1. Introduction
White root rot disease (WRD) is one of the most detrimental diseases in rubber plantations in Indonesia, India, Malaysia, Sri Lanka, Thailand, West and Central Africa [
1]. This disease has been reported to cause about 3–15% of loss on production every year, particularly in small holder plantations [
2]. According to the survey conducted by Sail et al. [
3] in 2009, 43% of the small holder plantations in Malaysia were seriously infected with WRD. In Thailand, WRD is responsible for causing almost half of the yield losses in old rubber plantations, and killing rubber trees irrespective of age and health status [
4]. Plant varieties within a species can differ in their ability to defend themselves. Numerous management methods have been introduced including clone screening, whereby rubber clones with higher resistance against WRD were recommended and bred for large scale
Hevea plantation in Malaysia by the Rubber Research Institute of Malaysia (RRIM) [
5].
Efforts in developing varieties with higher resistance to WRD are still proceeded by many other countries, in the hope to find a solution to suppress this disease. Despite the efforts of introducing higher resistance clones,
Hevea plantations in the Southeast Asia are still facing significant economic loss due to the WRD, which reflects that the currently planted commercial clones are still susceptible to the WRD [
6]. In most cases, infection rates of the pathogen on the host plant are highly influenced by the environmental conditions and the capacity of the plant to defend itself. However, under different circumstances, such as different degrees of isolates virulence in combination with the adverse environmental conditions which favor the pathogen, the interaction between the same plant variety and pathogen species may cause different outcomes.
Rigidoporus microporus is a well-known fungal pathogen, causing white root disease (WRD) on more than 100 different tree species. The greatest loss caused by this pathogen was recorded in rubber tree (
Hevea brasiliensis) plantations [
7]. It was first identified in 1904 as a pathogen of rubber tree in a botanical garden of Singapore [
8]. Kaewchai et al. [
9] conducted a pathogenicity test of WRD for 32 isolates of
R. microporus obtained from two different provinces in Thailand. However, only 3 of the isolates obtained from Narathiwat Province were found to be highly virulent in causing WRD. Different isolates under the same pathogenic species may display different levels of virulence toward their host plant Lu et al. [
10], which complicates the whole management strategies of WRD. Hence, information regarding the strains of white root rot pathogen present in certain locations, their virulence level, and genetic variations are of extreme importance to facilitate the development of efficient disease management strategies.
In the early 1980s, RRIM600 was the most common rubber tree clone planted in Malaysia and was recommended for wide scale planting in other rubber growing countries due to its wide adaptability, even in sub-optimal areas [
11,
12]. The clone RRIM600 is introduced as a high-yield cultivar but is also reported to be highly susceptible to white root rot disease [
13]. Considering the significance of RRIM600 in the history of rubber tree breeding and its valuable phenotypic traits, the RRIM600 clone was used in this study. To date, information regarding the strains of white root rot pathogen present in Malaysia, their virulence level, and genetic variations are lacking. Therefore, isolates from symptomatic root tissues of rubber trees collected from different locations in Malaysia were characterized and their virulence against the rubber tree clone RRIM600 was determined in the nursery trial.
4. Discussion
Numerous reports have been published about the occurrence of white root rot disease on rubber trees from countries like Thailand, Sri Lanka, Indonesia, and Nigeria. However, only a few studies were conducted for the discovery and comparison of the white root rot pathogen isolates in Malaysia. Examining virulence profiles of R. microporus isolates collected around Malaysia in contrasting disease reactions will contribute to a better understanding of the interactions underlying tolerance and susceptibility of rubber clone RRIM600 to different R. microporus isolates causing WRD. Determination of the most virulent isolate in present study is vital and shall be taken into consideration for the selection of suitable pathogen isolate in the development of more effective control measures in combating tenacious R. microporus. Taken together, field evaluation through visual observation and laboratory assays has led to screening of the most virulent isolate. It has been many years since the last published paper on the virulence profiles of R. microporus isolates in Malaysia has been reported. This study represents the most current report on the characteristics and virulence degree of R. microporus isolates causing WRD on rubber trees in Malaysia, by assessing the disease severity through the observation of the above- and below-ground symptoms.
