Co-Infections by Fusarium circinatum and Phytophthora spp. on Pinus radiata: Complex Phenotypic and Molecular Interactions
Abstract
:1. Introduction
2. Results
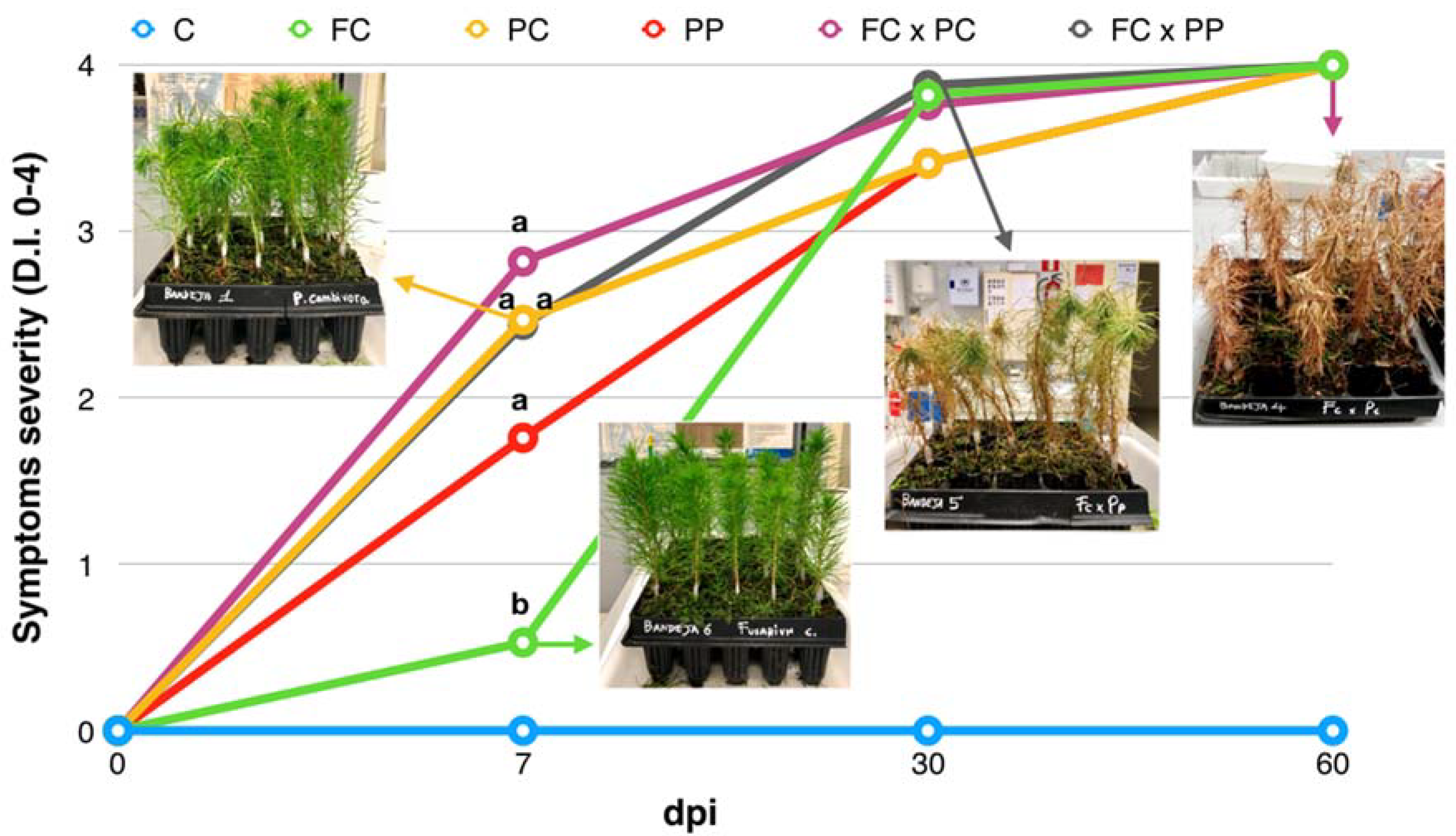
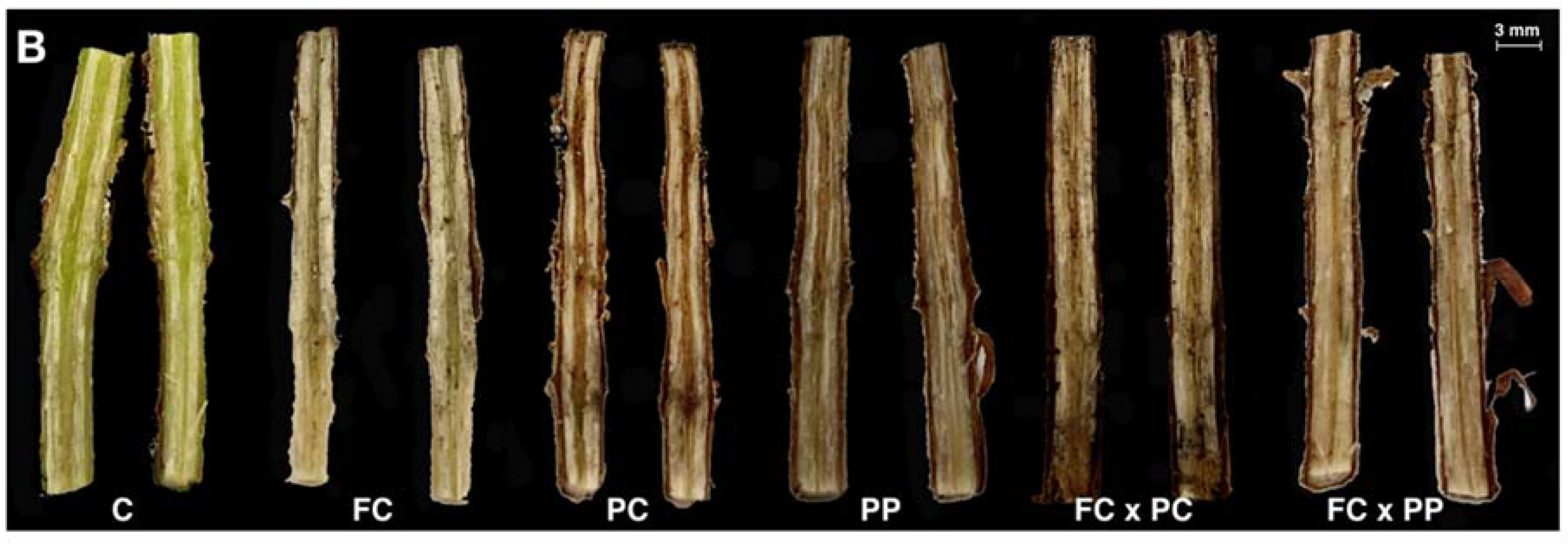
2.1. Symptom Progression
2.2. Housekeeping Gene Selection
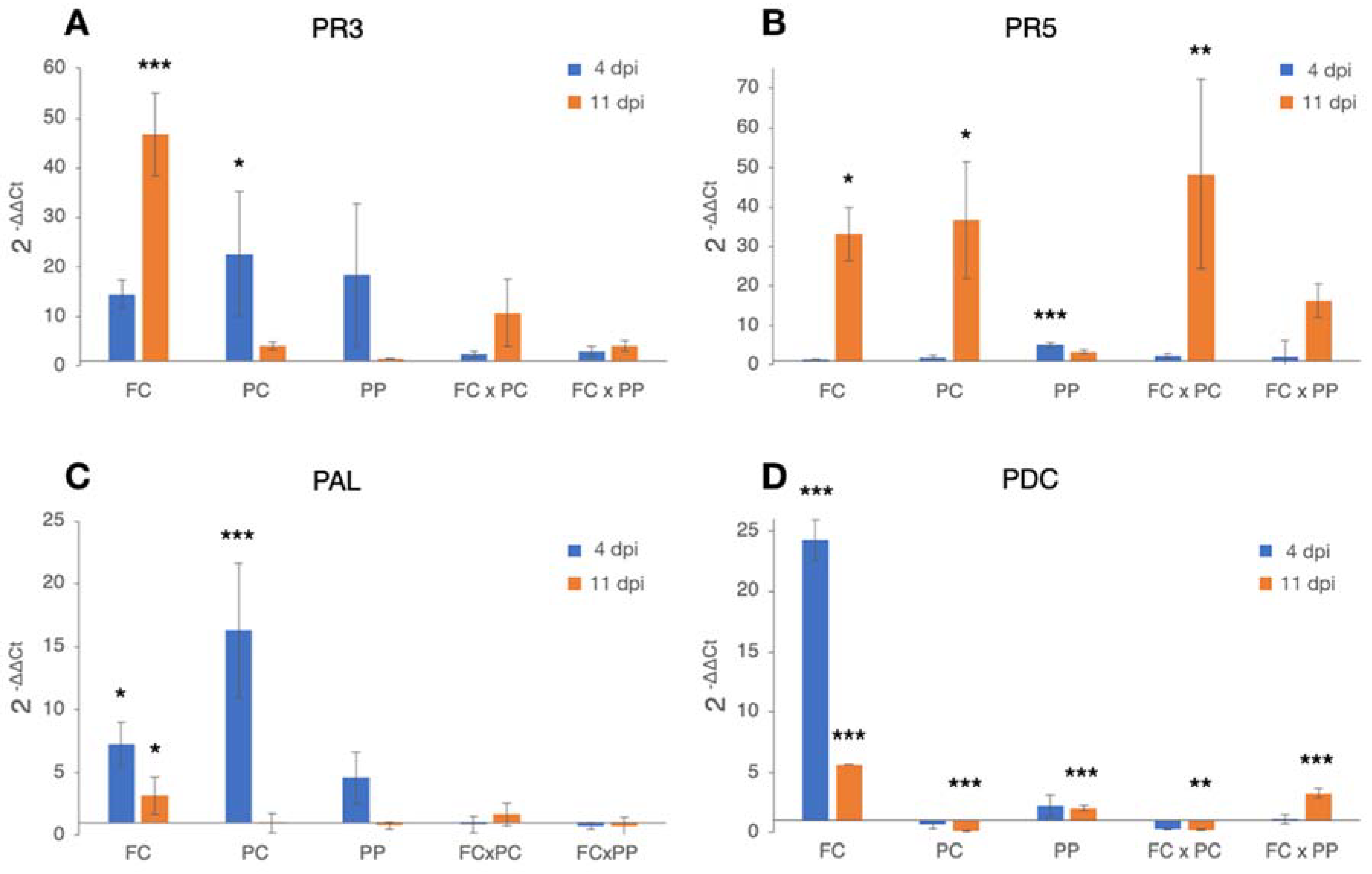
2.3. Differential Expression of Candidate Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Fungal Inoculum and Inoculation Methods
4.3. Evaluation of Symptoms and Internal Necrosis Length
4.4. Sample Collection, RNA Extraction, and cDNA Synthesis
4.5. Selection of Primers and Housekeeping Genes
4.6. Relative Expression of Candidate Genes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, D.L. In situ genetic conservation of a naturally restricted and commercially widespread species, Pinus radiata. For. Ecol. Manag. 2004, 197, 311–322. [Google Scholar] [CrossRef]
- Garbelotto, M. Molecular analysis to study invasions by forest pathogens: Examples from Mediterranean ecosystems. Phytopath. Medit. 2008, 47, 183–203. [Google Scholar]
- Guerrero, P.C.; Bustamante, R.O. Can native tree species regenerate in Pinus radiata plantations in Chile? Evidence from field and laboratory experiments. For. Ecol. Manag. 2007, 253, 97–102. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Hodge, G.; Dvorak, S. Differential responses of Central American and Mexican pine species and Pinus radiata to infection by the pitch canker fungus. New For. 2000, 19, 241–258. [Google Scholar] [CrossRef]
- EPPO. PM7/91(2) Fusarium circinatum (formerly Giberella circinata). Bull. OEPP/EPPO Bull. 2019, 49, 228–247. [Google Scholar] [CrossRef] [Green Version]
- Ioos, R.; Aloi, F.; Piškur, B.; Guinet, C.; Mullett, M.; Berbegal, M.; Bragança, H.; Cacciola, S.O.; Oskay, F.; Cornejo, C.; et al. Transferability of PCR-based diagnostic protocols: An international collaborative case study assessing protocols targeting the quarantine pine pathogen Fusarium circinatum. Sci. Rep. UK 2019, 9, 8195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, T.R.; Wikler, R.; Clark, K.R.; Okamoto, S.L.; Storer, D.; Bonello, A.J. Resistance to pitch canker disease, caused by Fusarium subglutinans f. sp. pini, in Monterey pine (Pinus radiata). Plant Pathol. 1998, 47, 706–711. [Google Scholar]
