Wheat Leaf Antioxidative Status—Variety-Specific Mechanisms of Zinc Tolerance during Biofortification
Abstract
:1. Introduction
2. Results
2.1. Zn Concentrations in Flag Leaves
2.2. Effects of Zn on the Products of Lipid Peroxidation
2.3. Effects of Zn on Total Glutathione Content and Related Enzymes
2.4. Effects of Zn on Antioxidant Enzyme Activities
2.5. Effects of Zn on Soluble Phenolic Content
3. Discussion
4. Materials and Methods
4.1. Soils and Treatments
- Control without Zn application;
- Zn-sulfate: application of 1.5 kg ha−1 Zn in the form of ZnSO4 × 7 H2O (6.6 kg ha−1);
- Zn-EDTA: application of 1.5 kg ha−1 of Zn in the form of Zn-EDTA (10 kg ha−1).
4.2. Analysis of the Zn Content
4.3. Determination of the Products of Lipid Peroxidation
4.4. Measurement of Total Glutathione Content
4.5. Extraction and Assays of Enzymes
4.6. Extraction and Determination of Total Soluble Phenols
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Zhang, W.J.; Li, W.; Lin, Z.P. The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 2009, 75, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Qian, J.; Hou, J.; Zhang, W.J.; Lu, J. Excess Zn alters the nutrient uptake and induces the antioxidative responses in submerged plant Hydrilla verticillata (L.f.) Royle. Chemosphere 2009, 76, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, V.; Lingua, G.; D’Agostino, G.; Carniato, F.; Roccotiello, E.; Berta, G. Effects of high zinc concentration on poplar leaves: A morphological and biochemical study. Environ. Exp. Bot. 2011, 71, 50–56. [Google Scholar] [CrossRef]
- Jain, R.; Srivastava, S.; Solomon, S.; Shrivastava, A.; Chandra, A. Impact of excess zinc on growth parameters, cell division, nutrient accumulation, photosynthetic pigments and oxidative stress of sugarcane (Saccharum spp.). Acta Physiol. Plant 2010, 32, 979–986. [Google Scholar] [CrossRef]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Biotechnol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Reichman, S. The Responses of Plants to Metal Toxicity: A Review Forusing on Copper, Manganese & Zinc; Australian Minerals & Energy Environment Foundation: Melbourne, Australia, 2002. [Google Scholar]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer International Publishing: Cham, Germany, 2014; Volume 232, pp. 1–44. [Google Scholar]
- Pinto, E.; Sigaud-kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal–induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Prasad, K.V.S.K.; Paradha Saradhi, P.; Sharmila, P. Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ. Exp. Bot. 1999, 42, 1–10. [Google Scholar] [CrossRef]