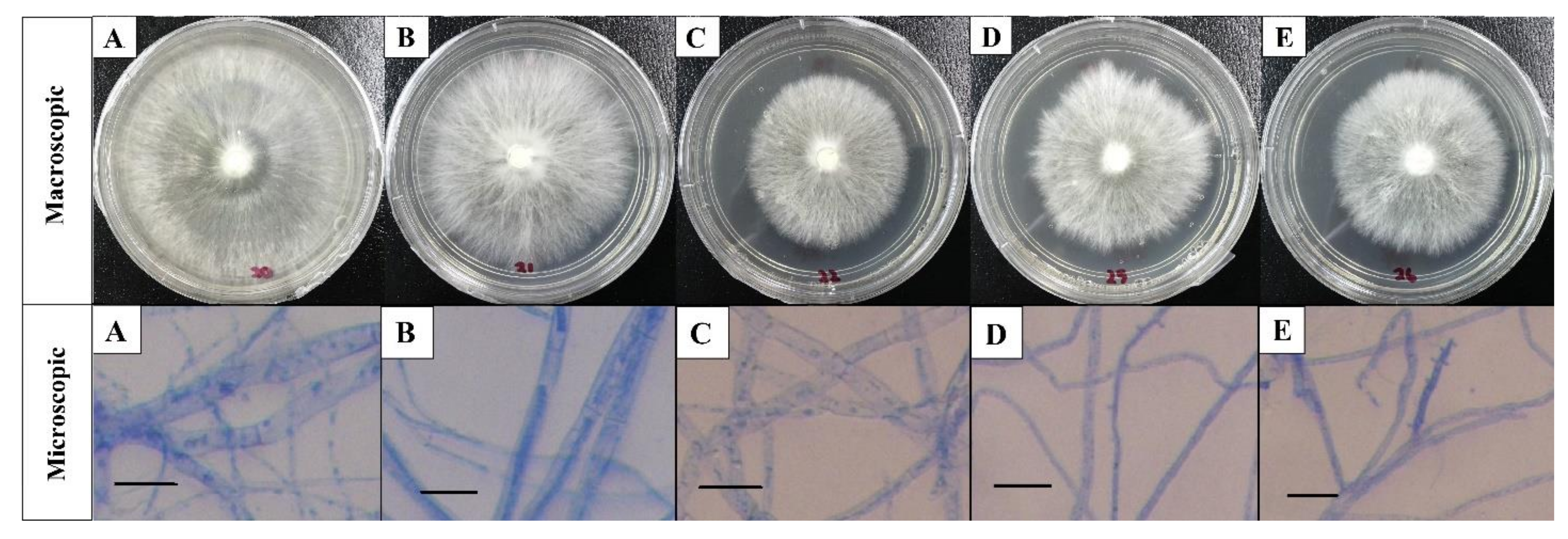
The macroscopic characteristics of the white color and relatively fluffy aerial mycelia, with the milky color on the reverse side of the culture plate in the present study, have led to the preliminary identification of the five white root rot pathogen isolates as
R. microporus which were also in agreement with previously reported description by Farhana et al. [
6], Hood [
19], Kaewchai and Soytong [
1], and Nandris et al. [
7]. Likewise, the microscopic results were similar to the findings by Kaewchai et al. [
9] and Kaewchai and Soytong [
1], who pointed out that the hypha of the fungal isolate of
R. microporus were hyaline, septate, and possessed many branches without the presence of clamp connection. In contrast, the above observation was differentiable from the work done by Farhana et al. [
6]. It was revealed that the
R. microporus isolate was collected from rubber tree clone RRIM2020, containing a hyphae with clamp connection under compound microscope. In fact, clamp connections are unique structures to the fungi under the phylum Basidiomycota where
R. microporus belongs to. Yet, the structure of clamp connection was not observed from any of the isolates in the present study. The function of clamp connections is to maintain the dikaryotic condition, and they were reported to form only in dikaryotic hyphae during their life cycle which was to ensure each of the cell is binucleate, but not all dikaryotic hyphae form them [
20]. The morphological studies of
R. microporus isolated from different rubber clones in Malaysia (RRIM2008, PB260, RRIM2024, PB350 RRIM600); the host from where the pathogen was isolated has provided information on the preliminary features of the white root rot pathogen.