- Garbelotto, M.; Smith, T.; Schweigkofler, W. Variation of spore dispersal of Fusarium circinatum, the causal agent of pine pitch canker, over a 12-month-period at two locations in Northern California. Phytopathology 2008, 98, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Hepting, G.H.; Roth, E.R. Pitch canker, a new disease of southern pines of forestry. J. For. 1946, 44, 742–744. [Google Scholar]
- Viljoen, A.; Wingfield, M.J.; Marasas, W.F.O. First report of Fusarium subglutinans f. sp. pini on seedlings in South Africa. Plant Dis. 1994, 78, 309–312. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, S.; Yang, S.; Lee, Y. First report of pitch canker disease on Pinus rigida in Korea. Plant Pathol. 2000, 16, 52–54. [Google Scholar]
- Thoungchaleun, V.; Kim, K.W.; Lee, D.K.; Chang, C.S.; Park, E.W. Pre-infection behavior of the pitch canker fungus Fusarium circinatum on pine stems. Plant Pathol. J. 2008, 24, 112–117. [Google Scholar] [CrossRef]
- Gordon, T.R.; Storer, A.J.; Wood, D.L. The pitch canker epidemic in California. Plant Dis. 2001, 85, 1128–1139. [Google Scholar] [CrossRef]
- Abad, G.Z. The taxonomy of Phytophthora: What is done and what is needed for the correct identification and diagnostics of species in the genus. In Proceedings of the 7th International Union of Forest Research Organizations, IUFRO Working Party 7-02-09 Meeting, Phytophthora in Forests and Natural Ecosystems, Esquel, Argentina, 10–14 November 2014. [Google Scholar]
- Ruano-Rosa, D.; Schena, L.; Agosteo, G.E.; Magnano di San Lio, G.; Cacciola, S.O. Phytophthora oleae sp. nov., causing fruit rot of olive in southern Italy. Plant Pathol. 2018, 67, 1362–1373. [Google Scholar] [CrossRef]
- Brasier, C.M. Phytophthora Pathogens of Trees: Their Rising Profile in Europe; Forestry Commission: Stockport, UK, 1999; p. 30. [Google Scholar]
- Jung, T.; Pérez-Sierra, A.; Duran, A.; Horta Jung, M.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil-and airborne Phytophthora species in forests and woodlands. Persoonia 2018, 40, 180–220. [Google Scholar] [CrossRef] [Green Version]
- Riolo, M.; Aloi, F.; La Spada, F.; Sciandrello, S.; Moricca, S.; Santilli, E.; Pane, A.; Cacciola, S.O. Diversity of Phytophthora communities across different types of Mediterranean vegetation in a nature reserve area. Forests 2020, 11, 853. [Google Scholar] [CrossRef]
