Acceleration in Germination Sensu stricto Plays a Central Role on Seedling Vigor in Post-Germination
Abstract
:1. Introduction
2. Material and Methods
2.1. Biological Model
2.2. Water Dynamics in Germinating Seeds
Modelling the Water Dynamics in Germinating Seeds
2.3. Seed Germination Assays
2.4. Seedling Development Assays
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef] [Green Version]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [Green Version]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edje, O.T.; Burris, J.S. Effects of soybean seed vigor on field performance 1. Agron. J. 1971, 63, 536–538. [Google Scholar] [CrossRef]
- Caverzan, A.; Giacomin, R.; Müller, M.; Biazus, C.; Lângaro, N.C.; Chavarria, G. How does seed vigor affect soybean yield components? Agron. J. 2018, 110, 1318–1327. [Google Scholar] [CrossRef]
- Elias, S.G.; Copeland, L.O.; McDonald, M.B.; Baalbaki, R.Z. Seed Testing: Principles and Practices; Michigan State University Press: East Lansing, MI, USA, 2012. [Google Scholar]
- ISTA. International Rules for Seed Testing. 2019. Available online: https://www.seedtest.org/en/international-rules-_content---1--1083.html (accessed on 9 October 2021).
- Penfield, S.; King, J. Towards a systems biology approach to understanding seed dormancy and germination. Proc. R. Soc. B Biol. Sci. 2009, 276, 3561–3569. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-Oliveira, J.P.; Ranal, M.A. Sample size and water dynamics on germinating diaspores: The first step for physiological and molecular studies on the germination process. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2017, 152, 840–847. [Google Scholar] [CrossRef]
- Leopold, A.C. Volumetric components of seed imbibition. Plant Physiol. 1983, 73, 677–680. [Google Scholar] [CrossRef] [Green Version]
- Vertucci, C.W.; Leopold, A.C. Dynamics of imbibition by soybean embryos. Plant Physiol. 1983, 72, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Vertucci, C.W. The kinetics of seed imbibition: Controlling factors and relevance to seedling vigor. In Seed Moisture. CSSA Special Publication No. 14., 1st ed.; Stanwood, P.C., McDonald, M.B., Eds.; Crop Science Society of America Inc.: Madison, WI, USA, 1989; pp. 93–115. [Google Scholar]
- McDonald, M.B.; Vertucci, C.W.; Roos, E.E. Soybean seed imbibition: Water absorption by seed parts. Crop Sci. 1988, 28, 993–997. [Google Scholar] [CrossRef]
- Meyer, C.J.; Steudle, E.; Peterson, C.A. Patterns and kinetics of water uptake by soybean seeds. J. Exp. Bot. 2006, 58, 717–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manz, B.; Müller, K.; Kucera, B.; Volke, F.; Leubner-Metzger, G. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiol. 2005, 138, 1538–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, K.; Koizumi, M.; Ishida, N.; Kano, H. Water uptake by dry beans observed by micro-magnetic resonance imaging. Ann. Bot. 2006, 98, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtyla, Ł.; Garnczarska, M.; Zalewski, T.; Bednarski, W.; Ratajczak, L.; Jurga, S. A comparative study of water distribution, free radical production and activation of antioxidative metabolism in germinating pea seeds. J. Plant Physiol. 2006, 163, 1207–1220. [Google Scholar] [CrossRef]
- Munz, E.; Rolletschek, H.; Oeltze-Jafra, S.; Fuchs, J.; Gündel, A.; Neuberger, T.; Ortleb, S.; Jakob, P.M.; Borisjuk, L.; Oeltze-Jafra, S. A functional imaging study of germinating oilseed rape seed. New Phytol. 2017, 216, 1181–1190. [Google Scholar] [CrossRef] [Green Version]
- De Medeiros, A.D.; Pinheiro, D.T.; Xavier, W.A.; da Silva, L.J.; Dias, D.C.F.D.S. Quality classification of Jatropha curcas seeds using radiographic images and machine learning. Ind. Crops Prod. 2020, 146, 112162. [Google Scholar] [CrossRef]
- Ribeiro-Oliveira, J.P. Dinâmica da Água em Diásporos de Espécies de Interesse Agrícola (Water Dynamics on Diaspores of Agricultural Interest); Universidade Federal de Uberlândia: Uberlandia, Brazil, 2015. [Google Scholar]
- Ribeiro-Oliveira, J.P.; Ranal, M.A.; Boselli, M.A. Water dynamics on germinating diaspores: Physiological perspectives from biophysical measurements. Plant Phenom. 2020, 2020, 5196176. [Google Scholar] [CrossRef]
- USDA. Oilseeds: World Markets and Trade. Circ. Ser. FOP 06-17. Glob. Oilseed Consum. Contin. to Grow Despite Slowing Trade Prod; USDA: Washington, DC, USA, 2019.
