Impact of Environmental Factors on Stilbene Biosynthesis
Abstract
:1. Introduction
2. Biosynthesis of Stilbenes and Stilbenoids
2.1. Stilbene Synthase
2.2. Glucosylation/Deglucosylation
2.3. Methylation
2.4. Prenylation
2.5. Oligomerization
3. Impact of Environmental Factors on the Biosynthesis of Stilbenes
3.1. UV Radiation
3.2. Light
3.3. Temperature
3.4. Wounding
3.5. Biotic Stress
3.6. Elicitation
3.7. Other Environmental Factors
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4CL | 4-Coumarate:CoA ligase |
| CDs | Cyclodextrins |
| CHS | Chalcone synthase |
| CNL | Cinnamate:CoA ligase |
| C4H | Cinnamate 4-hydroxylase |
| DPS | Dihydropinosylvin synthase |
| GFP | Green fluorescent protein |
| GPP | Geranyl diphosphate |
| GUS | β-Glucuronidase |
| LED | Light emitting diode |
| LOX | Lipoxygenase |
| MeJa | Methyl jasmonate |
| OGlu | O-β-D-glucopyranoside |
| OMT | O-Methyltransferase |
| PAL | Phenylalanine ammonia-lyase |
| Phe | Phenylalanine |
| PKSs | Polyketide synthase superfamily |
| PMT | Pinosylvin O-methyltransferase |
| PS | Pinosylvin synthase |
| PTAL | Bifunctional phenylalanine/tyrosine ammonia-lyase |
| ROMT | Resveratrol O-methyltransferase |
| ROS | Reactive oxygen species |
| RS | Resveratrol synthase |
| STS | Stilbene synthase |
| TAL | Tyrosine ammonia-lyase |
| Tyr | Tyrosine |
| UV | Ultraviolet radiation |
References
- Roupe, K.A.; Remsberg, C.M.; Yáñez, J.A.; Davies, N.M. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar] [CrossRef] [PubMed]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Merillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Waffo Teguo, P.; Fauconneau, B.; Deffieux, G.; Huguet, F.; Vercauteren, J.; Mérillon, J.M. Isolation, identification, and antioxidant activity of three stilbene glucosides newly extracted from Vitis vinifera cell cultures. J. Nat. Prod. 1998, 61, 655–657. [Google Scholar] [CrossRef]
- Privat, C.; Telo, J.P.; Bernardes-Genisson, V.; Vieira, A.; Souchard, J.P.; Nepveu, F. Antioxidant properties of trans-ε-viniferin as compared to stilbene derivatives in aqueous and nonaqueous media. J. Agric. Food Chem. 2002, 50, 1213–1217. [Google Scholar] [CrossRef]
- Biais, B.; Krisa, S.; Cluzet, S.; Da Costa, G.; Waffo-Teguo, P.; Mérillon, J.M.; Richard, T. Antioxidant and cytoprotective activities of grapevine stilbenes. J. Agric. Food Chem. 2017, 65, 4952–4960. [Google Scholar] [CrossRef]
- Albert, S.; Horbach, R.; Deising, H.B.; Siewert, B.; Csuk, R. Synthesis and antimicrobial activity of (E) stilbene derivatives. Bioorg. Med. Chem. 2011, 19, 5155–5166. [Google Scholar] [CrossRef]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suga, T.; Ohta, S.; Munesada, K.; Ide, N.; Kurokawa, M.; Shimizu, M.; Ohta, E. Endogenous pine wood nematicidal substances in pines, Pinus massoniana, P. strobus and P. palustris. Phytochemistry 1993, 33, 1395–1401. [Google Scholar] [CrossRef]
- Torres, P.; Avila, J.G.; de Vivar, A.R.; García, A.M.; Marín, J.C.; Aranda, E.; Céspedes, C.L. Antioxidant and insect growth regulatory activities of stilbenes and extracts from Yucca periculosa. Phytochemistry 2003, 64, 463–473. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Li, X.J.; Zhao, C.Y.; Lu, Y.; Li, W.Q.; Liu, Z.L.; Feng, G.; Yang, L. Synthesis and insect antifeedant activity of stilbene derivatives against Brontispa longissima larvae. Med. Chem. Res. 2013, 22, 2196–2206. [Google Scholar] [CrossRef]
- Hansen, S.C.; Stolter, C.; Imholt, C.; Jacob, J. Plant secondary metabolites as rodent repellents: A systematic review. J. Chem. Ecol. 2016, 42, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.P.; Wieland, G.D.; Reichardt, P.B.; Lewis, V.E.; McCarthy, M.C. Pinosylvin methyl ether deters snowshoe hare feeding on green alder. Science 1983, 222, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.P.; Reichardt, P.B.; Bryant, J.P. Pinosylvin and pinosylvin methyl ether as feeding deterrents in green alder. J. Chem. Ecol. 1986, 12, 2117–2131. [Google Scholar] [CrossRef]
- Virjamo, V.; Julkunen-Tiitto, R.; Henttonen, H.; Hiltunen, E.; Karjalainen, R.; Korhonen, J.; Huitu, O. Differences in vole preference, secondary chemistry and nutrient levels between naturally regenerated and planted Norway spruce seedlings. J. Chem. Ecol. 2013, 39, 1322–1334. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Hasan, M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Vervandier-Fasseur, D.; Latruffe, N. The potential use of resveratrol for cancer prevention. Molecules 2019, 24, 4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, R.; Ebrahimzadeh, M.A. Resveratrol–A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef]
- Yang, M.F.; Yao, X.; Chen, L.M.; Gu, J.Y.; Yang, Z.H.; Chen, H.F.; Zheng, X.; Zheng, Z.T. Synthesis and biological evaluation of resveratrol derivatives with anti-breast cancer activity. Arch. Pharm. (Weinheim) 2020, 353, e2000044. [Google Scholar] [CrossRef]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.K.; Luo, J.Y.; Lau, C.W.; Chen, Z.Y.; Tian, X.Y.; Huang, Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2020, 177, 1258–1277. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Choi, W.; Ju Son, D.; Sung Kim, Y.; Kim, M.K.; Yoon, B.E.; Pyee, J.; Hong, J.T.; Go, M.Y.; et al. Antiatherogenic effect of resveratrol attributed to decreased expression of ICAM-1 (Intercellular adhesion Molecule-1) mechanistic link from focal adhesion to monocyte adhesion. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, C.X.; Liu, Y.M.; Chen, K.L.; Chen, G. A comparative study of anti-aging properties and mechanism: Resveratrol and caloric restriction. Oncotarget 2017, 8, 65717. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef]
- Zu, Y.; Wang, S. Resveratrol-loaded liposomes: Browning subcutaneous white adipose tissue for combating obesity in C57BL/6 J mice. Curr. Dev. Nutr. 2020, 4, 1709. [Google Scholar] [CrossRef]
- Kim, H.; Seo, K.H.; Yokoyama, W. Chemistry of pterostilbene and its metabolic effects. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Deng, R.; Li, E.T.; Shen, J.; Wang, M. Pinosylvin provides neuroprotection against cerebral ischemia and reperfusion injury through enhancing PINK1/Parkin mediated mitophagy and Nrf2 pathway. J. Funct. Foods 2020, 71, 104019. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Jiang, Y.; Zhang, B.; Yang, H.; Ma, T. Resveratrol dimer trans-ε-viniferin prevents rotaviral diarrhea in mice by inhibition of the intestinal calcium-activated chloride channel. Pharmacol. Res. 2018, 129, 453–461. [Google Scholar] [CrossRef]
- Nivelle, L.; Aires, V.; Rioult, D.; Martiny, L.; Tarpin, M.; Delmas, D. Molecular analysis of differential antiproliferative activity of resveratrol, epsilon viniferin and labruscol on melanoma cells and normal dermal cells. Food Chem. Toxicol. 2018, 116, 323–334. [Google Scholar] [CrossRef]
- Global Resveratrol Market Research Report 2020. Industry Research, 2020. Available online: https://www.industryresearch.co/global-resveratrol-market-15064120 (accessed on 1 December 2020).
- Huang, H.; Liu, R.; Ou, W. A mini review on the chemical synthesis of resveratrol. Mini Rev. Org. Chem. 2020, 17, 546–558. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, Y.; Wang, F.; Zhang, S.; Xu, H. Non-food renewable and bioactive forest products for pest management: Valuation of agricultural properties of podophyllotoxin analogs derived from Podophyllum hexandrum as botanical pesticides. Ind. Crops Prod. 2020, 153, 112608. [Google Scholar] [CrossRef]
- Donati, L.; Ferretti, L.; Frallicciardi, J.; Rosciani, R.; Valletta, A.; Pasqua, G. Stilbene biosynthesis and gene expression in response to methyl jasmonate and continuous light treatment in Vitis vinifera cv. Malvasia del Lazio and Vitis rupestris Du Lot cell cultures. Physiol. Plant. 2019, 166, 646–662. [Google Scholar] [CrossRef]
- Huber, R.; Marcourt, L.; Schnee, S.; Michellod, E.; Wolfender, J.L.; Gindro, K.; Queiroz, E.F. Biotransformations with the enzymatic secretome of Botrytis cinerea combined with organic solvents for the generation of novel complex stilbene derivatives. Planta Med. 2019, 85, 1446–1447. [Google Scholar]
- Wang, C.; Zhi, S.; Liu, C.; Xu, F.; Zhao, A.; Wang, X.; Ren, Y.; Li, Z.; Yu, M. Characterization of stilbene synthase genes in mulberry (Morus atropurpurea) and metabolic engineering for the production of resveratrol in Escherichia coli. J. Agric. Food Chem. 2017, 65, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Szczepańska, P.; Yuzbashev, T.; Lazar, Z.; Ledesma-Amaro, R. De novo production of resveratrol from glycerol by engineering different metabolic pathways in Yarrowia lipolytica. Metab. Eng. Commun. 2020, 11, e00146. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Emiliani, G.; Fondi, M.; Fani, R.; Gribaldo, S. A horizontal gene transfer at the origin of phenylpropanoid metabolism: A key adaptation of plants to land. Biol. Direct 2009, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, C.; Zhao, G.; Ning, Y. Interactions between plant proteins/enzymes and other food components, and their effects on food quality. Crit. Rev. Food Sci. Nutr. 2017, 57, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; Reid, P.D.; Marsh, H.V. L-phenylalanine ammonia-lyase (maize) evidence for a common catalytic site for L-phenylalanine and L-tyrosine. Plant Physiol. 1971, 48, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Rösler, J.; Krekel, F.; Amrhein, N.; Schmid, J. Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol. 1997, 113, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- Ferrer, J.L.; Austin, M.B.; Stewart, C., Jr.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef] [Green Version]
- Rupprich, N.; Hildebrand, H.; Kindl, H. Substrate specificity in vivo and in vitro in the formation of stilbenes. Biosynthesis of rhaponticin. Arch. Biochem. Biophys. 1980, 200, 72–78. [Google Scholar] [CrossRef]
- Schöppner, A.; Kindl, H. Purification and properties of a stilbene synthase from induced cell suspension cultures of peanut. J. Biol. Chem. 1984, 259, 6806–6811. [Google Scholar] [PubMed]
- Bais, A.J.; Murphy, P.J.; Dry, I.B. The molecular regulation of stilbene phytoalexin biosynthesis in Vitis vinifera during grape berry development. Funct. Plant Biol. 2000, 27, 425–433. [Google Scholar] [CrossRef]
- Samappito, S.; Page, J.E.; Schmidt, J.; De-Eknamkul, W.; Kutchan, T.M. Aromatic and pyrone polyketides synthesized by a stilbene synthase from Rheum tataricum. Phytochemistry 2003, 62, 313–323. [Google Scholar] [CrossRef]
- Fliegmann, J.; Schröder, G.; Schanz, S.; Britsch, L.; Schröder, J. Molecular analysis of chalcone and dihydropinosylvin synthase from Scots pine (Pinus sylvestris), and differential regulation of these and related enzyme activities in stressed plants. Plant Mol. Biol. 1992, 18, 489–503. [Google Scholar] [CrossRef]
- Kodan, A.; Kuroda, H.; Sakai, F. A stilbene synthase from Japanese red pine (Pinus densiflora): Implications for phytoalexin accumulation and down-regulation of flavonoid biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 3335–3339. [Google Scholar] [CrossRef] [Green Version]
- Raiber, S.; Schröder, G.; Schröder, J. Molecular and enzymatic characterization of two stilbene synthases from Eastern white pine (Pinus strobus) A single Arg/His difference determines the activity and the pH dependence of the enzymes. FEBS Lett. 1995, 361, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef]
- Tropf, S.; Lanz, T.; Rensing, S.A.; Schröder, J.; Schröder, G. Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J. Mol. Evol. 1994, 38, 610–618. [Google Scholar] [CrossRef]
- Parage, C.; Tavares, R.; Rety, S.; Baltenweck-Guyot, R.; Poutaraud, A.; Renault, L.; Heintz, D.; Lugan, R.; Marais, G.A.; Aubourg, S.; et al. Structural, functional, and evolutionary analysis of the unusually large stilbene synthase gene family in grapevine. Plant Physiol. 2012, 160, 1407–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Sanchez, I.J.; Verpoorte, R. Plant polyketide synthases: A fascinating group of enzymes. Plant Physiol. Biochem. 2009, 47, 167–174. [Google Scholar] [CrossRef]
