A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity
Abstract
:1. Introduction
2. Results
2.1. Distillation and Physical Properties
2.2. Chemical Analysis of the EO
2.3. Enantioselective Evaluation of the EO
2.4. AChE Inhibition Activity
3. Discussion
3.1. The Chemical Composition
3.2. The Enantiomeric Evaluation
3.3. The Cholinergic Activity
4. Materials and Methods
4.1. General Information
4.2. Plant Material
4.3. Distillation of the EO and GC Sample Preparation
4.4. GC-MS Qualitative Analyses
4.5. GC-FID Quantitative Analyses
4.6. Enantioselective Analysis of the EO
4.7. AChE Inhibition Spectrophotometric Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittermeier, R.A.; Myers, N.; Thomsen, J.B.; Da Fonseca, G.A.; Olivieri, S. Biodiversity hotspots and major tropical wilderness areas: Approaches to setting conservation priorities. Conserv. Biol. 1998, 12, 516–520. [Google Scholar] [CrossRef]
- Araujo-Baptista, L.; Vimos-Sisa, K.; Cruz-Tenempaguay, R.; Falconí-Ontaneda, F.; Rojas-Fermín, L.; González-Romero, A. Componentes químicos y actividad antimicrobiana del aceite esencial de Lasiocephalus ovatus (Asteraceae) que crece en Ecuador. Acta Biol. Colomb. 2020, 25, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Ministerio del Ambiente del Ecuador. Quinto Informe Nacional para el Convenio sobre la Diversidad Biológica. Available online: https://www.ambiente.gob.ec/wp-content/uploads/downloads/2015/06/QUINTO-INFORME-BAJA-FINAL-19.06.2015.pdf (accessed on 27 September 2021).
- Convention on Biological Diversity. 6th National Report for the Convention on Biological Diversity. Available online: https://www.cbd.int/nr6/ (accessed on 27 September 2021).
- Malagón, O.; Ramírez, J.; Andrade, J.M.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and ethnopharmacology of the Ecuadorian flora. A review. Nat. Prod. Commun. 2016, 11, 297–314. [Google Scholar] [CrossRef] [Green Version]
- Zapata, B.; Duran, C.; Stashenko, E.; Betancur-Galvis, L.; Mesa-Arango, A.C. Actividad antimicótica y citotóxica de aceites esenciales de plantas de la familia Asteraceae. Rev. Iberoam. Micol. 2010, 27, 101–103. [Google Scholar] [CrossRef]
- Ruiz, C.; Díaz, C.; Rojas, R. Composición Química de Aceites Esenciales de 10 Plantas aromáticas peruanas. Rev. Soc. Quím. Perú 2015, 81, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Duschatzky, C.B.; Possetto, M.L.; Talarico, L.B.; García, C.C.; Michis, F.; Almeida, N.V.; de Lampasona, M.P.; Schuff, C.; Damonte, E.B. Evaluation of chemical and antiviral properties of essential oils from South American plants. Antivir. Chem. Chemother. 2005, 16, 247–251. [Google Scholar] [CrossRef]