- Jung, T.; Horta Jung, M.; Cacciola, S.O.; Cech, T.; Bakonyi, J.; Seress, D.; Mosca, S.; Schena, L.; Seddaiu, S.; Pane, A.; et al. Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus 2017, 8, 219–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbelotto, M.; Frankel, S.; Scanu, B. Soil- and waterborne Phytophthora species linked to recent outbreaks in Northern California restoration sites. Calif. Agric. 2018, 72, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Scott, P.; Bader, M.K.-F.; Burgess, T.; Hardy, G.; Williams, N. Global biogeography and invasion risk of the plant pathogen genus Phytophthora. Environ. Sci. Policy 2019, 101, 175–182. [Google Scholar] [CrossRef]
- Jung, T.; La Spada, F.; Pane, A.; Aloi, F.; Evoli, M.; Horta Jung, M.; Scanu, B.; Faedda, R.; Rizza, C.; Puglisi, I.; et al. Diversity and distribution of Phytophthora species in protected natural areas in Sicily. Forests 2019, 10, 259. [Google Scholar] [CrossRef] [Green Version]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: Saint Paul, MN, USA, 1996; p. 562. [Google Scholar]
- Marçais, B.; Caël, O.; Delatour, C. Interaction between root rot basidiomycetes and Phytophthora species on pedunculate oak. Plant Pathol. 2011, 60, 296–303. [Google Scholar] [CrossRef]
- Migliorini, D.; Tondini, E.; Luchi, N.; Ghelardini, L.; Capretti, P.; Santini, A. Detection of Phytophthora species on different woody species in nurseries. In Proceedings of the 7th International Union of Forest Research Organizations, IUFRO Working Party 7-02-09 Meeting, Phytophthora in Forests and Natural Ecosystems, Esquel, Argentina, 10–14 November 2014. [Google Scholar]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday Kaya, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Santilli, E.; Riolo, M.; La Spada, F.; Pane, A.; Cacciola, S.O. First report of root rot caused by Phytophthora bilorbang on Olea europaea in Italy. Plants 2020, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Chavarriaga, D.; Bodles, W.J.A.; Leifert, C.; Belbahri, L.; Woodward, S. Phytophthora cinnamomi and other fine root pathogens in north temperate pine forests. FEMS Microbiol. Lett. 2007, 276, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.; Burgess, T.I. Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov. Persoonia 2009, 22, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Tkaczyk, M.; Sikora, K.; Nowakowska, J.; Ani´sko, E.; Oszako, T.; Belbahri, L.; Milenković, I. Four different Phytophthora species that are able to infect Scots pine seedlings in laboratory conditions. Folia For. Pol. Ser. 2016, 58, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Cleary, M.; Blomquist, M.; Vetukuri, R.R.; Böhlenius, H.; Witzell, J. Susceptibility of common tree species in Sweden to Phytophthora cambivora, P. plurivora and P. cactorum. For. Pathol. 2017, 47, e12329. [Google Scholar] [CrossRef]
