1. Introduction
Carbon dioxide (CO
2) is the most important anthropogenic greenhouse gas, which has increased since the pre-industrial period from 280 to 416 µmol mol
−1 (ppm) in 2019 [
1]. Its atmospheric concentration is expected to rise to ca. 700 ppm at the end of this century [
2]. Besides this CO
2 increase, the average air temperature has risen about 0.9 °C since the late nineteenth century, most of that warming took place in the past 35 years [
3]. According to the Intergovernmental Panel on Climate Change [
4], the increase of global mean temperature by the end of the 21st century (2081–2100), relative to the period (1986–2005), will range from 0.3 °C to 4.8 °C. With these climate projections, the viticulture suitability may be greatly affected in most of the regions of the world [
5,
6,
7], which will need to adapt to changing climatic conditions to continue the production of quality wines at economically sustainable yields [
8,
9,
10].
The increase in global mean temperature is exerting a profound influence on grapevine physiology [
10]. For this reason, during the last decade, the consequences of warming on yield and berry quality have been extensively investigated with different approaches. One of the most important consequences linked to the increased ambient temperatures is the shifting of dates and the shortening of the phenological stages [
11,
12,
13]. Moreover, high temperature has a great impact on berry composition, which lead to the production of unbalanced red wines with high alcohol levels, the reduction of acidity, and changes of the phenolic composition of berries [
14,
15,
16,
17,
18,
19,
20], all of which, have a noticeable impact on the organoleptic properties that distinguish each variety. On the other hand, most studies focused on the influence of elevated CO
2 on grapevines show increased vegetative and fruit biomass due to higher rates of photosynthesis, with little repercussions on the quality of grapes and wine [
21,
22,
23,
24,
25]. However, the effect of elevated CO
2 stimulating grapevine production were attenuated when elevated CO
2 was combined with high temperature [
21,
26]. Within a global change scenario, the ideal grape composition for some specific wines is more difficult to achieve [
27].
The wine industry uses a limited number of
Vitis vinifera L. genotypes, thus in most grape-growing regions, the spread of world-renowned varieties has caused a considerable loss of autochthones grapevine genotypes traditionally grown [
