1. Introduction
Salvia is a member of the Lamiaceae, a family represented by 45 genera. Its fragrant plants are used in folk medicines around the world, and many of its species are important in the pharmacology, perfume, cosmetics, and food industries because they contain volatile and aromatic oils [
1,
2].
Salvia is native to Middle Eastern and Mediterranean regions and contains more than 900 species spread throughout the tropical and temperate regions of Europe and Asia [
3]. Several reports indicate that leaf essential oil extracts of
S. officinalis L. (SO) have specific spasmolytic, astringent, antimicrobial, antihidrotic, antioxidant, and hepatoprotective effects, in addition to sensory, antigenotoxic, and chemopreventive activity [
4,
5,
6]. These plants areherbaceous perennial ornamentals. They are encountered in many areas and regions in Taiwan where daily temperatures exceed 33 °C in summer from June to September, resulting in heat stress, inhibited plant growth, and poor quality and quantity. Currently, no method, apart from field observations, has been developed for phenotyping high-temperature susceptibility in garden sage plants; thus, the ability to display fresh-market sage production is of great importance commercially. There is considerable pressure on the floriculture industry to produce ornamental plants more efficiently, and as the landscaping demand for sage plants is increasing, any method to improve heat tolerance in the hot summer season is important to Taiwan nurserymen. Moreover, understanding sustainable heat tolerance informs assessments of performance and the adaptation of sage plants to certain growing areas, breeding programs, and scheduling of culture techniques and management. The responses of
Salvia species to water stresses [
7,
8], salt stresses [
9,
10], and ozone-induced oxidative stresses [
11] are reported, but there is limited information available regarding the morphology and physiology of sage plants grown under high-temperature stress.
Plants have been developed with different adaptive mechanisms to cope with heat stress by inducing physiological responses; hence, understanding the physiological mechanisms of resistant
Salvia species in response to heat stress is important. Heat stress reduces the ability of photosynthesis to utilize incident photons leading to photoinhibition, a concomitant reduction in the quantum yield of photochemistry, and a decrease in chlorophyll fluorescence (ChlF). ChlF measurements, such as the maximal quantum yield of PSII photochemistry (Fv/Fm), is a noninvasive technique that has been widely used in a range of photosynthetic organisms and tissues to study functional changes in the photosynthetic apparatus under different heat stress conditions in controlled environments and in the field [
12]. Reflectance spectra are altered when stress occurs, and these alterations can be used to calculate different vegetation indices such as the adjusted normalized difference vegetation index (NDVI), which has been linked to photosynthetic light-use efficiency [
13]. The soil-plant analysis development (SPAD) meter assesses total Chl contents and photosynthetic capacity and is widely used for the rapid, accurate, and nondestructive measurement of Chl concentrations in leaves. The effective management of these parameters in response to chemical treatments provides a better understanding of the photosynthetic characteristics of sage plants grown in high temperatures.
Heat’s unfavorable effects can be alleviated by thermo-tolerance induced by the exogenous application of plant growth regulators and osmoprotectants, or by the gradual application of temperature stress. Both salicylic acid (SA) and calcium chloride (CaCl
2) are recognized as signaling molecules for their roles in plant adaptation to changing environments, influencing various stress responses and regulating the physiological and biochemical mechanisms of plants adapted to adverse environmental conditions [
14,
15]. Applying SA to
S. nemorosa under drought stress alleviates oxidative stress by maintaining cell membrane stability and enhancing photosynthetic capacity and antioxidant defense mechanisms [
16]. In addition, several studies show the remedial effect of SA on the harmful effects of salinity on sage [
17], strawberry [
18], and corn [
19], and attribute the positive effects of SA under saline stress in improving growth by stimulating photosynthetic capacity and protecting performance parameters. Palta [
20] found a decrease in Ca
2+ concentration in potato leaves exposed to heat stress, but the Ca
2+ concentration during heat stress in the leaves of plants pre-applied with CaCl
2 was maintained at the same level as before the heat stress, suggesting that holding a certain Ca
2+ level in guard cells to maintain normal stomatal function allows plants to avoid heat stress effects by dissipating heat through transpiration. The combined applications of SA and CaCl
2 improve growth and mineral nutrition in tomatoes during salt stress [
21]. Studies of SA and CaCl
2, individually or combined, in wheat under salt stress reveal that combined applications proved more effective in reducing oxidative stress [
22]. Furthermore, the use of SA and CaCl
2 protected
Rhododendron from injuries induced by heat stress [
23]. Lateral bud sprouting and new leaves in poinsettia (
Euphorbia pulcherrima Willd.) cultivars were increased by SA and CaCl
2 applications in response to heat stress as a result of enhanced catalase activity and reduced malondialdehyde (MDA) levels [
24]. However, only a limited number of studies have investigated the heat resistance of sage plants, and the influence of SA and CaCl
2 pretreatment on sage heat tolerance has never been reported.