- Wilches, I.; Tobar, V.; Peñaherrera, E.; Cuzco, N.; Jerves, L.; Vander-Heyden, Y.; León-Tamariz, F.; Vila, E. Evaluation of anti-inflammatory activity of the methanolic extract from Jungia rugosa leaves in rodents. J. Ethnopharmacol. 2015, 173, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Acosta-León, K.L.; Tacuamán-Jácome, S.E.; Vinueza-Tapia, D.R.; Vélez-Correal, F.X.; Pilco-Bonilla, G.A.; García-Veloz, M.J.; Abdo-López, S.P. Hypoglycemic activity of Jungia rugosa on induced diabetic mice (Mus musculus). Pharmacologyonline 2019, 1, 239–245. [Google Scholar]
- Jorgensen, P.; Leon-Yanez, S. Catalogue of the Vascular Plants of Ecuador; Monogram St. Louis: St. Louis, MO, USA, 1999; Volume 75, p. 291. [Google Scholar]
- De la Torre, L.; Navarrete, H.; Muriel, P.M.; Macía, M.J.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador: Quito, Ecuador; Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Aarhus, Denmark, 2008; p. 228. [Google Scholar]
- Graf, B.L.; Rojas-Silva, P.; Baldeón, M.E. Discovering the Pharmacological Potential of Ecuadorian Market Plants using a Screens-to-Nature Participatory Approach. J. Biodivers. Biopros. Dev. 2016, 3, 1000156. [Google Scholar] [CrossRef] [Green Version]
- Enciso, E.; Arroyo, J. Efecto antiinflamatorio y antioxidante de los flavonoides de las hojas de Jungia rugosa Less (matico de puna) en modelo experimental en ratas. Fac. Med. 2011, 72, 231. [Google Scholar] [CrossRef] [Green Version]
- Criollo, K.; Molina, N. Evaluación de la Estabilidad de Extractos Obtenidos a Partir de Distintos Procesos de Secado de Jungia Rugosa. Bachelor’s Thesis, Universidad de Cuenca, Cuenca, Ecuador, 2008. [Google Scholar]
- Campoverde, J.; Verdugo, M. Determinación del Efecto Cicatrizante de las Hojas de Carne Humana (Jungia cf. rugosa). Bachelor’s Thesis, Universidad de Cuenca, Cuenca, Ecuador, 2008. [Google Scholar]
- Tropicos.org. Missouri Botanical Garden. Available online: https://www.tropicos.org (accessed on 31 August 2021).
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Gilardoni, G. Chemical, enantioselective, and sensory analysis of a cholinesterase inhibitor essential oil from Coreopsis triloba SF Blake (Asteraceae). Plants 2019, 8, 448. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, B. Actividad Antioxidante y Citotóxica de 35 Plantas Medicinales de la Cordillera Negra. Master’s Thesis, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2017. [Google Scholar]