28]. Given the future climatic forecasts, it has been proposed the varietal and clonal selection, as well as diversification of wines produced, as valuable tools to regulate a too much accelerated and/or unbalanced grape ripening process [
12]. In this view, recent studies have shown that the intra-varietal diversity of commercial varieties could be exploited to maintain grape quality under future climate conditions [
18,
29,
30]. On the other hand, in the last decade, there has been a renewed interest in recovering and studying local ancient cultivars to preserve the genetic resources of each grape-growing region [
31,
32,
33,
34]. This trend could be a valuable strategy to sustain higher genetic diversity, as well as product diversity in the markets. To this end, we have recently characterized the oenological potential of some ancient grapevine varieties under greenhouse conditions [
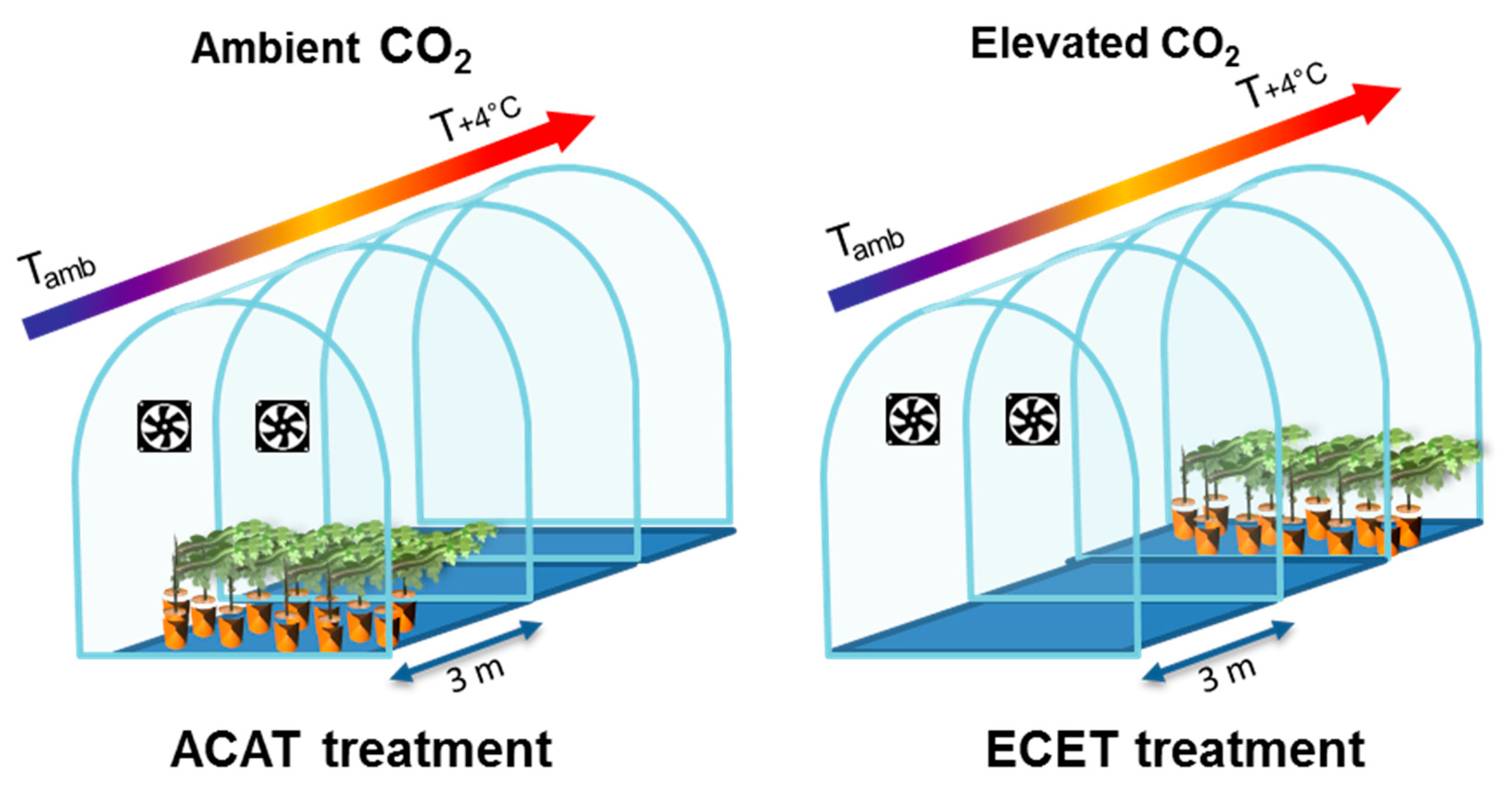
35]. To learn more about some of these varieties, under the present study we undertook a characterization of their response to some climate change conditions, in terms of plant growth and fruit composition. Therefore, this work aimed to determine if the biodiversity hidden within local grapevine varieties could be used as a potential tool to help viticulture to adapt to the future climate scenario. The study was performed in potted vines grown in temperature gradient greenhouses (TGGs) facilities, where an elevated CO
2 treatment of 700 ppm and elevated temperature treatment of +4 °C relative to ambient were imposed, simulating, at least in part, the likely climate conditions around 2100 [
2].
3. Discussion
The adaption capacity of ancient grapevine varieties to climatic conditions has been suggested as a valuable tool to exploit the grapevine diversity in relation to traits that are impacted by climate change, such as phenology, the accumulation of sugars, organic acids, and phenolic compounds [
35,
36,
37]. The hypotheses raised in this study were: (i) the genotypes studied performed differently according to the parameters analyzed; (ii) the distinctive properties of each genotype can evolve differently in the new climate scenario. The seven genotypes from old varieties tested are fully distinguished by features as the length of the reproductive cycle, bunch and berry mass, total soluble solids, TPI, and anthocyanin content (
Figure S1). According to our results, TEMP, GRA72, and GRA63 stood out for having higher anthocyanin content, color density, and TPI, whereas TV, AMB, PAS, and TOR were characterized by presenting longer veraison to maturity and fruit set to maturity periods.
