0.4 (2.6–3.9) | 3.3 ± 0.4 (2.8–4.0) | 3.1 ± 0.2 (3.0–3.3) |
| V or T | 63.0–68.0 | 20–33 | 62.3, 65.5 | 21.9, 28.6 | 62.1 (58.9–63.3) | 62 ± 2 (59–64) | 33 ± 5 (25–42) | 61.5 ± 1.9 (59.0–64.0) | 25.0 ± 4.1 (20.9–29.1) |
| o | 139.0–188.0 | - | 126.5, 142.9 | 138.5, 141.0 | 134.4 (122.0–140.1) | 152 ± 15 (126–183) | 171 ± 15 (142–188) | 135.8 ± 17.4 (116.0–156.0) | 147.1 ± 8.5 (137.5–154.0) |
| DGO | - | - | 21.5, 25.0 | 18.0, 19.0 | - | 25 ± 2 (22–27) | 23 ± 2 (19.0–26.0) | 24.0 ± 1.4 (22.0–26.0) | 21.0 ± 1.0 (20.0–22.0) |
| Stylet length | 18.0–22.0 | 13.0–16.0 | 17.0, 17.5 | 13.0, 13.5 | 19.7 (18.2–21.1) | 16 ± 1 (15–18) | 14 ± 1 (12–15) | 17.8 ± 1.7 (16.0–20.0) | 15.0 ± 1.0 (14.0–16.0) |
| Lip region width | - | - | - | 5.0, 6.5 | - | - | - | 6.6 ± 0.7 (6.0–7.0) | 6.3 ± 0.6 (6.0–7.0) |
| Tail length | - | - | 41.5, 42.0 | 39.0, 43.0 | - | 34 ± 4 (26–40) | 34 ± 2 (30–36) | 30.8 ± 3.5 (27.0–36.0) | 31.3 ± 1.5 (30.0–33.0) |
| h | 13.0–18.0 | 15.0–23.0 | 18.5 | 10.0, 12.0 | 14.6 11.5–18.2) | 10 ± 1 (9–12) | 11 ± 1 (10–12) | 10.5 ± 1.4 (9.0–12.0) | 10.0 ± 1.0 (9.0–11.0) |
| Spicule length | - | 21.0–24.0 | - | 21.0, 21.5 | - | 22 ± 2 (19–25) | - | 21.3 ± 1.5 (20.0–23.0) | |
| Gubernaculum length | - | 8.0–10.0 | - | 6.5, 7.0 | - | 9 ± 1 (8–10) | - | 9.0 ± 1.0 (8.0–10.0) | |
| Cultivated Olive, Huévar del Aljarafe, Seville Province, Spain [3] | Cultivated Olive, Petrokefali, Crete, Greece [9] | Cultivated Olive, Limnes, Crete, Greece [9] | ||||
|---|---|---|---|---|---|---|
| Character/Ratio | Immature Females | Males | Immature Females | Males | Immature Females | Male |
| n | 10 | 10 | 10 | 5 | 10 | 10 |
| L a | 484 ± 30 (432–520) | 478 ± 31 (432–514) | 467 ± 27 (432–506) | 468 ± 31 (433–503) | 488 ± 31 (428–526) | 463 ± 33 (418–516) |
| a | 29.7 ± 1.5 (27.6–32.1) | 29.9 ± 1.4 (27.6–31.9) | 28.9 ± 1.9 (26.1–31.6) | 30.8 ± 2.1 (27.1–32.0) | 29.7 ± 1.0 (28.5–31.4) | 28.2 ± 1.8 (26.1–31.3) |
| b | 3.7 ± 0.3 (3.3–4.3) | 3.6 ± 0.3 (3.0–4.1) | 3.6 ± 0.3 (3.3–4.3) | 3.4 ± 0.3 (3.1–3.6) | 3.7 ± 0.2 (3.4–4.1) | 3.6 ± 0.3 (3.2–4.0) |
| c | 15.4 ± 1.2 (14.1–17.6) | 14.8 ± 0.7 (13.4–15.4) | 15.3 ± 0.9 (13.8–17.0) | 14.7 ± 0.9 (13.1–15.4) | 15.4 ± 1.0 (14.1–17.1) | 13.8 ± 1.3 (11.6–15.4) |