- Pandith, S.A.; Ramazan, S.; Khan, M.I.; Reshi, Z.A.; Shah, M.A. Chalcone synthases (CHSs): The symbolic type III polyketide synthases. Planta 2020, 251, 15. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.B.; Bowman, M.E.; Ferrer, J.L.; Schröder, J.; Noel, J.P. An aldol switch discovered in stilbene synthases mediates cyclization specificity of type III polyketide synthases. Chem. Biol. 2004, 11, 1179–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liang, J.; Chen, H.; Ding, G.; Ma, B.; He, N. Evolutionary and functional analysis of mulberry type III polyketide synthases. BMC Genom. 2016, 17, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, G.; Brown, J.W.; Schröder, J. Molecular analysis of resveratrol synthase: cDNA, genomic clones and relationship with chalcone synthase. Europ. J. Biochem. 1988, 172, 161–169. [Google Scholar] [CrossRef]
- Melchior, F.; Kindl, H. Coordinate-and elicitor-dependent expression of stilbene synthase and phenylalanine ammonia-lyase genes in Vitis cv. Optima. Arch. Biochem. Biophys. 1991, 288, 552–557. [Google Scholar] [CrossRef]
- Preisig-Müller, R.; Schwekendiek, A.; Brehm, I.; Reif, H.J.; Kindl, H. Characterization of a pine multigene family containing elicitor-responsive stilbene synthase genes. Plant Mol. Biol. 1999, 39, 221–229. [Google Scholar] [CrossRef]
- Warren, R.L.; Keeling, C.I.; Yuen, M.M.; Raymond, A.; Taylor, G.A.; Vandervalk, B.P.; Mohamadi, H.; Paulino, D.; Chiu, R.; Jackman, S.D.; et al. Improved white spruce (Picea glauca) genome assemblies and annotation of large gene families of conifer terpenoid and phenolic defense metabolism. Plant J. 2015, 83, 189–212. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Ralph, S.G.; Bohlmann, J.; Fenning, T.M.; Gershenzon, J.; Schmidt, A. Biosynthesis of the major tetrahydroxystilbenes in spruce, astringin and isorhapontin, proceeds via resveratrol and is enhanced by fungal infection. Plant Physiol. 2011, 157, 876–890. [Google Scholar] [CrossRef] [Green Version]
- Christine, K.Y.; Springob, K.; Schmidt, J.; Nicholson, R.L.; Chu, I.K.; Yip, W.K.; Lo, C. A stilbene synthase gene (SbSTS1) is involved in host and nonhost defense responses in Sorghum. Plant Physiol. 2005, 138, 393–401. [Google Scholar]
- Zhu, F.; Han, J.; Liu, S.; Chen, X.; Varshney, R.K.; Liang, X. Cloning, expression pattern analysis and subcellular localization of resveratrol synthase gene in peanut (Arachis hypogaea L.). Am. J. Plant Sci. 2014, 5, 3619–3631. [Google Scholar] [CrossRef] [Green Version]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Haberer, G.; Hellsten, U.; Mitros, T.; Poliakov, A.; et al. The Sorghum bicolor genome and the diversification of grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Sharma, I.; Kumari, N.; Sharma, V. Defense gene expression in Sorghum bicolor against Macrophomina phaseolina in leaves and roots of susceptible and resistant cultivars. J. Plant Interact. 2014, 9, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.G.; Choi, S.C.; Kang, Y.; Kim, K.M.; Kang, C.S.; Kim, C. Constructing a reference genome in a single lab: The possibility to use oxford nanopore technology. Plants 2019, 8, 270. [Google Scholar] [CrossRef] [Green Version]
- Sparvoli, F.; Martin, C.; Scienza, A.; Gavazzi, G.; Tonelli, C. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.). Plant Mol. Biol. 1994, 24, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Ge, H.; Howard, S.; Qiu, W. Transcriptional expression of stilbene synthase genes are regulated developmentally and differentially in response to powdery mildew in Norton and Cabernet Sauvignon grapevine. Plant Sci. 2012, 197, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; He, M.; Cao, J.; Wang, H.; Ding, J.; Jiao, Y.T.; Li, R.M.; He, J.; Wang, D.; Wang, Y. The comparative analysis of the potential relationship between resveratrol and stilbene synthase gene family in the development stages of grapes (Vitis quinquangularis and Vitis vinifera). Plant Physiol. Biochem. 2014, 74, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Gachon, C.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef]
- Wang, J.; Hou, B. Glycosyltransferases: Key players involved in the modification of plant secondary metabolites. Front. Biol. China 2009, 4, 39–46. [Google Scholar] [CrossRef]
- Lepak, A.; Gutmann, A.; Kulmer, S.T.; Nidetzky, B. Creating a water-soluble resveratrol-based antioxidant by site-selective enzymatic glucosylation. ChemBioChem 2015, 16, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoda, K.; Kubota, N.; Uesugi, D.; Kobayashi, Y.; Hamada, H.; Hamada, H. Glycosylation of Stilbene Compounds by Cultured Plant Cells. Molecules 2020, 25, 1437. [Google Scholar] [CrossRef] [Green Version]
- Navarro, G.; Martínez-Pinilla, E.; Ortiz, R.; Noé, V.; Ciudad, C.J.; Franco, R. Resveratrol and related stilbenoids, nutraceutical/dietary complements with health-promoting actions: Industrial production, safety, and the search for mode of action. Compr. Rev. Food Sci. Food Saf. 2018, 17, 808–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, L.; Guo, Y.X.; Dong, Y.S.; Zhang, D.J.; Xiu, Z.L. Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2007, 75, 763. [Google Scholar] [CrossRef]
- Park, K.T.; Kim, J.K.; Lim, Y.H. Deglycosylation of stilbene glucoside compounds improves inhibition of 3-hydroxy-3-methylglutaryl coenzyme a reductase and squalene synthase activities. Food Sci. Biotechnol. 2014, 23, 647–651. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Aleynova, O.A.; Grigorchuk, V.P.; Dubrovina, A.S. Stilbene accumulation and expression of stilbene biosynthesis pathway genes in wild grapevine Vitis amurensis Rupr. Planta 2017, 245, 151–159. [Google Scholar] [CrossRef]
- Colombo, F.; Di Lorenzo, C.; Regazzoni, L.; Fumagalli, M.; Sangiovanni, E.; de Sousa, L.P.; Bavaresco, L.; Tomasi, D.; Bosso, A.; Aldini, G.; et al. Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties. Food Funct. 2019, 10, 1797–1807. [Google Scholar] [CrossRef]
- Romero-Pérez, A.I.; Lamuela-Raventós, R.M.; Andrés-Lacueva, C.; de la Torre-Boronat, M.C. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 2001, 49, 210–215. [Google Scholar] [CrossRef]
- Boubakri, H.; Poutaraud, A.; Wahab, M.A.; Clayeux, C.; Baltenweck-Guyot, R.; Steyer, D.; Marcic, C.; Mliki, A.; Soustre-Gacougnolle, I. Thiamine modulates metabolism of the phenylpropanoid pathway leading to enhanced resistance to Plasmopara viticola in grapevine. BMC Plant Biol. 2013, 13, 1–15. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.H.; Bersch, A.M.; Santos, R.O.; Trindade, S.C.; Costa, E.L.; Peres, M.M.; Malmann, C.A.; Schneider, M.; Bochi, V.C.; Sautter, C.K.; et al. Postharvest UV-C irradiation stimulates the non-enzymatic and enzymatic antioxidant system of ‘Isabel’hybrid grapes (Vitis labrusca× Vitis vinifera L.). Food Res. Int. 2017, 102, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Choi, S.J.; Baek, K.H. Application of ultraviolet c irradiation for the increased production of secondary metabolites in plants. J. Anim. Plant Sci. 2020, 30, 1082–1091. [Google Scholar]
- Souid, I.; Toumi, I.; Hermosín-Gutiérrez, I.; Nasri, S.; Mliki, A.; Ghorbel, A. The effect of salt stress on resveratrol and piceid accumulation in two Vitis vinifera L. cultivars. Physiol. Mol. Biol. Plants 2019, 25, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.G.; Decendit, A.; Papastamoulis, Y.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit increases stilbene metabolism in Cabernet Sauvignon berries. J. Agric. Food Chem. 2011, 59, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Villangó, S.; Szekeres, A.; Bencsik, O.; Láposi, R.; Pálfi, Z.; Zsófi, Z. The effect of postveraison water deficit on the phenolic composition and concentration of the Kékfrankos (Vitis vinifera L.) berry. Sci. Hort. 2016, 209, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Bowles, D.; Lim, E.K.; Poppenberger, B.; Vaistij, F.E. Glycosyltransferases of lipophilic small molecules. Annu. Rev. Plant Biol. 2006, 57, 567–597. [Google Scholar] [CrossRef]
- Hall, D.; De Luca, V. Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J. 2007, 49, 579–591. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef]
- Romero-Pérez, A.I.; Ibern-Gómez, M.; Lamuela-Raventós, R.M.; de la Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Concenco, F.I.; Brotto, G.F.; Nora, L. Grape wine and juice: Comparison on resveratrol levels. Int. J. Adv. Res. Sci. Eng. Technol. 2019, 6, 368–386. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Wendelin, S.; Eder, R. Effects of various vinification techniques on the concentration of cis-and trans-resveratrol and resveratrol glucoside isomers in wine. Am. J. Enol. Vitic. 1997, 48, 214–219. [Google Scholar]
- Kostadinović, S.; Wilkens, A.; Stefova, M.; Ivanova, V.; Vojnoski, B.; Mirhosseini, H.; Winterhalter, P. Stilbene levels and antioxidant activity of Vranec and Merlot wines from Macedonia: Effect of variety and enological practices. Food Chem. 2012, 135, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Gaensly, F.; Agustini, B.C.; da Silva, G.A.; Picheth, G.; Bonfim, T.M.B. Autochthonous yeasts with β-glucosidase activity increase resveratrol concentration during the alcoholic fermentation of Vitis labrusca grape must. J. Funct. Foods 2015, 19, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.P.; Wang, R.; Huang, C.Y.; Lai, J.T.; Lo, Y.C.; Huang, S.T. Characterization of an extracellular β-glucosidase from Dekkera bruxellensis for resveratrol production. J. Food Drug Anal. 2018, 26, 163–171. [Google Scholar] [CrossRef]
- Chen, M.; Li, D.; Gao, Z.; Zhang, C. Enzymatic transformation of polydatin to resveratrol by piceid-β-d-glucosidase from Aspergillus oryzae. Bioprocess Biosyst. Eng. 2014, 37, 1411–1416. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Mulla, D.; Praznik, W.; Viernstein, H.; Mueller, M. Bioconversion of piceid to resveratrol by selected probiotic cell extracts. Bioprocess Biosyst. Eng. 2016, 39, 1879–1885. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Kim, M.; Cho, S.G.; Kim, M.K.; Kim, S.W.; Lim, Y.H. Biotransformation of mulberroside A from Morus alba results in enhancement of tyrosinase inhibition. J. Ind. Microbiol. Biotechnol. 2010, 37, 631–637. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, N.; Lim, Y.H. Evaluation of the antibacterial activity of rhapontigenin produced from rhapontin by biotransformation against Propionibacterium acnes. J. Microbiol. Biotechnol. 2010, 20, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Komaikul, J.; Kitisripanya, T.; Inyai, C.; Likhitwitayawuid, K.; Sritularak, B.; Tanaka, H.; Putalun, W. Phytostilbenoid production in white mulberry (Morus alba L.) cell culture using bioreactors and simple deglycosylation by endogenous enzymatic hydrolysis. In Vitro Cell. Dev. Biol. Plant 2019, 55, 199–208. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Cluzet, S.; Palos Pinto, A.; Dufour, M.C.; Corio-Costet, M.F.; Mérillon, J.M. Pinus pinaster Knot: A source of polyphenols against Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 8884–8891. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; Leborgne, C.; Waffo-Téguo, P.; Pedrot, E.; Richard, T.; Mérillon, J.M.; Valls Fonayet, J. Separation and isolation of major polyphenols from maritime pine (Pinus pinaster) knots by two-step centrifugal partition chromatography monitored by LC-MS and NMR spectroscopy. J. Sep. Sci. 2020, 43, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Sati, S.C.; Sati, N.; Sati, O.P. Bioactive constituents and medicinal importance of genus Alnus. Pharmacogn. Rev. 2011, 5, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshadri, T.R. Polyphenols of Pterocarpus and Dalbergia woods. Phytochemistry 1972, 11, 881–898. [Google Scholar] [CrossRef]
- Maurya, R.; Ray, A.B.; Duah, F.K.; Slatkin, D.J.; Schiff, P.L., Jr. Constituents of Pterocarpus marsupium. J. Nat. Prod. 1984, 47, 179–181. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Langcake, P.; Cornford, C.A.; Pryce, R.J. Identification of pterostilbene as a phytoalexin from Vitis vinifera leaves. Phytochemistry 1979, 18, 1025–1027. [Google Scholar] [CrossRef]
- Jeandet, P.; Douillet-Breuil, A.C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef]