- Sechi, C.; Seddaiu, S.; Linaldeddu, B.T.; Franceschini, A.; Scanu, B. Dieback and mortality of Pinus radiata trees in Italy associated with Phytophthora cryptogea. Plant Dis. 2014, 98, 159. [Google Scholar] [CrossRef]
- Elvira-Recuenco, M.; Cacciola, S.O.; Sanz-Ros, A.V.; Garbelotto, M.; Aguayo, J.; Solla, A.; Mullett, M.; Drenkhan, T.; Oskay, F.; Aday Kaya, A.G.; et al. Potential interactions between invasive Fusarium circinatum and other pine pathogens in Europe. Forests 2020, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Landa, B.B.; Arias-Giraldo, L.F.; Henricot, B.; Montes-Borrego, M.; Shuttleworth, L.A.; Pérez-Sierra, A. Diversity of Phytophthora species detected in disturbed and undisturbed British soils using high-throughput sequencing targeting ITS rRNA and COI mtDNA regions. Forests 2021, 12, 229. [Google Scholar] [CrossRef]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef]
- Fiorilli, V.; Catoni, M.; Lanfranco, L.; Zabet, N.R. Interactions of Plants with Bacteria and Fungi: Molecular and Epigenetic Plasticity of the Host. Front. Plant Sci. 2020, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Morse, A.M.; Nelson, C.D.; Covert, S.F.; Holliday, A.G.; Smith, K.E.; Davis, J.M. Pine genes regulated by the necrotrophic pathogen Fusarium circinatum. TAG Theor. Appl. Genet. Theor. Angew. Genet. 2004, 109, 922–932. [Google Scholar] [CrossRef]
- Quesada, T.; Gopal, V.; Cumbie, W.P.; Eckert, A.J.; Wegrzyn, J.L.; Neale, D.B.; Goldfarb, B.; Huber, D.A.; Casella, G.; Davis, J.M. Association mapping of quantitative disease resistance in a natural population of loblolly pine (Pinus taeda L.). Genetics 2010, 186, 677–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Veluthakkal, R.; Dasgupta, M. Pathogenesis-related genes and proteins in forest tree species. Trees 2010, 24, 993–1006. [Google Scholar] [CrossRef]
- Pascual, M.B.; El-Azaz, J.; Fernando, N.; Cañas, R.A.; Avila, C.; Cánovas, F.M. Biosynthesis and metabolic fate of phenylalanine in conifers. Front. Plant Sci. 2016, 7, 1030. [Google Scholar] [CrossRef] [Green Version]
- Donoso, A.; Rodriguez, V.; Carrasco, A.; Ahumada, R.; Sanfuentes, E.; Valenzuela, S. Relative expression of seven candidate genes for pathogen resistance on Pinus radiata infected with Fusarium circinatum. Physiol. Mol. Plant Pathol. 2015, 92, 42–50. [Google Scholar] [CrossRef]