| c’ | 3.1 ± 0.3 (2.6–4.0) | 3.0 ± 0.1 (2.8–3.1) | 3.3 ± 0.3 (2.8–4.0) | 3.0 ± 0.2 (2.8–3.3) | 3.1 ± 0.4 (2.6–3.8) | 2.9 ± 0.1 (2.8–3.1) |
| V or T | 62.6 ± 2.2 (59.0–66.0) | 27.1 ± 3.8 (21.1–32.2) | 61.4 ± 2.0 (58.0–64.0) | 31.8 ± 1.3 (30.0–33.0) | 62.4 ± 1.9 (60.0–65.0) | 31.3 ± 5.3 (21.5–37.1) |
| o | 138.0 ± 10.4 (126.3–156.3) | 135.1 ± 3.8 (129.4–140.0) | 129.8 ± 16.6 (105.0–156.3) | 142.5 ± 7.2 (133.3–153.0) | 128.8 ± 8.4 (116.7–138.0) | 140.3 ± 5.2 (133.3–147.0) |
| DGO | 23.8 ± 1.3 (22.0–26.0) | 21.6 ± 1.1 (20.0–23.0) | 23.8 ± 1.5 (21.0–26.0) | 20.8 ± 1.3 (20.0–23.0) | 23.6 ± 1.3 (21.0–25.0) | 21.6 ± 1.7 (19.0–24.0) |
| Stylet length | 17.3 ± 1.2 (16.0–19.0) | 16.0 ± 0.9 (15.0–17.0) | 18.5 ± 1.7 (16.0–21.0) | 14.6 ± 0.5 (14.0–15.0) | 17.4 ± 1.4 (15.5–20.0) | 15.4 ± 0.5 (15.0–16.0) |
| Lip region width | 6.6 ± 0.6 (6.0–7.5) | 6.5 ± 0.5 (6.0–7.0) | 6.7 ± 0.7 (6.0–7.5) | 6.6 ± 0.5 (6.0–7.0) | 6.7 ± 0.8 (6.0–8.0) | 6.5 ± 0.6 (6.0–7.5) |
| Tail length | 31.5 ± 3.2 (27.0–37.0) | 32.4 ± 2.3 (29.0–35.0) | 30.6 ± 2.8 (27.0–36.0) | 31.4 ± 0.9 (30.0–32.0) | 31.8 ± 3.0 (28.0–37.0) | 33.6 ± 2.4 (29.0–37.0) |
| h | 10.8 ± 1.6 (9.0–13.0) | 10.6 ± 0.5 (10.0–11.0) | 10.4 ± 1.1 (9.0–12.0) | 10.2 ± 1.3 (9.0–11.0) | 10.6 ± 1.3 (9.0–12.0) | 10.5 ± 0.8 (9.0–12.0) |
| Spicule length | - | 22.6 ± 1.4 (21.0–24.0) | - | 21.8 ± 1.3 (20.0–23.0) | - | 22.4 ± 1.4 (20.0–24.0) |
| Gubernaculum length | - | 10.0 ± 0.9 (9.0–11.0) | - | 9.2 ± 0.8 (8.0–10.0) | - | 10.2 ± 0.8 (9.0–11.0) |
| Potato, Burera, North Rwanda [14] | Almond-Peach Rootstock, Montañana, Zaragoza, Spain, This Study | Corn, Bečej, Vojvodina, Serbia, This Study | Corn, Moretta, Cuneo, Italy, This Study | |||||
|---|---|---|---|---|---|---|---|---|
| Character/Ratio | Immature Females | Males | Immature Females | Males | Immature Females | Males | Immature Females | Males |
| n | 6 | 1 | 10 | 10 | 10 | 10 | 10 | 10 |
| L a | 403 ± 8 (395–416) | 462 | 461 ± 36 (401–517) | 482 ± 33 (410–533) | 425 ± 13 (401–440) | 461 ± 39 (400–505) | 427 ± 11 (411–441) | 436.7 ± 28 (405–483) |