There are several physiological stress markers that can be measured in plants to provide insight as to how cell membranes behave under heat-stress conditions, and the percent relative injury (RI%) is one of the most commonly used parameters. The ability to quantify high-temperature stress and rapidly screen sage cultivars for high-temperature susceptibility is dependent on the temperature and duration of treatment. Previously, we analyzed and maximized the differences in RI% among five commercial sage cultivars for the differences in high-temperature tolerances among cultivars and optimizing the stress duration. We compared potted plants subjected to stress from 10 to 60 min intervals at 30, 35, 40, 45, 50, and 55 °C to maximize the differences in RI% among cultivars and achieve maximum separations based on cultivars having significantly different performances. In general, all differences detected between cultivars corresponded to appearance, with S. elegans Vahl (SE) having a superior performance over SO plants under 55 °C for 30 min, which was sufficient to detect differences among cultivars with known differences in summer landscape performances. None of the SE plants showed any visible signs of stress immediately after heat chamber treatment.
The hypothesis of this experiment was that the effects of heat stress on SE and SO genotypes could be lessened by pretreatment with SA and CaCl2 under growth chamber conditions because it might protect cell membranes from the adverse effects of heat-shock stress. Thus, we tried to determine how SA and CaCl2 affected the physiology and morphology of SE and SO species under an induced heat-shock stress, as their tolerance responses were directly linked to the coordinated responses of physiological parameters and resulted from the effectiveness of CaCl2 and SA in alleviating the inhibitory effects of heat-shock stress.
3. Discussion
Sage plants are economically important ornamental flowers and are traded worldwide as potted plants. High-temperature stress is a major risk to fresh-market
Salvia production in Taiwan, and heat intolerance is a major constraint in sage cultivation. Heat stress affects the phenotype of a plant, causing leaf etiolation and wilting; it also alters the anatomy, physiology, and photosynthetic capability of plants [
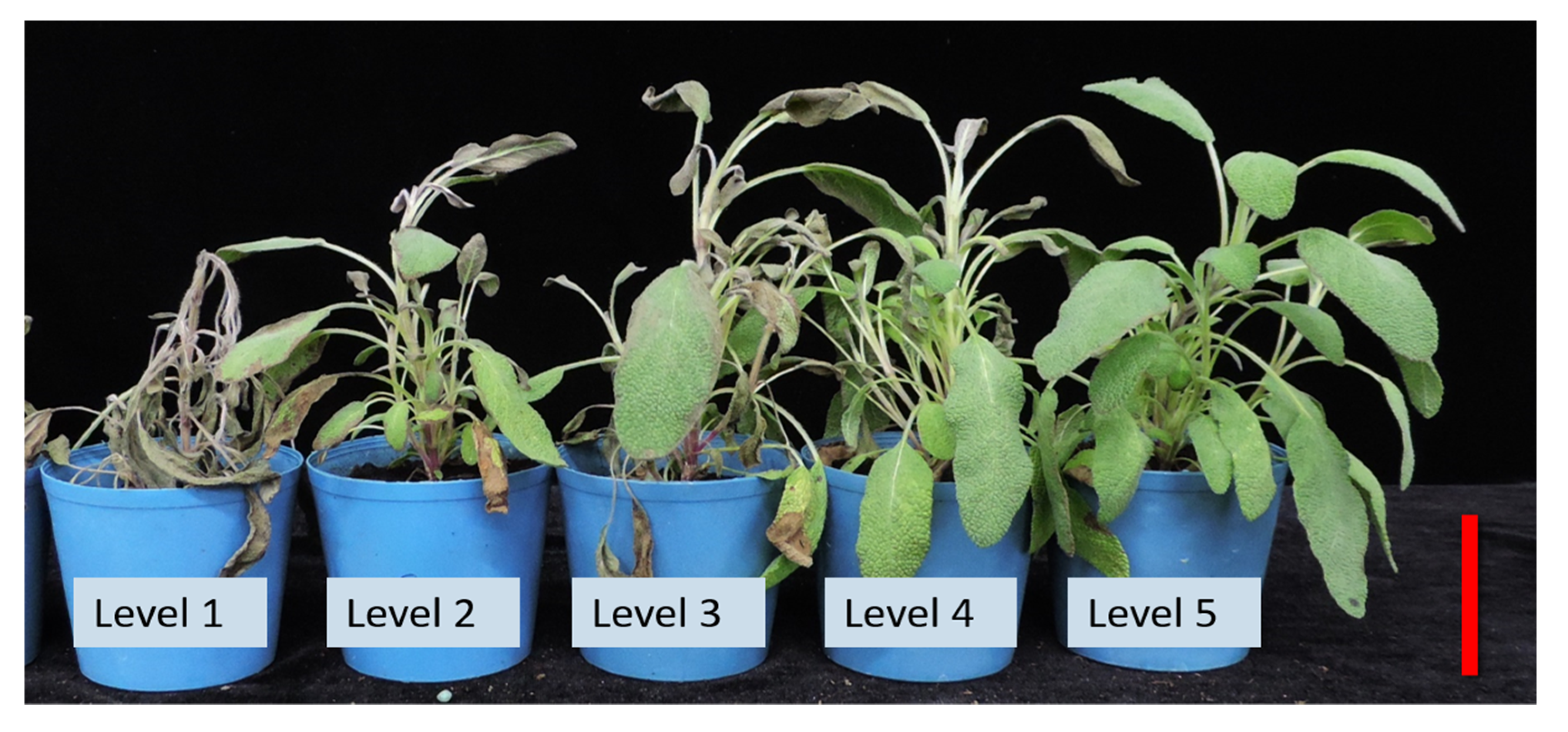