- Štolfa, I.; Pfeiffer, T.Ž.; Špoljarić, D.; Teklić, T.; Lončarić, Z. Heavy metal-induced oxidative stress in plants: Response of the antioxidative system. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Germany, 2015; pp. 127–163. [Google Scholar]
- Barrameda-Medina, Y.; Montesinos-Pereira, D.; Romero, L.; Blasco, B.; Ruiz, J.M. Role of GSH homeostasis under Zn toxicity in plants with different Zn tolerance. Plant Sci. 2014, 227, 110–121. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of fertilizers with zinc: An excellent investment for humanity and crop production in India. J. Trace Elem. Med. Biol. 2009, 23, 281–289. [Google Scholar] [CrossRef]
- Nakandalage, N.; Nicolas, M.; Norton, R.M.; Hirotsu, N.; Milham, P.J.; Seneweera, S. Improving rice zinc biofortification success rates through genetic and crop management approaches in a changing environment. Front. Plant Sci. 2016, 7, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Sun, D.; Wang, C.; Ding, H.; Qin, H.; Hou, J.; Huang, X.; Xie, Y.; Guo, T. Physiological responses and yield of wheat plants in zinc-mediated alleviation of drought stress. Front. Plant Sci. 2017, 8, 860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.P.B.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ekiz, H.; Torun, B.; Gultekin, I.; Karanlik, S.; Bagci, S.A.; Cakmak, I. Effect of different zinc application methods on grain yield and zinc concentration in wheat cultivars grown on zinc-deficient calcareous soils. J. Plant Nutr. 1997, 20, 461–471. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. N. Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Zain, M.; Khan, I.; Khan Qadri, R.W.; Ashraf, U.; Hussain, S.; Minhas, S.; Siddiquei, A.; Jahangir, M.M.; Bashir, M. Foliar application of micronutrients enhances wheat growth, yield and related attributes. Am. J. Plant Sci. 2015, 06, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Nadgorska-Socha, A.; Ptasinski, B.; Kita, A. Heavy metal bioaccumulation and antioxidative responses in Cardaminopsis arenosa and Plantago lanceolata leaves from metalliferous and non-metalliferous sites: A field study. Ecotoxicology 2013, 22, 1422–1434. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Blazquez, N.; Garcia-Gomez, C.; Fernandez, M.D. Influence of Zn-contaminated soils in the antioxidative defence system of wheat (Triticum aestivum) and maize (Zea mays) at different exposure times: Potential use as biomarkers. Ecotoxicology 2015, 24, 279–291. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Jia, L.; Chen, H.; Wei, X. Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol. Environ. Saf. 2013, 89, 150–157. [Google Scholar] [CrossRef]