- Pant, P.; Sut, S.; Castagliuolo, I.; Gandin, V.; Maggi, F.; Gyawali, R.; Dall’Acqua, S. Sesquiterpene rich essential oil from Nepalese Bael tree (Aegle marmelos (L.) Correa) as potential antiproliferative agent. Fitoterapia 2019, 138, 104266. [Google Scholar] [CrossRef]
- Montalván, M.; Peñafiel, M.A.; Ramírez, J.; Cumbicus, N.; Bec, N.; Larroque, C.; Bicchi, C.; Gilardoni, G. Chemical Composition, Enantiomeric Distribution, and Sensory Evaluation of the Essential Oils Distilled from the Ecuadorian Species Myrcianthes myrsinoides (Kunth) Grifo and Myrcia mollis (Kunth) DC. (Myrtaceae). Plants 2019, 8, 511. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Cumbicus, N.; Gilardoni, G. A novel chemical profile of a selective in vitro cholinergic essential oil from Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae), a native Andean species of Ecuador. Molecules 2021, 26, 45. [Google Scholar] [CrossRef]
- Armijos, C.; Gilardoni, G.; Amay, L.; Lozano, A.; Bracco, F.; Ramirez, J.; Bec, N.; Larroque, C.; Vita Finzi, P.; Vidari, G. Phytochemical and ethnomedicinal study of Huperzia species used in the traditional medicine of Saraguros in Southern Ecuador; AChE and MAO inhibitory activity. J. Ethnopharmacol. 2016, 193, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Benyelles, B.; Allali, H.; Dib, M.E.A.; Djabou, N.; Paolini, J.; Costa, J. Chemical Composition Variability of Essential Oils of Daucus gracilis Steinh. from Algeria. Chem. Biodivers. 2017, 14, e1600490. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-1932633219. [Google Scholar]
- Saroglou, V.; Marin, P.; Rancic, A.; Veljic, M.; Skaltsa, H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem. Syst. Ecol. 2007, 35, 146–152. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, A.; Tomi, P.; Bernardini, A.; Morelli, I.; Flamini, G.; Cioni, P.; Usaï, M.; Marchetti, M. A comparative study of volatile constituents of two Helichrysum italicum (Roth) Guss. Don Fil subspecies growing in Corsica (France), Tuscany and Sardinia (Italy). Flavour Fragr. J. 2003, 18, 487–491. [Google Scholar] [CrossRef]
- Smadja, J.; Rondeau, P.; Shum Cheong Sing, A. Volatile constituents of five Citrus Petitgrain essential oils from Reunion. Flavour Fragr. J. 2005, 20, 399–402. [Google Scholar] [CrossRef]