- Vek, V.; Poljanšek, I.; Humar, M.; Willför, S.; Oven, P. In vitro inhibition of extractives from knotwood of Scots pine (Pinus sylvestris) and black pine (Pinus nigra) on growth of Schizophyllum commune, Trametes versicolor, Gloeophyllum trabeum and Fibroporia vaillantii. Wood Sci. Technol. 2020, 54, 1645–1662. [Google Scholar] [CrossRef]
- Hart, J.H. Role of phytostilbenes in decay and disease resistance. Annu. Rev. Phytopathol. 1981, 19, 437–458. [Google Scholar] [CrossRef]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; An, C.H.; Woo, S.G.; Jeong, H.J.; Kim, Y.M.; Park, S.J.; Yoon, B.D.; Kim, C.Y. Production of pinostilbene compounds by the expression of resveratrol O-methyltransferase genes in Escherichia coli. Enzyme Microb. Technol. 2014, 54, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Pan, Z.; Polashock, J.J.; Dayan, F.E.; Mizuno, C.S.; Snook, M.E.; Liu, C.J.; Baerson, S.R. In planta production of the highly potent resveratrol analogue pterostilbene via stilbene synthase and O-methyltransferase co-expression. Plant Biotechnol. J. 2012, 10, 269–283. [Google Scholar] [CrossRef]
- Martínez-Márquez, A.; Morante-Carriel, J.A.; Palazon, J.; Bru-Martínez, R. Rosa hybrida orcinol O-methyl transferase-mediated production of pterostilbene in metabolically engineered grapevine cell cultures. New Biotechnol. 2018, 42, 62–70. [Google Scholar] [CrossRef]
- Purwanto, R.; Hori, K.; Yamada, Y.; Sato, F. Unraveling additional O-methylation steps in benzylisoquinoline alkaloid biosynthesis in California poppy (Eschscholzia californica). Plant Cell Physiol. 2017, 58, 1528–1540. [Google Scholar] [CrossRef] [Green Version]
- Nakatsubo, T.; Kitamura, Y.; Sakakibara, N.; Mizutani, M.; Hattori, T.; Sakurai, N.; Shibata, D.; Suzuki, S.; Umezawa, T. At5g54160 gene encodes Arabidopsis thaliana 5-hydroxyconiferaldehyde O-methyltransferase. J. Wood Sci. 2008, 54, 312–317. [Google Scholar] [CrossRef]
- Shimizu, T.; Lin, F.; Hasegawa, M.; Okada, K.; Nojiri, H.; Yamane, H. Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J. Biol. Chem. 2012, 287, 19315–19325. [Google Scholar] [CrossRef] [Green Version]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Baerson, S.R.; Dayan, F.E.; Rimando, A.M.; Nanayakkara, N.D.; Liu, C.J.; Schröder, J.; Fishbein, M.; Pan, Z.; Kagan, I.A.; Pratt, L.H.; et al. A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs. J. Biol. Chem. 2008, 283, 3231–3247. [Google Scholar] [CrossRef] [Green Version]
- Koeduka, T.; Hatada, M.; Suzuki, H.; Suzuki, S.; Matsui, K. Molecular cloning and functional characterization of an O-methyltransferase catalyzing 4′-O-methylation of resveratrol in Acorus calamus. J. Biosci. Bioeng. 2019, 127, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Chiron, H.; Drouet, A.; Claudot, A.C.; Eckerskorn, C.; Trost, M.; Heller, W.; Ernst, D.; Sandermann, H. Molecular cloning and functional expression of a stress-induced multifunctional O-methyltransferase with pinosylvin methyltransferase activity from Scots pine (Pinus sylvestris L.). Plant Mol. Biol. 2000, 44, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Paasela, T.; Lim, K.J.; Pietiäinen, M.; Teeri, T.H. The O-methyltransferase PMT 2 mediates methylation of pinosylvin in Scots pine. New Phytol. 2017, 214, 1537–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pailee, P.; Sangpetsiripan, S.; Mahidol, C.; Ruchirawat, S.; Prachyawarakorn, V. Cytotoxic and cancer chemopreventive properties of prenylated stilbenoids from Macaranga siamensis. Tetrahedron 2015, 71, 5562–5571. [Google Scholar] [CrossRef]
- Leláková, V.; Béraud-Dufour, S.; Hošek, J.; Šmejkal, K.; Prachyawarakorn, V.; Pailee, P.; Widmann, C.; Václavík, J.; Coppola, T.; Mazella, J.; et al. Therapeutic potential of prenylated stilbenoid macasiamenene F through its anti-inflammatory and cytoprotective effects on LPS-challenged monocytes and microglia. J. Ethnopharmacol. 2020, 263, 113147. [Google Scholar] [CrossRef]
- Biondi, D.M.; Rocco, C.; Ruberto, G. New dihydrostilbene derivatives from the leaves of Glycyrrhiza glabra and evaluation of their antioxidant activity. J. Nat. Prod. 2003, 66, 477–480. [Google Scholar] [CrossRef]
- Biondi, D.M.; Rocco, C.; Ruberto, G. Dihydrostilbene derivatives from Glycyrrhiza glabra leaves. J. Nat. Prod. 2005, 68, 1099–1102. [Google Scholar] [CrossRef]
- Ye, R.; Fan, Y.H.; Ma, C.M. Identification and enrichment of α-glucosidase-inhibiting dihydrostilbene and flavonoids from Glycyrrhiza uralensis leaves. J. Agric. Food Chem. 2017, 65, 510–515. [Google Scholar] [CrossRef]
- Meng, H.C.; Zhu, S.; Fan, Y.H.; Ye, R.; Hattori, M.; Komatsu, K.; Ma, C.M. Discovery of prenylated dihydrostilbenes in Glycyrrhiza uralensis leaves by UHPLC-MS using neutral loss scan. Ind. Crops Prod. 2020, 152, 112557. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Potter, T.L.; Horn, B.W. Prenylated stilbenes from peanut root mucilage. Phytochem Anal. 2006, 17, 312–322. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Neff, S.A.; Gloer, J.B. New stilbenoids from peanut (Arachis hypogaea) seeds challenged by an Aspergillus caelatus strain. J. Agric. Food Chem. 2009, 57, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S.; Neff, S.A.; Gloer, J.B. New dimeric stilbenoids from fungal-challenged peanut (Arachis hypogaea) seeds. J. Agric. Food Chem. 2010, 58, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S. Production of phytoalexins in peanut (Arachis hypogaea) seed elicited by selected microorganisms. J. Agric. Food Chem. 2013, 61, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S.; Krausert, N.M.; Gloer, J.B. New monomeric stilbenoids from peanut (Arachis hypogaea) seeds challenged by an Aspergillus flavus strain. J. Agric. Food Chem. 2016, 64, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, L.; Huang, D. Food grade fungal stress on germinating peanut seeds induced phytoalexins and enhanced polyphenolic antioxidants. J. Agric. Food Chem. 2011, 59, 5993–6003. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.; Lye, P.Y.; Wong, S.K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar] [PubMed]
- Yang, T.; Fang, L.; Sanders, S.; Jayanthi, S.; Rajan, G.; Podicheti, R.; Thallapuranam, S.K.; Mockaitis, K.; Medina-Bolivar, F. Stilbenoid prenyltransferases define key steps in the diversification of peanut phytoalexins. J. Biol. Chem. 2018, 293, 28–46. [Google Scholar] [CrossRef] [Green Version]
- Aisyah, S.; Gruppen, H.; Slager, M.; Helmink, B.; Vincken, J.P. Modification of prenylated stilbenoids in peanut (Arachis hypogaea) seedlings by the same fungi that elicited them: The fungus strikes back. J. Agric. Food Chem. 2015, 63, 9260–9268. [Google Scholar] [CrossRef]
- Aguamah, G.E.; Langcake, P.; Leworthy, D.P.; Page, J.A.; Pryce, R.J.; Strange, R.N. Two novel stilbene phytoalexins from Arachis hypogaea. Phytochemistry 1981, 20, 1381–1383. [Google Scholar] [CrossRef]
- Wotton, H.R.; Strange, R.N. Circumstantial evidence for phytoalexin involvement in the resistance of peanuts to Aspergillus flavus. Microbiology 1985, 131, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Cooksey, C.J.; Garratt, P.J.; Richards, S.E.; Strange, R.N. A dienyl stilbene phytoalexin from Arachis hypogaea. Phytochemistry 1988, 27, 1015–1016. [Google Scholar] [CrossRef]
- Péresse, T.; Jézéquel, G.; Allard, P.M.; Pham, V.C.; Huong, D.T.; Blanchard, F.; Bignon, J.; Lévaique, H.; Wolfender, J.-L.; Litaudon, M.; et al. Cytotoxic prenylated stilbenes isolated from Macaranga tanarius. J. Nat. Prod. 2017, 80, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, W.J.; Araya-Cloutier, C.; Bijlsma, J.; de Swart, A.; Sanders, M.G.; de Waard, P.; Gruppen, H.; Vincken, J.P. Antibacterial prenylated stilbenoids from peanut (Arachis hypogaea). Phytochem. Lett. 2018, 28, 13–18. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhu, W.; Liu, S.; Guan, Q.; Chen, X.; Huang, W.; Yang, B.; Tian, J.; Tian, J. Molecular characterization of a geranyl diphosphate-specific prenyltransferase catalyzing stilbenoid prenylation from Morus alba. Plant Cell Physiol. 2018, 59, 2214–2227. [Google Scholar] [CrossRef] [PubMed]
- Munakata, R.; Olry, A.; Karamat, F.; Courdavault, V.; Sugiyama, A.; Date, Y.; Krieger, C.; Silie, P.; Foureau, E.; Papon, V.; et al. Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis. New Phytol. 2016, 211, 332–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, M.; Alcántara, J.; Barceló, A.R. Oxidation of trans-resveratrol by a hypodermal peroxidase isoenzyme from Gamay rouge grape (Vitis vinifera) berries. Am. J. Enol. Vitic. 1997, 48, 33–38. [Google Scholar]
- Lin, M.; Yao, C.S. Natural oligostilbenes. Stud. Nat. Prod. Chem. 2006, 33, 601–644. [Google Scholar]
- Chiou, W.F.; Shen, C.C.; Chen, C.C.; Lin, C.H.; Huang, Y.L. Oligostilbenes from the roots of Vitis thunbergii. Planta Med. 2009, 75, 856–859. [Google Scholar] [CrossRef]
- Shu, N.; Zhou, H.; Hu, C. Simultaneous determination of the contents of three stilbene oligomers in Caragana sinica collected in different seasons using an improved HPLC method. Biol. Pharm. Bull. 2006, 29, 608–612. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Endo, H.; Oyama, M.; Iinuma, M. Novel isolation of stilbenoids with enantiomeric and meso forms from a Cyperus rhizome. Phytochem. Lett. 2012, 5, 267–270. [Google Scholar] [CrossRef]
- Abe, N.; Ito, T.; Oyama, M.; Sawa, R.; Takahashi, Y.; Iinuma, M. Resveratrol derivatives from Vatica albiramis. Chem. Pharm. Bull. 2011, 59, 452–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimokawa, Y.; Hirasawa, Y.; Kaneda, T.; Hadi, A.H.A.; Morita, H. Cuspidans A and B, two new stilbenoids from the bark of Gnetum cuspidatum. Chem. Pharm. Bull. 2012, 60, 790–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.N.; Peng, Y.; Xu, L.J.; Liu, Z.A.; Gu, J.; Zhong, A.G.; Xiao, P.G. Three new oligostilbenes from the seeds of Paeonia suffruticosa. Chem. Pharm. Bull. 2010, 58, 843–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Q.; Tan, J.J.; Tan, C.H.; Jiang, S.H.; Zhu, D.Y. Halophilols A and B, two new stilbenes from Iris halophila. Planta Med. 2003, 69, 779–781. [Google Scholar] [PubMed]
- Syah, Y.M.; Achmad, S.A.; Ghisalberti, E.L.; Hakim, E.H.; Makmur, L.; Soekamto, N.H. A stilbene dimer, andalasin B, from the root trunk of Morus macroura. J. Chem. Res. 2004, 5, 339–340. [Google Scholar] [CrossRef]
- Douillet-Breuil, A.C.; Jeandet, P.; Adrian, M.; Bessis, R. Changes in the phytoalexin content of various Vitis spp. in response to ultraviolet C elicitation. J. Agric. Food Chem. 1999, 47, 4456–4461. [Google Scholar] [CrossRef]
- Wang, X.F.; Yao, C.S. Naturally active oligostilbenes. J. Asian Nat. Prod. Res. 2016, 18, 376–407. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanaka, T.; Nakaya, K.I.; Iinuma, M.; Takahashi, Y.; Naganawa, H.; Ohyama, M.; Nakanishi, Y.; Bastow, K.F.; Lee, K.H. A new resveratrol octamer, vateriaphenol A, in Vateria indica. Tetrahedron Lett. 2001, 42, 5909–5912. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.N.; Lou, H.X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef]
- Pezet, R.; Perret, C.; Jean-Denis, J.B.; Tabacchi, R.; Gindro, K.; Viret, O. Delta-viniferin, a resveratrol dehydrodimer: One of the major stilbenes synthesized by stressed grapevine leaves. J. Agric. Food Chem. 2003, 51, 5488–5492. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. Oxidative dimerization of 4-hydroxystilbenes in vitro: Production of a grapevine phytoalexin mimic. J. Chem. Soc. Chem. Commun. 1977, 7, 208–210. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Li, G.P.; Kang, Y.L.; Teng, B.H.; Yao, C.S. Biomimetic synthesis of resveratrol trimers catalyzed by horseradish peroxidase. Molecules 2017, 22, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezet, R. Purification and characterization of a 32-kDa laccase-like stilbene oxidase produced by Botrytis cinerea Pers.: Fr. FEMS Microbiol. Lett. 1998, 167, 203–208. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Zhu, X.; Li, S.; Wang, Z.; Wang, L.; Li, Z.; Chen, G. Using laccases in the nanoflower to synthesize viniferin. Catalysts 2017, 7, 188. [Google Scholar] [CrossRef] [Green Version]
- Ros Barceló, A.; Pomar, F.; López-Serrano, M.; Pedreno, M.A. Peroxidase: A multifunctional enzyme in grapevines. Funct. Plant Biol. 2003, 30, 577–591. [Google Scholar] [CrossRef]