- El-Nasharty, A.; Rezk, A.; Abou El-Nour, E.; Nofal, O. Utilization efficiency of zinc by some wheat cultivars under stress condition of zinc deficiency. World Appl. Sci. J. 2013, 25, 1485–1489. [Google Scholar]
- White, P.; Broadley, M. Physiological limits to zinc biofortification of edible crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowack, B.; Schwyzer, I.; Schulin, R. Uptake of Zn and Fe by wheat (Triticum aestivum var. Greina) and transfer to the grains in the presence of chelating agents (ethylenediaminedisuccinic acid and ethylenediaminetetraacetic acid). J. Agric. Food Chem. 2008, 56, 4643–4649. [Google Scholar] [CrossRef] [PubMed]
- Weckx, J.J.; Clijsters, H.M. Zn phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol. Biochem. 1997, 35, 405–410. [Google Scholar]
- Panda, S.K.; Chaudhury, I.; Khan, M.H. Heavy metals induce lipid peroxidation and affect antioxidants in wheat leaves. Biol. Plant. 2003, 46, 289–294. [Google Scholar] [CrossRef]
- D’souza, R.M.; Devaraj, V. Induction of oxidative stress and antioxidative mechanisms in hyacinth bean under zinc stress. Afr. Crop Sci. J. 2012, 20, 17–19. [Google Scholar]
- Youssef, M.M.; Azooz, M.M. Biochemical studies on the effects of zinc and lead on oxidative stress, antioxidant enzymes and lipid peroxidation in okra (Hibiscus esculentus cv. Hassawi). Sci. Int. 2013, 1, 29–38. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Javed, M.T.; Ali, Q.; Haider, M.Z.; Irshad, S.; Rizwan, M.; et al. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the toxic effect of excessive copper concentrations on growth, gaseous exchange and chloroplast ultrastructure of Corchorus capsularis L. and improves copper accumulation capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Pilon-Smits, E.A.H.; Tarun, A.S.; Weber, S.U.; Jouanin, L.; Terry, N. Cadmium tolerance and accumulation in indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1999, 121, 1169–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, L.E.; Burkhead, J.L.; Hale, K.L.; Terry, N.; Pilon, M.; Pilon-Smits, E.A.H. Analysis of transgenic indian mustard plants for phytoremediation of metal-contaminated mine tailings. J. Environ. Qual. 2003, 32, 432–440. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Kömives, T.; Gullner, G.; Gyulai, G.; Kiss, J.; Heszky, L.; Radimszky, L.; Rennenberg, H. Ability of transgenic poplars with elevated glutathione content to tolerate zinc (2+) stress. Environ. Int. 2005, 31, 251–254. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Štolfa, I.; Špoljarić Maronić, D.; Žuna Pfeiffer, T.; Lončarić, Z. Glutathione and related enzymes in response to abiotic stress. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Germany, 2016; pp. 183–211. [Google Scholar]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [Green Version]