- Skaltsa, H.; Demetzos, C.; Lazari, D.; Sokovic, M. Essential oil analysis and antimicrobial activity of eight Stachys species from Greece. Phytochemistry 2003, 64, 743–752. [Google Scholar] [CrossRef]
- Boti, J.; Muselli, A.; Tomi, F.; Koukoua, G.; N’Guessan, T.; Costa, J.; Casanova, J. Combined analysis of Cymbopogon giganteus Chiov. leaf oil from Ivory Coast by GC/RI, GC/MS and 13C-NMR. Comptes Rendus Chimie 2006, 9, 164–168. [Google Scholar] [CrossRef]
- Duquesnoy, E.; Castola, V.; Casanova, J. Composition and chemical variability of the twig oil of Abies alba Miller from Corsica. Flavour Fragr. J. 2007, 22, 293–299. [Google Scholar] [CrossRef]
- Cavalli, J.; Tomi, F.; Bernardini, A.; Casanova, J. Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003, 18, 532–538. [Google Scholar] [CrossRef]
- Grujic-Jovanovic, S.; Skaltsa, H.; Marin, P.; Sokovic, M. Composition and antibacterial activity of the essential oil of six Stachys species from Serbia. Flavour Fragr. J. 2004, 19, 139–144. [Google Scholar] [CrossRef]
- Hachicha, S.; Skanji, T.; Barrek, S.; Ghrabi, Z.; Zarrouk, H. Composition of the essential oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007, 22, 101–104. [Google Scholar] [CrossRef]
- Wedge, D.; Klun, J.; Tabanca, N.; Demirci, B.; Ozek, T.; Husnu Can Baser, K.; Liu, Z.; Zhang, S.; Cantrell, C.; Zhang, J. Bioactivity-Guided Fractionation and GC/MS Fingerprinting of Angelica sinensis and Angelica archangelica Root Components for Antifungal and Mosquito Deterrent Activity. J. Agric. Food Chem. 2009, 57, 464–470. [Google Scholar] [CrossRef]
- Martinez, J.; Rosa, P.; Menut, C.; Leydet, A.; Brat, J.; Pallet, D.; Meireles, M. Valorization of Brazilian Vetiver (Vetiveria zizanioides (L.) Nash ex Small) Oil. J. Agric. Food Chem. 2004, 52, 6578–6584. [Google Scholar] [CrossRef]
- Maksimovic, M.; Vidic, D.; Milos, M.; Solic, M.; Abadzic, S.; Siljak-Yakovlev, S. Effect of the environmental conditions on essential oil profile in two Dinaric Salvia species: S. brachyodon Vandas and S. officinalis L. Biochem. Syst. Ecol. 2007, 35, 473–478. [Google Scholar] [CrossRef]
- Chéraif, I.; Jannet, H.; Hammami, M.; Khouja, M.L.; Mighri, Z. Chemical composition and antimicrobial activity of essential oils of Cupressus arizonica Greene. Biochem. Syst. Ecol. 2007, 35, 813–820. [Google Scholar] [CrossRef]