- Takaya, Y.; Terashima, K.; Ito, J.; He, Y.H.; Tateoka, M.; Yamaguchi, N.; Niwa, M. Biomimic transformation of resveratrol. Tetrahedron 2005, 61, 10285–10290. [Google Scholar] [CrossRef]
- Wilkens, A.; Paulsen, J.; Wray, V.; Winterhalter, P. Structures of two novel trimeric stilbenes obtained by horseradish peroxidase catalyzed biotransformation of trans-resveratrol and (−)-ε-viniferin. J. Agric. Food Chem. 2010, 58, 6754–6761. [Google Scholar] [CrossRef]
- Sako, M.; Hosokawa, H.; Ito, T.; Iinuma, M. Regioselective oxidative coupling of 4-hydroxystilbenes: Synthesis of resveratrol and ε-viniferin (E)-dehydrodimers. J. Org. Chem. 2004, 69, 2598–2600. [Google Scholar] [CrossRef]
- Natori, Y.; Ito, M.; Anada, M.; Nambu, H.; Hashimoto, S. Catalytic asymmetric synthesis of (−)-E-δ-viniferin via an intramolecular C–H insertion of diaryldiazomethane using Rh2 (S-TFPTTL) 4. Tetrahedron Lett. 2015, 56, 4324–4327. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lucini, L.; Busconi, M.; Flamini, R.; De Rosso, M. Wine resveratrol: From the ground up. Nutrients 2016, 8, 222. [Google Scholar] [CrossRef] [Green Version]
- Flamini, R.; Zanzotto, A.; de Rosso, M.; Lucchetta, G.; Dalla Vedova, A.; Bavaresco, L. Stilbene oligomer phytoalexins in grape as a response to Aspergillus carbonarius infection. Physiol. Mol. Plant Pathol. 2016, 93, 112–118. [Google Scholar] [CrossRef]
- Sarig, P.; Zutkhi, Y.; Monjauze, A.; Lisker, N.; Ben-Arie, R. Phytoalexin elicitation in grape berries and their susceptibility to Rhizopus stolonifer. Physiol. Mol. Plant Pathol. 1997, 50, 337–347. [Google Scholar] [CrossRef]
- Cantos, E.; García-Viguera, C.; de Pascual-Teresa, S.; Tomás-Barberán, F.A. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J. Agric. Food Chem. 2000, 48, 4606–4612. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Parpinello, G.P.; Tornielli, G.B.; Ferrarini, R.; Giulivo, C. Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. J. Agric. Food Chem. 2001, 49, 5531–5536. [Google Scholar] [CrossRef] [PubMed]
- Moriartry, J.M.; Harmon, R.; Weston, L.A.; Bessis, R.; Breuil, A.C.; Adrian, M.; Jeandet, P. Resveratrol content of two Californian table grape cultivars. Vitis 2001, 40, 43–44. [Google Scholar]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J. Agric. Food Chem. 2002, 50, 6322–6329. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; Fernández, M.J.; Oliva, J.; Tomás-Barberán, F.A. Postharvest UV-C-irradiated grapes as a potential source for producing stilbene-enriched red wines. J. Agric. Food Chem. 2003, 51, 1208–1214. [Google Scholar] [CrossRef]
- Cantos, E.; Tomás-Barberán, F.A.; Martínez, A.; Espín, J.C. Differential stilbene induction susceptibility of seven red wine grape varieties upon post-harvest UV-C irradiation. Eur. Food Res. Technol. 2003, 217, 253–258. [Google Scholar] [CrossRef]
- González-Barrio, R.; Salmenkallio-Marttila, M.; Tomás-Barberán, F.A.; Cantos, E.; Espín, J.C. Etiology of UV-C-induced browning in var. Superior white table grapes. J. Agric. Food Chem. 2005, 53, 5990–5996. [Google Scholar] [CrossRef]
- González-Barrio, R.; Beltrán, D.; Cantos, E.; Gil, M.I.; Espín, J.C.; Tomás-Barberán, F.A. Comparison of ozone and UV-C treatments on the postharvest stilbenoid monomer, dimer, and trimer induction in var. ‘Superior’ white table grapes. J. Agric. Food Chem. 2006, 54, 4222–4228. [Google Scholar] [CrossRef]
- González-Barrio, R.; Vidal-Guevara, M.L.; Tomás-Barberán, F.A.; Espín, J.C. Preparation of a resveratrol-enriched grape juice based on ultraviolet C-treated berries. Innov. Food Sci. Emerg. Technol. 2009, 10, 374–382. [Google Scholar] [CrossRef]
- Sánchez, J.J.; Corral, E.C.; Orea, J.M.; Delgado, M.S.; Ureña, A.G. Elicitation of trans-resveratrol by laser resonant irradiation of table grapes. Appl. Phys. B 2007, 87, 559–563. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Puertas, B.; Fernández, M.I.; Palma, M.; Cantos-Villar, E. Induction of stilbenes in grapes by UV-C: Comparison of different subspecies of Vitis. Innov. Food. Sci. Emerg. Technol. 2010, 11, 231–238. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, N.; Kim, C.T.; Maeng, J.S.; Pyee, J. Quantitative evaluation of resveratrol enrichment induced by UV stimulus in harvested grapes. Food Sci. Biotechnol. 2012, 21, 597–601. [Google Scholar] [CrossRef]
- Crupi, P.; Pichierri, A.; Basile, T.; Antonacci, D. Postharvest stilbenes and flavonoids enrichment of table grape cv Redglobe (Vitis vinifera L.) as affected by interactive UV-C exposure and storage conditions. Food Chem. 2013, 141, 802–808. [Google Scholar] [CrossRef]
- Freitas, P.M.; López-Gálvez, F.; Tudela, J.A.; Gil, M.I.; Allende, A. Postharvest treatment of table grapes with ultraviolet-C and chitosan coating preserves quality and increases stilbene content. Postharvest Biol. Technol. 2015, 105, 51–57. [Google Scholar] [CrossRef]
- Yin, X.; Singer, S.D.; Qiao, H.; Liu, Y.; Jiao, C.; Wang, H.; Li, Z.; Fei, Z.; Wang, Y.; Fan, C.; et al. Insights into the mechanisms underlying ultraviolet-C induced resveratrol metabolism in grapevine (V. amurensis Rupr.) cv.“Tonghua-3”. Front. Plant Sci. 2016, 7, 503. [Google Scholar] [CrossRef] [Green Version]
- Sheng, K.; Zheng, H.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol. Techol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Kong, Q.; Deng, R.; Li, X.; Zeng, Q.; Zhang, X.; Yu, X.; Ren, X. Based on RNA-Seq analysis identification and expression analysis of Trans-scripusin A synthesize-related genes of UV-treatment in postharvest grape fruit. Arch. Biochem. Biophys. 2020, 690, 108471. [Google Scholar] [CrossRef]
- Sales, J.M.; Resurreccion, A.V.A. Maximising resveratrol and piceid contents in UV and ultrasound treated peanuts. Food Chem. 2009, 117, 674–680. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, M.R.; Chun, J.C.; Yun, S.J. Resveratrol accumulation and resveratrol synthase gene expression in response to abiotic stresses and hormones in peanut plants. Plant Sci. 2003, 164, 103–209. [Google Scholar] [CrossRef]
- Rudolf, J.R.; Resurreccion, A.V. Elicitation of resveratrol in peanut kernels by application of abiotic stresses. J. Agric. Food Chem. 2005, 53, 10186–10192. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Chang, E.; Li, M.; Ji, J.; Yao, X.; Bartish, I.V.; Liu, J.; Ma, J.; Chen, L.; Jiang, Z.; et al. Transcriptome characterization of Gnetum parvifolium reveals candidate genes involved in important secondary metabolic pathways of flavonoids and stilbenoids. Front. Plant Sci. 2016, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Liu, C.; Chang, E.; Ji, J.; Yao, X.; Yue, J.; Bartish, I.V.; Chen, L.; Jiang, Z.; Shi, S. High temperature and UV-C treatments affect stilbenoid accumulation and related gene expression levels in Gnetum parvifolium. Electron. J. Biotechnol. 2017, 25, 43–49. [Google Scholar] [CrossRef]
- Pastore, C.; Dal Santo, S.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Filippetti, I.; Tornielli, G.B. Whole plant temperature manipulation affects flavonoid metabolism and the transcriptome of grapevine berries. Front. Plant Sci. 2017, 8, 929. [Google Scholar] [CrossRef]
- Wang, L.; An, M.; Huang, W.; Zhan, J. Melatonin and phenolics biosynthesis-related genes in Vitis vinifera cell suspension cultures are regulated by temperature and copper stress. Plant Cell Tiss. Org. Cult. 2019, 138, 475–488. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Romero, I.; Jiménez, J.B.; Orea, J.M.; González-Urena, A.; Escribano, M.I.; Merodio, C. Involvement of the phenylpropanoid pathway in the response of table grapes to low temperature and high CO2 levels. Postharvest Biol. Technol. 2007, 46, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Ballesta, M.T.; Alvarez, I.; Escribano, M.I.; Merodio, C.; Romero, I. Effect of high CO2 levels and low temperature on stilbene biosynthesis pathway gene expression and stilbenes production in white, red and black table grape cultivars during postharvest storage. Plant Physiol. Biochem. 2020, 151, 334–341. [Google Scholar] [CrossRef]
- Degu, A.; Ayenew, B.; Cramer, G.R.; Fait, A. Polyphenolic responses of grapevine berries to light, temperature, oxidative stress, abscisic acid and jasmonic acid show specific developmental-dependent degrees of metabolic resilience to perturbation. Food Chem. 2016, 212, 828–836. [Google Scholar] [CrossRef]
- Tassoni, A.; Durante, L.; Ferri, M. Combined elicitation of methyl-jasmonate and red light on stilbene and anthocyanin biosynthesis. J. Plant Physiol. 2012, 169, 775–781. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.A.; Choi, S.J.; Yun, H.K. Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic. Environ. Biotechnol. 2015, 56, 36–43. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.A.; Yun, H.K. Inhibition of Botrytis cinerea and accumulation of stilbene compounds by light-emitting diodes of grapevine leaves and differential expression of defense-related genes. Eur. J. Plant Pathol. 2015, 143, 753–765. [Google Scholar] [CrossRef]
- Taurino, M.; Ingrosso, I.; D’amico, L.; Domenico, S.D.; Nicoletti, I.; Corradini, D.; Santino, A.; Giovinazzo, G. Jasmonates elicit different sets of stilbenes in Vitis vinifera cv. Negramaro cell cultures. SpringerPlus 2015, 4, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andi, S.A.; Gholami, M.; Ford, C.M. The effect of methyl jasmonate and light irradiation treatments on the stilbenoid biosynthetic pathway in Vitis vinifera cell suspension cultures. Nat. Prod. Res. 2018, 32, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Yang, J.; Zhang, D.; Cai, Q.; Zhou, D.; Tu, S.; Liu, Q.; Tu, K. Effects of white LED light and UV-C radiation on stilbene biosynthesis and phytochemicals accumulation identified by UHPLC–MS/MS during peanut (Arachis hypogaea L.) germination. J. Agric. Food Chem. 2020, 68, 5900–5909. [Google Scholar] [CrossRef]
- Houillé, B.; Besseau, S.; Courdavault, V.; Oudin, A.; Glévarec, G.; Delanoue, G.; Guerin, L.; Simkin, J.A.; Papon, N.; Clastre, M.; et al. Biosynthetic origin of E-resveratrol accumulation in grape canes during postharvest storage. J. Agric. Food Chem. 2015, 63, 1631–1638. [Google Scholar] [CrossRef]
- Vannozzi, A.; Wong, D.C.J.; Höll, J.; Hmmam, I.; Matus, J.T.; Bogs, J.; Ziegler, T.; Dry, I.; Barcaccia, G.; Lucchin, M. Combinatorial regulation of stilbene synthase genes by WRKY and MYB transcription factors in grapevine (Vitis vinifera L.). Plant Cell Physiol. 2018, 59, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.R.; Innocenti, M.; Mulinacci, N.; Melani, F.; Valletta, A.; Sciandra, I.; Pasqua, G. Enhancement of viniferin production in Vitis vinifera L. cv. Alphonse Lavallée cell suspensions by low-energy ultrasound alone and in combination with methyl jasmonate. J. Agric. Food Chem. 2012, 60, 11135–11142. [Google Scholar] [CrossRef]
- Yin, X.; Huang, L.; Zhang, X.; Guo, C.; Wang, H.; Li, Z.; Wang, X. Expression patterns and promoter characteristics of the Vitis quinquangularis VqSTS36 gene involved in abiotic and biotic stress response. Protoplasma 2017, 254, 2247–2261. [Google Scholar] [CrossRef]
- Chiron, H.; Drouet, A.; Lieutier, F.; Payer, H.D.; Ernst, D.; Sandermann, H. Gene induction of stilbene biosynthesis in Scots pine in response to ozone treatment, wounding, and fungal infection. Plant Physiol. 2000, 124, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Lim, K. Scots Pine (Pinus sylvestris L.) Heartwood Formation and Wounding Stress: A View from the Transcriptome. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2017. [Google Scholar]
- Johansson, S.M.; Lundgren, L.N.; Asiegbu, F.O. Initial reactions in sapwood of Norway spruce and Scots pine after wounding and infection by Heterobasidion parviporum and H. annosum. For. Pathol. 2004, 34, 197–210. [Google Scholar] [CrossRef]
- Billet, K.; Houillé, B.; Besseau, S.; Mélin, C.; Oudin, A.; Papon, N.; Courdavault, V.; Clastre, M.; Giglioli-Guivarc’h, N.; Lanoue, A. Mechanical stress rapidly induces E-resveratrol and E-piceatannol biosynthesis in grape canes stored as a freshly-pruned byproduct. Food Chem. 2018, 240, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.K.; Strange, R.N. Phytoalexin accumulation in groundnuts in response to wounding. Plant Sci. 1991, 78, 157–163. [Google Scholar] [CrossRef]