- Cuypers, A.; Vangronsveld, J.; Clijsters, H. The redox status of plant cells (AsA and GSH) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant Physiol. Biochem. 2001, 39, 657–664. [Google Scholar] [CrossRef]
- Di Baccio, D.; Kopriva, S.; Sebastiani, L.; Rennenberg, H. Does glutathione metabolism have a role in the defence of poplar against zinc excess? New Phytol. 2005, 167, 73–80. [Google Scholar] [CrossRef]
- Madhava Rao, K.V.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Anjum, N.A.; Umar, S.; Ahmad, A.; Iqbal, M. Responses of components of antioxidant system in moongbean genotypes to cadmium stress. Commun. Soil Sci. Plant Anal. 2008, 39, 2469–2483. [Google Scholar] [CrossRef]
- Ekmekçi, Y.; Tanyolaç, D.; Ayhan, B. A crop tolerating oxidative stress induced by excess lead: Maize. Acta Physiol. Plant 2009, 31, 319–330. [Google Scholar] [CrossRef]
- Yannarelli, G.G.; Fernández-Alvarez, A.J.; Santa-Cruz, D.M.; Tomaro, M.L. Glutathione reductase activity and isoforms in leaves and roots of wheat plants subjected to cadmium stress. Phytochemistry 2007, 68, 505–512. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycine betaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants 2010, 16, 259–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halušková, L.u.; Valentovičová, K.; Huttová, J.; Mistrík, I.; Tamás, L. Effect of abiotic stresses on glutathione peroxidase and glutathione S-transferase activity in barley root tips. Plant Physiol. Biochem. 2009, 47, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Ikehara, N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997, 115, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Takahama, U.; Oniki, T. Flavonoids and some other phenolics as substrates of peroxidase: Physiological significance of the redox reactions. J. Plant Res. 2000, 113, 301–309. [Google Scholar] [CrossRef]
- Rai, V.; Vajpayee, P.; Singh, S.N.; Mehrotra, S. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci. 2004, 167, 1159–1169. [Google Scholar] [CrossRef]
- Pandolfini, T.; Gabbrielli, R.; Comparini, C. Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum. Plant Cell Environ. 2006, 15, 719–725. [Google Scholar] [CrossRef]