| a | 24.3 ± 0.7 (23.5–25.5) | 29.2 | 27.6 ± 2.4 (25.1–31.9) | 28.1 ± 1.2 (26.5–30.4) | 26.2 ± 1.4 (24.3–28.2) | 25.5 ± 1.6 (22.1–27.3) | 25.9 ± 1.7 (23.0–28.0) | 27.6 ± 1.8 (25.3–31.2) |
| b | 4.1 ± 0.1 (4.0–4.3) | 4.1 | 3.1 ± 0.3 (2.6–3.6) | 4.1 ± 0.2 (3.8–4.6) | 3.5 ± 0.4 (2.9–4.2) | 3.9 ± 0.4 (3.4–4.6) | 3.6 ± 0.4 (2.9–4.2) | 3.9 ± 0.2 (3.0–3.3) |
| c | 13.1 ± 0.7 (12.4–14.2) | 13.6 | 12.6 ± 1.3 (11.0–14.9) | 13.7 ± 1.7 (11.0–17.6) | 12.6 ± 0.7 (12.0–14.0) | 15.3 ± 1.4 (13.7–17.9) | 12.6 ± 0.8 (11.8–14.5) | 13.2 ± 0.9 (12.3–14.6) |
| c’ | 2.9 ± 0.3 (2.5–3.3) | 2.5 | 3.5 ± 0.3 (2.8–4.0) | 2.9 ± 0.3 (2.4–3.6) | 3.7 ± 0.2 (3.4–3.9) | 2.7 ± 0.2 (2.4–3.0) | 3.6 ± 0.2 (3.2–3.8) | 3.1 ± 0.1 (3.0–3.3) |
| V or T | 63.3 ± 1.2 (62.1–64.9) | - | 61.1 ± 0.9 (59.6–62.7) | 27.1 ± 5.3 (18.7–35.1) | 59.9 ± 0.6 (59–61) | 30.5 ± 3 (26–35) | 60.1 ± 1.0 (58.0–61.8) | 23.4 ± 2.1 (21.5–26.0) |
| o | - | - | 127.4 ± 9.6 (112.5–141.2) | 137.4 ± 15.4 (117.9–163.0) | 120 ± 7 (113.3–135.5) | 131 ± 10 (102–133) | 118.0 ± 7.9 (106.3–129.0) | 122.7 ± 7.9 (114.3–143.0) |
| DGO | 20.2 ± 1.6 (18.0–22.0) | - | 20.8 ± 2.4 (17.5–24.0) | 19.1 ± 2.1 (16.0–22.0) | 17.9 ± 1.6 (16.0–21.0) | 18.6 ± 1.1 (17.0–21.0) | 17.7 ± 1.5 (16.0–20.0) | 17.4 ± 1.0 (16.0–20.0) |
| Stylet length | 17.3 ± 0.3 (17.0–18.0) | 16.8 | 16.3 ± 0.8 (15.0–17.0) | 13.9 ± 0.3 (13.5–14.5) | 14.9 ± 0.6 (14.0–16.0) | 14.2 ± 0.3 (13.5–14.5) | 15.0 ± 0.7 (14.0–16.0) | 14.2 ± 0.4 (13.5–15.0) |
| Lip region width | - | - | 6.6 ± 0.4 (6.0–7.0) | 6.1 ± 0.4 (5.5–6.50) | 6.5 ± 0.3 (6.0–7.0) | - | 6.5 ± 0.3 (6.0–7.0) | 6.5 ± 0.4 (6.0–7.0) |
| Tail length | 30.9 ± 1.3 (29.0–32.0) | 34.0 | 37.1 ± 5.1 (27.0–41.5) | 35.6 ± 5.0 (25.5–43.0) | 33.8 ± 1.5 (31.0–35.5) | 30.3 ± 4.1 (23.5–35.0) | 33.9 ± 2.0 (30.0–36.5) | 33.6 ± 1.3 (32.0–36.0) |
| h | - | - | 12.3 ± 1.6 (9.5–14.0) | 11.6 ± 2.0 (7.5–14.5) | 12.2 ± 1.4 (10.5–15.0) | 8.7 ± 1.2 (7.0–10.0) | 12.5 ± 1.7 (10.5–15.0) | 8.5 ± 0.9 (7.0–10.0) |
| Spicule length | - | - | - | 21.9 ± 1.2 (20.0–23.5) | - | 22.3 ± 1.3 (20.0–24.5) | - | 22.9 ± 0.7 (22.0–24.0) |
| Gubernaculum length | - | - | - | 9.2 ± 1.0 (8.0–11.0) | - | 8.0 ± 0.7 (7.0–9.0) | - | 7.7 ± 0.3 (7.0–8.0) |