- Kundakovic, T.; Fokialakis, N.; Kovacevic, N.; Chinou, I. Essential oil composition of Achillea lingulata and A. umbellata. Flavour Fragr. J. 2007, 22, 184–187. [Google Scholar] [CrossRef]
- Duquesnoy, E.; Dinh, N.; Castola, V.; Casanova, J. Composition of a Pyrolytic oil from Cupressus funebris Endl. of Vietnamese origin. Flavour Fragr. J. 2006, 21, 453–457. [Google Scholar] [CrossRef]
- Paolini, J.; Tomi, P.; Bernardini, A.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed analysis of the essential oil from Cistus albidus L. by combination of GC/RI, GC/MS and 13CNMR spectroscopy. Nat. Prod. Res. Former. Nat. Prod. Lett. 2008, 22, 1270–1278. [Google Scholar]
- Cozzani, S.; Muselli, A.; Desjobert, J.; Bernardini, A.; Tomi, F.; Casanova, J. Chemical composition of essential oil of Teucrium polium subsp. capitatum (L.) from Corsica. Flavour Fragr. J. 2005, 20, 436–441. [Google Scholar] [CrossRef]
- Boti, J.; Koukoua, G.; N’Guessan, T.; Muselli, A.; Bernardini, A.; Casanova, J. Composition of the leaf, stem bark and root bark oils of Isolona cooperi investigated by GC (retention index), GC-MS and 13C-NMR spectroscopy. Phytochem. Anal. 2005, 16, 357–363. [Google Scholar] [CrossRef]
- Tunalier, Z.; Kirimer, J.; Husnu Can Baser, K. Wood Essential Oils of Juniperus foetidissima Willd. Holzforschung 2003, 57, 140–144. [Google Scholar] [CrossRef]
- Choi, H.; Sawamura, M. Composition of the Essential Oil of Citrus tamurana Hort. ex Tanaka (Hyuganatsu). J. Agric. Food Chem. 2000, 48, 4868–4873. [Google Scholar] [CrossRef]
- Khaoukha, G.; Jemia, M.B.; Amira, S.; Laouer, H.; Bruno, M.; Scandolera, E.; Senatore, F. Characterisation and antimicrobial activity of the volatile components of the flowers of Magydaris tomentosa (Desf.) DC. collected in Sicily and Algeria. Nat. Prod. Res. Former. Nat. Prod. Lett. 2014, 28, 1152–1158. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Aćimović, M.; Ljujić, J.; Vulić, J.; Zheljazkov, V.D.; Pezo, L.; Varga, A.; Tumbas Šaponjac, V. Helichrysum italicum (Roth) G. Don Essential Oil from Serbia: Chemical Composition, Classification and Biological Activity—May It Be a Suitable New Crop for Serbia? Agronomy 2021, 11, 1282. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, P.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Yakoubi, R.; Megateli, S.; Sadok, T.H.; Bensouici, C.; Bağci, E. A synergistic interaction of Algerian essential oils of Laurus nobilis L., Lavandula stoechas L. and Mentha pulegium L. on anticholinesterase and antioxidant activities. Biocatal. Agric. Biotechnol. 2021, 31, 101891. [Google Scholar] [CrossRef]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour. Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