- UNAV. Soybeans: The Other Strategic Commodity of South America. 2019. Available online: https://www.unav.edu/web/global-affairs/detalle/-/blogs/soybeans-the-other-strategic-commodity-of-south-america (accessed on 7 September 2021).
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2018: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change; ISAAA Brief No. 54; ISAAA: Ithaca, NY, USA, 2018. [Google Scholar]
- Ribeiro-Oliveira, J.P.; Ranal, M.A. Sample size in studies on the germination process. Botany 2016, 94, 103–115. [Google Scholar] [CrossRef]
- ISTA. ISTA Method Validation for Seed Testing; ISTA: Bassersdorf, Switzerland, 2007. [Google Scholar]
- ISO. ISO 5725-2. Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 2: Basic Method for the Determination of Repeatability and Reproducibility of a Standard Measurement Method, 1st ed.; ISO: Geneva, Switzerland, 1994. [Google Scholar]
- Hirsch, M.W.; Smale, S.; Devaney, R.L. Differential Equations, Dynamical Systems, and an Introduction to Chaos; Academic Press: Cambrigde, MA, USA, 2013. [Google Scholar]
- Buckland, S.T. Algorithm AS 214: Calculation of Monte Carlo confidence intervals. J. R. Stat. Soc. Ser. C Appl. Stat. 1985, 34, 296. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerial Recips in Fortran; Cambridge University Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Crank, J. The mathematics of diffusion. In The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Brazil. Regras para Análise de Sementes, 3rd ed.; MAPA/ACS, Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2009.
- Jackson, C.; Esnouf, M.P.; Winzor, D.J.; Duewer, D.L. Defining and measuring biological activity: Applying the principles of metrology. Accredit. Qual. Assur. 2007, 12, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Marcos Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef] [Green Version]