| Corn, Le Sen, Landes, France, This Study | Wheat, Mihail Kogalniceau, Romania, This Study | Corn, Létavertes, Hajdú-Bihar, Hungary, This Study | Corn, Möckmühl, Heilbronn, Germany, This Study | |||||
|---|---|---|---|---|---|---|---|---|
| Character/Ratio | Immature Females | Males | Immature Females | Males | Immature Females | Males | Immature Females | Males |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| L a | 428 ± 22 (396–473) | 444 ± 21 (411–477) | 435 ± 29 (387–468) | 435 ± 25 (409–485) | 430 ± 14 (412–458) | 432 ± 19.3 (411–474) | 422 ± 16 (409–429) | 417 ± 7 (409–429) |
| a | 26.3 ± 1.3 (24.2–28.3) | 28.3 ± 1.8 (25.7–31.2) | 27.3 ± 1.4 (24.9–29.1) | 28.1 ± 1.8 (25.6–30.3) | 27.0 ± 0.8 (25.8–28.3) | 28.9 ± 2.5 (26.3–32.2) | 27.3 ± 0.7 (26.2–28.3) | 27.6 ± 1.6 (25.6–29.8) |
| b | 3.6 ± 0.4 (3.2–4.3) | 3.9 ± 0.2 (3.6–4.1) | 3.7 ± 0.3 (3.3–4.2) | 3.8 ± 0.2 (3.5–4.0) | 3.7 ± 0.1 (3.6–4.0) | 3.8 ± 0.1 (3.5–4.0) | 3.6 ± 0.1 (3.4–3.8) | 3.7 ± 0.2 (3.4–4.1) |
| c | 12.8 ± 0.8 (11.4–14.3) | 13.5 ± 0.5 (12.6–14.0) | 14.0 ± 0.9 (12.6–15.5) | 13.0 ± 0.5 (12.6–14.0) | 14.1 ± 0.4 (13.5–14.8) | 14.1 ± 1.0 (12.4–15.4) | 14.0 ± 0.2 (13.7–14.3) | 13.1 ± 1.0 (11.6–15.3) |
| c’ | 3.2 ± 0.2 (2.9–3.4) | 3.0 ± 0.2 (2.7–3.2) | 3.0 ± 0.2 (2.8–3.3) | 3.1 ± 0.2 (2.8–3.3) | 3.0 ± 0.1 (2.9–3.3) | 2.9 ± 0.2 (2.7–3.3) | 3.0 ± 0.1 (2.9–3.3) | 3.1 ± 0.2 (2.8–3.3) |
| V or T | 60.5 ± 0.8 (59.5–62.0) | 31.0 ± 6.8 (26.5–43.0) | 60.3 ± 0.9 (58.1–61.5) | 32.2 ± 3.1 (29.5–37.1) | 60.6 ± 0.5 (60.0–61.5) | 33.9 ± 7.4 (27.0–48.1) | 60.6 ± 0.5 (60.0–61.5) | 33.9 ± 7.4 (27.0–48.1) |
| o | 120.1 ± 7.3 (106.7–130.0) | 117.6 ± 6.9 (107.1–129.0) | 124.0 ± 9.7 (106.7–135.5) | 124.5 ± 8.5 (113.3–138.0) | 121.3 ± 8.8 (106.7–137.9) | 125.5 ± 7.6 (115.4–138.0) | 125.0 ± 7.5 (113.3–135.7) | 121.5 ± 6.6 (107.1–129.0) |
| DGO | 17.9 ± 1.5 (16.0–20.0) | 16.8 ± 0.91 (15.0–18.0) | 19.0 ± 1.9 (16.0–21.0) | 17.5 ± 0.6 (17.0–18.5) | 18.3 ± 1.3 (16.0–20.0) | 17.3 ± 1.0 (15.0–18.5) | 18.6 ± 1.1 (17.0–20.0) | 17.0 ± 0.8 (15.0–18.0) |