- Righetti, L.; Franceschetti, M.; Ferri, M.; Tassoni, A.; Bagni, N. Resveratrol production in Vitis vinifera cell suspensions treated with several elicitors. Caryologia 2007, 60, 169–171. [Google Scholar]
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Biochem. 2008, 46, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lijavetzky, D.; Almagro, L.; Belchi-Navarro, S.; Martínez-Zapater, J.M.; Bru, R.; Pedreño, M.A. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes 2008, 1, 132. [Google Scholar] [CrossRef] [Green Version]
- Ferri, M.; Tassoni, A.; Franceschetti, M.; Righetti, L.; Naldrett, M.J.; Bagni, N. Chitosan treatment induces changes of protein expression profile and stilbene distribution in Vitis vinifera cell suspensions. Proteomics 2009, 9, 610–624. [Google Scholar] [CrossRef]
- Santamaria, A.R.; Antonacci, D.; Caruso, G.; Cavaliere, C.; Gubbiotti, R.; Laganà, A.; Valletta, A.; Pasqua, G. Stilbene production in cell cultures of Vitis vinifera L. cvs Red Globe and Michele Palieri elicited by methyl jasmonate. Nat. Prod. Res. 2010, 24, 1488–1498. [Google Scholar] [CrossRef]
- Mihai, R.; Cristina, S.; Helepciuc, F.; Brezeanu, A.; Stoian, G. Biotic and abiotic elicitors induce biosynthesis and accumulation of resveratrol with antitumoral activity in the long-term Vitis vinifera L. callus cultures. Rom. Biotechnol. Lett. 2011, 16, 6683–6689. [Google Scholar]
- Ferri, M.; Dipalo, S.C.; Bagni, N.; Tassoni, A. Chitosan elicits mono-glucosylated stilbene production and release in fed-batch bioreactor cultures of grape cells. Food Chem. 2011, 124, 1473–1479. [Google Scholar] [CrossRef]
- Santamaria, A.R.; Mulinacci, N.; Valletta, A.; Innocenti, M.; Pasqua, G. Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia. J. Agric. Food Chem. 2011, 59, 9094–9101. [Google Scholar] [CrossRef] [PubMed]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Caia, Z.; Knorra, D.; Smetanskaa, I. Enhanced anthocyanins and resveratrol production in Vitis vinifera cell suspension culture by indanoyl-isoleucine, N-linolenoyl-L-glutamine and insect saliva. Enzym. Microb. Technol. 2012, 50, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Ma, L.; Xi, H.F.; Duan, W.; Wang, J.F.; Li, S.H. Individual and combined effects of CaCl2 and UV-C on the biosynthesis of resveratrols in grape leaves and berry skins. J. Agric. Food Chem. 2013, 61, 7135–7141. [Google Scholar] [CrossRef]
- Vuong, T.V.; Franco, C.; Zhang, W. Treatment strategies for high resveratrol induction in Vitis vinifera L. cell suspension culture. Biotechnol. Rep. 2014, 1–2, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Almagro, L.; Belchí-Navarro, S.; Martínez-Marquez, A.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and coronatine. Plant Physiol. Biochem. 2015, 97, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef]
- Erte, E.; Vural, N.; Mehmetoğlu, Ü.; Güvenç, A. Optimization of an abiotic elicitor (ultrasound) treatment conditions on trans-resveratrol production from Kalecik Karası (Vitis vinifera L.) grape skin. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Yang, M.H.; Kuo, C.H.; Hsieh, W.C.; Ku, K.L. Investigation of microbial elicitation of trans-resveratrol and trans-piceatannol in peanut callus led to the application of chitin as a potential elicitor. J. Agric. Food Chem. 2010, 58, 9537–9541. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Nopo-Olazabal, C.; Condori, J.; Nopo-Olazabal, L.; Balmaceda, C.; Medina-Bolivar, F. Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J. Agric. Food Chem. 2015, 63, 3942–3950. [Google Scholar] [CrossRef]
- Tang, K.; Zhan, J.C.; Yang, H.R.; Huang, W.D. Changes of resveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings. J. Plant Physiol. 2010, 167, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Nakabayashi, R.; Ogata, Y.; Sakurai, N.; Tokimatsu, T.; Goto, S.; Suzuki, M.; Jasinski, M.; Martinoia, E.; Otagaki, S.; et al. Multiomics in grape berry skin revealed specific induction of the stilbene synthetic pathway by ultraviolet-C irradiation. Plant Physiol. 2015, 168, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Matus, J.T. Transcriptomic and metabolomic networks in the grape berry illustrate that it takes more than flavonoids to fight against ultraviolet radiation. Front. Plant Sci. 2016, 7, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, D.; Yang, Z.; Zeng, Q.; Luo, Y.; He, N. Flavones produced by mulberry flavone synthase Type I constitute a defense line against the ultraviolet-B stress. Plants 2020, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Yao, W.; Wang, L.; Wang, Y. Dynamic translocation of stilbene synthase VpSTS29 from a Chinese wild Vitis species upon UV irradiation. Phytochemistry 2019, 159, 137–147. [Google Scholar] [CrossRef]
- Leonelli, F.; Valletta, A.; Migneco, L.M.; Marini Bettolo, R. Stemarane diterpenes and diterpenoids. Int. J. Mol. Sci. 2019, 20, 2627. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.H.; Pandurangan, M.; Kim, D.H.; Venkatesh, J.; Patel, R.V.; Mistry, B.M. A rich source of potential bioactive compounds with anticancer activities by Catharanthus roseus cambium meristematic stem cell cultures. J. Ethnopharmacol. 2018, 217, 107–117. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S.; Kataria, S.; Joshi, J.; Datta, S.; Vairale, M.G.; Veer, V. A review on responses of plants to UV-B radiation related stress. In UV-B Radiation: From Environmental Stressor to Regulator of Plant Growth; John Wiley & Sons: West Sussex, UK, 2017; Volume 75. [Google Scholar]
- Hollósy, F. Effects of ultraviolet radiation on plant cells. Micron 2002, 33, 179–197. [Google Scholar] [CrossRef]
- Tyunin, A.P.; Kiselev, K.V. Alternations in VaSTS gene cytosine methylation and t-resveratrol production in response to UV-C irradiation in Vitis amurensis Rupr. cells. Plant Cell Tiss. Org. Cult. 2016, 124, 33–45. [Google Scholar] [CrossRef]
- Wang, W.; Tang, K.; Yang, H.R.; Wen, P.F.; Zhang, P.; Wang, H.L.; Huang, W.D. Distribution of resveratrol and stilbene synthase in young grape plants (Vitis vinifera L. cv. Cabernet Sauvignon) and the effect of UV-C on its accumulation. Plant Physiol. Biochem. 2010, 48, 142–152. [Google Scholar] [CrossRef]
- Fritzemeier, K.H.; Rolfs, C.H.; Pfau, J.; Kindl, H. Action of ultraviolet-C on stilbene formation in callus of Arachis hypogaea. Planta 1983, 159, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, K.V.; Grigorchuk, V.P.; Ogneva, Z.V.; Suprun, A.R.; Dubrovina, A.S. The effect of ultraviolet-C and precursor feeding on stilbene biosynthesis in spruce Picea jezoensis. J. Plant Physiol. 2019, 234, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, J.; Wu, X.; Wang, Y.; Lin, Y.; Wu, D.; Zhang, H.; Qin, J. Molecular analysis of UV-C induced resveratrol accumulation in Polygonum cuspidatum Leaves. Int. J. Mol. Sci. 2019, 20, 6185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höll, J.; Vannozzi, A.; Czemmel, S.; D’Onofrio, C.; Walker, A.R.; Rausch, T.; Lucchin, M.; Boss, P.K.; Dry, I.B.; Bogs, J. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 2013, 25, 4135–4149. [Google Scholar] [CrossRef] [Green Version]
- Berli, F.; D’Angelo, J.; Cavagnaro, B.; Bottini, R.; Wuilloud, R.; Silva, M.F. Phenolic composition in grape (Vitis vinifera L. cv. Malbec) ripened with different solar UV-B radiation levels by capillary zone electrophoresis. J. Agric. Food Chem. 2008, 56, 2892–2898. [Google Scholar] [CrossRef]
- Carbonell-Bejerano, P.; Diago, M.P.; Martínez-Abaigar, J.; Martínez-Zapater, J.M.; Tardáguila, J.; Núñez-Olivera, E. Solar ultraviolet radiation is necessary to enhance grapevine fruit ripening transcriptional and phenolic responses. BMC Plant Biol. 2014, 14, 183. [Google Scholar] [CrossRef]
- Li, X.; Zheng, X.; Yan, S.; Li, S. Effects of salicylic acid (SA), ultraviolet radiation (UV-B and UV-C) on trans-resveratrol inducement in the skin of harvested grape berries. Front. Agric. China 2008, 2, 77–81. [Google Scholar] [CrossRef]
- Zinser, C.; Ernst, D.; Sandermann, H., Jr. Induction of stilbene synthase and cinnamyl alcohol dehydrogenase mRNAs in Scots pine (Pinus sylvestris L.) seedlings. Planta 1998, 204, 169–176. [Google Scholar] [CrossRef]
- Zinser, C.; Jungblut, T.; Heller, W.; Seidlitz, H.K.; Schnitzler, J.P.; Ernst, D.; Sandermann, H., Jr. The effect of ozone in Scots pine (Pinus sylvestris L.): Gene expression, biochemical changes and interactions with UV-B radiation. Plant Cell Environ. 2000, 23, 975–982. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Imaizumi, T.; Yamauchi, M.; Sekiya, M.; Shimonishi, Y.; Tanaka, F. Responses of phytonutrients and tissue condition in persimmon and cucumber to postharvest UV-C irradiation. Postharvest Biol. Technol. 2018, 145, 33–40. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Close, D.C.; McArthur, C.; Hagerman, A.E.; Davies, N.W.; Beadle, C.L. Phenolic acclimation to ultraviolet-A irradiation in Eucalyptus nitens seedlings raised across a nutrient environment gradient. Photosynthetica 2007, 45, 36–42. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, C.H.; Li, H.J.; Hsiao, C.Y.; Su, C.C.; Lee, P.L.; Hung, C.F. Protective effects of resveratrol against UVA-induced damage in ARPE19 cells. Int. J. Mol. Sci. 2015, 16, 5789–5802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotilainen, T.; Tegelberg, R.; Julkunen-Tiitto, R.; Lindfors, A.; Aphalo, P.J. Metabolite specific effects of solar UV-A and UV-B on alder and birch leaf phenolics. Glob. Chang. Biol. 2008, 14, 1294–1304. [Google Scholar] [CrossRef]
- Kim, T.E.; Pyee, J.H.; Cho, Y.J. Effect of ultraviolet irradiation on the stilbenoid content of blueberry leaves. J. Food Process Eng. 2020, 43, e13546. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Yang, T.; Ma, H.; Zhang, J.; Wu, T.; Song, T.; Tian, J.; Yao, Y. Systematic identification of long noncoding RNA s expressed during light-induced anthocyanin accumulation in apple fruit. Plant J. 2019, 100, 572–590. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of light on secondary metabolites in selected leafy greens: A review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Sams, C.E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef] [Green Version]
- Mayhew, P.J.; Jenkins, G.B.; Benton, T.G. A long-term association between global temperature and biodiversity, origination and extinction in the fossil record. Proc. Royal Soc. B 2008, 275, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayhew, P.J.; Bell, M.A.; Benton, T.G.; McGowan, A.J. Biodiversity tracks temperature over time. PNAS 2012, 109, 15141–15145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Zhou, X.; Wang, T.; Wang, G.; Cao, F. Regulation of flavonoid metabolism in ginkgo leaves in response to different day-night temperature combinations. Plant Physiol. Biochem. 2020, 147, 133–140. [Google Scholar] [CrossRef]
- Lecourieux, D.; Kappel, C.; Claverol, S.; Pieri, P.; Feil, R.; Lunn, J.E.; Bonneu, M.; Wang, L.; Gomès, E.; Delrot, S.; et al. Proteomic and metabolomic profiling underlines the stage-and time-dependent effects of high temperature on grape berry metabolism. J. Integr. Plant Biol. 2020, 62, 1132–1158. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Sales, C.; Beltrán, J.; Gómez-Cadenas, A.; Arbona, V. Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front. Plant Sci. 2017, 7, 1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenoni, S.; Fasoli, M.; Guzzo, F.; Dal Santo, S.; Amato, A.; Anesi, A.; Commisso, M.; Herderich, M.; Ceoldo, S.; Avesani, L.; et al. Disclosing the molecular basis of the postharvest life of berry in different grapevine genotypes. Plant Physiol. 2016, 172, 1821–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (Vitis vinifera) fruit. BMC Plant Biol. 2014, 14, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Vastano, B.C.; Chen, Y.; Zhu, N.; Ho, C.T.; Zhou, Z.; Rosen, R.T. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J. Agric. Food Chem. 2000, 48, 253–256. [Google Scholar] [CrossRef]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb. et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef]