- TeKrony, D.M. Accelerated aging test: Principles and procedures. Seed Technol. 2005, 27, 135–146. [Google Scholar]
- Egli, D.B.; Tekrony, D.M. Relationship beween soybean seed vigor and yield 1. Agron. J. 1979, 71, 755–759. [Google Scholar] [CrossRef]
- Paula, A.D.M.d. Vigor Relativo: Uma Nova Abordagem para Classificação de Lotes de Sementes; Universidade Federal de Uberlândia: Uberlandia, Brazil, 2020. [Google Scholar]
- Santana, D.; Ranal, M. Linear correlation in experimental design models applied to seed germination. Seed Sci. Technol. 2006, 34, 233–239. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 207–219. [Google Scholar]
- Šidák, Z. Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc. 1967, 62, 626–633. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Nikolaeva, M.G. Factors controlling the seed dormancy pattern. In The Physiology and Biochemistry of Seed Dormancy and Germination, 1st ed.; Kahn, A.A., Ed.; North-Holland Publishing: Amsterdam, The Netherlands, 1977; pp. 51–74. [Google Scholar]
- Davis, J.A. Elementary Survey Analysis, 1st ed.; Prentice-Hall: Hoboken, NJ, USA, 1971. [Google Scholar]
- Vivas, P.; Resende, L.R.B., Jr.; Guimarães, R.; Azevedo, R.; Da Silva, E.; Toorop, P. Biospeckle activity in coffee seeds is associated non-destructively with seedling quality. Ann. Appl. Biol. 2017, 170, 141–149. [Google Scholar] [CrossRef]
- Bewley, J.D. Breaking down the walls—A role for endo-β-mannanase in release from seed dormancy? Trends Plant Sci. 1997, 2, 464–469. [Google Scholar] [CrossRef]
- Sliwinska, E.; Bassel, G.W.; Bewley, J.D. Germination of arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J. Exp. Bot. 2009, 60, 3587–3594. [Google Scholar] [CrossRef] [Green Version]
- Heading, R.C.; Nimmo, J.; Prescott, L.F.; Tothill, P. The dependence of paracetamol absorption on the rate of gastric emptying. Br. J. Pharmacol. 1973, 47, 415–421. [Google Scholar] [CrossRef]
