Arctium lappa and Arctium tomentosum, Sources of Arctii radix: Comparison of Anti-Lipoxygenase and Antioxidant Activity as well as the Chemical Composition of Extracts from Aerial Parts and from Roots
Abstract
:1. Introduction
2. Results and discussion
2.1. Evaluation of Lipoxygenase Activity Inhibition Ability in Cell-Free System
2.2. Evaluation of ROS Scavenging in Cell-Free Systems
2.2.1. Scavenging of DPPH
2.2.2. Scavenging of the Superoxide Anion
2.2.3. Scavenging of Hydrogen Peroxide
2.3. Phytochemical Analysis
2.3.1. Total Content of Phenolic Compounds
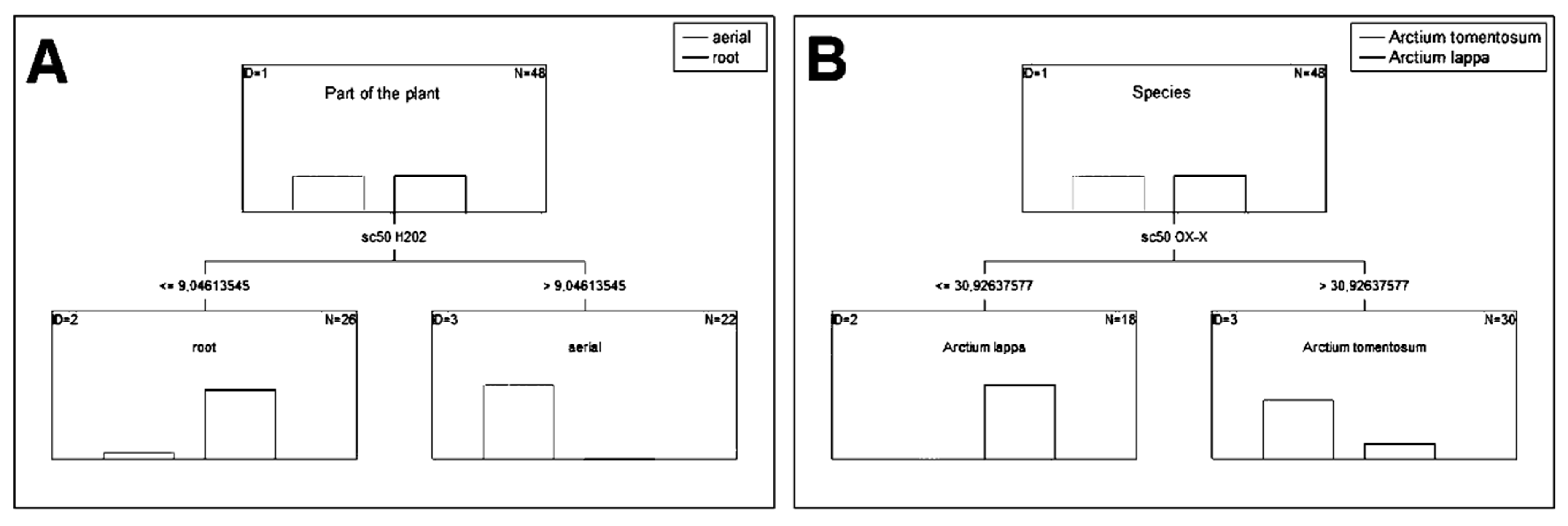
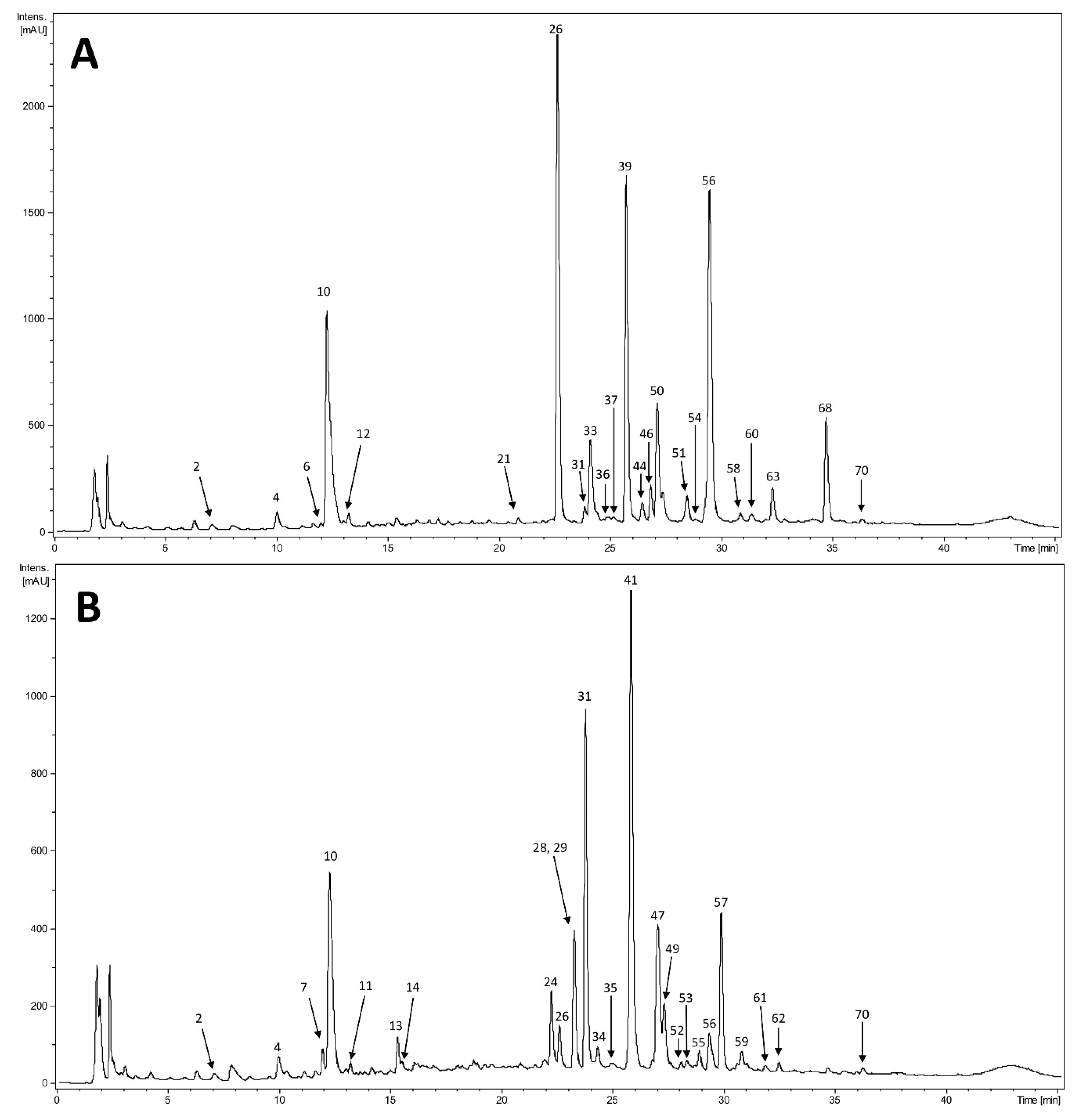
2.3.2. HPLC–DAD–MSn
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Extracts Preparation
3.3. Evaluation of Lipoxygenase Activity Inhibition Ability in Cell-Free System
3.4. Evaluation of ROS Scavenging in Cell-Free Systems
3.4.1. Scavenging of DPPH
3.4.2. Scavenging of the Superoxide Anion
3.4.3. Scavenging of Hydrogen Peroxide
3.5. Phytochemical Analysis
3.5.1. Total Content of Phenolic Compounds
3.5.2. HPLC–DAD–MSn
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wink, M.; van Wyk, B.E. Rośliny Lecznicze Świata, 1st ed.; MedPharm: Wrocław, Poland, 2004; ISBN 9788360466513. (In Polish) [Google Scholar]
- Sederski, M.E. Prawie Wszystko o Ziołach i Ziołolecznictwie, 3rd ed.; Mateusz E: Podkowa Leśna, Poland, 2017; ISBN 9788394554019. (In Polish) [Google Scholar]
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet: Dietary Guidelines and Impact on Health and Disease; Romagnolo, D.F., Selmin, O.I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319279671. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2015; Volume 9, ISBN 9789401795104. [Google Scholar]
- De la Rosa, L.A.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G. Fruit and Vegetable Phytochemicals; Blackwell Publishing: Hoboken, NJ, USA, 2010; ISBN 9780813803203. [Google Scholar]
- Silva, M.; Silva, L.R. Hydroxycinnamic Acids (HCAS): Structure, Biological Properties and Health Effects; Nova Science Publishers: New York, NY, USA, 2015; ISBN 9781634833554. [Google Scholar]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2019, 100, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Anioł-Kwiatkowska, J.; Kwiatkowski, S.; Berdowski, W. Rośliny Lecznicze: Atlas; Arkady: Warsaw, Poland, 1993; ISBN 8321336345. (In Polish) [Google Scholar]
- Ożarowski, A.; Jaroniewski, W. Rośliny Lecznicze i Ich Praktyczne Zastosowanie; Zw. Zaw.: Warsaw, Poland, 1987; ISBN 9788320204728. (In Polish) [Google Scholar]
- Carlotto, J.; Da Silva, L.M.; Dartora, N.; Maria-Ferreira, D.; Sabry, D.D.A.; Filho, A.P.S.; Werner, M.F.d.P.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M.; et al. Identification of a dicaffeoylquinic acid isomer from Arctium lappa with a potent anti-ulcer activity. Talanta 2015, 135, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Carlotto, J.; de Souza, L.M.; Baggio, C.H.; Werner, M.F.D.P.; Maria-Ferreira, D.; Sassaki, G.L.; Iacomini, M.; Cipriani, T.R. Polysaccharides from Arctium lappa L.: Chemical structure and biological activity. Int. J. Biol. Macromol. 2016, 91, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; Zhang, Z.; Xu, J.; Xie, Z.; Slavin, M.; Gao, X. In vitro and in vivo antioxidant activity of a fructan from the roots of Arctium lappa L. Int. J. Biol. Macromol. 2014, 65, 446–453. [Google Scholar] [CrossRef]