- Harju, A.; Venalainen, M. Stilbenes as constitutive and induced protection compounds in Scots pine (Pinus sylvestris L.). Gen. Tech. Rep. PSW-GTR 2012, 240, 20–26. [Google Scholar]
- Sullivan, T.P.; Crump, D.R.; Wieser, H.; Dixon, E.A. Influence of the plant antifeedant, pinosylvin, on suppression of feeding by snowshoe hares. J. Chem. Ecol. 1992, 18, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, R.; Schöppner, A.; Kindl, H. Stilbene synthase from seedlings of Pinus sylvestris: Purification and induction in response to fungal infection. Mol. Plant Microbe Interact. 1990, 3, 444–449. [Google Scholar] [CrossRef]
- Langcake, P.; McCarthy, W. The relationship of resveratrol production to infection of grapevine leaves by Botrytis cinerea. Vitis 1979, 18, 244–253. [Google Scholar]
- Jeandet, P.; Bessis, R.; Sbaghi, M.; Meunier, P. Production of the phytoalexin resveratrol by grapes as a response to Botrytis attack under natural conditions. J. Phytopathol. 1995, 143, 135–139. [Google Scholar] [CrossRef]
- Bavaresco, L.; Pettegolli, D.; Cantü, E.; Fregoni, M.; Chiusa, G.; Trevisan, M. Elicitation and accumulation of stilbene phytoalexins in grapevine berries infected by Botrytis cinerea. Vitis 1997, 36, 77–83. [Google Scholar]
- Bézier, A.; Lambert, B.; Baillieul, F. Study of defense-related gene expression in grapevine leaves and berries infected with Botrytis cinerea. Eur. J. Plant Pathol. 2002, 108, 111–120. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Fung, R.W.; Gonzalo, M.; Fekete, C.; Kovacs, L.G.; He, Y.; Marsh, E.; McIntyre, L.M.; Schachtman, D.P.; Qiu, W. Powdery mildew induces defense-oriented reprogramming of the transcriptome in a susceptible but not in a resistant grapevine. Plant Physiol. 2008, 146, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Schnee, S.; Viret, O.; Gindro, K. Role of stilbenes in the resistance of grapevine to powdery mildew. Physiol. Mol. Plant Pathol. 2008, 72, 128–133. [Google Scholar] [CrossRef]
- Bavaresco, L.; Vezzulli, S.; Battilani, P.; Giorni, P.; Pietri, A.; Bertuzzi, T. Effect of ochratoxin A-producing Aspergilli on stilbenic phytoalexin synthesis in grapes. J. Agric. Food Chem. 2003, 51, 6151–6157. [Google Scholar] [CrossRef]
- Vezzulli, S.; Battilani, P.; Bavaresco, L. Stilbene-synthase gene expression after Aspergillus carbonarius infection in grapes. Am. J. Enol. Vitic. 2007, 58, 132–134. [Google Scholar]
- Martin, N.; Vesentini, D.; Rego, C.; Monteiro, S.; Oliveira, H.; Ferreira, R.B. Phaeomoniella chlamydospora infection induces changes in phenolic compounds content in Vitis vinifera. Phytopathol. Mediterr. 2009, 48, 101–116. [Google Scholar]
- Paul, B.; Chereyathmanjiyil, A.; Masih, I.; Chapuis, L.; Benoît, A. Biological control of Botrytis cinerea causing grey mould disease of grapevine and elicitation of stilbene phytoalexin (resveratrol) by a soil bacterium. FEMS Microbiol. Lett. 1998, 165, 65–70. [Google Scholar] [CrossRef]
- Verhagen, B.; Trotel-Aziz, P.; Jeandet, P.; Baillieul, F.; Aziz, A. Improved resistance against Botrytis cinerea by grapevine-associated bacteria that induce a prime oxidative burst and phytoalexin production. Phytopathology 2011, 101, 768–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruau, C.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Baillieul, F.; Aziz, A. Pseudomonas fluorescens PTA-CT2 triggers local and systemic immune response against Botrytis cinerea in grapevine. Mol. Plant Microbe Interact. 2015, 28, 1117–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.M.; Cha, M.; Bajpai, V.K.; Baek, K.H. Production of a major stilbene phytoalexin, resveratrol in peanut (Arachis hypogaea) and peanut products: A mini review. Rev. Environ. Sci. Biotechnol. 2013, 12, 209–221. [Google Scholar] [CrossRef]
- Sobolev, V.S. Localized production of phytoalexins by peanut (Arachis hypogaea) kernels in response to invasion by Aspergillus species. J. Agric. Food Chem. 2008, 56, 1949–1954. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Tassoni, A.; Fornalè, S.; Franceschetti, M.; Musiani, F.; Michael, A.J.; Perry, B.; Bagni, N. Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol. 2005, 166, 895–906. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Park, S.H.; Park, S.C.; Kim, S.; Kim, T.H.; Lee, J.; Kim, S.W.; Ryu, Y.B.; Jeong, C.J.; Kim, C.Y. Induced extracellular production of stilbenes in grapevine cell culture medium by elicitation with methyl jasmonate and stevioside. Bioresour. Bioprocess. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Delaunois, B.; Farace, G.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ. Sci. Pollut. Res. 2014, 21, 4837–4846. [Google Scholar] [CrossRef]
- Iriti, M.; Rossoni, M.; Borgo, M.; Faoro, F. Benzothiadiazole enhances resveratrol and anthocyanin biosynthesis in grapevine, meanwhile improving resistance to Botrytis cinerea. J. Agric. Food Chem. 2004, 52, 4406–4413. [Google Scholar] [CrossRef] [PubMed]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Crespo-Villegas, O.; Garde-Cerdán, T. Elicitors used as a tool to increase stilbenes in grapes and wines. Food Res. Int. 2017, 98, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Donnez, D.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Carbonell-Bejerano, P.; Belchí-Navarro, S.; Bru, R.; Martínez-Zapater, J.M.; Lijavetzky, D.; Pedreño, M.A. Dissecting the transcriptional response to elicitors in Vitis vinifera cells. PLoS ONE 2014, 9, e109777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.; Figueiras, A.; Gallardo, E.; Nerín, C.; Domingues, F.C. Strategies to improve the solubility and stability of stilbene antioxidants: A comparative study between cyclodextrins and bile acids. Food Chem. 2014, 145, 115–125. [Google Scholar] [CrossRef]
- Bru, R.; Sellés, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef]
- Martinez-Esteso, M.J.; Sellés-Marchart, S.; Vera-Urbina, J.C.; Pedreño, M.A.; Bru-Martinez, R. Changes of defense proteins in the extracellular proteome of grapevine (Vitis vinifera cv. Gamay) cell cultures in response to elicitors. J. Proteom. 2009, 73, 331–341. [Google Scholar] [CrossRef]
- Martínez-Márquez, A.; Morante-Carriel, J.A.; Ramírez-Estrada, K.; Cusidó, R.M.; Palazon, J.; Bru-Martínez, R. Production of highly bioactive resveratrol analogues pterostilbene and piceatannol in metabolically engineered grapevine cell cultures. Plant Biotechnol. J. 2016, 14, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Lambert, C.; Lemaire, J.; Auger, H.; Guilleret, A.; Reynaud, R.; Clément, C.; Courot, E.; Taidi, B. Optimize, modulate, and scale-up resveratrol and resveratrol dimers bioproduction in Vitis labrusca L. cell suspension from flasks to 20 L bioreactor. Plants 2019, 8, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somboon, T.; Chayjarung, P.; Pilaisangsuree, V.; Keawracha, P.; Tonglairoum, P.; Kongbangkerd, A.; Wongkrajang, K.; Limmongkon, A. Methyl jasmonate and cyclodextrin-mediated defense mechanism and protective effect in response to paraquat-induced stress in peanut hairy root. Phytochemistry 2019, 163, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wongshaya, P.; Chayjarung, P.; Tothong, C.; Pilaisangsuree, V.; Somboon, T.; Kongbangkerd, A.; Limmongkon, A. Effect of light and mechanical stress in combination with chemical elicitors on the production of stilbene compounds and defensive responses in peanut hairy root culture. Plant Physiol. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Komaikul, J.; Kitisripanya, T.; Likhitwitayawuid, K.; Sritularak, B.; Tanaka, H.; Putalun, W. Improvement of stilbenoid production by 2-hydroxypropyl-β-cyclodextrin in white mulberry (Morus alba L.) callus cultures. Nat. Prod. Res. 2019, 33, 2762–2769. [Google Scholar] [CrossRef]
- Inyai, C.; Boonsnongcheep, P.; Komaikul, J.; Sritularak, B.; Tanaka, H.; Putalun, W. Alginate immobilization of Morus alba L. cell suspension cultures improved the accumulation and secretion of stilbenoids. Bioprocess Biosyst. Eng. 2019, 42, 131–141. [Google Scholar] [CrossRef]
- Inyai, C.; Yusakul, G.; Komaikul, J.; Kitisripanya, T.; Likhitwitayawuid, K.; Sritularak, B.; Putalun, W. Improvement of stilbene production by mulberry Morus alba root culture via precursor feeding and co-elicitation. Bioprocess Biosyst. Eng. 2020. [Google Scholar] [CrossRef]
- Lanz, T.; Schröder, G.; Schröder, J. Differential regulation of genes for resveratrol synthase in cell cultures of Arachis hypogaea L. Planta 1990, 181, 169–175. [Google Scholar] [CrossRef]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E.; Fernández-Marín, M.I.; Puertas, B.; Serrano-Albarrán, M.J. Optimising UV-C preharvest light for stilbene synthesis stimulation in table grape: Applications. Innov. Food Sci. Emerg. Technol. 2015, 29, 222–229. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E.; Puertas, B.; Richard, T. Daily preharvest UV-C light maintains the high stilbenoid concentration in grapes. J. Agric. Food Chem. 2016, 64, 5139–5147. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Sady, S.; Sielicka, M. The stilbene profile in edible berries. Phytochem. Rev. 2019, 18, 37–67. [Google Scholar] [CrossRef] [Green Version]
- Segade, S.R.; Vincenzi, S.; Giacosa, S.; Rolle, L. Changes in stilbene composition during postharvest ozone treatment of ‘Moscato bianco’ winegrapes. Food Res. Int. 2019, 123, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Bashir, T.; Bae, H. Use of ultrasonication technology for the increased production of plant secondary metabolites. Molecules 2017, 22, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potrebko, I.; Resurreccion, A.V. Effect of ultraviolet doses in combined ultraviolet−ultrasound treatments on trans-resveratrol and trans-piceid contents in sliced peanut kernels. J. Agric. Food Chem. 2009, 57, 7750–7756. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Baek, K.H. Induction of resveratrol biosynthesis in grape skins and leaves by ultrasonication treatment. Korean J. Hort. Sci. Technol. 2013, 31, 496–502. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.; Shi, A.; Liu, L.; Wang, Q. Preparation of resveratrol-enriched and poor allergic protein peanut sprout from ultrasound treated peanut seeds. Ultrason. Sonochem. 2016, 28, 334–340. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef]
- Herrera, J.C.; Hochberg, U.; Degu, A.; Sabbatini, P.; Lazarovitch, N.; Castellarin, S.D.; Fait, A.; Alberti, G.; Peterlunger, E. Grape metabolic response to postveraison water deficit is affected by interseason weather variability. J. Agric. Food Chem. 2017, 65, 5868–5878. [Google Scholar] [CrossRef]
- Hochberg, U.; Degu, A.; Cramer, G.R.; Rachmilevitch, S.; Fait, A. Cultivar specific metabolic changes in grapevines berry skins in relation to deficit irrigation and hydraulic behavior. Plant Physiol. Biochem. 2015, 88, 42–52. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.; Degu, A.; Herrera, J.C.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Multi-omics and integrated network analyses reveal new insights into the systems relationships between metabolites, structural genes, and transcriptional regulators in developing grape berries (Vitis vinifera L.) exposed to water deficit. Front. Plant Sci. 2017, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Corso, M.; Vannozzi, A.; Maza, E.; Vitulo, N.; Meggio, F.; Pitacco, A.; Telatin, A.; D’Angelo, M.; Feltrin, E.; Negri, A.S.; et al. Comprehensive transcript profiling of two grapevine rootstock genotypes contrasting in drought susceptibility links the phenylpropanoid pathway to enhanced tolerance. J. Exp. Bot. 2015, 66, 5739–5752. [Google Scholar] [CrossRef] [PubMed]
- Pinasseau, L.; Vallverdú-Queralt, A.; Verbaere, A.; Roques, M.; Meudec, E.; Le Cunff, L.; Péros, J.-P.; Ageorges, A.; Sommerer, N.; Boulet, J.-C.; et al. Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics. Front. Plant Sci. 2017, 8, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosemann, D.; Heller, W.; Sandermann, H. Biochemical plant responses to ozone: II. Induction of stilbene biosynthesis in Scots pine (Pinus sylvestris L.) seedlings. Plant Physiol. 1991, 97, 1280–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, R.B. Effects of exposure to high ozone concentrations on stilbenes in Sitka spruce (Picea sitchensis (Bong.) Carr.) bark and on its lignification response to infection with Heterobasidion annosum (Fr.) Bref. Physiol. Mol. Plant Pathol. 1996, 48, 117–129. [Google Scholar] [CrossRef]