25].Visual appearance is the most important criterion to be considered for ornamental plants, and the ability of sage plants to maintain visual quality can make this ornamental plant a potential candidate for re-vegetating public and private lands. Furthermore, in the floriculture industry, the need for heat-tolerant plant cultivars is increasing because of rising global temperatures. In trying to understand the responses to high temperature, leaf-monitoring photosynthetic parameters were identified and characterized after heat stress, and the effects of high temperature on the appearance and physiological characteristics of two sage cultivars were examined in this study. The experiment was conducted to test the efficacy of chemical applications for improving appearance by enhancing the tolerance capacity of plants against high-temperature stress, reduce the impact of heat stress during vegetative growth stages, and develop a strategy to improve the heat tolerance of sage plants. Comparing the appearance ratings of all tested plants, the SE genotype was more tolerant (range 3.9~5.0) relative to the SE genotype (range 2.6~4.8) in all chemical treatments and controls (
Table 3), displaying normal growth and development (except in the 800μM SA treatment) under heat stress. SE plants tended to be unaffected and exhibited adaptive morphologic plasticity. In contrast, the leaves of untreated SO plants turned brown and withered, in particular, their lower leaves looked epinastic, chlorotic, and senescent, plants defoliated with different levels of injury and damage, and damage was irreversible. However, after chemical treatments, evaluations 3 days post heat stress also revealed no visible signs of high-temperature damage, e.g., shoot burn or leaf necrosis (
Figure 1). Thus, SO plants treated with exogenous SA and CaCl
2, alone or in combination were markedly protected from heat-induced growth inhibition. SO plant control groups had the lowest scores, compared with treatments, and a few of the SO control plants after 3d of high-temperature stress clearly showed heat stress phenotypes with differing levels of severity, including plant death, indicating the highest degree of injury among all treatments. Compared with single chemical treatment and controls, all combined chemical treatments induced more new leaves in the SO cultivar (photos not shown), suggesting that CaCl
2 and SA acted as primary signaling molecules for regulating new leaves in response to heat stress. Further work needs to be conducted to confirm whether SA and CaCl
2 have potential use for increasing the dry weight production of these sage plants.