- Gilardoni, G.; Matute, Y.; Ramírez, J. Chemical and Enantioselective Analysis of the Leaf Essential Oil from Piper coruscans Kunth (Piperaceae), a Costal and Amazonian Native Species of Ecuador. Plants 2020, 9, 791. [Google Scholar] [CrossRef]
- Ramírez, J.; Andrade, M.D.; Vidari, G.; Gilardoni, G. Essential Oil and Major Non-Volatile Secondary Metabolites from the Leaves of Amazonian Piper subscutatum. Plants 2021, 10, 1168. [Google Scholar] [CrossRef]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
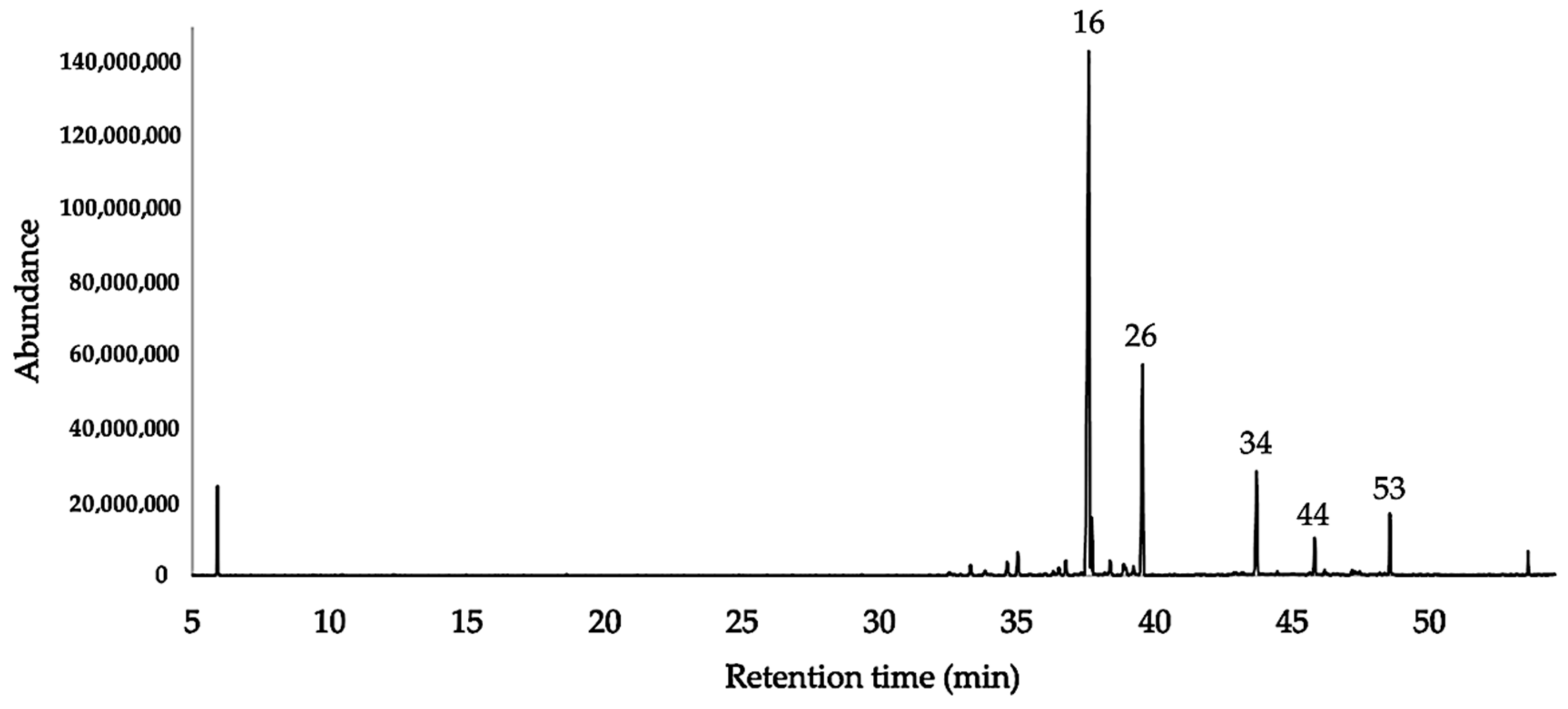
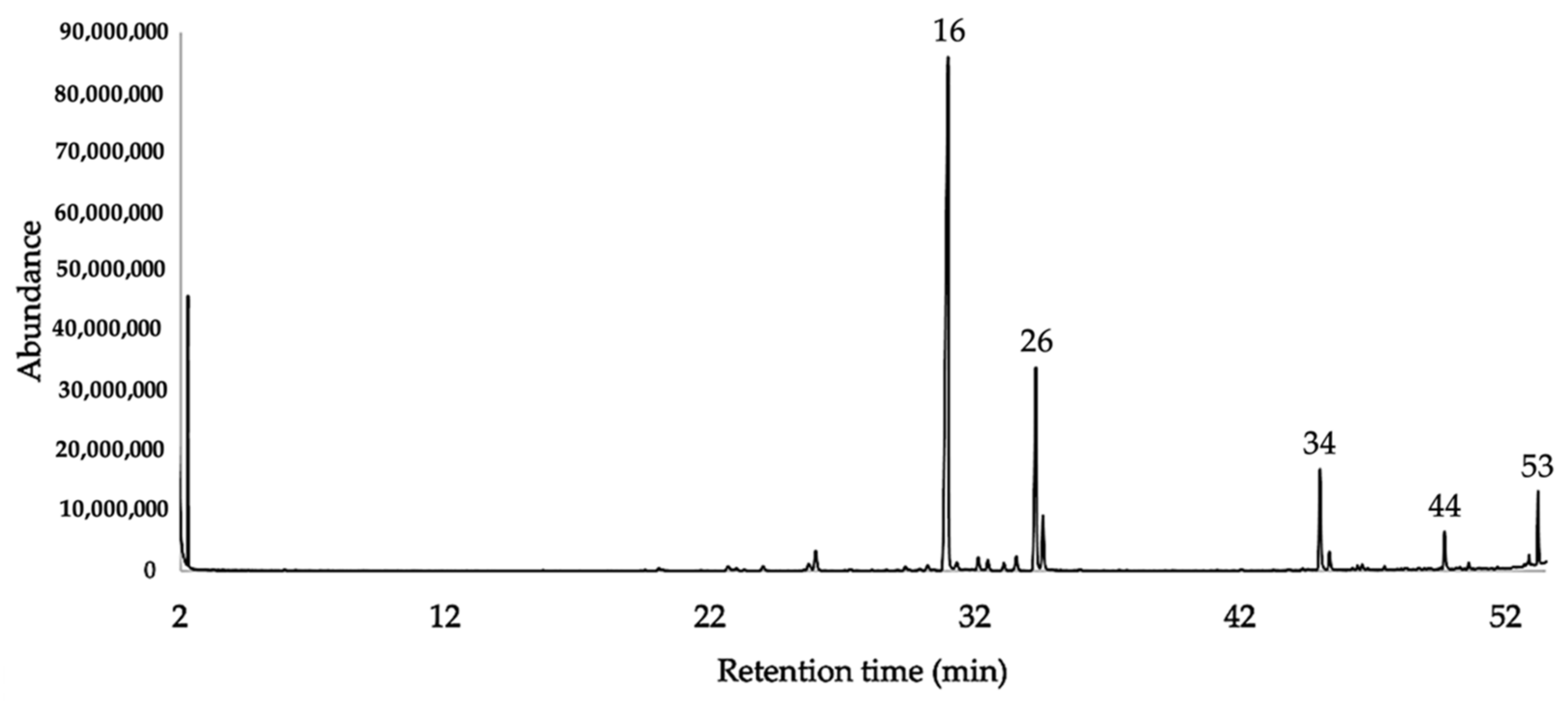
| N. | Compounds | DB-5ms | HP-INNOWax | DB-5ms | HP-INNOWax | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LRI 1 | LRI | Ref. | LRI 1 | LRI | Ref. | (%) 2 | σ | (%) 2 | σ | ||
| 1 | α-copaene | 1370 | 1374 | [25] | 1464 | 1489 | [26] | 0.2 | 0.01 | 0.3 | 0.01 |
| 2 | β-cubebene | 1378 | 1387 | [25] | 1536 | 1542 | [27] | trace | 0.02 | trace | - |
| 3 | 7-epi-sesquithujene | 1385 | 1390 | [25] | 1576 | - | 0.9 | 0.02 | trace | - | |
| 4 | Italicene | 1395 | 1405 | [25] | 1525 | 1536 | [28] | 0.3 | 0.08 | 0.5 | 0.02 |
| 5 | α-chamipinene | 1397 | 1396 | [25] | 1552 | - | trace | 0.05 | trace | - | |
| 6 | undetermined (MW 204) | 1412 | - | 1549 | - | 1.1 | 0.02 | 0.4 | 0.01 | ||
| 7 | α-cis-bergamotene | 1420 | 1411 | [25] | 1584 | 1577 | [28] | 1.6 | 0.03 | 1.7 | 0.05 |
| 8 | α-trans-bergamotene | 1430 | 1432 | [25] | 1530 | 1560 | [29] | 0.1 | 0.01 | 0.3 | 0.02 |
| 9 | seychellene | 1442 | 1444 | [25] | 1663 | - | 0.1 | - | trace | - | |
| 10 | α-humulene | 1448 | 1452 | [25] | 1652 | 1667 | [27] | 1.1 | 0.01 | 0.5 | 0.01 |
| 11 | allo-aromadendrene | 1451 | 1458 | [25] | 1626 | 1637 | [30] | 0.1 | 0.02 | trace | - |
| 12 | (E)-β-farnesene | 1453 | 1454 | [25] | 1669 | 1664 | [27] | trace | - | 0.8 | 0.02 |
| 13 | 6-demethoxy ageratochromene | 1458 | 1461 | [25] | 2083 | 2075 | [31] | 0.9 | 0.02 | 1.4 | 0.02 |
| 14 | 2-epi-(E)-caryophyllene | 1466 | 1465 | [32] | 1674 | 1669 | [32] | 0.1 | 0.01 | trace | - |
| 15 | ishwarane | 1471 | 1465 | [25] | 1609 | 1636 | [33] | 0.1 | 0.07 | trace | - |
| 16 | γ-curcumene | 1476 | 1481 | [25] | 1685 | 1692 | [27] | 47.1 | 0.70 | 49.7 | 0.40 |