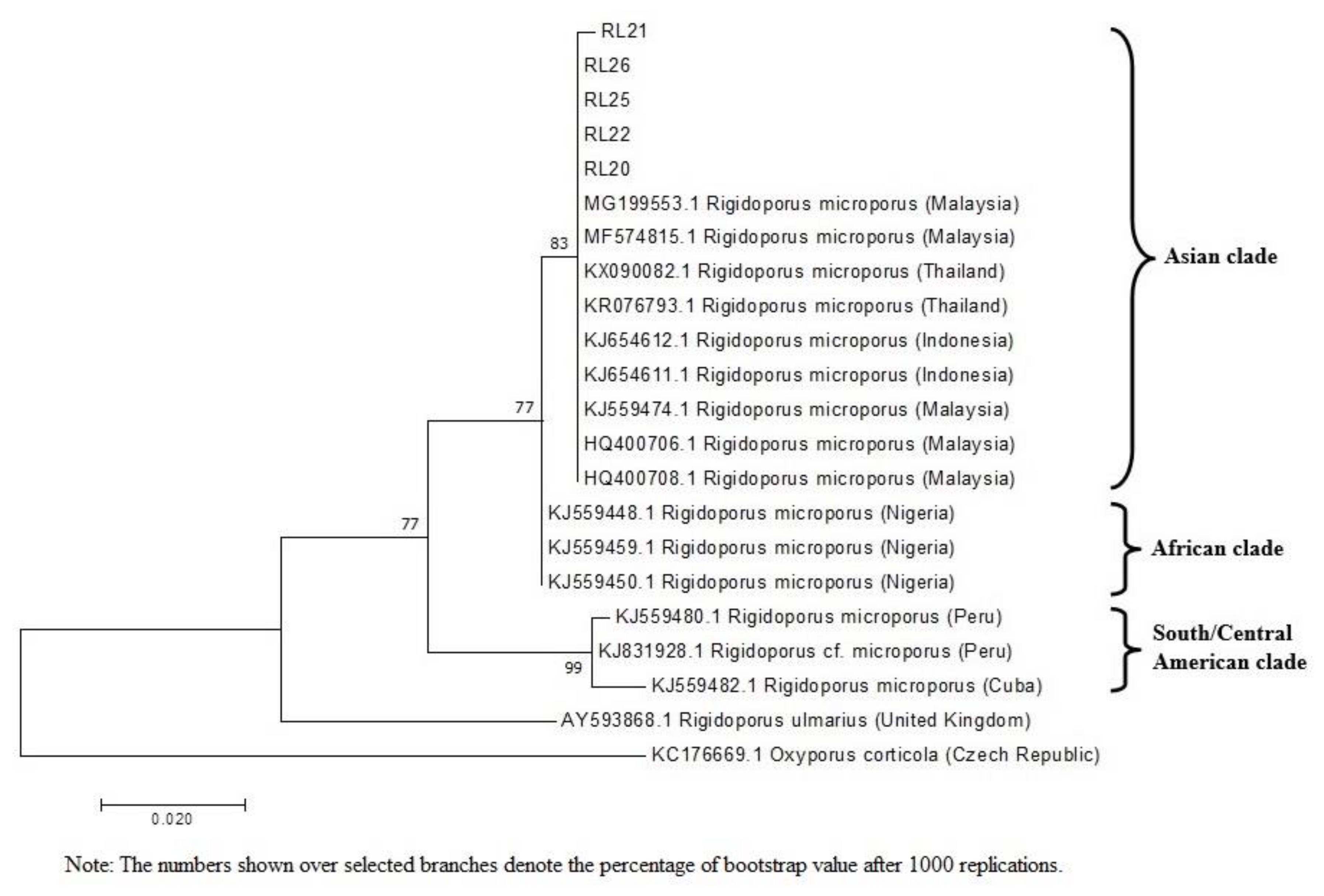
The results of phylogenetic relationship in the present study demonstrated that the five isolates obtained from different rubber plantations in Malaysia were closely related to the isolates from Asian clade with 83% bootstrap support for their genetic similarity. Isolates from Asian clade were more closely related to the isolate from African clade but further away from those in South and Central America. It was clearly shown that the African and Asian clades were well distinguished from the clade consisting of isolates from South and Central America. Oghenekaro and partners [
21] reported high levels of WRD occurrence in Asian and African rubber plantations where majority of the world’s rubber plantations are located such as Thailand, Indonesia, Sri Lanka, Nigeria, and Malaysia. On the contrary, this pathogen is less copious in native habitats of the rubber tree. According to Oghenekome [
22], WRD is not a serious problem in rubber plantations in the South Americas, for example, Brazil, which was the native habitat of the rubber tree. Plantations represent even-aged monocultures and are more susceptible to disease than native forests, where epidemics are restricted by the age structure and the diversity of the plant community [
23].
The RRIM600 cultivar in this study has clearly shown the symptoms of WRD infection just three months after inoculation, with foliar discoloration and root rotting incidences. These symptoms were similar with the previous works published by Kaewchai and Soytong [
1] and Farid et al. [
24], where the leaves turned yellow, and the infected roots turned into a darker color. The pathogenicity study revealed that RRIM600 rubber clone died in less than two weeks after the seedlings showed yellowing foliar symptom 3 months after inoculation. Omorusi [
25] reported that the foliage symptoms usually appear only when the host is untreatable due to the fast and increased rate of disease infection. The discoloration of the leaves was usually linked to the disruption of the root function in the host. In root disease infection, wilting and defoliation of leaves are considered symptoms of advanced disease development [
24].