| Stylet length | 14.9 ± 0.6 (14.0–16.0) | 14.3 ± 0.9 (14.0–15.0) | 15.3 ± 0.5 (14.5–16.0) | 14.1 ± 0.6 (13.0–15.0) | 15.1 ± 0.4 (14.5–15.5) | 13.8 ± 0.5 (13.0–14.5) | 14.9 ± 0.6 (14.0–15.5) | 14.0 ± 0.4 (13.5–15.0) |
| Lip region width | 6.6 ± 0.3 (6.0–7.0) | 6.5 ± 0.3 (6.0–7.0) | 6.6 ± 0.2 (6.5–7.0) | 6.5 ± 0.4 (6.0–7.0) | 6.7 ± 0.3 (6.5–7.0) | 6.6 ± 0.4 (6.0–7.0) | 6.6 ± 0.3 (6.0–7.0) | 6.4 ± 0.5 (6.0–7.0) |
| Tail length | 33.5 ± 1.4 (31.0–35.5) | 33.3 ± 0.7 (32.0–34.0) | 31.0 ± 1.5 (29.0–34.0) | 31.8 ± 1.9 (32.0–38.0) | 30.6 ± 1.3 (29.0–33.0) | 31.2 ± 2.3 (28.0–36.0) | 30.1 ± 1.0 (28.0–31.0) | 32.5 ± 2.0 (28.0–36.0) |
| h | 11.4 ± 0.8 (10.5–12.5) | 10.0 ± 1.5 (8.0–15.5) | 10.4 ± 0.6 (9.5–11.5) | 10.3 ± 1.8 (8.0–12.5) | 10.1 ± 0.4 (9.5–10.5) | 9.9 ± 0.6 (9.0–11.0) | 10.0 ± 0.4 (9.5–10.5) | 9.7 ± 1.3 (8.0–12.5) |
| Spicule length | - | 22.5 ± 0.6 (22.0–23.5) | - | 22.2 ± 0.3 (22.0–23.0) | - | 21.8 ± 2.5 (21.0–22.5) | - | 21.6 ± 0.8 (20.0–22.5) |
| Gubernaculum length | - | 7.8 ± 0.3 (7.5–8.0) | - | 7.7 ± 0.4 (7.0–8.0) | - | 7.5 ± 0.4 (7.0–8.0) | - | 7.4 ± 0.4 (7.0–8.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomares-Rius, J.E.; Clavero-Camacho, I.; Archidona-Yuste, A.; Cantalapiedra-Navarrete, C.; León-Ropero, G.; Braun Miyara, S.; Karssen, G.; Castillo, P. Global Distribution of the Reniform Nematode Genus Rotylenchulus with the Synonymy of Rotylenchulus macrosoma with Rotylenchulus borealis. Plants 2021, 10, 7. https://doi.org/10.3390/plants10010007
Palomares-Rius JE, Clavero-Camacho I, Archidona-Yuste A, Cantalapiedra-Navarrete C, León-Ropero G, Braun Miyara S, Karssen G, Castillo P. Global Distribution of the Reniform Nematode Genus Rotylenchulus with the Synonymy of Rotylenchulus macrosoma with Rotylenchulus borealis. Plants. 2021; 10(1):7. https://doi.org/10.3390/plants10010007
Chicago/Turabian StylePalomares-Rius, Juan E., Ilenia Clavero-Camacho, Antonio Archidona-Yuste, Carolina Cantalapiedra-Navarrete, Guillermo León-Ropero, Sigal Braun Miyara, Gerrit Karssen, and Pablo Castillo. 2021. "Global Distribution of the Reniform Nematode Genus Rotylenchulus with the Synonymy of Rotylenchulus macrosoma with Rotylenchulus borealis" Plants 10, no. 1: 7. https://doi.org/10.3390/plants10010007