- Su, S.; Wink, M. Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry 2015, 117, 340–350. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.Y.; Leung, G.P.H.; Yu, P.H.F.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef]
- De Almeida, A.B.A.; Sánchez-Hidalgo, M.; Martín, A.R.; Luiz-Ferreira, A.; Trigo, J.R.; Vilegas, W.; Dos Santos, L.C.; Souza-Brito, A.R.M.; De La Lastra, C.A. Anti-inflammatory intestinal activity of Arctium lappa L. (Asteraceae) in TNBS colitis model. J. Ethnopharmacol. 2013, 146, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef]
- Haghi, G.; Hatami, A.; Mehran, M. UPLC and HPLC of caffeoyl esters in wild and cultivated Arctium lappa L. Food Chem. 2013, 138, 321–326. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of five new classes of chlorogenic acids in burdock (Arctium lappa L.) roots by liquid chromatography/tandem mass spectrometry. Food Funct. 2011, 2, 63–71. [Google Scholar] [CrossRef]
- Kuo, D.H.; Hung, M.C.; Hung, C.M.; Liu, L.M.; Chen, F.A.; Shieh, P.C.; Ho, C.T.; Way, T. Der Body weight management effect of burdock (Arctium lappa L.) root is associated with the activation of AMP-activated protein kinase in human HepG2 cells. Food Chem. 2012, 134, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, K.; Schliemann, W.; Strack, D. Isolation and identification of arctiin and arctigenin in leaves of burdock (Arctium lappa L.) by polyamide column chromatography in combination with HPLC-ESI/MS. Phytochem. Anal. 2005, 16, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Duh, P. Antioxidant activity of burdock (Arctium lappa Linné): Its scavenging effect on free-radical and active oxygen. J. Am. Oil Chem. Soc. 1998, 75, 455–461. [Google Scholar] [CrossRef]
- Wang, B.S.; Yen, G.C.; Chang, L.W.; Yen, W.J.; Duh, P. Der Protective effects of burdock (Arctium lappa Linne) on oxidation of low-density lipoprotein and oxidative stress in RAW 264.7 macrophages. Food Chem. 2007, 101, 729–738. [Google Scholar] [CrossRef]
- Ji, K.Y.; Jang, J.H.; Lee, E.H.; Kim, S.M.; Song, H.W.; Yang, W.K.; Kim, H.Y.; Kim, K.H.; Lee, Y.S.; Kim, D.S.; et al. Canavalia gladiata and Arctium lappa extracts ameliorate dextran sulphate sodium-induced inflammatory bowel disease by enhancing immune responses. J. Funct. Foods 2018, 45, 24–33. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Wang, C.; Jiang, Q.; Zhang, L.; Cao, Y.; Zhong, W.; Wang, C. Protective effects of Arctium lappa L. root extracts (AREs) on high fat diet induced quail atherosclerosis. BMC Complement. Altern. Med. 2016, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pomari, E.; Stefanon, B.; Colitti, M. Effect of Arctium lappa (burdock) extract on canine dermal fibroblasts. Vet. Immunol. Immunopathol. 2013, 156, 159–166. [Google Scholar] [CrossRef]
- Ghorat, F.; Azizkhani, M.; Naji, S.; Ranjbary, A.G.; Doostishoar, F. Histopathological evaluation of Burdock (Arctium lappa) root hydroalcoholic extract on wound healing. Iran. Red Crescent Med. J. 2017, 19, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Kim, D.O.; Heo, H.J. Melanogenesis regulatory activity of the ethyl acetate fraction from Arctium lappa L. leaf on α-MSH–induced B16/F10 melanoma cells. Ind. Crops Prod. 2019, 138, 111581. [Google Scholar] [CrossRef]