A growing body of evidence shows that grapevine phenology is hastened by elevated temperatures [
12,
13,
38] and high atmospheric CO
2 concentration [
13,
39]. Warmer temperature joint to elevated CO
2 concentrations also produced, in general, a faster grapevine development but the effect had different intensity depending on the commercial clone tested [
39,
40,
41]. By contrast, in our study, the ECET treatment did not modify the length of phenological phases in the genotypes from old varieties tested being. TEMP was the earlier ripening variety and TOR the latest ripening regardless of the treatment applied (
Table 1). A long-term study from Biasi et al. [
36] shows that climate change modified the phenology of autochthonous grapevine varieties to a lesser extent than that of international varieties, which agrees partially with our results. However, it cannot discard that the artificial conditions of the TGGs and the relatively short duration of elevated CO
2 treatment of our study had also influenced the results. In this last aspect, Edwards et al. [
23] indicated that whereas elevated temperature impacted phenology from the onset of treatment, the effects of elevated CO
2 only started to be detected after three years of treatment.
Most studies have reported that the effects of elevated CO
2 on grapevines are more related to bunch and berry mass than to fruit quality. In general, increasing CO
2 led to increasing of the bunch and berry mass [
21,
22,
24,
25] and this effect was maintained when elevated CO
2 interacted with high temperature [
26]. Our results are in contrast with those of Kizildeniz et al. [
26] since that ECET treatment resulted in a general reduction of bunch mass and compactness, and berry mass (
Table 2). Those discrepancies could be explained, at least partially, by differences in the ambient temperatures of each growing season (five heatwaves recorded in the ECET treatment), which could have become the main factor contributing to the reduction of yield detected in our study [
29,
40]. Regarding the genotypes, TV, GRA72 and GRA63 were the most affected by ECET treatment in terms of yield, whereas no changes were detected in bunch or berry characteristics of TEMP, PAS, AMB, and TOR. These results confirm a broad range of responses to changes in environmental conditions among the genotypes from old varieties assessed (
Figure 1). In addition, ECET treatment only reduced the relative skin mass in GRA72. This change could have relevance because the structure of berry skin plays a key role as a constitutive defense barrier against pathogens (for example, fungi) that try to invade grapes. It has been found that warm conditions favoring the development of fungi can also reduce the reinforcement of the berry skin, thus increasing the susceptibility of fruits to be penetrated by fungi growing on their surface [
42].
The rise of air temperature affects gene expression and enzymatic activity of primary and secondary metabolism of grape berries, which could have a considerable impact on must and wine characteristics [
19]. The main effects of warming on berry composition include reduction of anthocyanin content [
14,
20,
29], fall of organic acids [
15], and changes in the composition of phenolic compounds, mainly anthocyanins and flavonols [
18,
43]. In addition, high temperatures hasten the accumulation of sugars in the must, leading to the elaboration of wines with higher alcohol content [
11]. However, the extent of all described impacts on berry quality differs among varieties [
14,
20] and even, among clones within the same variety [
18,
29,
30]. Until now, all these studies have focused on commercial varieties, so studying the behavior of old varieties can provide valuable information to assess their use in a future climate scenario. In fact, our results suggest that, under ECET conditions, the old grapevine genotypes could respond quite distinctly than the reported commercial ones. So, under ECET, the accumulation of sugars was significantly decreased in some cases (TEMP, TV, and GRA72), and no changes were detected in the others (GRA63, PAS, AMB, and TOR) (
Figure 2). These results contrast with general observations that sugar accumulation is largely increased by warm temperatures, such as occurred in commercial clones of Tempranillo [
29], Chardonnay, Shiraz, and Cabernet Sauvignon [
44]. In addition, it has been demonstrated that increasing CO
2 has little effect on total soluble solids in commercial varieties like Riesling and Cabernet Sauvignon [
25] and Tempranillo [
26]. However, in our case, it is possible that the effect of elevated temperature was compensated when the temperature interacted with elevated CO
2. Moreover, it should be taken into account that in the present study, the harvest dates were based on both the total soluble solids and titratable acidity, leading to an early harvest of some genotypes to avoid the excessive loss of acidity (see Material and Methods section). Although comparisons of the behavior of potted plants grown under our artificial conditions with field grapevines are not direct, our results suggest that, under the future climatic scenario, some of the old genotypes tested would be able to control better the sugar accumulation rates than some commercial genotypes grown in similar artificial conditions [
26,
29] (
Figure 2).