- Sgarbi, E.; Fornasiero, R.B.; Lins, A.P.; Bonatti, P.M. Phenol metabolism is differentially affected by ozone in two cell lines from grape (Vitis vinifera L.) leaf. Plant Sci. 2003, 165, 951–957. [Google Scholar] [CrossRef]
- Schubert, R.; Fischer, R.; Hain, R.; Schreier, P.H.; Bahnweg, G.; Ernst, D.; Sandermann, H., Jr. An ozone-responsive region of the grapevine resveratrol synthase promoter differs from the basal pathogen-responsive sequence. Plant Mol. Biol. 1997, 34, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, R.P.; Kasurinen, A.; Häikiö, E.; Holopainen, J.K.; Julkunen-Tiitto, R.; Holopainen, T.; Kivimäenpää, M. Combined effects of elevated ozone, temperature, and nitrogen on stem phenolic concentrations of Scots pine (Pinus sylvestris) seedlings. Can. J. For. Res. 2019, 49, 246–255. [Google Scholar] [CrossRef]
- Ismail, A.; Riemann, M.; Nick, P. The jasmonate pathway mediates salt tolerance in grapevines. J. Exp. Bot. 2012, 63, 2127–2139. [Google Scholar] [CrossRef] [Green Version]
- Kostopoulou, Z.; Therios, I.; Molassiotis, A. Resveratrol and its combination with α-tocopherol mediate salt adaptation in Citrus seedlings. Plant Physiol. Biochem. 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Speiser, C.; Smetanska, I. Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl. Biochem. Biotech. 2013, 171, 330–340. [Google Scholar] [CrossRef]
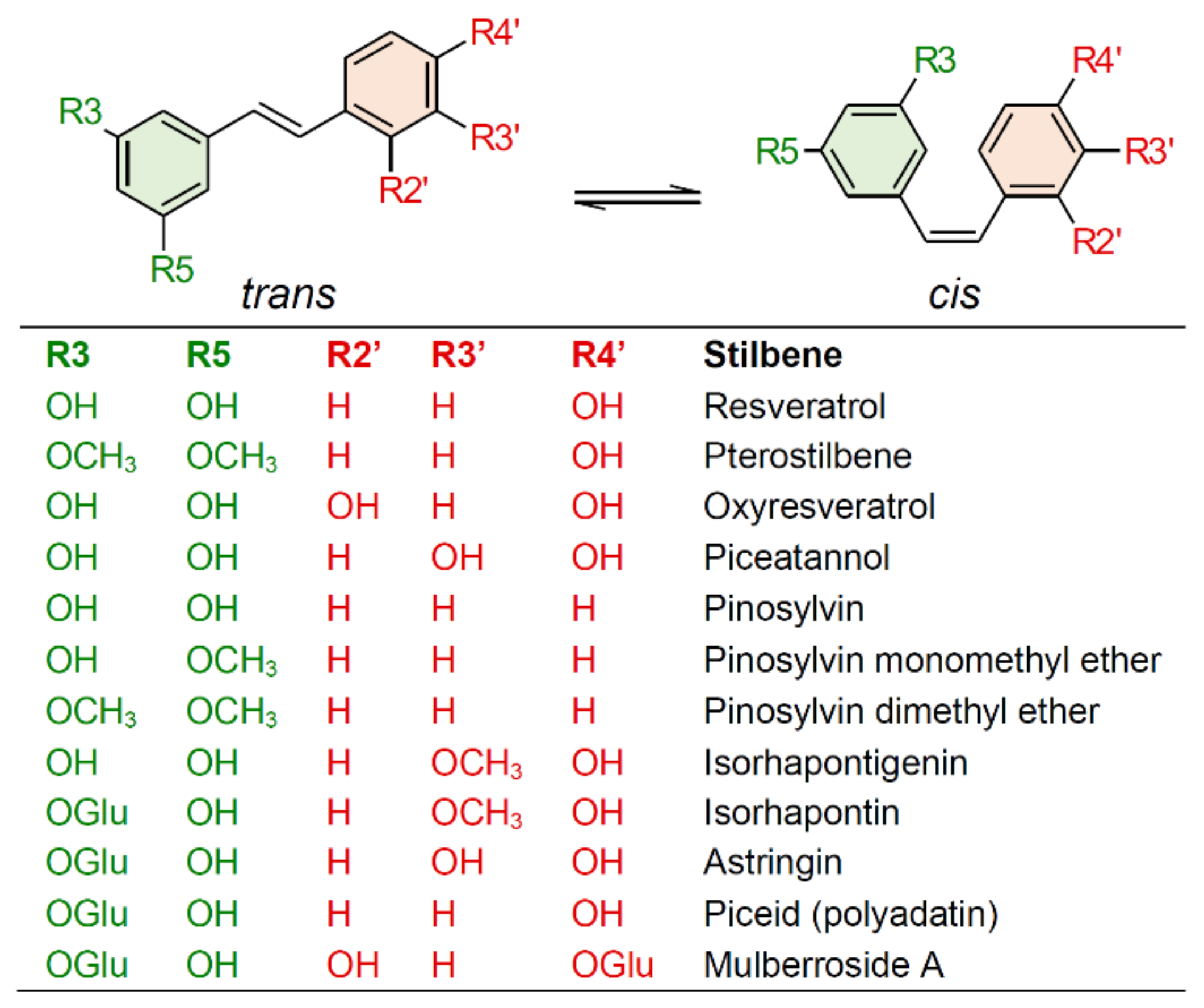
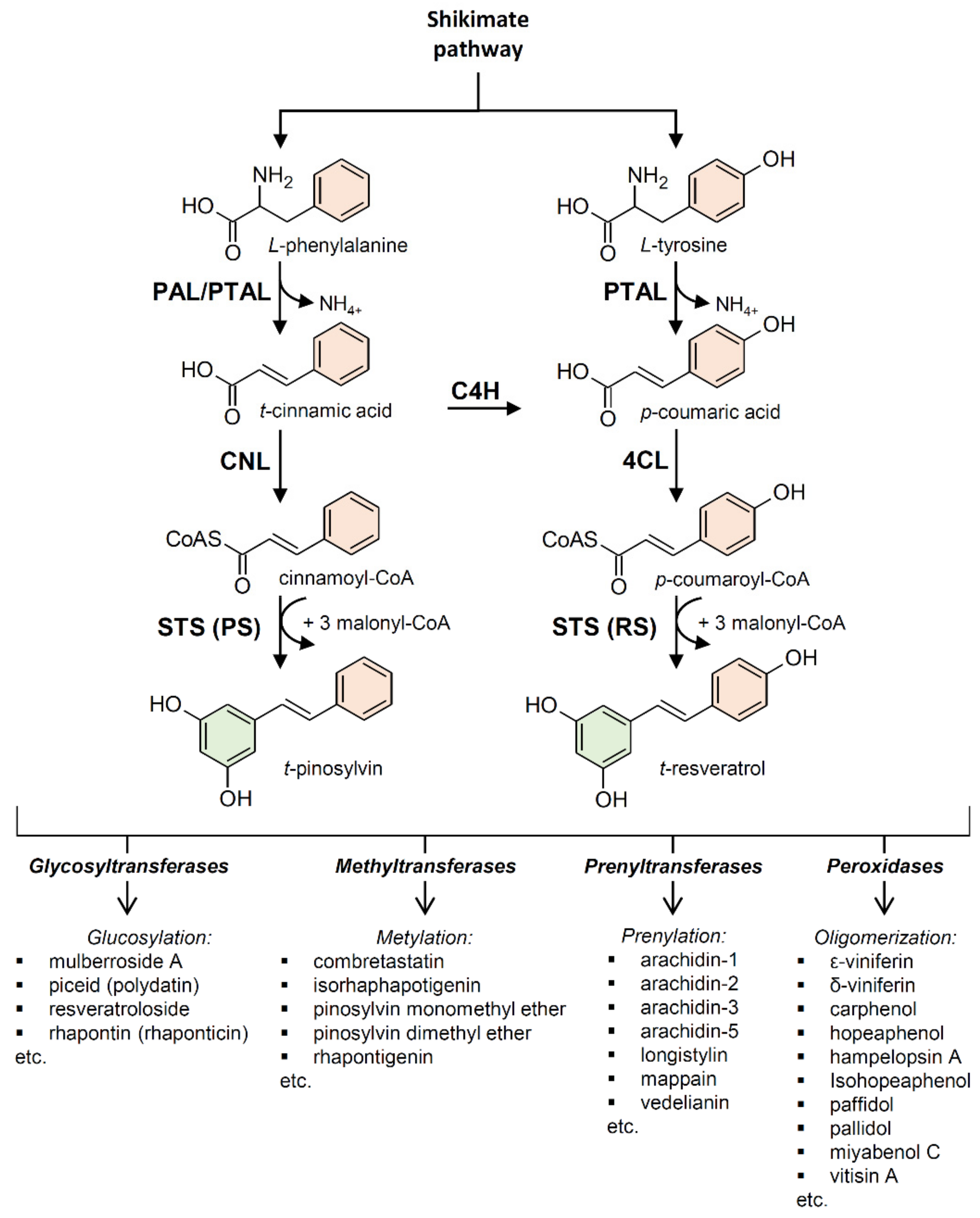
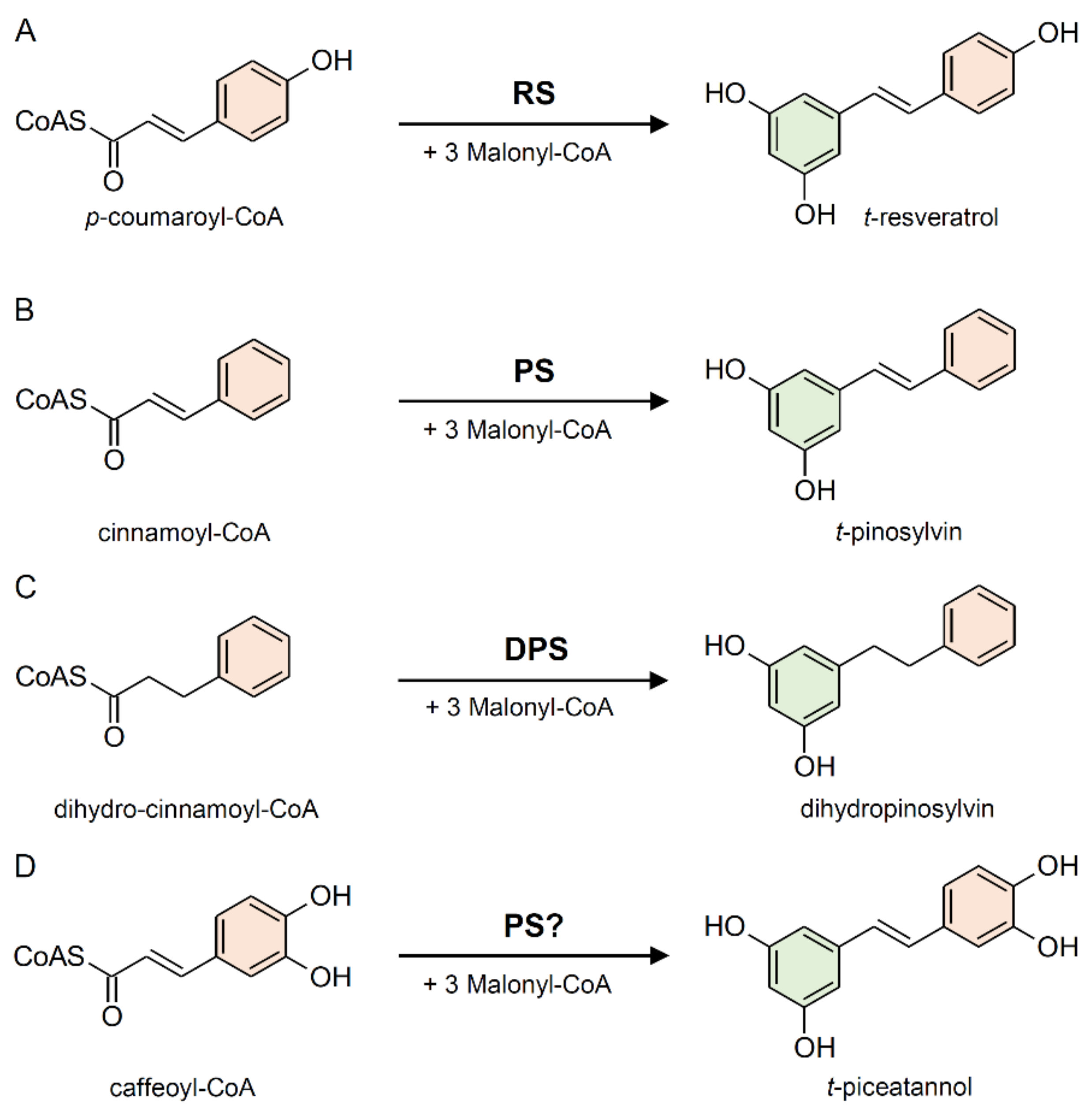
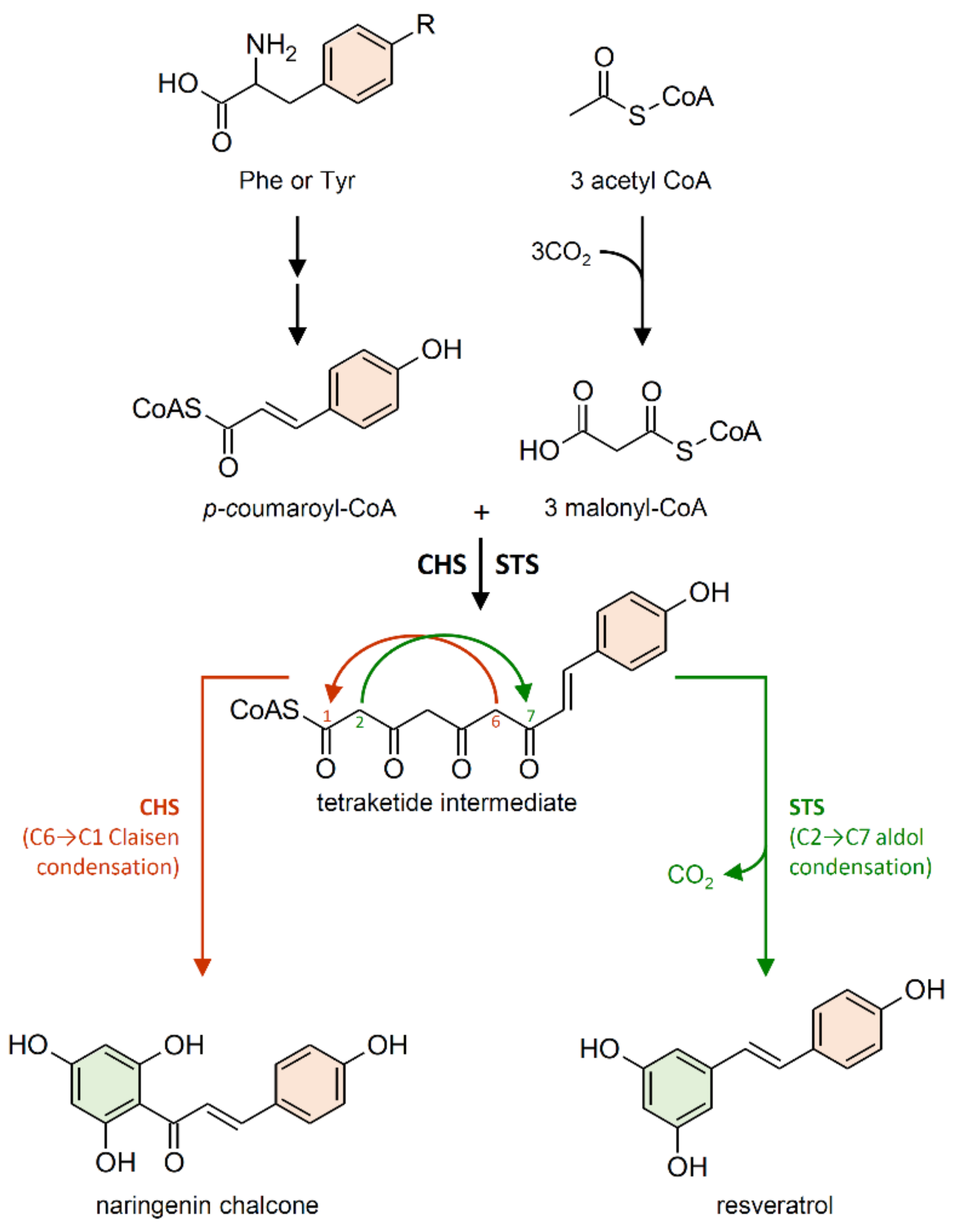

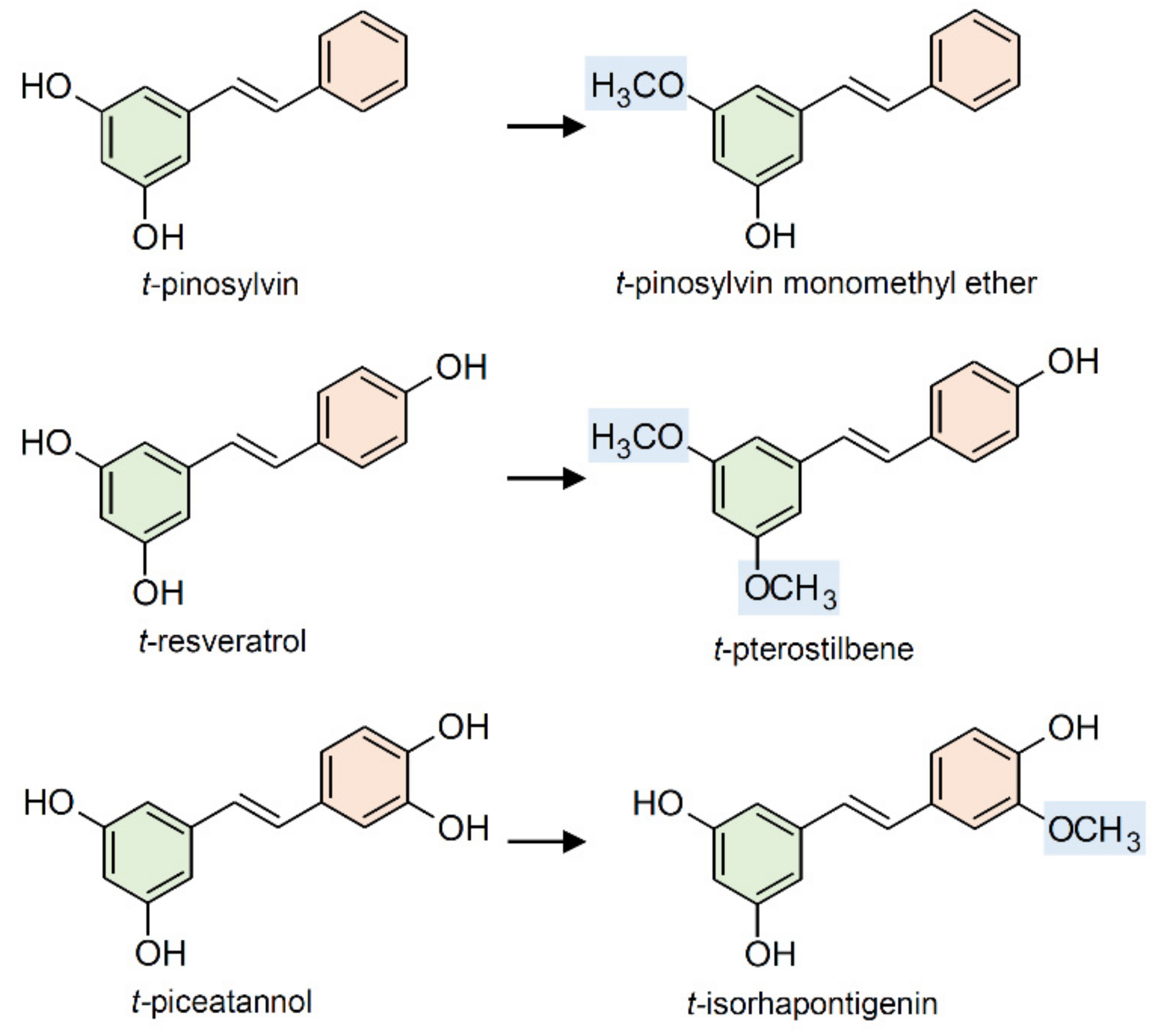
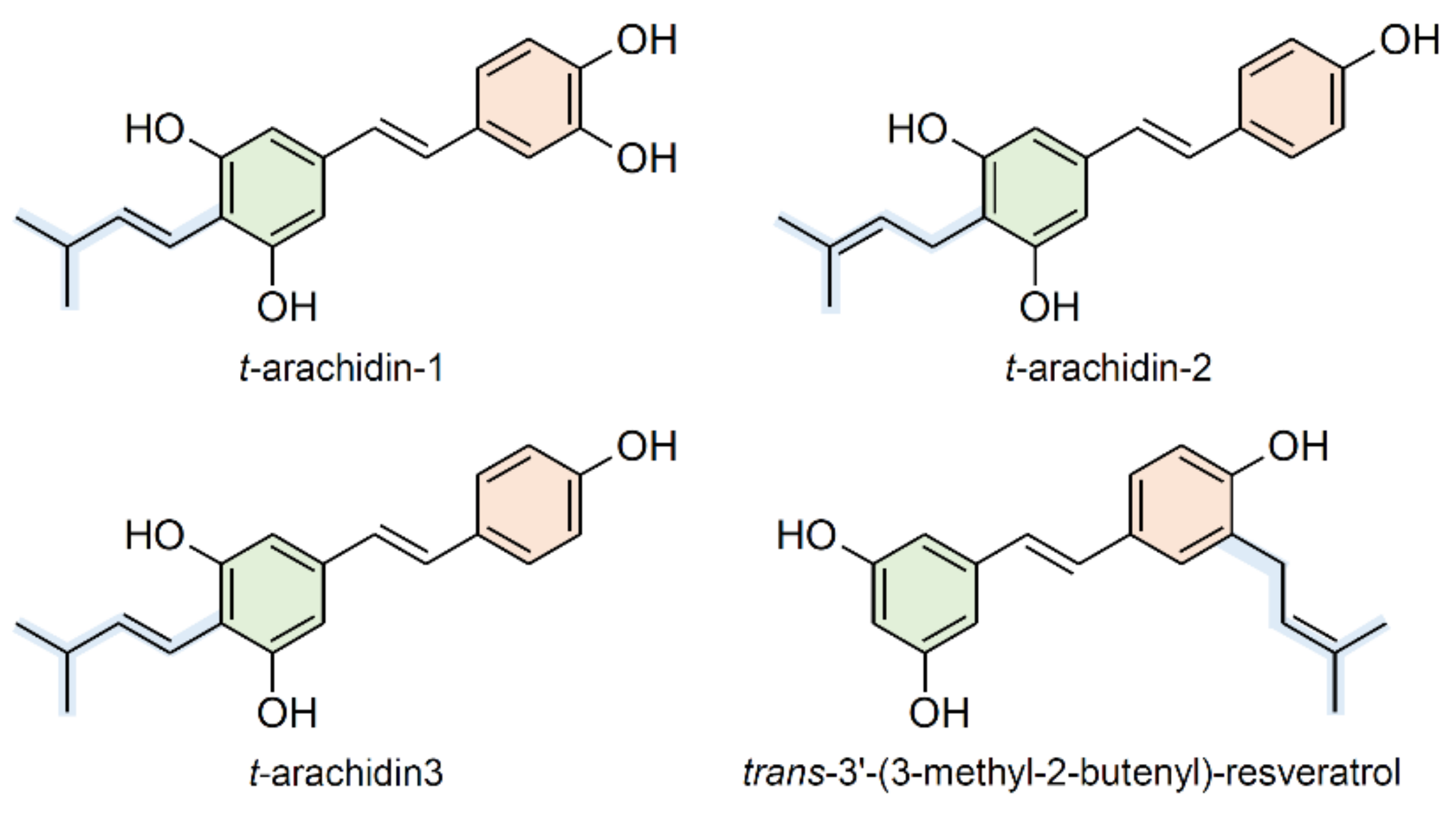
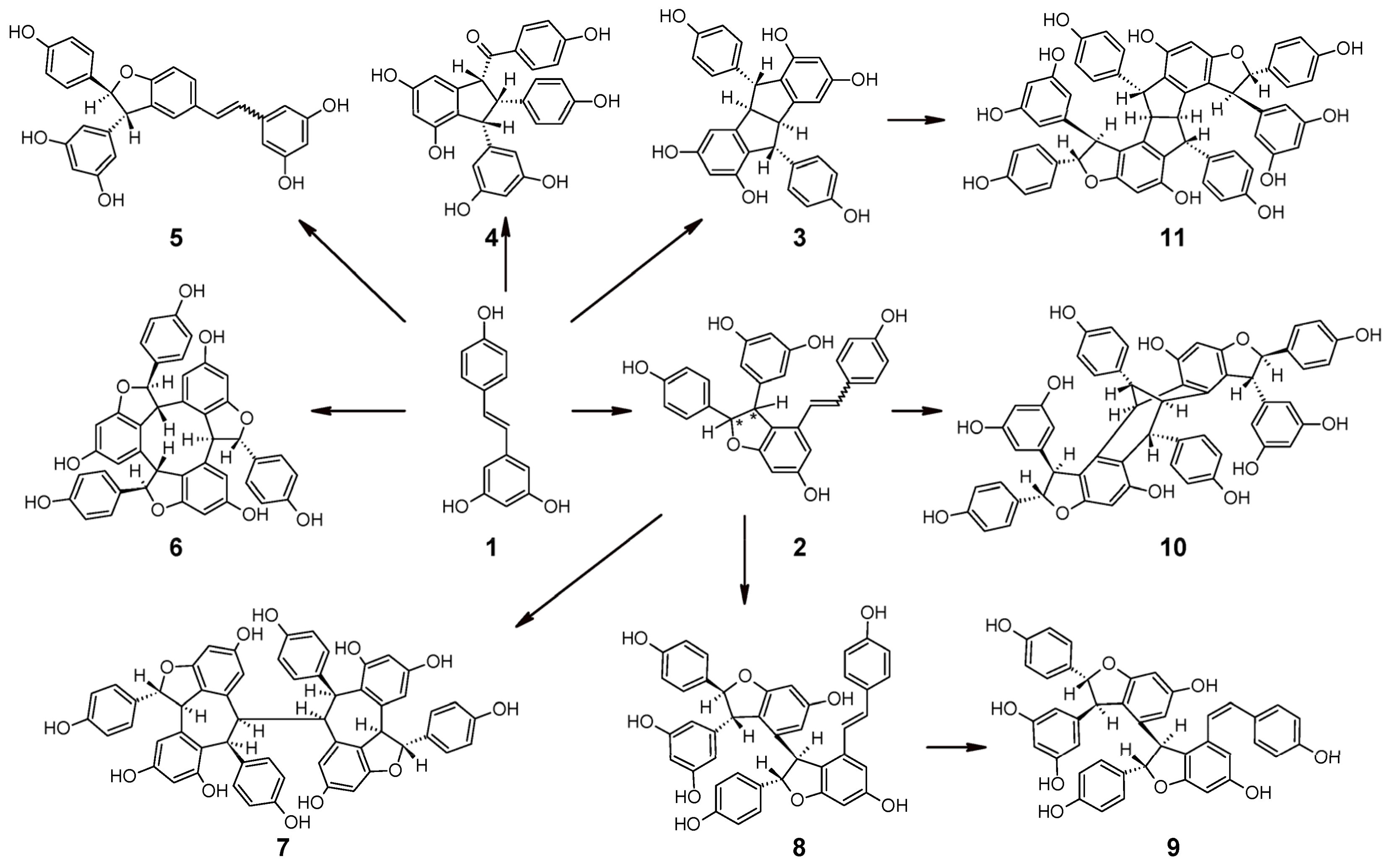
| Species/Cultivar/Variety | Treatment/s | Metabolites | Results | Reference |
|---|---|---|---|---|
| Vitis vinifera cvs. Alphonse Lavallée, Dan Ben-Hanna, Dabuki, Early Superior, Flame seedless, Kishmish, Muscat Hamburg, Perlette, Spring Blush, Superior, Thompson seedless, Zeiny, Gamay, Gamaret, Pinot, Shasla | Irradiation of grape berries with UV-C or inoculation of grape berries with Rhizopus stolonifer | Stilbenes (resveratrol and pterostilbene) | Increased stilbene accumulation, greater with UV-C compared to fungal inoculum | [183] |
| V. vinifera cv. Napoleon | Irradiation of grape berries with UV-C or UV-B | Stilbenes (resveratrol and piceid); anthocyanins; flavonoids; hydroxycinnamic acids (caffeoyltartaric acid and chlorogenic acid) | Increased stilbene accumulation, greater with UV-C compared to UV-B (3 and 2-fold, respectively) | [184] |
| V. vinifera cv. Corvina | Irradiation of grape berries with UV-B and wilting at different temperatures | Stilbenes (trans and cis-resveratrol, trans and cis-piceid); total polyphenols, flavonoids, anthocyanins, catechins, and proanthocyanidins | Enhanced stilbene accumulation and STS gene expression | [185] |
| V. vinifera cvs. Black Corinth and Flame seedless | Irradiation of grape berries with UV-C | Resveratrol; total anthocyanins | Greater resveratrol increase (4-fold) in cv. Flame Seedless. Lower increase in cv. Black Corinth. Negative relationship between resveratrol synthesis and anthocyanin concentration | [186] |
| V. vinifera cv. Flame seedless, Red Globe, Crimson seedless, Napoleon, Superior seedless, Moscatel Italica, Dominga | Irradiation of grape berries with UV-C | Trans- and cis-resveratrol, trans-piceatannol, trans-piceid, trans-astringin, α-viniferin, ε-viniferin | Increased stilbene concentration, with higher accumulation of trans-resveratrol, trans-piceatannol, and viniferins | [187] |
| V. vinifera cv. Monastrell | Irradiation of grape berries with UV-C, followed by traditional maceration | Stilbenes (trans-resveratrol, trans-piceatannol); anthocyanins; flavonols; flavanols (total catechins); hydroxycinnamic acids (p-coumaroyltartaric acid) | Increased in trans-piceatannol and trans-resveratrol content (1.5 and 2-fold, respectively) in wines without impacting standard oenological parameters | [188] |
| V. vinifera cvs. Tempranillo, Cabernet-Sauvignon, Merlot, Syrah, Monastrell, Garnacha, Cariñena | Irradiation of grape berries with UV-C | Trans-resveratrol, trans-piceatannol, α-viniferin, ε-viniferin | Increased concentrations of trans-resveratrol, trans-piceatannol, and viniferins in grape skins of all varieties, except Monastrell, in which only trans-piceatannol concentration increased | [189] |
| V. vinifera cv. Superior | Irradiation of grape berries with UV-C | Trans-resveratrol, trans-piceid, trans-piceatannol, viniferins (resveratrol dehydrodimers and dehydrotrimers) | Increased trans-resveratrol accumulation (10-fold); induction of trans-piceid, trans-piceatannol, and viniferins (not detected in control grapes) | [190] |
| V. vinifera cv. Superior | Comparison of UV-C and ozone (O3) treatments on grape berries | Trans-resveratrol, piceatannol, and viniferins (resveratrol dehydrodimers and dehydrotrimers) | Increased accumulation of stilbenes after both UV-C and O3 treatments. O3 more effective than UV-C in inducing the accumulation of viniferins | [191] |
| V. vinifera cv. Superior | Irradiation of grape berries with UV-C, followed by maceration with Na2S2O5 and enzymes | Stilbenes (trans-resveratrol; trans-piceid; trans-piceatannol, viniferins); hydroxycinnamic acids; flavonols; flavanols (catechins and procyanidins) | Increased stilbene concentration (35-fold) in grape juice under optimum conditions (maceration for 2 h at 45 °C with 0.2% Na2S2O5 using UV-C-treated grape berries) | [192] |
| V. vinifera cv. Red Globe | Irradiation of grape berries with UV-B nanosecond laser pulses | Trans-resveratrol | Increased trans-resveratrol accumulation (6-fold) in grape berries subjected to a resonant wavelength of the compound (302.1 nm) | [193] |
| V. vinifera sylvestris var. V9. V15, V16; V. vinifera sativa var. Merlot, Syrah, Graciano, Tempranillo, Palomino fino, Palomino negro, Tintilla de Rota; V. vinifera sativa hybrid Orion, Regent | Irradiation of grape berries with UV-C | Trans-resveratrol, piceatannol, ε-viniferin, δ-viniferin | Increased stilbene concentration, with differences depending on variety and campaign, but not on subspecies | [194] |
| V. vinifera × V. labrusca cv. Kyoho | Irradiation of grape berries with UV-C and storage at different temperatures (0 °C or 20 °C) | Resveratrol | Increased resveratrol concentration, especially in UV-treated grapes stored at high temperature | [195] |
| V. vinifera cv. Redglobe | Irradiation of grape berries with UV-C and storage at different temperatures (25 °C or 4 °C) | Stilbenes (trans-resveratrol, cis- and trans-piceid); flavonols; anthocyanins; flavanols (catechins) | Increased concentration of cis- and trans-piceid after UV-C treatment and cold storage | [196] |
| V. vinifera cv. Crimson | Treatment of grape berries with UV-C and chitosan, followed by storage at different temperatures | Trans-resveratrol | Increased resveratrol content in grapes and lower susceptibility to fungal decay after UV-C treatment combined with chitosan coating followed by storage at 20 °C for 24 h before refrigerated storage | [197] |
| V. amurensis cv. Tonghua-3 | Treatment of grape berries with UV-C | Trans- and cis-resveratrol | Increased accumulation of stilbene compounds, up-regulation of multiple STS genes, down-regulation of CHS genes | [198] |
| V. vinifera × V. labrusca cv. Summer Black | Treatment of grape berries with UV-B or UV-C | Stilbenes (trans-resveratrol, trans-piceid); gallic acid; hydroxycinnamic acids (caffeic acid, trans-ferulic acid); flavanols [(+)-catechin, (−)-epicatechin, epicatechin gallate] | Increased accumulation of phenolic compounds and STS gene expression, more induced by UV-C than UV-B | [199] |
| V. vinifera cv. Kyoho | Irradiation of grape berries with UV-B | Trans-resveratrol, trans-scirpusin A, trans-ε-viniferin, trans-δ-viniferin, trans-pterostilbene | Increased production of the analyzed stilbenes, up-regulation of stilbene biosynthetic genes | [200] |
| Arachis hypogaea cv. Georgia green | Treatment of peanuts with UV-C or ultrasonication | Trans-resveratrol, trans-piceid | Increased resveratrol, piceid, and total stilbene concentration, more induced by ultrasound than UV-C | [201] |
| A. hypogaea var. Jinpoong | Leaves subjected to UV-C, wounding, paraquat, H2O2, salicylic acid, jasmonic acid ethephon, abscisic acid | Resveratrol | Maximum resveratrol increases in response to UV (over 200-fold), followed by paraquat (20-fold) and wounding, H2O2, salicylic acid, jasmonic acid, and ethephon (between 2- and 9-fold) | [202] |
| A. hypogaea Georgia green | Treatment of peanuts with UV-C | Trans-resveratrol | Increased trans-resveratrol accumulation (10-fold) | [203] |
| Gnetum parvifolium | Treatment of 1-year-old seedlings with high temperature (40 °C) and UV-C treatments | Resveratrol and piceatannol | Both high temperature and UV-C strongly induce the expression of PAL, C4H-, 4CL-, and STS-like genes, but only UV-C enhance stilbene accumulation | [204] |
| Gnetum parvifolium | Treatment of 1-year-old seedlings with high temperature (40 °C) and UV-C treatments | Resveratrol and piceatannol | Both high temperature and UV-C strongly induce the expression of PAL, C4H, 4CL, STS, and CYP genes. High temperatures do not affect stilbene accumulation in stems but decrease stilbene concentration in roots at 3 h. UV-C irradiation induces total stilbene accumulation in stems but not in roots. | [205] |
| Pinus sylvestris | Treatment of needles from 5-years-old plantlets with UV-C | Pinosylvin and pinosylvin monomethylether | Induction of PMT2 expression | [134] |
| V. vinifera cv. Sangiovese | Potted vines grown in air-conditioned greenhouses under high temperature or low temperature regimes (26 and 21 °C as average and 42 and 35 °C as maximum air daily temperature, respectively) | Stilbenes | Increased expression of STS and PAL genes under low temperatures | [206] |
| V. vinifera cv. Cabernet Sauvignon | Treatment of cell suspension cultures with high temperature (38 °C) or low temperature (16 °C) and CuSO4 | Stilbenes | Downregulation of STS expression under both low and high temperature and upregulation of STS expression in response to CuSO4 | [207] |
| V. vinifera cv. Cardinal | Treatment of grape berries with low temperature (0 °C) and high CO2 levels (20%) | Trans-resveratrol; total anthocyanins | Low temperature reduces trans-resveratrol content in both treated and non-treated grapes, although the decrease is higher in CO2-treated grapes | [208] |
| V. vinifera cvs. Dominga, Superior seedless, Autumn Royal, Red Globe | Treatment of grape berries with low temperature (0 °C) and high CO2 levels (3 days) | Resveratrol, resveratrol-glucoside, trans-piceatannol, z-miyabenol, pallidol | Stilbene accumulation in response to low temperature and CO2 is cultivar dependent. High CO2 levels activate stilbene pathways in cv. Dominga. Low temperature increase stilbenes biosynthesis in cv. Red Globe. Stilbene accumulation is independent of the atmosphere storage in cvs. Superior Seedless and Autumn Royal | [209] |
| V. vinifera cv. Shiraz | Treatment of grape berries with high light (2500 μmol m−2 s−1), high temperature (40 °C), oxidative stress (120 μM menadione), 3.026 mM abscisic acid, and 200 μM jasmonic acid (JA) | Resveratrol, piceid, and viniferin | At the pre-veraison stage, an increase in anthocyanins levels is accompanied by a declining stilbene accumulation in response to JA, menadione, and high light. At the veraison stage, mild change in anthocyanin levels in response to all the treatments is accompanied by stilbene accumulation | [210] |
| V. vinifera cv. Barbera | Treatment of cell suspensions with red LED light (1.34 μE m−2 s−1, 625 ± 10 nm) and 10 μM methyl-jasmonate (MeJa) | Stilbenes (cis- and trans-piceid, cis- and trans-resveratrol, cis- and trans-resveratroloside); catechins; anthocyanins | Strong increase in total stilbenes induced by MeJa, whose effect is enhanced by a red light. Increase in total anthocyanins in response to MeJa, used alone or in combination with a red light. Decrease in catechins under red light; increase in response to MeJa alone or in combination with red light | [211] |