- Knott, A.; Reuschlein, K.; Mielke, H.; Wensorra, U.; Mummert, C.; Koop, U.; Kausch, M.; Kolbe, L.; Peters, N.; Stäb, F.; et al. Natural Arctium lappa fruit extract improves the clinical signs of aging skin. J. Cosmet. Dermatol. 2008, 7, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.C.; Baggio, C.H.; Freitas, C.S.; Lepieszynski, J.; Mayer, B.; Twardowschy, A.; Missau, F.C.; dos Santos, É.P.; Pizzolatti, M.G.; Marques, M.C.A. Gastroprotective activity of the chloroform extract of the roots from Arctium lappa L. J. Pharm. Pharmacol. 2008, 60, 795–801. [Google Scholar] [CrossRef] [PubMed]
- El-Kott, A.F.; Bin-Meferij, M.M. Use of Arctium lappa Extract Against Acetaminophen-Induced Hepatotoxicity in Rats. Curr. Ther. Res. Clin. Exp. 2015, 77, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.C.; Lin, C.H.; Lin, C.C.; Lin, Y.H.; Chen, C.F.; Chen, I.C.; Wang, L.Y. Hepatoprotective effects of Arctium lappa Linne on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. J. Biomed. Sci. 2002, 9, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.M.; Allemand, A.; Mendes, D.A.G.B.; dos Santos, A.C.; André, E.; de Souza, L.M.; Cipriani, T.R.; Dartora, N.; Marques, M.C.A.; Baggio, C.H.; et al. Ethanolic extract of roots from Arctium lappa L. accelerates the healing of acetic acid-induced gastric ulcer in rats: Involvement of the antioxidant system. Food Chem. Toxicol. 2013, 51, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.M.; Burci, L.D.M.; Crestani, S.; de Souza, P.; da Silva, R.d.C.M.V.d.A.F.; Dartora, N.; de Souza, L.M.; Cipriani, T.R.; da Silva-Santos, J.E.; André, E.; et al. Acid-gastric antisecretory effect of the ethanolic extract from Arctium lappa L. root: Role of H+, K+-ATPase, Ca2+ influx and the cholinergic pathway. Inflammopharmacology 2018, 26, 521–530. [Google Scholar] [CrossRef]
- Liu, H.C.; Ku, M.K.; Chung, F.Y.; Lin, C.C.; Lin, S.R. Effectiveness of great burdock essence compounds in the adjuvant treatment of gastric ulcer patients infected with Helicobacter pylori. Genomic Med. Biomark. Health Sci. 2012, 4, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.C.; Lin, L.F.; Yeh, C.S.; Lin, Y.L.; Chang, H.J.; Lin, S.R.; Chang, M.Y.; Hsiao, C.P.; Lee, S.C. Burdock essence promotes gastrointestinal mucosal repair in ulcer patients. Fooyin J. Health Sci. 2010, 2, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Ju, J.; Wang, K.; Gu, C.; Feng, Y. Evaluation of hypoglycemic activity of total lignans from Fructus Arctii in the spontaneously diabetic Goto-Kakizaki rats. J. Ethnopharmacol. 2014, 151, 548–555. [Google Scholar] [CrossRef]
- Xu, Z.; Gu, C.; Wang, K.; Ju, J.; Wang, H.; Ruan, K.; Feng, Y. Arctigenic acid, the key substance responsible for the hypoglycemic activity of Fructus Arctii. Phytomedicine 2015, 22, 128–137. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, D.H.; Cho, G.H.; Kim, J.S.; Kang, D.G.; Lee, H.S. Arctium lappa ameliorates endothelial dysfunction in rats fed with high fat/cholesterol diets. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, N.; Kan, J.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Liu, J.; Qian, C.; Jin, C. Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, N.; Kan, J.; Sun, R.; Tang, S.; Wang, Z.; Chen, M.; Liu, J.; Jin, C. Anti-inflammatory activity of alkali-soluble polysaccharides from Arctium lappa L. and its effect on gut microbiota of mice with inflammation. Int. J. Biol. Macromol. 2020, 154, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Lv, W.; Ma, C.; Wang, Z.; Chen, S. Assessment of antibacterial activity of fractions from burdock leaf against food-related bacteria. Food Control 2010, 21, 1272–1278. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Tang, Y.; Chen, X. The effect of burdock leaf fraction on adhesion, biofilm formation, quorum sensing and virulence factors of Pseudomonas aeruginosa. J. Appl. Microbiol. 2017, 122, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Sui, S.; Huang, J.; Bai, J.P.; Ren, T.S.; Zhao, Q.C. Neuroprotective effects of Arctium lappa L. roots against glutamate-induced oxidative stress by inhibiting phosphorylation of p38, JNK and ERK 1/2 MAPKs in PC12 cells. Environ. Toxicol. Pharmacol. 2014, 38, 189–198. [Google Scholar] [CrossRef]
- Tian, X.; Guo, L.P.; Hu, X.L.; Huang, J.; Fan, Y.H.; Ren, T.S.; Zhao, Q.C. Protective Effects of Arctium lappa L. Roots Against Hydrogen Peroxide-Induced Cell Injury and Potential Mechanisms in SH-SY5Y Cells. Cell. Mol. Neurobiol. 2015, 35, 335–344. [Google Scholar] [CrossRef]
- Kwon, Y.K.; Choi, S.J.; Kim, C.R.; Kim, J.K.; Kim, Y.J.; Choi, J.H.; Song, S.W.; Kim, C.J.; Park, G.G.; Park, C.S.; et al. Antioxidant and cognitive-enhancing activities of Arctium lappa L. roots in Aβ1-42-induced mouse model. Appl. Biol. Chem. 2016, 59, 553–565. [Google Scholar] [CrossRef]
- Rutkowski, L. Klucz do Oznaczania roślin Naczyniowych Polski Niżowej; PWN: Warsaw, Poland, 1998; ISBN 8301122188. (In Polish) [Google Scholar]
- European Medicines Agency. Community Herbal Monograph on Arctium lappa L., Radix; EMA/HMPC/246763/2009; European Medicines Agency: Amsterdam, The Netherlands, 2010; Volume 44. [Google Scholar]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131, 33–55. [Google Scholar] [CrossRef]
- Jarić, S.; Kostić, O.; Mataruga, Z.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 2018, 211, 311–328. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, H.; Ge, L.; Gong, H.; Tian, S. Determination of arctiin and arctigenin contents in Arctium tomentosum Mill. by HPLC method. J. Chem. 2011, 8, 372–377. [Google Scholar] [CrossRef]
- Strawa, J.; Jakimiuk, K.; Waluk, M.; Poslednik, M.; Nazaruk, J.; Tomczyk, M. Phytochemical examination of wolly burdock Arctium tomentosum leaves and flower heads. Chem. Nat. Compd. 2020, 56, 345–347. [Google Scholar] [CrossRef]
- Halliwell, B. Disease: Some New Concepts. FASEB J. 1987, 1987, 358–364. [Google Scholar] [CrossRef]
- Sugiura, Y.; Torii, T.; Matsuda, K.; Yamada, Y. Anti-allergic effects of extracts from commercial products of cooked burdock. Food Sci. Technol. Res. 2009, 15, 423–426. [Google Scholar] [CrossRef] [Green Version]
- Chagas-Paula, D.A.; Oliveira, T.B.; Faleiro, D.P.V.; Oliveira, R.B.; Da Costa, F.B. Outstanding Anti-inflammatory Potential of Selected Asteraceae Species through the Potent Dual Inhibition of Cyclooxygenase-1 and 5-Lipoxygenase. Planta Med. 2015, 81, 1296–1307. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Bazylko, A.; Piwowarski, J.P.; Filipek, A.; Bonarewicz, J.; Tomczyk, M. In vitro antioxidant and anti-inflammatory activities of extracts from Potentilla recta and its main ellagitannin, agrimoniin. J. Ethnopharmacol. 2013, 149, 222–227. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Jakiw, L.; Khlebnikov, A.I.; Blaskovich, C.L.; Jutila, M.A.; Quinn, M.T. Immunomodulatory Activity of Oenothein B Isolated from Epilobium angustifolium. J. Immunol. 2009, 183, 6754–6766. [Google Scholar] [CrossRef] [Green Version]