As time passes following heat stress, the SPAD and NVDI values of all control plants displayed remarkable decreases, compared with plants treated with 100~400 μM SA (
Table 1). In addition, the lowest and highest RI% values for all plants were detected in the 100 μM SA treatment and controls (
Table 2). SO plant SPAD, NVDI, Fv/Fm, and RI values tended to be more sensitive to 100μMand 200 μM SA treatments than the 800 μM SA treatment. Although our study did not reveal a specific dosage for elevating SA in sage plants, it would appear that 100μMand 200 μM SA may be suitable. In addition, visual deterioration in SO plants increased as SA concentration increased (
Table 3), and there were visually significant reductions in plant growth. Yadegari [
26] showed that at low concentrations, while SA had a stimulating effect at higher concentrations, it reduced essential oil content in
S. officinalis. Furthermore, Es-sbihi et al. [
27] also reported that a low (0.5 mM) SA application to
S. officinalis reduced plant Na
+ content, improved growth, and increased nutrient (calcium, potassium, and phosphorus) levels, chlorophyll, essential oil content, and peltate gland density. SO plants are clearly highly tuned to the absolute levels of SA because a small amount of change can result in drastically different responses. Manipulating SA homeostasis by altering the concentration of SA could be an important strategy for altering the behavior and survival of SO plants under heat stress. As a consequence, SA may play an important role in the photosynthetic system under heat stress, but too high a concentration may destroy the photosynthetic ability to remediate leaf damage. In addition to SA application, significantly higher SPAD but lower RI values of SE plants were detected in the 5 mM CaCl
2 treatment, compared with controls after heat stress, and significantly higher NDVI and Fv/Fm but lower RI values for SO plants after heat stress were also observed in the 5 mM CaCl
2 treatment, compared with controls (
Table 4 and
Table 5). Goswami et al. [
28] reported that a foliar spray of Ca
2+ (10 mM) prior to heat stress (42 °C) at the grain-filling stage proved beneficial for the growth of wheat. Ca
2+ ions protect cell membranes from heat injury, possibly by directly binding to cell membranes to reduce their fluidity under high temperatures. Therefore, these physiological parameters are suitable for evaluating the phenotypes of these plants under high-temperature stress and can help in the advanced interpretation of the photochemical process in sage plants. In particular, the SPAD, NVDI, Fv/Fm, and RI values of SO plants treated with 100 μM SA or 5 mM CaCl
2 indicated increases in Chl content and photosynthetic capacity and reduced cell membranes to result in photoinhibitory effects, compared with controls. It is possible that Ca
2+ and SA could be important modulators of the cellular signaling of transduction events following heat stress injury, and the development of short-term heat stress in leaves is more gradual or perhaps delayed by SA and CaCl
2 treatments. However, these data still reflect the physiological attributes that contribute to our perception of plant ecophysiology and subsequent growth in outdoor planting sites.
The role of genetic variation is apparent from the observations of variation in the response of different species growing side-by-side in the same treatment and time duration. Plants adapt their photosynthesis to a certain degree in response to prevailing temperatures, and the sensitivity of photosynthesis to high temperatures varies between sage cultivars. When different chemical treatments across varieties were compared, SE plants exhibited higher NDVI values than SO plants under identical treatment at same time durations (
Table 1,
Table 4 and
Table 6), implying that their genotypes exhibited different abilities and specificities in photosynthetic light-use efficiency. Thus, the NDVI of SE had better temperature tolerance than SO. The Fv/Fm reduction indicates that an important portion of the photosynthesis system (PS)II reaction center was damaged, as the Fv/Fm value in healthy, uninhibited leaves is typically 0.8.This value may be strongly depressed after exposure to heat stress, which precipitates the suppression of the electron transfer chain [
29]. The highest Fv/Fm value (0.71) of SO plants with 100 μM SA treatment 3d after heat stress was close to 0.8 (
Table 1), indicating that there was no long-term photoinhibition, and 30 °C was still suitable for the growth of these plants. However, lower Fv/Fm values were observed in untreated control plants, compared with chemically treated plants (
Table 4 and
Table 6). The photoinhibition of photosynthesis is characterized by a reduction in the quantum yield of photochemistry and reduced ChlF, which entails both the inhibition of PSII and increased thermal de-excitation of excited Chl [
30]. Compared with controls, the pre-application of 100 μM SA + 5 mM CaCl
2, followed by high temperature seemed to provide better adaptation to heat stress, and the induced heat stress tolerance may be directly linked to the coordinated response of Fv/Fm and the direct implication in the regulation of Chl content, which could help create better future agricultural methods in relation to current global warming predictions. In addition, lower RI% and better appearance led to greater heat alleviation in SO plants subjected to chemical treatments (except for 800 μM SA treatment), compared with untreated control plants (
Table 2,
Table 3,
Table 5 and
Table 7). Thermal damage to cell membranes was characterized by a marked increase in the RI%. High temperatures weaken cell membranes, which leads to the leakage of electrolytes from the cell.