It was interesting to note that those RRIM600 rubber seedlings infected with R. microporus isolate RL21, which is originally from Sarawak, had caused 83.33% of mortality on rubber seedlings in the sixth month after inoculation. As opposed to isolate RL21, the rest of the isolates have demonstrated a lower percentage of mortality rate on the rubber seedlings. These results explained that different isolates of the same species may cause different level of damage to the host, although the isolates are genetically closely related.
Despite being driven by the biotic factor (host and pathogen), Prasetyo et al. [
26] stated that the development of the disease is also depends on abiotic factors such as humidity, temperature, pH, soil porosity, and soil characteristics. The cultural characteristics among
R. microporus isolates proved to be quite variable in terms of their mycelial growth rates under different culture conditions. The effect of pH on the mycelial growth of
R. microporus isolates in this study was conducted up to pH 7.0, as soil in Malaysia is usually acidic in nature [
27]. The present in vitro study has revealed the significant effect of pH value on the growth rate of
R. microporus. The highest mycelial growths for
R. microporus isolates were recorded at pH 6.5 and the lowest mycelial growths were recorded at pH 3.5. Liyanage et al. [
28] found that low pH value inhibited the growth of
R. microporus. According to the findings by Rodesuchit et al. [
29], Semangun [
30], and Wahyuni et al. [
31], the growth of
R. microporus is favored by neutral pH (it can grow well in pH 6–7), whereas the fungal growth is slowed and suppressed at pH 4 and lower. Dede et al. [
32] also reported that WRD occurrence are higher in areas with higher pH values than in areas with lower pH values, even within a rubber plantation. In the field, estate farmers have been amending the soil with sulfur to inhibit the growth of
R. microporus with the means of reducing the use of pesticides or before the advent of fungicides. With very little detrimental impacts on the plant, the addition of sulfur can increase the acidity of the soil and is believed to help in slowing down the growth of
R. microporus [
33]. However, using pH to control the spread of
R. microporus may not be an effective method, as detrimental effects of radial growth rate reduction were only observed in pH 3.5. Decent mycelial growth of
R. microporus isolates were observed on media with pH higher than 3.5, especially isolate RL21 and RL22, which revealed their ability to tolerate wide range of pH.
Temperature is the most important environmental factor for regulating the growth and reproduction of fungi through its effect on conidial germination and appressorium development, and mycelial growth in this study. According to Oghenekaro et al. [
34],
R. microporus isolate (MS564b) collected from the sapwood of wild
Hevea brasiliensis showed the highest mean growth on MEA at 25 °C, while the rest of the isolates (MUCL45064, ED310, and M13) have the maximum hyphae growth at 30 °C. From this study, it was clear that the growth of
R. microporus isolates were harshly affected with temperature lower than 25 °C and above 30 °C. Mycelial growth for all isolates were inhibited when the temperature exceed 40 °C.
Liyanage et al. [
28] reported that all the eleven isolates of
R. microporus isolated in Sri Lanka grows best in continuous darkness except one isolate which grew well under both light and dark condition. In fact, some rubber planters are still implementing a long-adopted labor-intensive procedure to prevent WRD. The tap and part of lateral roots are expose to sunlight for few days prior to be painted with protective fungicides and covered back with fresh soil. This conventional prevention technique aims to create the environmental conditions that do not favor the disease development by combination of direct light exposure and high temperature which help to reduce the WRD occurrence [
25]. However, such treatment is only complementary and not a substitute for complete elimination of the source of infection. As we could see from this study, light or dark incubation condition has no effect on the radial growth rate of all the isolates in this study except RL22, which showed a slightly lower growth rate when exposed under continuous light incubation. This explains that light has a very low inhibitory effect on
R. microporus isolates found in this study.
Identifying the most virulent isolate will help to facilitate the long-needed studies on resistance research and host-related interactions on how the fungus is able to facilitate the decay of rubber tree. R. microporus isolate RL21 was found to have the highest growth adaptability to a wide range of culture conditions and this may cause difficulties to control the levels of pathogen inoculum in soil under real field conditions. The high disease severity index on rubber seedlings by isolate RL21 also depicted the potential of this isolate to be more harmful to rubber trees. Further information on this pathogen in Malaysia rubber plantations will be necessary for the successful management of WRD caused by R. microporus. A common strategy for controlling WRD is to reduce inoculum in the soil. Therefore, an appropriate technique for measuring the amount of R. microporus in the soil is essential for studying the effectiveness of different strategies for reducing soil inoculum levels.