- O’Dowd, Y.; Driss, F.; Dang, P.M.C.; Elbim, C.; Gougerot-Pocidalo, M.A.; Pasquier, C.; El-Benna, J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: Scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem. Pharmacol. 2004, 68, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bădărau, A.S.; Swamy, M.K.; Shaw, S.; Maggi, F.; Da Silva, L.E.; López, V.; Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Arctium Species Secondary Metabolites Chemodiversity and Bioactivities. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]



| Species | Natural Site | Geographical Coordinates | Specimens of Raw Material | Part of the Plant | Abbreviation | Mass of Plant Material (g) | Mass of Lyophilized Extracts (g) |
|---|---|---|---|---|---|---|---|
| Arctium lappa | Jaszczurowa | 49°53′03″ N; 21°33′40″ E | AL/16/J | Aerial parts | ALAPJ | 30.00 | 6.69 |
| Roots | ALRJ | 30.00 | 4.45 | ||||
| Wola Wyżna A | 49°23′30″ N; 21°52′24″ E | AL/17/WA | Aerial parts | ALAPWA | 36.70 | 2.24 | |
| Roots | ALRWA | 19.28 | 2.65 | ||||
| Wola Wyżna B | 49°23′30″ N; 21°52′24″ E | AL/17/WB | Aerial parts | ALAPWB | 30.00 | 2.23 | |
| Roots | ALRWB | 14.10 | 2.95 | ||||
| Jaśliska | 49°23′38″ N; 21°52′45″ E | AL/17/ZJ | Aerial parts | ALAPZJ | 28.90 | 2.30 | |
| Roots | ALRZJ | 13.70 | 2.55 | ||||
| Arctium tomentosum | Czudec | 49°56′44″ N; 21°50′17″ E | AT/16/C | Aerial parts | ATAPC | 30.00 | 5.96 |
| Roots | ATRC | 30.00 | 4.06 | ||||
| Kołaczyce | 49°48′30″ N; 21°26′25″ E | AT/16/K | Aerial parts | ATAPK | 23.90 | 4.35 | |
| Roots | ATRK | 17.77 | 2.75 | ||||
| Strzyżów | 49°52′15″ N; 21°47′28″ E | AT/16/S | Aerial parts | ATAPS | 16.90 | 3.64 | |
| Roots | ATRS | 28.83 | 3.88 | ||||
| Strzyżów, Zadworze st. | 49°52′15″ N; 21°47′28″ E | AT/16/Z | Aerial parts | ATAPZ | 26.80 | 5.40 | |
| Roots | ATRZ | 30.00 | 6.94 |
| Species | Sample | LOX Inhibition ± SD (%) | SC50 ± SD (μg·mL−1) | Total Phenolic Content ± SD (mg·g−1) | |||
|---|---|---|---|---|---|---|---|
| 200 μg·mL−1 | 400 μg·mL−1 | DPPH | O2•− | H2O2 | |||
| Arctium lappa | ALAPJ | 8.85 ± 0.84 | 22.58 ± 1.38 | 56.70 ± 3.40 | 42.44 ± 2.47 | 15.79 ± 1.11 | 74.15 ± 7.49 |
| ALAPWA | 11.46 ± 2.04 | 38.45 ± 1.69 | 29.28 ± 2.97 | 26.00 ± 4.70 | 8.68 ± 0.37 | 131.14 ± 9.35 | |
| ALAPWB | 7.80 ± 2.19 | 24.58 ± 1.49 | 36.48 ± 3.22 | 15.42 ± 3.16 | 9.53 ± 0.45 | 101.67 ± 7.27 | |
| ALAPZJ | 7.12 ± 2.59 | 28.87 ± 2.45 | 30.48 ± 3.56 | 20.41 ± 4.12 | 10.62 ± 0.62 | 119.16 ± 7.29 | |
| ALRJ | 9.53 ± 0.94 | 41.15 ± 1.49 | 31.96 ± 2.56 | 29.82 ± 2.57 | 5.12 ± 0.18 | 147.14 ± 11.88 | |
| ALRWA | 11.42 ± 1.89 | 31.09 ± 2.78 | 26.78 ± 3.19 | 29.57 ± 4.13 | 5.66 ± 0.29 | 137.43 ± 9.74 | |
| ALRWB | 7.16 ± 2.01 | 22.71 ± 2.15 | 31.14 ± 3.02 | 20.17 ± 3.79 | 6.32 ± 0.37 | 116.61 ± 9.90 | |
| ALRZJ | 8.87 ± 2.47 | 31.27 ± 2.61 | 29.03 ± 3.38 | 30.25 ± 4.50 | 5.54 ± 0.28 | 135.87 ± 11.46 | |
| Arctium tomentosum | ATAPC | 5.42 ± 1.10 | 13.12 ± 0.85 | 74.19 ± 4.38 | 75.88 ± 6.37 | 80.36 ± 6.97 | 51.55 ± 7.05 |
| ATAPK | 9.74 ± 1.09 | 23.39 ± 2.38 | 43.28 ± 1.85 | 35.56 ± 2.47 | 28.64 ± 2.13 | 85.61 ± 9.28 | |
| ATAPS | 15.77 ± 1.12 | 36.18 ± 1.34 | 38.48 ± 2.37 | 36.21 ± 2.71 | 18.07 ± 1.31 | 97.84 ± 8.10 | |
| ATAPZ | 11.49 ± 1.08 | 21.20 ± 1.09 | 54.87 ± 2.80 | 47.68 ± 5.86 | 45.15 ± 5.52 | 79.09 ± 6.30 | |
| ATRC | 8.49 ± 0.74 | 25.85 ± 1.72 | 40.70 ± 2.64 | 49.11 ± 6.39 | 5.83 ± 0.20 | 95.45 ± 9.06 | |
| ATRK | 9.90 ± 0.83 | 38.47 ± 2.49 | 28.70 ± 2.93 | 37.26 ± 6.08 | 6.97 ± 0.61 | 145.09 ± 11.69 | |
| ATRS | 8.53 ± 0.62 | 16.41 ± 0.83 | 59.94 ± 3.94 | 58.91 ± 4.32 | 8.38 ± 0.66 | 71.74 ± 4.14 | |