- Urquhart, J. The odds of the three nons when an aptly prescribed medicine isn′t working: Non-compliance, non-absorption, non-response. Br. J. Clin. Pharmacol. 2002, 54, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Onsager, L. Reciprocal relations in irreversible processes. II. Phys. Rev. 1931, 38, 2265–2279. [Google Scholar] [CrossRef] [Green Version]
- Ranal, M.A.; De Santana, D.G. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, W.R.; Ranal, M.A.; de Santana, D.G.; Nogueira, A.P.O. Germination and emergence measurements could group individuals and species? Braz. J. Bot. 2015, 38, 457–468. [Google Scholar] [CrossRef]
- Kranner, I.; Minibayeva, F.; Beckett, R.; Seal, C. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef]
- Larcher, W. The environment of plants. In Physiological Plant Ecology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–67. [Google Scholar]
- Vanwallendael, A.; Soltani, A.; Emery, N.C.; Peixoto, M.M.; Olsen, J.; Lowry, D.B. A molecular view of plant local adaptation: Incorporating stress-response networks. Annu. Rev. Plant Biol. 2019, 70, 559–583. [Google Scholar] [CrossRef] [Green Version]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro-Oliveira, J.P.; Ranal, M.A.; De Santana, D.G.; Pereira, L.A. Sufficient sample size to study seed germination. Aust. J. Bot. 2016, 64, 295–301. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds; Springer: New York, NY, USA, 2013. [Google Scholar]
- Selye, H. A syndrome produced by diverse nocuous agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2016, 68, 765–783. [Google Scholar] [CrossRef]
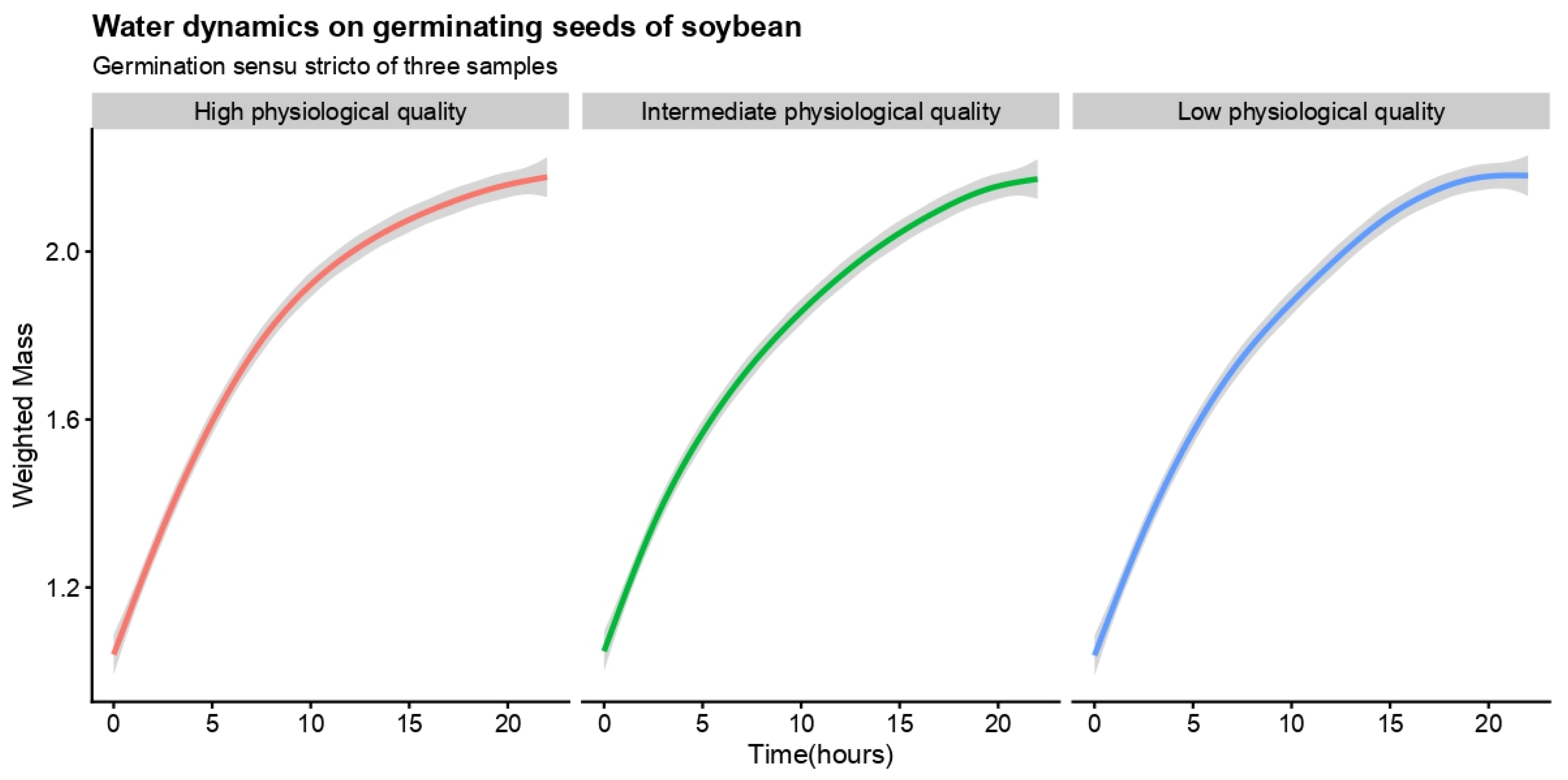
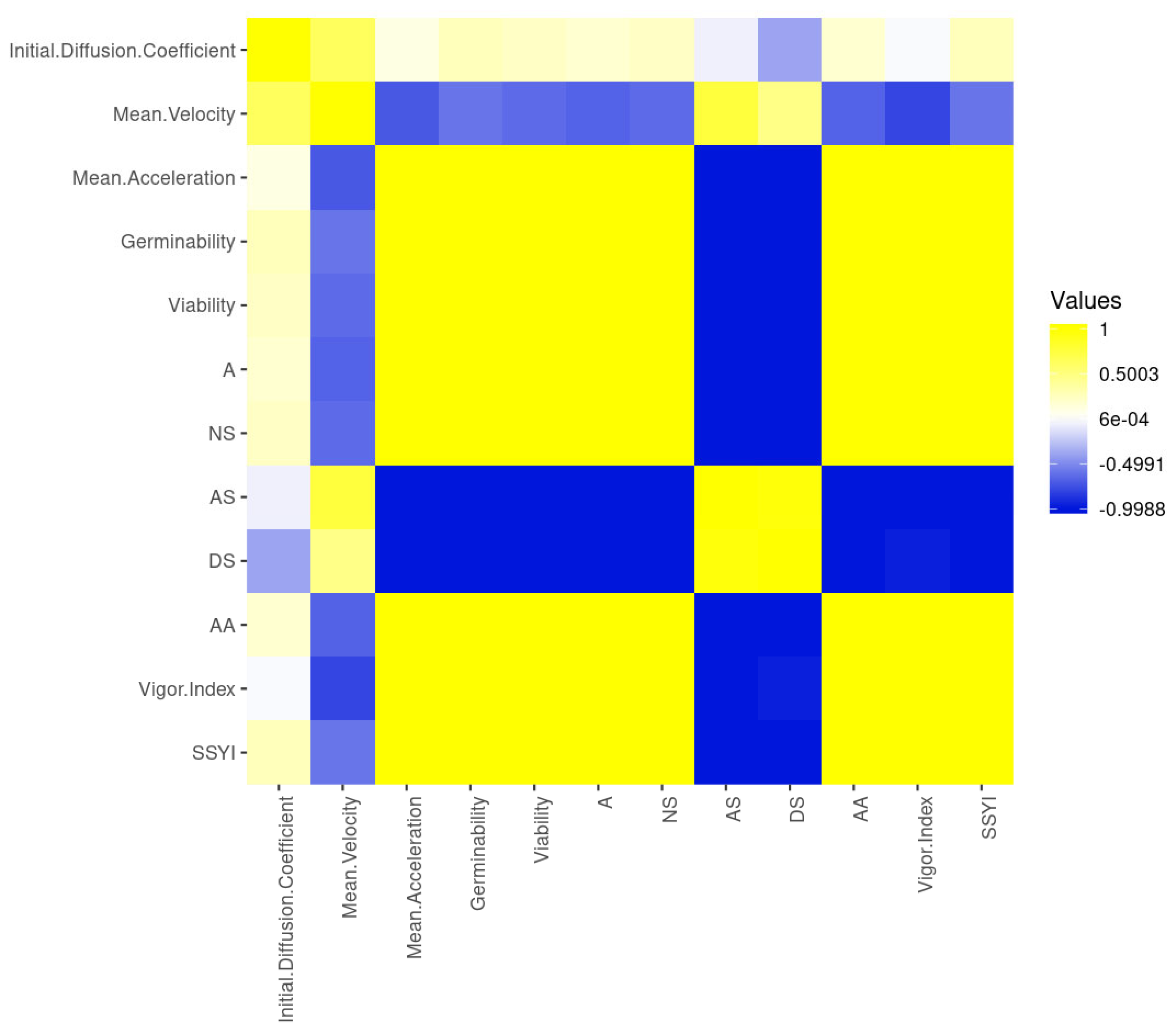
| Seed-Seedling Transition Step | Seed Sample | ||||||
|---|---|---|---|---|---|---|---|
| Germination sensu stricto | Low physiological quality | 0.0014 | 0.055 (0.0279; 0.0822) | −0.0184 (−0.0391; 0.0298) | |||
| Intermediate physiological quality | 0.0018 | 0.058 (0.0278; 0.0882) | −0.0182 (−0.0468; 0.0105) | ||||