| 17 | ar-curcumene | 1479 | 1479 | [25] | 1768 | 1774 | [27] | 3.4 | 0.34 | 4.2 | 0.32 |
| 18 | γ-muurolene | 1486 | 1478 | [25] | 1692 | 1690 | [27] | 0.1 | 0.07 | 0.8 | 0.02 |
| 19 | β-selinene | 1489 | 1489 | [25] | 1710 | 1717 | [27] | 0.3 | 0.04 | 1.3 | 0.02 |
| 20 | α-zingiberene | 1491 | 1493 | [25] | 1698 | 1713 | [34] | 0.1 | 0.11 | trace | - |
| 21 | epi-cubebol | 1493 | 1493 | [25] | 1943 | 1928 | [35] | 1.0 | 0.13 | trace | - |
| 22 | β-bisabolene | 1505 | 1505 | [25] | 1719 | 1728 | [27] | 0.9 | 0.02 | 1.0 | 0.02 |
| 23 | α-cuprenene | 1506 | 1505 | [25] | 1733 | 1759 | [36] | 0.5 | 0.01 | 0.6 | 0.01 |
| 24 | δ-amorphene | 1511 | 1511 | [25] | 1704 | 1710 | [37] | trace | - | trace | - |
| 25 | γ-cadinene | 1513 | 1513 | [25] | 1744 | 1763 | [27] | 0.7 | 0.01 | 0.8 | 0.01 |
| 26 | β-sesquiphellandrene | 1521 | 1521 | [25] | 1762 | 1771 | [27] | 17.0 | 0.20 | 17.9 | 0.16 |
| 27 | 8,14-cedranoxide | 1549 | 1541 | [25] | 1842 | 1858 | [38] | 0.1 | - | trace | - |
| 28 | cis-muurol-5-en-4-α-ol | 1569 | 1559 | [25] | 2210 | 2221 | [39] | 0.1 | 0.02 | trace | - |
| 29 | spathulenol | 1575 | 1577 | [25] | 2141 | 2140 | [40] | 0.1 | - | 0.5 | 0.01 |
| 30 | allo-cedrol | 1588 | 1589 | [25] | 2261 | - | 0.1 | 0.03 | trace | - | |
| 31 | sesquithuriferol | 1605 | 1604 | [25] | 2125 | 2113 | [41] | 0.3 | 0.01 | 0.3 | 0.01 |
| 32 | isolongifolan-7-α-ol | 1609 | 1618 | [25] | 2117 | - | 0.3 | 0.01 | 0.2 | 0.11 | |
| 33 | cis-isolongifolanone | 1613 | 1612 | [25] | 2168 | - | 0.2 | 0.01 | 0.3 | 0.01 | |
| 34 | undetermined (MW 220) | 1627 | - | 2070 | - | 6.7 | 0.10 | 7.2 | 0.14 | ||
| 35 | 3-iso-thujopsanone | 1632 | 1641 | [25] | 2106 | - | 0.2 | 0.13 | trace | - | |
| 36 | allo-aromadendrene epoxide | 1634 | 1639 | [25] | 2096 | 2095 | [42] | trace | - | trace | - |
| 37 | epi-α-muurolol | 1642 | 1640 | [25] | 2194 | 2186 | [27] | 0.1 | 0.01 | 0.1 | 0.01 |
| 38 | 3-thujopsanone | 1650 | 1653 | [25] | 2265 | - | 0.3 | - | 0.2 | 0.01 | |
| 39 | α-cadinol | 1653 | 1652 | [25] | 2244 | 2227 | [27] | 0.2 | 0.02 | trace | - |
| 40 | 14-hydroxy-9-epi-(E)-caryophyllene | 1658 | 1668 | [25] | 2110 | - | 0.1 | 0.04 | trace | - | |
| 41 | 7-epi-α-eudesmol | 1664 | 1662 | [25] | 2209 | 2205 | [43] | 0.2 | 0.01 | trace | - |
| 42 | bulnesol | 1667 | 1670 | [25] | 2204 | 2200 | [44] | 0.2 | 0.01 | trace | - |
| 43 | 8-cedren-13-ol | 1685 | 1688 | [25] | 2335 | 2359 | [45] | 0.4 | 0.03 | trace | - |