| ATRZ | 7.56 ± 0.77 | 20.54 ± 1.40 | 44.62 ± 2.08 | 46.70 ± 5.08 | 8.10 ± 0.48 | 93.16 ± 6.68 | |
| No. | Compound | Rt (min) | UV–Vis maxima (nm) | [M-H]- m/z | MS2 ions | MS3 ions | NL (amu) | AL- APWA | ATAPZ | ALRWA | ATRZ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | caffeoylquinic acid derivative | 5.2 | 240, 301sh, 324 | 451 | 353b, 191 | 191b, 179 | + | + | |||
| 2 | 3-O-caffeoylquinic acid * (neochlorogenic acid) | 7.2 | 305sh, 321 | 353 | 191b, 179 | 191 | 162 | + | + | + | |
| 3 | caffeoylfumaroylmaloylquinic acid | 8.8 | 241sh, 305sh, 327 | 567 | 469b, 387, 307 | 353, 307b, 277, 191 | 162 | + | + | ||
| 4 | caffeoylquinic acid dimer | 10.1 | 248sh, 286, 328 | 705 | 595, 513b, 339, 229 | + | + | + | |||
| 5 | dicaffeoylfumaroylquinic acid | 10.3 | 300sh, 315 | 613 | 515b, 409 | 353, 323b, 191, 179 | 162 | + | |||
| 6 | undefined compound | 11.8 | 217, 265 | 643 | 545b, 203 | 341b, 203 | + | ||||
| 7 | undefined compound | 11.9 | 217, 265 | 537 | 375b, 345, 327 | 345b, 327 | 162 | + | |||
| 8 | undefined compound | 12.0 | 217, 265 | 643 | 545b, 203 | 341b, 203 | + | ||||
| 9 | undefined compound | 12.0 | 218, 265 | 503 | 341b, 281, 179 | 281b, 251, 179 | 162 | + | |||
| 10 | 5-O-caffeoylquinic acid # (chlorogenic acid) | 12.4 | 235, 306sh, 320 | 353 | 191b, 179 | 191 | 162 | + | + | + | + |
| 11 | 4-O-caffeoylquinic acid * (cryptochlorogenic acid) | 13.3 | 306sh, 323 | 353 | 191, 179, 173b, 135 | 191 | 162 | + | + | ||
| 12 | 4-O-caffeoylquinic acid derivative | 13.4 | 306sh, 324 | 593 | 353, 239b | 191, 179, 173b, 155 | 162 | + | + | ||
| 13 | undefined compound | 15.3 | 280, 308sh | 433 | 221b, 177 | + | + | ||||
| 14 | undefined compound | 15.3 | 280, 308sh | 485 | 467, 399, 305b, 189 | + | |||||
| 15 | 5-O-p-coumaroylquinic acid * | 16.4 | 301sh, 311 | 337 | 191b, 163 | 191 | 146 | + | |||
| 16 | dicaffeoylfumaroylquinic acid | 17.7 | 239sh, 301sh, 320 | 613 | 515b | 353b, 335, 191, 179 | 162 | + | + | ||
| 17 | 5-O-feruloylquinic acid * | 18.2 | 297 | 367 | 191b, 173 | 191 | 176 | + | |||
| 18 | caffeoylquinic acid derivative | 20 | 300sh, 317 | 649 | 533, 487b, 451, 371, 353, 335 | 371b, 353, 289, 191 | 162 | + | |||
| 19 | dicaffeoylquinic acid derivative | 20.1 | 300sh, 317 | 955 | 839, 613, 515b, 341 | + | |||||
| 20 | tricaffeoylquinic acid derivative | 20.4 | 302sh, 319 | 839 | 677, 647, 515b, 323 | 515b, 485, 323 | 162 | + | |||
| 21 | caffeoylquinic acid derivative | 20.9 | 308sh, 327 | 651 | 553b | 453, 391, 353b, 291, 191 | + | ||||
| 22 | dicaffeoylmaloylquinic acid derivative | 21.5 | 234, 303sh, 321 | 793 | 631, 613, 515b, 497, 341 | 515b, 341 | 162 | + | |||
| 23 | caffeoylmaloylquinic acid | 21.7 | 232, 303sh, 319 | 469 | 419, 388, 323 | + | + | ||||
| 24 | quercetin derivative | 22.3 | 255, 352 | 609 | 343, 301b, 271 | + | |||||
| 25 | undefined compound | 22.6 | 303sh, 321 | 431 | 351, 263b, 247, 121 | + | |||||
| 26 | quercetin rhamnohexoside | 22.8 | 255, 353 | 609 | 343, 301b, 255 | + | + | ||||
| 27 | tricaffeoylquinic acid derivative | 23.1 | 234, 303sh, 321 | 793 | 677, 631b, 515, 469, 353 | (677) 515b, 353, 335 | + | + | |||
| (631) 515b, 439, 341 | 162 | ||||||||||
| 28 | quercetin 3-O-galactoside (hyperoside) # | 23.3 | 255, 352 | 463 | 343, 301b, 179 | + | |||||
| 29 | apigenin derivative | 23.4 | 255, 264sh, 352, 374sh | 449 | 269b, 225, 207 | + | |||||