| V. labruscana cvs. Campbell Early and Kyoho | Treatment of grape berries and leaves with fluorescent white light and purple, blue, and red LED lights | Cis- and trans-resveratrol, cis- and trans-piceid, cis- and trans-piceatannol | Increased accumulation of stilbenes (mainly trans- and cis-piceid) and induction of stilbene biosynthetic genes in response to red and blue LED light | [212,213] |
| V. vinifera cv. Negramaro | Light-exposed and dark-maintained cell cultures | Trans-resveratrol, trans-piceid, cis-ε-viniferin, trans-ε-viniferin, trans-δ-viniferin | Higher levels of trans-resveratrol and viniferins under darkness; higher levels of trans-piceid under light | [214] |
| V. vinifera cv. Shahani | High-level white light irradiation (10,000 lux) and MeJa (25, 50, 100 and 200 μM) | Stilbenes (trans-resveratrol, trans-piceid); total phenols; total flavonoids | Inhibitory effect of high light on stilbene biosynthesis; 50 μM MeJa is optimal for efficient production of total phenols, flavonoids, and stilbenes | [215] |
| V. vinifera cv. Malvasia; V. rupestris Du Lut | Light-exposed and dark-maintained cell cultures | Trans-resveratrol, trans-piceid, trans-ε-viniferin, trans-δ-viniferin | Increase in stilbene content under light conditions | [40] |
| Arachis hypogaea | White LED light and UV-C radiation during peanut germination | Stilbenes (resveratrol, piceid, piceatannol); total phenols; total flavonoids | White light significantly induces stilbene accumulation by upregulating the expression of genes and enzymes involved in the stilbene biosynthetic pathway. UV-C is more effective than white light in promoting stilbene accumulation | [216] |
| V. vinifera cvs. Cabernet Franc, Chardonnay, Chenin, Malbec (Côt), Gamay, Grolleau, Pinot Noir, Sauvignon Blanc | Wounding (stem pruning) | Trans-resveratrol, trans-piceatannol, trans-ε-viniferin, ampelopsin A, trans-miyabenol C, cis- and trans-vitisin B, hopeaphenol, isohopeaphenol | Induction of trans-resveratrol and trans-piceatannol during the first 6 weeks of storage at 20 °C | [217] |
| V. vinifera cv. Pinot Noir | Wounding (leaf discs) | Stilbenes | Increase in transcription levels of several STS genes | [60] |
| V. vinifera cv. Pinot Noir | Wounding (leaf discs) | Stilbenes | Increased transcript level of VviSTS29, -41, and -48, coupled with the induction of WRKY and R2R3-MYB transcription factors | [218] |
| V. vinifera cv. Alphonse Lavallée | Mechanical stress (low-energy ultrasound) alone or in combination with MeJa on cell suspension cultures | Trans-resveratrol, trans-piceid, trans-ε-viniferin, trans-δ-viniferin | Increase in trans-ε-viniferin production in response to ultrasounds. Increase in trans-δ-viniferin in response to ultrasound and MeJa co-treatment | [219] |
| V. quinquangularis | Wounding, exogenous stress-associated hormones, and biotic stress in leaves of transgenic tobacco transformed with VqSTS36 promoter fused to the GUS reporter gene | Stilbenes | Induction of VqSTS36 promoter activity in response to wounding, salicylic acid, and inoculation with the phytopathogenic fungus Erysiphe cichoracearum | [220] |
| Pinus sylvestris | Wounding of stem-phloem alone or in combination with fungal infection | Stilbenes | Transient increase in STS and PMT expression in response to wounding, more pronounced with wounding in combination with fungal inoculation | [221] |
| P. sylvestris | Wounding of seedlings | Pinosylvin and pinosylvin monomethyl ether | Upregulation of stilbene biosynthetic genes including PMT2 during heartwood formation and in response to stress | [222] |
| P. sylvestris | Wounding and infection of seedlings with Heterobasidion parviporum or H. annosum | Pinosylvin and pinosylvin monomethylether | Significantly higher amounts of stilbenes 10 days after treatment. Greater increase in infected than in just wounded samples | [223] |
| P. sylvestris | Wounding 5-years-old seedlings | Pinosylvin and pinosylvin monomethylether | Induction of PMT2 | [134] |
| V. vinifera cv. Pinot Noir | Mechanical wounding on freshly pruned canes | Trans-resveratrol and trans-piceatannol | Transient expression of PAL and STS genes, followed by a rapid accumulation of stilbenes | [224] |
| Arachis hypogaea | Wounding stress (cotyledons) | Resveratrol, arachidin-3, arachidin-4 | Induction of all analyzed stilbenes | [225] |
| A. hypogaea | Wounding stress (size reduction, grinding, chopping, slicing, ultrasound) | Trans-resveratrol | Slicing produces the highest increase of trans-resveratrol accumulation | [203] |
| Pinus sylvestris | Ozone fumigation (saplings grown in phytotron) | Stilbenes | Enhanced STS and PMT transcript levels in needles but not in healthy phloem | [221] |
| V. quinquangularis (accession Shang-24; powdery mildew (PM) resistant); V. pseudoreticulata (accession Hunan-1; PM susceptible) | Infection by Uncinula necator (sin. Erysiphe necator) | Stilbenes | VqSTS36 transcript levels increase substantially following PM infection | [220] |
| V. vinifera cv. Barbera | Elicitation of cell suspension cultures with salicylic acid, Na-orthovanadate, jasmonates, chitosan, D-glucosamine, N-acetyl-D-glucosamine, ampicillin, rifampicin | Trans- and cis-resveratrol | Induction of ex-novo synthesis of stilbenes stilbene synthase protein by MeJa and chitosan | [226] |
| V. vinifera cv. Gamay Fréaux var. Teinturier | Elicitation of cell suspension cultures with MeJa in combination with sucrose | Stilbenes (trans-resveratrol and piceids); total anthocyanins | Induction of PAL, CHS, STS, UDP-glucose: flavonoid-O-glucosyltransferase, proteinase inhibitor and chitinase gene expression. Enhanced accumulation of piceids and anthocyanins in cells, and trans-resveratrol and piceids in culture medium | [227] |
| V. vinifera cv. Monastrell albino | Elicitation of cell suspension cultures with MeJa and cyclodextrin (CDs) used independently or in combination | Trans-resveratrol | Induction of stilbene biosynthetic gene expression by MeJa and CDs when used independently. Enhanced trans-resveratrol production in CDs-treated cells but not in MeJa-treated cells | [228] |
| V. vinifera cv. Barbera | Elicitation of cell suspension cultures with chitosan | Trans- and cis-resveratrol | Induction of trans-resveratrol production and STS gene expression | [229] |
| V. vinifera cvs. Red Globe and Michele Palieri | Elicitation of calli with MeJa | Trans-piceid, resveratrol glucoside, cis-piceid, resveratrol diglucoside, resveratrol dimer monoglucosides, resveratrol dimer diglucosides, resveratrol dimer triglucosides, resveratrol dimer tetraglucosides, picetannol monoglycosylated, picetannol diglycosylated | Enhanced production of stilbenes, mainly trans-piceid and ε-viniferin | [230] |
| V. vinifera cv. Isabelle | Elicitation of calli with biotic (fungal extract of Fusarium oxysporum) and abiotic (mannitol, abscisic acid, jasmonic acid) elicitors | Trans-resveratrol | Optimum accumulation of trans-resveratrol with a combined treatment of mannitol (2 mM) and jasmonic acid (40 µM) | [231] |
| V. vinifera cv. Barbera | Elicitation of cell suspension cultures with chitosan | Mono-glucosylated derivatives resveratrol (trans- and cis-piceid and trans- and cis-resveratroloside) | Increased in trans-resveratrol endogenous accumulation and decreased release into the culture medium. De-novo synthesis and/or accumulation of STS proteins. No influence on cis-resveratrol and on resveratrol mono-glucosides | [232] |
| V. vinifera cv. Italia | Elicitation of calli and cell suspension cultures with MeJa, jasmonic acid or chitosan | Trans-resveratrol, piceid trans-δ-viniferin, trans-ε-viniferin | Induction of trans-resveratrol, piceid, and viniferins by jasmonates. Jasmonic acid enhances simultaneously δ- and ε-viniferin biosynthesis, whereas MeJa stimulates preferentially δ-viniferin production. | [233] |
| V. vinifera cv. Monastrell | Elicitation of cell suspension cultures with MeJa, cyclodextrins, and UV-C used independently or in combination | Trans-resveratrol | Highest increase in trans-reveratrol production was obtained with the combined use of MeJa, cyclodextrins, and an optimal sucrose concentration. Greatest release of trans-resveratrol into the culture medium is achieved with the combined use of MeJa, cyclodextrin, and UV-C | [234] |
| V. vinifera cv. Gamay Fréaux | Elicitation of cell suspension cultures with indanoyl-isoleucine (In-Ile), N-linolenoyl-l-glutamine (Lin-Gln), and insect saliva (from Manduca sexta larvae) | 3-O-Glucosyl-resveratrol; 4-(3,5-dihydroxy-phenyl)-phenol; total anthocyanins | Increased accumulation of phenolic acids, particularly 3-O-glucosyl-resveratrol, in response to In-Ile, Lin-Gln, and saliva | [235] |
| V. vinifera cv. Hongbaladuo; V. vinifera × V. amurensis cv. Beihong | Treatment of leaves and berries with CaCl2 and UV-C used alone or in combination | Cis- and trans-resveratrol | Increased resveratrol content with single treatments, greater increase with combined treatment | [236] |
| V. vinifera cv. Gamay Fréaux | Elicitation of cell suspension cultures with jasmonic acid, salicylic acid, β-glucan, and chitosan | Stilbenes (trans-resveratrol and trans-piceid); total anthocyanins | Increased resveratrol production with co-treatment with jasmonic acid and β-glucan | [237] |
| V. vinifera cv. Negramaro | Elicitation of cell cultures with chitosan, MeJa, jasmonic acid, coronatine, and 12-oxo-phytodienoic acid | Trans-resveratrol, trans-piceid, cis-ε-viniferin, trans-ε-viniferin, trans-δ-viniferin | MeJa is the most effective in inducing trans-resveratrol the biosynthesis, while 12-oxo-phytodienoic acid, jasmonic acid, and coronatine are the most effective in inducing the biosynthesis of viniferins | [214] |
| V. vinifera cv. Monastrell | Elicitation cell suspension cultures with cyclodextrins and coronatine | Trans-resveratrol | Induction of stilbene biosynthetic genes by cyclodextrins and/or coronatine. Maximum level of trans-resveratrol production and secretion into the culture medium with co-treatment with 50 mM cyclodextrins and 1 μM coronatine | [238] |
| V. vinifera cv. Tempranillo | Foliar application of MeJa, chitosan, and yeast extract | Stilbenes (trans- and cis-piceid and trans- and cis-resveratrol); flavonols; anthocyanins; hydroxybenzoic acids; hydroxycinnamic acids | MeJa and yeast extract improve both grape and wine anthocyanin content. Stilbene content is clearly improved by yeast extract | [239] |
| Vitis vinifera L. cv. Kalecik Karası | Elicitation of grape berries with ultrasound | Trans-resveratrol | About 20-fold increase in trans-resveratrol content in grape skin | [240] |
| Arachis hypogaea cv. Tainan No. 14 | Elicitation of calli with bacteria and fungi (both viable and autoclaved) or with chitin | Trans-resveratrol and trans-piceatannol | Induction of stilbene biosynthesis by fungi (both viable and autoclaved) and chitin | [241] |
| A. hypogaea cv. Hull line 3 | Elicitation of hairy root cultures with MeJa and methyl-β-cyclodextrin | Trans-resveratrol, trans-piceatannol, trans-arachidin-1 and trans-arachidin-3 | Co-treatment with MeJa and cyclodextrin led to high levels of stilbenes in the culture medium | [242] |
| Arachis hypogaea cv. Georgia green | Treatment of peanuts with ultrasonication or UV-C | Trans-resveratrol, trans-piceid | Increased resveratrol, piceid, and total stilbene concentration, more induced by ultrasound than UV-C | [201] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants 2021, 10, 90. https://doi.org/10.3390/plants10010090
Valletta A, Iozia LM, Leonelli F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants. 2021; 10(1):90. https://doi.org/10.3390/plants10010090
Chicago/Turabian StyleValletta, Alessio, Lorenzo Maria Iozia, and Francesca Leonelli. 2021. "Impact of Environmental Factors on Stilbene Biosynthesis" Plants 10, no. 1: 90. https://doi.org/10.3390/plants10010090
APA StyleValletta, A., Iozia, L. M., & Leonelli, F. (2021). Impact of Environmental Factors on Stilbene Biosynthesis. Plants, 10(1), 90. https://doi.org/10.3390/plants10010090