- Sasaki, M.; Yamamoto, Y.; Matsumoto, H. Lignin deposition induced by aluminum in wheat (Triticum aestivum) roots. Physiol. Plant. 2006, 96, 193–198. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia obovata under Cd and Zn stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–214. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–53063. [Google Scholar]
- Trierweiler, J.F.; Lindsay, W.L. EDTA-ammonium carbonate soil test for zinc. Soil Sci. Soc. Am. J. 1969, 33, 49–54. [Google Scholar] [CrossRef]
- Akerboom, T.P.M.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Siegel, B.Z.; Galston, A.W. The isoperoxidases of Pisum Sativum. Plant Physiol. 1967, 42, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Foyer, C.H. Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 1978, 139, 9–17. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Simons, P.C.; Vander Jagt, D.L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal. Biochem. 1977, 82, 334–341. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]

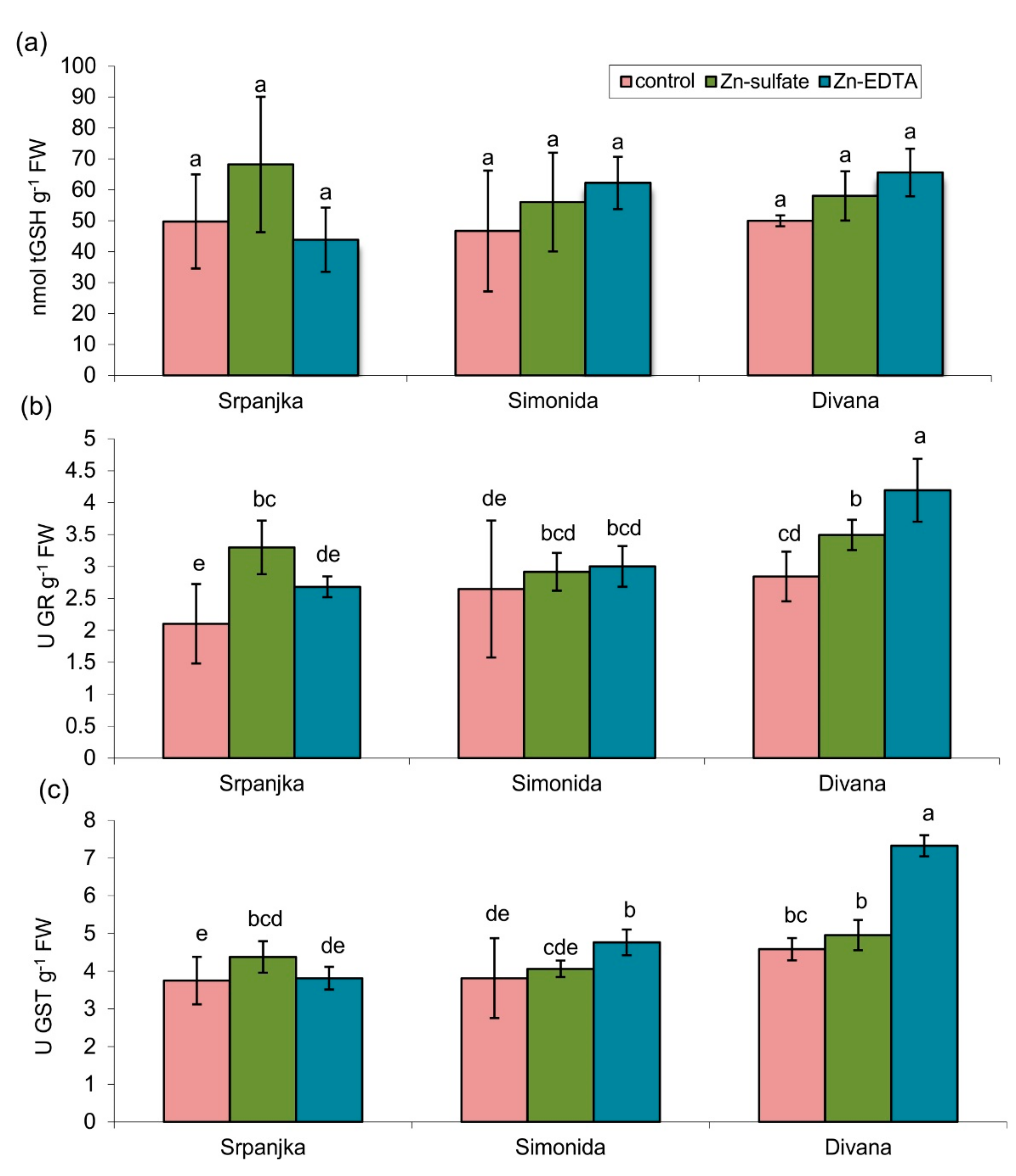
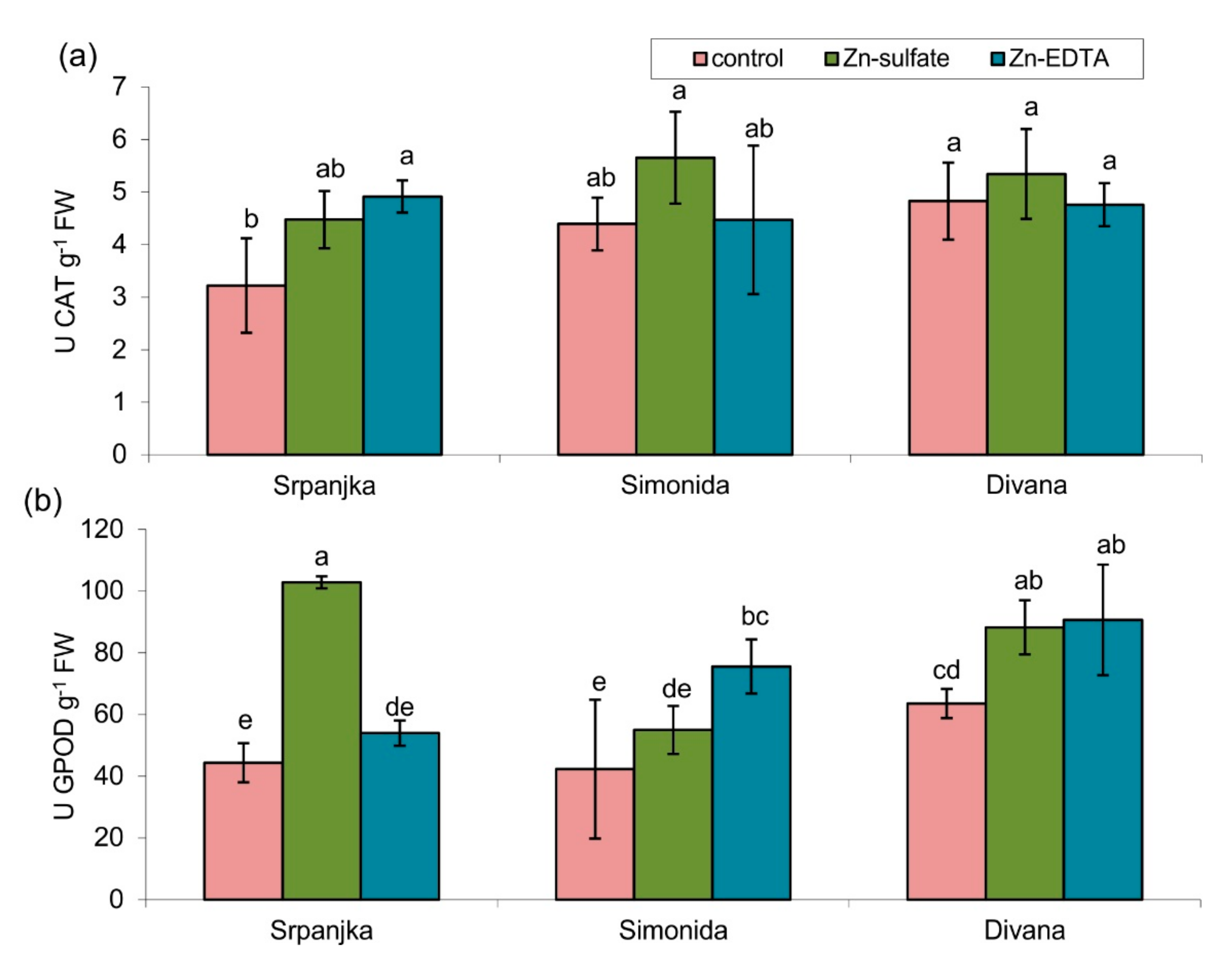

| Treatment | Srpanjka | Simonida | Divana |
|---|---|---|---|
| Control | 12.14 ± 0.35 b | 23.59 ± 10.12 b | 13.05 ± 0.81 b |
| Zn-sulfate | 48.25 ± 20.22 b | 23.35 ± 5.36 b | 24.29 ± 3.57 b |
| Zn-EDTA | 152.43 ± 19.07 a | 148.00 ± 34.09 a | 194.98 ± 53.25 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štolfa Čamagajevac, I.; Vuković, R.; Vuković, K.; Vuković, A.; Ivezić, V.; Žuna Pfeiffer, T.; Krstin, L.; Lončarić, Z. Wheat Leaf Antioxidative Status—Variety-Specific Mechanisms of Zinc Tolerance during Biofortification. Plants 2021, 10, 2223. https://doi.org/10.3390/plants10102223
Štolfa Čamagajevac I, Vuković R, Vuković K, Vuković A, Ivezić V, Žuna Pfeiffer T, Krstin L, Lončarić Z. Wheat Leaf Antioxidative Status—Variety-Specific Mechanisms of Zinc Tolerance during Biofortification. Plants. 2021; 10(10):2223. https://doi.org/10.3390/plants10102223
Chicago/Turabian StyleŠtolfa Čamagajevac, Ivna, Rosemary Vuković, Kristina Vuković, Ana Vuković, Vladimir Ivezić, Tanja Žuna Pfeiffer, Ljiljana Krstin, and Zdenko Lončarić. 2021. "Wheat Leaf Antioxidative Status—Variety-Specific Mechanisms of Zinc Tolerance during Biofortification" Plants 10, no. 10: 2223. https://doi.org/10.3390/plants10102223
APA StyleŠtolfa Čamagajevac, I., Vuković, R., Vuković, K., Vuković, A., Ivezić, V., Žuna Pfeiffer, T., Krstin, L., & Lončarić, Z. (2021). Wheat Leaf Antioxidative Status—Variety-Specific Mechanisms of Zinc Tolerance during Biofortification. Plants, 10(10), 2223. https://doi.org/10.3390/plants10102223