| High physiological quality | 0.0016 | 0.055 (0.0355; 0.0074) | −0.0164 (−0.0310; −0.0019) | ||||
| Embryo protrusion | G (%) | V (%) | A (%) | ||||
| Low physiological quality | 28.00 ± 3.8 c | 48.00 ± 4.2 c | 26.36 ± 0.36 c | ||||
| Intermediate physiological quality | 54.00 ± 4.2 b | 62.00 ± 4.1 b | 65.66 ± 0.39 b | ||||
| High physiological quality | 98.00 ± 1.2 a | 98.00 ± 1.2 a | 99.94 ± 0.02 a | ||||
| Post-germination (seedling development) | NS (%) | AS (%) | DS (%) | AA (%) | SVI (%) | SSYI (%) | |
| Low physiological quality | 24.00 ± 3.4 c | 25.00 ± 0.008 b | 51.00 ± 0.008 c | 16.00 ± 3.7 c | 80.00 ± 3.6 b | 71.43 ± 3.81 c | |
| Intermediate physiological quality | 48.00 ± 4.0 b | 27.00 ± 3.61 b | 23.00 ± 3.66 b | 34.00 ± 4.0 b | 77.27 ± 3.4 b | 81.48 ± 3.29 b | |
| High physiological quality | 98.00 ± 1.0 a | 1.00 ± 3.7 a | 1.00 ± 4.21 a | 94.00 ± 2.0 a | 95.92 ± 1.7 a | 100.00 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro-Oliveira, J.P.; Bosseli, M.A.; da Silva, E.A.A. Acceleration in Germination Sensu stricto Plays a Central Role on Seedling Vigor in Post-Germination. Plants 2021, 10, 2151. https://doi.org/10.3390/plants10102151
Ribeiro-Oliveira JP, Bosseli MA, da Silva EAA. Acceleration in Germination Sensu stricto Plays a Central Role on Seedling Vigor in Post-Germination. Plants. 2021; 10(10):2151. https://doi.org/10.3390/plants10102151
Chicago/Turabian StyleRibeiro-Oliveira, João Paulo, Marco Aurélio Bosseli, and Edvaldo Aparecido Amaral da Silva. 2021. "Acceleration in Germination Sensu stricto Plays a Central Role on Seedling Vigor in Post-Germination" Plants 10, no. 10: 2151. https://doi.org/10.3390/plants10102151
APA StyleRibeiro-Oliveira, J. P., Bosseli, M. A., & da Silva, E. A. A. (2021). Acceleration in Germination Sensu stricto Plays a Central Role on Seedling Vigor in Post-Germination. Plants, 10(10), 2151. https://doi.org/10.3390/plants10102151