| 44 | cyperotundone | 1690 | 1695 | [25] | 2474 | - | 2.5 | 0.04 | 2.5 | 0.28 | |
| 45 | zizanal | 1701 | 1697 | [25] | 2450 | - | 0.6 | 0.01 | 1.4 | 0.07 | |
| 46 | cis-thujopsenal | 1705 | 1708 | [25] | 2294 | - | trace | - | trace | - | |
| 47 | 14-hydroxy-α-humulene | 1713 | 1713 | [25] | - | - | 0.1 | - | trace | - | |
| 48 | vetiselinenol | 1718 | 1730 | [25] | 2445 | - | 0.2 | - | trace | - | |
| 49 | γ-costol | 1742 | 1745 | [25] | 2337 | - | 0.3 | 0.01 | trace | - | |
| 50 | xanthorrhizol | 1749 | 1751 | [25] | 2657 | 2674 | [42] | 0.6 | 0.02 | 1.1 | 0.27 |
| 51 | cedryl acetate | 1776 | 1767 | [25] | 2132 | 2150 | [46] | 0.2 | 0.02 | 0.3 | 0.01 |
| 52 | 8-cedren-13-ol acetate | 1782 | 1788 | [25] | 2248 | - | 0.3 | 0.09 | trace | - | |
| 53 | undetermined (MW 262) | 1789 | - | 2272 | - | 4.7 | 0.18 | 3.3 | 0.07 | ||
| 54 | 8-α-acetoxyelemol | 1793 | 1792 | [25] | - | - | trace | - | trace | - | |
| 55 | undetermined (MW 280) | 2029 | - | - | - | 1.3 | 0.30 | trace | - | ||
| 56 | n-tricosane | 2301 | 2300 | [25] | 2300 | 2300 | [47] | 0.2 | 0.07 | trace | - |
| monoterpene hydrocarbons | - | - | |||||||||
| oxygenated monoterpenes | - | - | |||||||||
| sesquiterpene hydrocarbons | 75.8% | 80.8% | |||||||||
| oxygenated sesquiterpenes | 22.3% | 18.8% | |||||||||
| others | 0.2% | trace | |||||||||
| total | 98.3% | 99.6% | |||||||||
| Sample | Enzymatic Inhibition (%) | σ |
|---|---|---|
| Galanthamine 1.0 µg/mL | 49.2 | 5.2 |
| Laurus nobilis EO 38 µg/mL | 38.8 | 4.2 |
| Jungia rugosa EO 38 µg/mL | 25.9 | 13.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvopiña, K.; Malagón, O.; Capetti, F.; Sgorbini, B.; Verdugo, V.; Gilardoni, G. A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants 2021, 10, 2102. https://doi.org/10.3390/plants10102102
Calvopiña K, Malagón O, Capetti F, Sgorbini B, Verdugo V, Gilardoni G. A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants. 2021; 10(10):2102. https://doi.org/10.3390/plants10102102
Chicago/Turabian StyleCalvopiña, Karyna, Omar Malagón, Francesca Capetti, Barbara Sgorbini, Verónica Verdugo, and Gianluca Gilardoni. 2021. "A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity" Plants 10, no. 10: 2102. https://doi.org/10.3390/plants10102102
APA StyleCalvopiña, K., Malagón, O., Capetti, F., Sgorbini, B., Verdugo, V., & Gilardoni, G. (2021). A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants, 10(10), 2102. https://doi.org/10.3390/plants10102102