- Bolton, M.D. Primary metabolism and plant defense-fuel for the fire. Mol. Plant Microbe Interact. 2009, 22, 487–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, X.Y.; Wang, J.R.; Ouellet, T.; Rocheleau, H.; Wei, Y.M.; Pu, Z.E.; Jiang, Q.T.; Lan, X.J.; Zheng, Y.L. Genome-wide identification and evaluation of novel internal control genes for Q-PCR based transcript normalization in wheat. Plant Mol. Biol. 2010, 74, 307–311. [Google Scholar] [CrossRef]
- Brunner, A.M.; Yakovlev, I.A.; Strauss, S.H. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Jarosova, J.; Kundu, J.K. Validation of reference genes as internal control for studying viral infections in cereals by quantitative real-time RT-PCR. BMC Plant Biol. 2010, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Amaral, J.; Correia, B.; António, C.; Rodrigues, A.M.; Gómez-Cadenas, A.; Valledor, L.; Hancock, R.D.; Alves, A.; Pinto, G. Pinus susceptibility to pitch canker triggers specific physiological responses in symptomatic plants: An integrated approach. Front. Plant Sci. 2009, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma counteracts the challenge of Phytophthora nicotianae infections on Tomato by modulating plant defense mechanisms and the expression of crinkler, necrosis-inducing Phytophthora protein 1, and cellulose-binding elicitor lectin pathogenic effectors. Front. Plant Sci. 2020, 11, 583539. [Google Scholar] [CrossRef] [PubMed]
- La Spada, F.; Aloi, A.; Coniglione, M.; Pane, A.; Cacciola, S.O. Natural biostimulants elicit plant immune system in an integrated management strategy of the postharvest green mold of orange fruits incited by Penicillium digitatum. Front. Plant Sci. 2021, 12, 684722. [Google Scholar] [CrossRef]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Dalio, R.J.D.; Máximo, H.J.; Oliveira, T.S.; Azevedo, T.M.; Felizatti, H.L.; Campos, M.A.; Machado, M.A. Molecular basis of Citrus sunki susceptibility and Poncirus trifoliata resistance upon Phytophthora parasitica attack. Mol. Plant Microb. Interact. 2018, 31, 386–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panabières, F.; Ali, G.S.; Allagui, M.B.; Dalio, R.J.D.; Gudmestad, N.C.; Kuhn, M.-L.; Gua Roy, S.; Schena, L.; Zampounis, A. Phytophthora nicotianae diseases worldwide: New knowledge of a long-recognised pathogen. Phytopath. Medit. 