Like sugars, titratable acidity reflects the degree of berry ripening. In grapes, the acidity depends on levels of both tartaric and malic acid, whose pathways can be connected [
45]. The general trend is that high temperatures accelerate the decrease of grape acidity during ripening, mainly because of the faster depletion of malic acid leading to potential effects on wine aging capacity [
46]. Thereby, it was reported that high temperatures produced malate losses in commercial varieties as Shiraz [
15], Cabernet Sauvignon, Chardonnay [
14], and Tempranillo [
29]. In addition, studies in which the elevated CO
2 was combined with high temperature have reported decreases of malic acid in different commercial clones of Tempranillo [
39,
41]. Our results partially agree with those described in commercial varieties because some old genotypes (TEMP, TV, and GRA63) also experienced significant decreases of titratable acidity in response to ECET treatment (
Figure 2).
Some research has indicated that, under a global warming scenario, the decoupling between the accumulation of primary metabolites (namely, sugars and organic acids) and the secondary metabolites (i.e., phenolic and aromatic compounds) in berries will be accentuated [
16,
30]. Among these secondary metabolites, berry phenolic compounds contribute to the organoleptic properties of the wine (i.e., taste, color, and aroma) and, in addition, they have benefits to human health due to their antioxidant capacity [
47,
48]. The phenolic compounds most reduced under elevated temperatures are anthocyanins and flavonols [
18,
41,
49], which lead to a decrease in the total phenolic content of berries. In our experimental conditions, the genotypes of old grapevine varieties displayed different trends since the ECET treatment caused the decrease of TPI and total anthocyanins in TEMP and TV, the maintenance of these compounds in GRA63, PAS, AMB, and TOR, and the improvement of anthocyanin content in GRA72 (
Figure 3). The reduction of phenolic content has been related to decreases in enzymatic activities involved in the phenylpropanoid pathway [
20]. Other studies confirmed the degradation of anthocyanins by peroxidases, which exhibit higher activity at elevated temperatures [
49,
50,
51]. In addition, the increase in the tonality index of TEMP, TV, and PAS points to significant modifications of must characteristics from these varieties that could be associated with differences in anthocyanin profiles (
Figure 2). Alterations in the relative abundance of different anthocyanin families are known to lead to different tonalities [
52]. Grape phenolics have a variable extraction potential (called extractability), whose assessment is based on anthocyanin extraction from the whole berries [
53]. Our research includes the measurement of extractable anthocyanins and estimations of cellular extractability of anthocyanins (EA) and seed maturity (SM) to estimate the impact of climate change on the oenological potential of each variety. Overall, EA and SM were little affected by the combination of elevated CO
2 and high temperature (
Table 4). However, under ECET treatment, EA increased in GRA72 and, especially in TV, indicating that the lower potential of color extraction of this genotype could get worse under ECET conditions (
Figure 3) [
35]. The SM was a stable property that only increased in TV under ECET conditions but staying within the average values of the assessed genotypes. High values of SM are typical of seeds with non-polymerized tannins, which give high green astringency to the resulting wine [
53]. This was the case of the TOR whose SM values were excessively high, regardless of the temperature and CO
2 condition (
Table 4).
Phenolic compounds have received considerable interest based on their antioxidant and free-radical-scavenging properties, catechins, proanthocyanidins, and anthocyanins being the most abundant antioxidants present in berries [
48,
54]. Our data show that, apart from GRA63, the total antioxidant capacity of old genotypes was lower than that of commercial clones of Tempranillo [
29]. So, GRA63 was the genotype with the highest antioxidant capacity whereas TOR was the genotype with the lowest values, which could be explained, at least in part, by the considerable difference in the anthocyanin content between both genotypes (
Table 4). A significant relationship between antioxidant capacity and anthocyanin content has been reported in different grapevine varieties [
55,
56,
57]. By contrast, under the ECET treatment imposed in our study, neither the decrease in total antioxidant capacity of GRA72 and TOR nor the improvement of this property in AMB did seem to be directly related to changes in TPI and/or anthocyanins (
Figure 3), suggesting that the antioxidant potential could be more related to specific phenolic compounds rather than to the total concentrations [
57].
Finally, it should be taken into account that the old genotypes assessed in the present study showed the different length of reproductive phases, which could have conditioned some responses to the combination of elevated CO
2 and high temperature in terms of berry quality (
Table 1). Thus, the harvest took out under quite different temperatures regardless of the CO
2 treatment applied, ranging from 36 °C in the middle of August (harvest of TEMP) to 29 °C at the end of October (harvest of TOR) (Figure 5). In addition, we have recorded that under ECET, 57% of the ripening period of TEMP occurred at temperatures above 35 °C, which could have accentuated, at least in part, the susceptibility of this genotype to imposed conditions.