| 30 | tricaffeoylquinic acid | 23.4 | 301sh, 321 | 677 | 515b, 485 | 353, 323b, 191 | 162 | + | + | ||
| 31 | quercetin 3-O-glucoside (isoquercitrin) # | 23.9 | 251, 305sh, 333 | 463 | 301b, 257, 179 | + | + | ||||
| 32 | dicaffeoyldimaloylquinic acid | 24 | 235sh, 302sh, 326 | 747 | 631b, 469 | 515b, 469, 353, 335 | + | ||||
| 33 | kaempferol 3-O-glucuronide # | 24.2 | 253, 343 | 461 | 357, 285b | + | |||||
| 34 | kaempferol rhamnohexoside | 24.4 | 265, 331 | 593 | 447, 327, 285b | 357, 285b | 146 | + | |||
| 35 | undefined compound | 24.7 | 265, 331 | 701 | 655, 509, 335b, 263 | + | |||||
| 36 | dicaffeoylmaloylquinic acid | 24.9 | 238, 304sh, 326 | 631 | 515, 469b, 353 | 353b, 173 | 162 | + | + | + | |
| 37 | tricaffeoylsuccinoylquinic acid | 25.3 | 296, 323 | 777 | 677, 615b, 515 | 515b, 453, 353 | 162 | + | + | ||
| 38 | dicaffeoylfumaroylquinic acid | 25.7 | 238sh, 305sh, 325 | 613 | 515b, 433 | 353b, 299, 203 | 162 | + | |||
| 39 | kaempferol rhamnohexoside | 25.8 | 264, 343 | 593 | 285 | + | |||||
| 40 | dicaffeoylfumaroylquinic acid | 25.8 | 237sh, 305sh, 325 | 613 | 515b, 433, 353 | 353b, 299, 203 | + | ||||
| 41 | quercetin malonylhexoside | 25.9 | 256, 353 | 549 | 505b, 463, 301 | + | |||||
| 42 | dicaffeoylfumaroylquinic acid | 26.1 | 236sh, 305sh, 325 | 613 | 515b | 353b, 335, 203, 191, 173 | 162 | + | |||
| 43 | dicaffeoylcoumaroylmaloylquinic acid | 26.2 | 242sh, 305sh, 324 | 777 | 631, 615, 515b | (631) 515 | 146 | + | |||
| (615) 515, 453, 353b | 162 | ||||||||||
| (515) 353, 191, 179, 173b | 162 | ||||||||||
| 44 | dicaffeoylmaloylquinic acid | 26.5 | 239sh, 306sh, 327 | 631 | 469b, 353, 191 | 353, 191, 173 | + | + | |||
| 45 | dicaffeoyldimaloylquinic acid | 26.7 | 241, 308, 332, 351 | 747 | 631b, 585, 469 | (631) 469b, 353 | 162 | + | |||
| (585) 469b, 353 | 162 | ||||||||||
| 46 | dicaffeoylmaloylquinic acid | 26.9 | 238sh, 306sh, 328 | 631 | 469b, 353, 191 | 353b, 307, 191 | 162 | + | + | ||
| 47 | kaempferol hexoside | 27.1 | 240sh, 265, 303sh, 328 | 447 | 327, 285b, 255 | + | |||||
| 48 | dicaffeoylmaloylquinic acid | 27.2 | 235, 303sh, 327 | 631 | 515, 469b, 353, 307, 191 | (515) 353b, 335, 191 | 162 | + | |||
| (469) 353b, 307, 191 | 162 | ||||||||||
| 49 | dicaffeoylquinic acid derivative | 27.2 | 237, 303sh, 327 | 767 | 515b, 353 | 353b, 335, 191 | 162 | + | + | ||
| 50 | dicaffeoylfumaroylquinic acid | 27.4 | 234sh, 304sh, 326 | 613 | 515b, 353 | 353b, 191 | 162 | + | |||
| 51 | dicaffeoyldimaloylquinic acid | 28.3 | 237, 306sh, 328 | 747 | 631, 585, 469b, 353 | (631) 469b, 353 | 162 | + | + | + | |
| (585) 469b, 353 | |||||||||||
| 52 | dicaffeoylmaloylquinic acid | 28.4 | 266, 282, 327 | 631 | 469b, 451, 353, 335 | 353b, 307, 191 | 162 | + | |||
| 53 | undefined compound | 28.4 | 266, 282, 327 | 417 | 327, 284b, 255 | 255 | 162 | + | |||
| 54 | apigenin 7-O-glucuronide # | 28.5 | 266, 331 | 445 | 269b, 175 | + | |||||
| 55 | quercetin malonylhexoside | 29 | 253, 335 | 549 | 505b | 463, 445, 301b | + | ||||
| 56 | dicaffeoylsuccinoylquinic acid | 29.6 | 241, 306sh, 327 | 615 | 515, 453b, 353, 191 | (515) 353b, 335, 299, 255, 203, 173 | 162 | + | + | + | + |
| (453) 353 | 162 | ||||||||||
| 57 | kaempferol malonylhexoside | 29.9 | 264, 343 | 533 | 489b, 285 | 285b, 255 | + | ||||
| 58 | dicaffeoylmaloylsuccinoylquinic acid | 30.9 | 237sh, 310sh, 328 | 731 | 569, 469b, 451, 353 | 489, 469b, 353, 289 | 162 | + | + | + | |
| 59 | caffeic acid derivative | 31 | 215, 245sh, 306sh, 328 | 459 | 297b, 179, 135 | 279, 179, 135b | 162 | + | + | ||