2016, 55, 20–40. [Google Scholar]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in plant disease complexes: A growing trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.M.; Silva, B.A.A.S.; Moreira, G.M.; Pfenning, L.H. Colletotrichum falcatum and Fusarium species induce symptoms of red rot in sugarcane in Brazil. Plant Path. 2021, 70, 1807–1818. [Google Scholar] [CrossRef]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Whitelaw-Weckert, M.A.; Sergeeva, V.; Priest, M.J. Botryosphaeria stevensii infection of Pinot Noir grapevines by soil-root transmission. Australas. Plant Path. 2006, 35, 369–371. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Mwangi, M.; Aigbe, S.O.; Leslie, J.F. Fusarium species from the cassava root rot complex in west Africa. Phytopathology 2006, 96, 673–676. [Google Scholar] [CrossRef] [Green Version]
- Belisario, A.; Maccaroni, M.; Coramusi, A.; Corazza, L.; Pryor, B.M.; Figuli, P. First report of Alternaria species groups involved in disease complexes of hazelnut and walnut fruit. Plant Dis. 2004, 88, 426. [Google Scholar] [CrossRef]
- Wing, K.B.; Pritts, M.P.; Wilcox, W.F. Strawberry black root rot: A review. Adv. Strawb. Res. 1994, 13, 13–19. [Google Scholar]
- Pemberton, I.J.; Smith, G.R.; Philley, G.L.; Rouquette, F.M.; Yuen, G.Y. First Report of Pythium ultimum, P. irregulare, Rhizoctonia solani AG 4 and Fusarium proliferatum from Arrowleaf clover (Trifolium vesiculosum): A disease complex. Plant Dis. 1998, 82, 128. [Google Scholar] [CrossRef] [PubMed]
- Garbelotto, M.; Gonthier, P. Biology, epidemiology and control of Heterobasidion species worldwide. Annu. Rev. Phytopath. 2013, 51, 39–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezos, D.; Martínez-Álvarez, P.; Diez, J.J.; Fernández, M.M. Association levels between Pityophthorus pubescens and Fusarium circinatum in pitch canker disease affected plantations in northern Spain. Entomol. Gen. 2016, 36, 43–54. [Google Scholar] [CrossRef]
- Bezos, D.; Martínez-Álvarez, P.; Fernández-Fernández, M.M.; Diez, J.J. Fungi and insect diversity associated with Pinus radiata in pitch-canker-affected stands. Int. For. Rev. 2014, 16, 336. [Google Scholar]
- Zhang, Y.; Xu, S.; Ding, P.; Wang, D.; Cheng, Y.T.; He, J.; Gao, M.; Xu, F.; Li, X.; Zhang, Y. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18220–18225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correll, J.C.; Gordon, T.R.; McCain, A.H.; Fox, J.W.; Koehler, C.S.; Wood, D.L.; Schultz, M.E. Pitch canker disease in California–Pathogenicity, distribution, and canker development on Monterey Pine (Pinus radiata). Plant Dis. 1991, 75, 676–682. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, D.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [Green Version]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Collinge, D.B.; Kragh, K.M.; Mikkelsen, J.D.; Nielsen, K.K.; Rasmussen, U.; Vad, K. Plant chitinases. Plant J. 1993, 3, 31–40. [Google Scholar] [CrossRef]
- Mauch, F.; Mauch-Mani, B.; Boller, T. Antifungal hydrolases in pea tissue: II. Inhibition of fungal growth by combinations of chitinase and beta-1,3-glucanase. Plant Physiol. 1988, 88, 936–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenier, J.; Potvin, C.; Trudel, J.; Asselin, A. Some thaumatin-like proteins hydrolyse polymeric b-1,3-glucans. Plant J. 1999, 19, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.K.; Selitrennikoff, C.P. Zeamatin, an antifungal protein from maize with membrane-permeabilizing activity. J. Gen. Microbiol. 1990, 136, 1771–1778. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.M.; Ellis, B.E. Fungal elicitor-mediated responses in pine cell cultures: III. Purification and characterization of phenylalanine ammonialyase. Plant Physiol. 1992, 98, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Dron, M.; Cramer, C.L.; Dixon, R.A.; Lamb, C.J. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J. Biol. 1989, 264, 14486–14492. [Google Scholar]
- Mithran, M.; Paparelli, E.; Novi, G.; Perata, P.; Loreti, E. Analysis of the role of the pyruvate decarboxylase gene family in Arabidopsis thaliana under low-oxygen conditions. Plant Biol. (Stutt.) 2013, 16, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Kürsteiner, O.; Dupuis, I.; Kuhlemeier, C. The pyruvate decarboxylase1 gene of Arabidopsis is required during anoxia but not other environmental stresses. Plant Physiol. 2003, 132, 968–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.K.; Holtzer, S.; Chacko, S.A.; Lin, Z.X.; Hoffman, R.K.; Holtzer, H. Phorbol esters selectively and reversibly inhibit a subset of myofibrillar genes responsible for the ongoing differentiation program of chick skeletal myotubes. Mol. Cell Biol. 1991, 11, 4473–4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, Y.-M.; Mun, J.-H.; Lee, I.; Woo, J.C.; Hong, C.B.; Kim, S.-G. Distinct roles of the first introns on the expression of Arabidopsis profilin gene family members. Plant Physiol. 2006, 140, 196–209. [Google Scholar] [CrossRef] [Green Version]

| Gene | Primer Sequence | GenBank ID | Functions/Putative Functions | References |