Different photosynthetic parameters acted differently under heat stress and to SA and CaCl2 treatments; however, each index or chemical is not necessarily equally significant in protecting against heat stress. The impacts of changing these parameters on the sage species were affected by SA and CaCl2 applications, and different species displayed variations in their photosynthetic systems. The different expressions of each species were associated with the heat stress response, and these controlled environmental conditions may also be more practical for sage growers. Our results suggest that photosynthetic parameters were heat-stress specific and not expressed solely in response to an increasing excess of photon energy, and they are suitable for evaluating the morphology and physiology of specific genotypes subjected to specific chemical treatments. Therefore, combining RI, SPAD, NVDI, and Fv/Fm values resulting from chemical treatment after a high-temperature treatment in a growth chamber can be used to select against the most susceptible plants. In other words, these identified systems can be a more efficient use of land for evaluating new material in the field and used for rapid monitoring and early detection of heat injury in vegetative stages. This means that hundreds of individual chemical-treated plants grown under heat stress can be cost-effectively screened daily, providing ample opportunity to discover individuals that manifest better spectral reflectance and physiological indicators.
Optimal chemical applications improve leaf appearance and stabilize the quality of sage plants. It is essential to determine the precise treatments to maximize sage plant appearance and physiological reactions when applying a particular combination of chemicals. SA is involved in Ca
2+-mediated signal transduction pathways in heat tolerance. Several studies established that Ca
2+ cooperates with SA by enhancing various types of resistance in plants. Chen and Kuc [
31] showed that Ca
2+ was able to regulate both intracellular and extracellular transportation of SA. Chen et al. [
32] reported that Ca
2+ controlled the movement of SA in and out of plant cells. Guo et al. [
33] demonstrated that SA treatment first led to Ca
2+ release from internal stores of
Salvia miltiorrhiza, and then a large amount of Ca
2+ effluxed from apoplasts in cell culture. Lan et al. [
34] illustrated that aluminum and SA increased cytosolic Ca
2+ concentrations in soybean roots and SA-mediated cellular Ca
2+ levels in soybean. Interestingly, the simultaneous addition of SA and Ca
2+ reduced NaCl-induced oxidative stress in wheat more effectively by preventing MDA accumulation [
22]. In our study, a strong synergistic interaction was observed between SA and CaCl
2 regarding RI, SPAD, NVDI, and Fv/Fm levels, where maximum and significantly increased SPAD, NVDI, and Fv/Fm levels and reduced RI% of SO plants were detected in 100 μΜ SA and 5 mM CaCl
2 combined treatments, compared with controls. Thus, the combined treatment has the ability to ameliorate heat stress and can be applied on a commercial scale to inform the development of rapid and precise management and integrated and quantitative measurements of bedded ornamental plants grown in plant factories or open fields to achieve maximum market benefit. Both SE and SO genotypes exhibita wide range of variability in appearance with desired chemical treatments and hence can be utilized directly or included in hybridization programs and prove useful in breeding programs for improving sage cultivars. For instance, subjecting SO plants to 100 μΜ SA and 5 mM CaCl
2 can result in a better morphological appearance that would be useful for ranking cultivars according to photosynthetic capacity. The knowledge acquired from this study can be directly translated into specific management practices for re-vegetation, landscaping, xeriscaping, and urban greening.