| 60 | coumaroylcaffeoylquinic acid | 31.4 | 304sh, 327 | 499 | 455, 353b, 337, 191 | + | + | + | |||
| 61 | caffeic acid derivative | 31.9 | 210, 324 | 557 | 459b, 297 | 297b, 179, 135 | + | ||||
| 62 | undefined compound | 32.5 | 266, 316 | 533 | 485b, 352, 315, 293 | + | |||||
| 63 | dicaffeoylsuccinoylfumaroylquinic acid | 32.4 | 236sh, 307sh, 328 | 713 | 615b, 453 | 515, 453, 353b, 191 | + | + | + | ||
| 64 | tricaffeoylquinic acid derivative | 33.5 | 308sh, 325 | 909 | 793b, 677, 613 | 515, 497b, 469, 353 | 162 | + | + | ||
| 65 | dicaffeoylmaloylquinic acid derivative | 33.9 | 232sh, 307sh, 326 | 793 | 631b, 613 | 515, 469b, 451, 353 | 162 | + | + | ||
| 66 | dicaffeoylmaloylquinic acid derivative | 34.2 | 241sh, 308sh, 325 | 793 | 631b, 613, 497, 469 | 515, 469b, 451, 353 | 162 | + | + | ||
| 67 | dicaffeoyldimaloylquinic acid derivative | 34.7 | 241, 306sh, 329 | 909 | 747b, 631, 585, 469, 353 | 631, 585, 469, 353 | 162 | + | + | ||
| 68 | dicaffeoyldissuccinoylquinic acid | 34.8 | 235, 306sh, 329 | 715 | 615, 553, 515, 453b, 353 | 453b, 353, 191 | 162 | + | + | ||
| 69 | dicaffeoylmaloylquinic acid derivative | 35.5 | 239sh, 307sh, 327 | 793 | 631b, 469, 353 | 469b, 451, 353, 191 | 162 | + | + | ||
| 70 | tricaffeoylmaloylquinic acid | 36.3 | 310sh, 326 | 793 | 631b, 469, 353, 277 | 469b, 353, 277, 191 | 162 | + | + | + | + |
| 71 | dicaffeoylmaloylsuccinoylquinic acid derivative | 38.2 | 238sh, 307sh, 328 | 893 | 731b, 631, 469, 353 | 631b, 469, 353 | 162 | + | + | ||
| 72 | tricaffeoylsuccinoylquinic acid | 38.5 | 241sh, 307sh, 325 | 777 | 615, 597, 515, 497b, 453, 353, 335 | 515, 453b, 353, 335 | 162 | + | + | ||
| 73 | dicaffeoylquinic acid derivative | 40.1 | 241sh, 305sh, 326 | 1071 | 909, 793b, 614, 515 | 793b, 613 | 162 | + | + | ||
| 74 | dicaffeoyldimaloylquinic acid derivative | 40.6 | 239sh, 305sh, 328 | 1071 | 909b, 748 | 747b, 632, 469, 353 | 162 | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowrońska, W.; Granica, S.; Dziedzic, M.; Kurkowiak, J.; Ziaja, M.; Bazylko, A. Arctium lappa and Arctium tomentosum, Sources of Arctii radix: Comparison of Anti-Lipoxygenase and Antioxidant Activity as well as the Chemical Composition of Extracts from Aerial Parts and from Roots. Plants 2021, 10, 78. https://doi.org/10.3390/plants10010078
Skowrońska W, Granica S, Dziedzic M, Kurkowiak J, Ziaja M, Bazylko A. Arctium lappa and Arctium tomentosum, Sources of Arctii radix: Comparison of Anti-Lipoxygenase and Antioxidant Activity as well as the Chemical Composition of Extracts from Aerial Parts and from Roots. Plants. 2021; 10(1):78. https://doi.org/10.3390/plants10010078
Chicago/Turabian StyleSkowrońska, Weronika, Sebastian Granica, Magdalena Dziedzic, Justyna Kurkowiak, Maria Ziaja, and Agnieszka Bazylko. 2021. "Arctium lappa and Arctium tomentosum, Sources of Arctii radix: Comparison of Anti-Lipoxygenase and Antioxidant Activity as well as the Chemical Composition of Extracts from Aerial Parts and from Roots" Plants 10, no. 1: 78. https://doi.org/10.3390/plants10010078
APA StyleSkowrońska, W., Granica, S., Dziedzic, M., Kurkowiak, J., Ziaja, M., & Bazylko, A. (2021). Arctium lappa and Arctium tomentosum, Sources of Arctii radix: Comparison of Anti-Lipoxygenase and Antioxidant Activity as well as the Chemical Composition of Extracts from Aerial Parts and from Roots. Plants, 10(1), 78. https://doi.org/10.3390/plants10010078