|---|---|---|---|---|
| Chitinase (PR3) | F: TGGCAACACGGACGCCCATT | HM219849.1 | Hydrolyzation of chitin. | [45,50,73,74] |
| R: ACCGGCGTCGTTTCTGTGCTT | ||||
| Thaumatin-like protein (PR5) | F: AGGAGCGCGTGTGATGCGTT | JQ015859.1 | Involved in cell wall damage and formation of pores on the plasma membrane. | [45,46,47,48,49,50,75,76] |
| R: TGAAAGTGCTGGTGGCGTCGT | ||||
| Phenylalanine ammonia-lyase (PAL) | F: TGCTGGCCACTGTGAAGCAGA | AY641535.1 | Lignin and phenolic accumulation in plants. Cinnamic acid synthesis | [45,46,47,48,49,50,77,78] |
| R: TCGCAGAAACGGCCTGGCAA | ||||
| Pyruvate decarboxylase (PDC) | F: CCCGCAAACAATGACGTGGGGT | JQ264496.1 | Involved in aerobic fermentation. | [45,46,47,48,49,50,79,80] |
| R: TGCGAGCAGATGGTCCAGCA | ||||
| Actin (ACT) | F: TGGACCTTGCTGGGCGTGATCT | GQ339779.1 | Major component of cytoskeleton microfilaments. | [45,46,47,48,49,50,81] |
| R: ACAATCTCGCGCTCTGCGGT | ||||
| β-Tubulin (TUB) | F: AAGGGGGTCAGTGTGGCAACCA | KM496536.1 | Structural units of the cytoskeleton microtubes | [45,46,47,48,49,50,82] |
| R: ACAGCCCGCGGAACAAACCT | ||||
| Ubiquitin (UBQ) | F: AGCCCTTATGCCGGAGGGGTTT | AF461687.1 | Participates in protein recognition by the proteasome | [45,47,50] |
| R: AGTGCGGGACTCCACTGTTCCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloi, F.; Zamora-Ballesteros, C.; Martín-García, J.; Diez, J.J.; Cacciola, S.O. Co-Infections by Fusarium circinatum and Phytophthora spp. on Pinus radiata: Complex Phenotypic and Molecular Interactions. Plants 2021, 10, 1976. https://doi.org/10.3390/plants10101976
Aloi F, Zamora-Ballesteros C, Martín-García J, Diez JJ, Cacciola SO. Co-Infections by Fusarium circinatum and Phytophthora spp. on Pinus radiata: Complex Phenotypic and Molecular Interactions. Plants. 2021; 10(10):1976. https://doi.org/10.3390/plants10101976
Chicago/Turabian StyleAloi, Francesco, Cristina Zamora-Ballesteros, Jorge Martín-García, Julio J. Diez, and Santa Olga Cacciola. 2021. "Co-Infections by Fusarium circinatum and Phytophthora spp. on Pinus radiata: Complex Phenotypic and Molecular Interactions" Plants 10, no. 10: 1976. https://doi.org/10.3390/plants10101976
APA StyleAloi, F., Zamora-Ballesteros, C., Martín-García, J., Diez, J. J., & Cacciola, S. O. (2021). Co-Infections by Fusarium circinatum and Phytophthora spp. on Pinus radiata: Complex Phenotypic and Molecular Interactions. Plants, 10(10), 1976. https://doi.org/10.3390/plants10101976










